Clinical Features of Esophageal Eosinophilia According to Endoscopic Phenotypes (original) (raw)
Abstract
Objective
Esophageal eosinophilia (EE), a histological hallmark of eosinophilic esophagitis, is classified into two endoscopic phenotypes: localized and diffuse EE. Our aim was to determine the prevalence of EE localized in the lower esophagus and to describe its clinical features in comparison with diffuse EE.
Methods
Data from 81 consecutive patients with EE were retrospectively investigated. EE was histologically defined as ≥15 eosinophils per high-power field. Based on the endoscopic appearance with a histological assessment, EE was classified as either diffuse or localized type. We compared the clinical features, including the medical treatment and natural course, between the two types.
Results
Of the 81 patients, 52 (64.2%) had diffuse EE, and 29 (35.8%) had localized EE. Among men patients, localized EE was significantly more common than diffuse EE. In localized EE, dysphagia and food impaction were less prevalent, and the presence of rings was significantly less common than in diffuse EE. Acid-suppressive therapy was administered to only 3 of the 29 patients with localized EE. In asymptomatic patients, especially those with localized EE, endoscopic abnormalities did not worsen but rather improved in some findings, such as with regard to furrows or exudate, during the natural course of three years without medical treatment.
Conclusion
Localized EE has a strong predilection for men patients and accounted for more than one third of all cases of EE. This condition appears to be less symptomatic and necessitates milder medical treatment than diffuse EE and might not worsen progressively.
Keywords: eosinophilic esophagitis, esophageal eosinophilia, endoscopic phenotype, localized esophageal eosinophilia, diffuse esophageal eosinophilia
Introduction
Eosinophilic esophagitis (EoE) is a Th2 cell-immune-mediated inflammatory disease characterized by symptoms of esophageal dysfunction and prominent esophageal eosinophilia (EE) (1). Since 1990, EoE has emerged as a major cause of dysphagia and food impaction, especially among Western populations (2,3). A large number of studies have elucidated the epidemiological features, diagnosis, molecular mechanisms, medical treatments, and prognosis of EoE to uncover the pathogenesis of this emerging disease; however, many aspects remain unclear (4). Furthermore, EoE is of considerable concern as an emerging non-acid related esophagitis in Asia, including Japan, as evidenced by a recent increase in reported cases (5-9).
Several endoscopic findings, including exudate, rings, edema, furrows, and strictures, have been reported to be characteristics of EoE (10). A meta-analysis by Kim et al. showed that at least one of these abnormalities was endoscopically detected in most patients when restricted to prospective studies (11). In a previous report on 33 patients with EE, our research group was the first to identify a subgroup of patients with EE in whom EoE-related endoscopy revealed that intense eosinophilic inflammation was localized in a small area of the lower esophagus (12). This group also found that the localized type of EE was associated with significantly fewer esophageal symptoms than was the diffuse type of EE, but that study consisted of nonconsecutively enrolled patients and included only 10 patients with localized EE.
Recently, Sawada et al. reported high rates of responsiveness to therapy with proton pump inhibitors (PPIs) in symptomatic patients with localized EE (13). However, little information about the proportion of cases of localized EE among all cases of EE is available, and its clinical features, including the practical medical treatment and natural course, compared with those of diffuse EE are unclear.
Given the above, we compared the clinical features of localized EE with those of diffuse EE in a cohort of consecutive patients with a diagnosis of EE.
Materials and Methods
This retrospective study included 81 consecutive patients who received a diagnosis of EE at 1 of 3 study hospitals in Yamagata and Miyagi Prefectures, Japan (25 at Yamagata University Hospital, 33 at JR Sendai Hospital, and 23 at Shinoda General Hospital) between July 2011 and July 2018.
The EE diagnosis was based on findings of an endoscopic examination performed for various upper gastrointestinal symptoms or periodic checkups for gastrointestinal malignancy. EE was histologically defined as the presence of ≥15 eosinophils per high-power field (HPF). The endoscopic phenotype was defined according to the endoscopic appearance, with supporting findings of the histological assessment, in reference to previous report as follows (12); The diffuse type was defined as a widespread area of EE involving one or more of three locations: upper, middle, and lower esophagus (Fig. 1). The localized type was defined as a small area of EE localized within 1 to 2 cm of the lower esophagus (Fig. 1). At least one biopsy sample was obtained from normal-appearing mucosa above the affected area in each patient, and insignificant eosinophilic accumulation (≤5 eosinophils/HPF) was confirmed histologically.
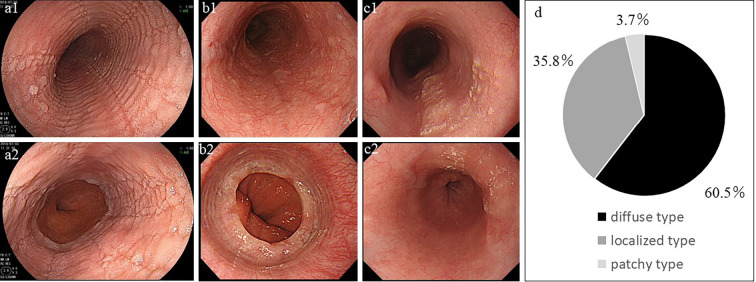
Figure 1.
The representative endoscopic images of diffuse EE (a1, a2), localized EE (b1, b2), and patchy-type EE (c1, c2); The proximal esophagus (a1, b1, c1) and distal esophagus (a2, b2, c2) are shown. In patients with diffuse EE (a1, a2), furrows, rings, and exudate were found in almost all areas of the esophagus. In patients with localized EE (b1, b2), furrows, rings, and exudate were observed only in the lower end of the esophagus. In patients with patchy-type EE (c1, c2), edematous mucosa was interspersed with exudate, furrows, or both, with an appearance resembling skip lesions in the esophagus and intervening normal-appearing mucosa. (d) The proportions of these three endoscopic phenotypes. EE: esophageal eosinophilia
Focal EE that was far away from the lower end of the esophagus was included in the “localized type” in our previous report (12), but in the present study, it was defined as patchy type and incorporated into the diffuse type in order to focus on the localized type at the lower end of the esophagus (Fig. 1).
The five endoscopic findings of EoE - rings, furrows exudate, edema (decreased vascularity), and stricture - were assessed qualitatively (for their absence or presence). In addition, they were originally and semiquantitatively scored as follows in reference to a previous report (10): For rings, furrows, and exudate, 0 indicated absent, 1 indicated mild, and 2 indicated severe; and for edema and stricture, 0 indicated absent, and 1 indicated present. Each endoscopic score and the sum of all scores (total endoscopic score; range, 0 to 8) were compared between the two groups. We also evaluated patients for the presence of hiatal hernia, defined as the presence of a gaping esophageal hiatus at a minimum width of the shaft of the peroral endoscope in the retroflexed position (14,15).
Endoscopic examinations at the three hospitals were performed by endoscopists familiar with EoE and certified by the Japanese Gastrointestinal Endoscopy Society (Y.A., T.K., and Y.S. in Yamagata University Hospital; Y.A. in Shinoda General Hospital; and R.K., S.U., and G.K. in JR Sendai Hospital). Furthermore, all endoscopic images were ultimately subjected to a detailed review and assessed by two endoscopists (K.T. and Y.A). When they disagreed on the assessment, agreement was obtained through discussion.
Medical treatments administered to patients with symptomatic EE, including PPIs, potassium-competitive acid blockers, and topical corticosteroids, were reviewed by assessing medical charts. Symptoms, abnormalities, and eosinophilic inflammation after the medical treatment documented in the records, including the endoscopy reports and pathological reports, were also reviewed. The disappearance of symptoms and eosinophilic infiltration, with ≤5 eosinophils/HPF after the standard dose of PPIs for at least eight weeks (or potassium-competitive acid blocker for at least four weeks), according to PPI treatment for erosive esophagitis, were assessed as clinical and histological remission, respectively (16,17).
This study was approved by the ethics committees of the participating hospitals and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before participation in the study.
Statistical analyses
Data are expressed as medians with ranges or as numbers with percentages. For comparisons between patients with diffuse and localized EE, continuous variables were analyzed with Wilcoxon's rank sum test or Wilcoxon's signed rank test, and categorical variables were analyzed with Fisher's exact probability tests. All statistical analyses were performed with the JMPⓇ software program, version 14.1.0 (SAS Institute, Cary, USA). A p value of <0.05 was considered statistically significant.
Results
Of the 81 patients with EE (median age, 49; range, 18 to 77 years), 69 were men, and 12 were women; 49 patients (60.5%) were classified as having diffuse EE, and 29 (35.8%) were classified as having localized EE. The remaining 3 patients (3.7%) exhibited focal edematous mucosa with exudate, furrows, or both, which were observed as skip lesions, away from the lower end of the esophagus. We designated these three patients as patchy type, which was promptly included as diffuse EE in the subsequent analyses (Fig. 1).
Table 1 summarizes the characteristics of the 81 patients. All but 1 patient with localized EE were men, which was significantly different from the gender ratio among patients with diffuse EE (p=0.0476). The frequencies of diagnostic opportunities (gastrointestinal screening, including periodic checkups or endoscopic examinations for any gastrointestinal symptoms), upper gastrointestinal symptoms, and PPI use at the time of the first diagnosis were not significantly different between the two groups. Bronchial asthma was significantly more common in the diffuse type (13.5%) than in the localized type (0%; p=0.046). Rings were significantly more prevalent among patients with diffuse EE than among those with localized EE (p=0.0004), whereas the remaining endoscopic findings were present to comparable degrees in both groups. The endoscopic score for rings and the total endoscopic score were significantly higher for patients with diffuse EE than for those with localized EE (for rings, p=0.0001; for total endoscopic score, p=0.018). The prevalence of hiatal hernia, which was similar in the two groups, was evaluated only in patients who underwent endoscopy with a regular transoral endoscope. The histological grade of EE did not differ markedly between the two groups.
Table 1.
Baseline Characteristics of Patients with Diffuse- and Localized-type Esophageal Eosinophilia.
| Diffuse EE | Localized EE | p value | |
|---|---|---|---|
| n | 52 | 29 | - |
| median age (range, years) | 48.5 (18-74) | 49 (33-77) | ns |
| gender | |||
| men (%) | 41 (78.8) | 28 (96.6)* | 0.0476* |
| allergic diseases (%) | |||
| bronchial asthma | 7 (13.5) | 0 | 0.0460* |
| allergic rhinitis | 20 (57.1) | 11 (37.9) | ns |
| atopic dermatitis | 3 (5.7) | 2 (6.9) | ns |
| any | 26 (50.0) | 13 (44.8) | ns |
| diagnostic opportunities | |||
| GI screening (%) | 43 (82.7) | 23 (79.3) | ns |
| GI symptoms (%) | 3 (5.8) | 4 (13.8) | ns |
| others (%) | 6 (11.5) | 2 (6.9) | ns |
| any upper GI symptoms | 19 (36.5) | 7(24.1) | ns |
| dysphagia (%) | 11 (21.2) | 2 (6.9) | ns |
| food impaction (%) | 6 (11.5) | 0 (0) | ns |
| heartburn (%) | 16 (30.7) | 7 (24.1) | ns |
| others (%) | 4 (7.7) | 1 (3.4) | ns |
| PPI use at the first diagnosis(%) | 2 (3.8) | 1 (3.4) | ns |
| History of esophageal dilatation | 0 | 0 | ns |
| prevalence of endoscopic findings | |||
| rings (%) | 36 (69.2) | 8 (27.6)* | 0.0004* |
| furrows (%) | 42 (80.7) | 27 (93.1) | ns |
| exudate (%) | 40 (76.9) | 23 (79.3) | ns |
| edema (decreased vascularity) (%) | 52 (100) | 29 (100) | ns |
| stricture (%) | 1 (1.9) | 0 (0) | ns |
| endoscopic score (median, range) | |||
| rings | 1 (0-2) | 0 (0-1) | 0.0001** |
| furrows | 2 (0-2) | 2 (0-2) | ns |
| exudate | 1 (0-1) | 1 (0-1) | ns |
| edema(decreased vascularity) | 1 (1-1) | 1 (1-1) | ns |
| stricture | 0 (0-1) | 0 (0-0) | ns |
| total | 4 (1-6) | 3 (2-5) | 0.018** |
| erosive esophagitis(%) | 6 (11.5) | 1 (3.4) | ns |
| hiatal hernia | 7/42*** (16.7) | 4/22*** (18.2) | ns |
| number of esophageal eosinophilia(/HPF) (median, range) | |||
| abnormal-appearing area | 62 (17-258) | 46 (16-162) | ns |
| normal-appearing mucosa | - | 0 (0-12) | - |
Next, we reviewed the medical treatment documented on medical charts. As first-line therapy, PPIs were administered orally for at least 8 weeks to 17 of 52 patients with diffuse EE but to only 2 of 29 patients with localized EE. In addition, two patients (one in the diffuse group and one in the localized group) took the standard dose of potassium-competitive acid blocker for four weeks because of concomitant peptic ulcer or severe heartburn based on the clinical judgment of the attending physician. In total, acid-suppressive therapy was performed in 18 of 52 diffuse patients (34.6%) and 3 of 29 localized EE (10.3%).
With acid-suppressive therapy, 11 of the 18 patients with diffuse EE achieved clinical remission, and 6 of those 11 patients were judged to have achieved clinical and histological remission. All three patients with localized EE achieved clinical remission, and two of the three achieved histological remission.
The endoscopic and histological findings before and after acid-suppressive therapy according to the two endoscopic phenotypes are shown in Table 2. The prevalence of furrows and exudate decreased significantly after acid-suppressive therapy in the diffuse type (p=0.0001, p=0.0377, respectively). The furrow score, total endoscopic score, and number of histological esophageal eosinophilia in the diffuse type were also decreased significantly after the therapy (p=0.0005, p=0.0012, p=0.002, respectively). In contrast, the endoscopic and histological findings tended to improve in the localized type after the therapy, although not to a significant degree. Topical steroid therapy with a liquid budesonide formulation was applied to none of the patients with localized EE and to five patients with diffuse EE who were unresponsive to PPI therapy. All 5 of those patients achieved clinical and histological remission within 8-12 weeks after the therapy.
Table 2.
Endoscopic and Histological Findings before and after Acid-suppressive Therapy according to Two Endoscopic Phenotypes.
| Diffuse EE | Localized EE | |||||
|---|---|---|---|---|---|---|
| before PPI | after PPI | p value | before PPI | after PPI | p value | |
| n | 18 | 15* | - | 3 | 3 | - |
| treatment period of PPI (weeks) | 8 (4-13)** | 8 (4-8)*** | ||||
| symptom improvement after PPI | 11 (61.1) | 2 (66.7) | ||||
| prevalence of endoscopic findings | ||||||
| rings (%) | 13 (72.2) | 9 (60.0) | ns | 1 (33.3) | 0 | ns |
| furrows (%) | 16 (88.9) | 3 (20.0) | 0.0001**** | 3 (100) | 0 | ns |
| exudate (%) | 14 (77.8) | 6 (40.0) | 0.0377**** | 3 (100) | 1 | ns |
| edema (decreased vascularity) (%) | 18(100) | 13 (86.7) | ns | 3 (100) | 1 (33.3) | ns |
| stricture (%) | 1 (5.6) | 1 (6.7) | ns | 0 | 0 | ns |
| endoscopic score (median, range) | ||||||
| rings | 1 (0-2) | 1 (0-2) | ns | 1 (0-1) | 0 (0-0) | ns |
| furrows | 2 (0-2) | 0 (0-2) | 0.0005***** | 2 (2-2) | 0 (0-0) | ns |
| exudate | 1 (0-1) | 0 (0-1) | ns | 1 (1-1) | 0 (0-1) | ns |
| edema (decreased vascularity) | 1 (1-1) | 1 (0-1) | ns | 1 (1-1) | 0 (0-1) | ns |
| stricture | 0 (0-1) | 0 (0-1) | ns | 0 (0-0) | 0 (0-0) | ns |
| total | 5 (2-6) | 2 (0-6) | 0.0012***** | 4 (4-5) | 0 (0-2) | ns |
| number of esophageal eosinophilia(/HPF) (median, range) | 70 (17-258) | 34 (0-297) | 0.002***** | 54 (46-66) | 0 (0-26) | ns |
| <15 eosinophils/HPF after PPI therapy | - | 6 (40.0) | - | - | 2 (66.7) | - |
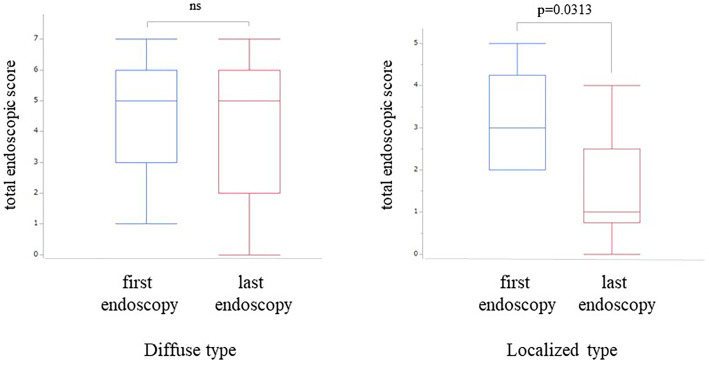
Next, we compared the patients according to the presence or absence of esophageal symptoms. There were no significant differences in the age, gender, allergic diseases, or endoscopic and histological findings between the symptomatic and asymptomatic patients (Table 3). To further evaluate the pathogenesis of the asymptomatic patients, we reviewed the endoscopic findings and rates of EE among asymptomatic patients who had long-term follow-up. Thirty-seven of 52 (71%) patients with diffuse EE and 16 of 29 (55%) patients with localized EE underwent follow-up esophagogastroduodenoscopy (EGD) after the initial diagnosis of EE. Since very few patients had a follow-up of more than five years, we considered a follow-up of more than three years as “long-term follow-up” in this study and analyzed the natural course of asymptomatic patients. When we compared the first and last endoscopy findings, none of the asymptomatic EE patients had progressed to symptomatic EE, at least in this study period. In 5 of 14 patients with the diffuse type and 1 of 7 patients with the localized type, PPIs were administered at the last follow-up endoscopy to maintain endoscopic and histological remission in the absence of esophageal symptoms. In the diffuse type, endoscopic exudate scores were significantly decreased at the last follow-up endoscopy compared to the first session (p=0.0313). In the localized type, the prevalence of furrows and total endoscopic scores were significantly decreased at the last follow-up endoscopy compared to the first session (p=0.0291, p=0.0156, respectively; Table 4). After excluding from both groups a total of 6 PPI users at the last follow-up endoscopy, the decreased total endoscopic score at the last endoscopy in the localized group remained significant (p=0.0313), although any significance was lost in the diffuse group (Fig. 2). The degree of histological esophageal eosinophilia tended to be lower at the last follow-up endoscopy in the localized type than in the diffuse type, but no statistical significance was found, regardless of the exclusion of PPI users.
Table 3.
Baseline Characteristics of Patients with Asymptomatic and Symptomatic EE.
| asymptomatic EE | symptomatic EE | p value | |
|---|---|---|---|
| n | 54 | 27 | - |
| median age (range, years) | 49 (33-74) | 48 (18-77) | ns |
| men (%) | 49 (90.7) | 20 (74.0) | ns |
| allergic diseases (%) | |||
| bronchial asthma | 3 (5.6) | 4 (14.8) | ns |
| allergic rhinitis | 20 (37.0) | 11 (40.7) | ns |
| atopic dermatitis, | 3 (5.6) | 2 (7.4) | ns |
| any | 27 (50.0) | 12(44.4) | ns |
| diagnostic opportunities | |||
| EGD for health checkup (%) | 47( 87.0) | 19 (70.4) | ns |
| EGD for GI symptoms (%) | 1 (1.9)* | 6 (22.2) | 0.0049** |
| others (%) | 6 (11.1) | 2 (7.4) | ns |
| upper GI symptoms | - | ||
| dysphagia (%) | - | 12 (22.2) | - |
| food Impaction (%) | - | 5 (9.3) | - |
| heartburn (%) | - | 20 (37.0) | - |
| others (%) | 1 (1.9)* | 3 (11.1) | - |
| PPI use at the first diagnosis (%) | 1 (1.9) | 1 (3.7) | ns |
| past history of esophageal dilatation | 0 | 0 | ns |
| endoscopic phenotypes | |||
| diffuse (%) | 29 (53.7) | 20 (74.1) | ns |
| localized (%) | 22 (40.7) | 7 (25.9) | ns |
| patchy (%) | 3 (5.6) | 0 | ns |
| prevalence of endoscopic findings | |||
| rings (%) | 28 (51.6) | 16 (59.3) | ns |
| furrows (%) | 45 (83.3) | 24 (88.8) | ns |
| exudate (%) | 43 (79.6) | 20 (74.1) | ns |
| edema (decreased vascularity) (%) | 54 (100) | 27 (100) | ns |
| stricture (%) | 0 (0) | 1 (3.7) | ns |
| endoscopic score (median, range) | |||
| rings | 1 (0-2) | 1 (0-2) | ns |
| furrows | 2 (0-2) | 2 (0-2) | ns |
| exudate | 1 (0-2) | 1 (0-1) | ns |
| edema (decreased vascularity) | 1 (1-1) | 1 (1-1) | ns |
| stricture | 0 (0-0) | 0 (0-1) | ns |
| total | 4 (1-7) | 4 (2-6) | ns |
| erosive esophagitis (%) | 6 (11.1) | 1 (1.2) | ns |
| hiatal hernia | 6/43***(14.0) | 5/21***(23.8) | ns |
| number of esophageal eosinophilia(/HPF) (median, range) | 57 (18-209) | 46 (16-258) | ns |
Table 4.
Endoscopic and Histological Findings at the First and Last Endoscopy Session in Asymptomatic EE with Long-term Follow up of More than Three Years.
| Diffuse EE | Localized EE | |||||
|---|---|---|---|---|---|---|
| first EGD | last EGD | p value | first EGD | last EGD | p value | |
| n | 14 | 14 | - | 7 | 7 | - |
| mean age at the first diagnosis (range, years) | 52 (37-74) | - | - | 52 (44-74) | - | - |
| mean number of follow-up endoscopy | 5 (3-7) | 4 (3-6) | ||||
| median follow up period (range, months) | 61 (36-82) | 49 (39-72) | ||||
| progression to symptomatic EE | - | 0 | - | - | 0 | - |
| PPI users (%) | 1 (7.1) | 5(35.7) | ns | 0 | 1 (14.3) | ns |
| prevalence of endoscopic findings | ||||||
| rings (%) | 8 (57.1) | 8 (57.1) | ns | 2 (28.6) | 1 (14.3) | ns |
| furrows (%) | 10 (71.4) | 7 (50.0) | ns | 6 (85.7) | 1 (14.3) | 0.0291* |
| exudate (%) | 12 (85.6) | 8 (57.1) | ns | 3 (42.9) | 1 (14.3) | ns |
| edema (decreased vascularity) (%) | 14 (100) | 12 (85.7) | ns | 7 (100) | 6 (85.7) | ns |
| stricture (%) | 0 (100) | 0 (100) | ns | 0 | 0 | ns |
| endoscopic score (median, range) | ||||||
| rings | 1 (0-2) | 1 (0-2) | ns | 0 (0-1) | 0 (0-1) | ns |
| furrows | 1.5 (0-2) | 0.5 (0.2) | ns | 2 (0-2) | 0 (0-2) | ns |
| exudate | 1 (0-2) | 1 (0-1) | 0.0313** | 0 (0-1) | 0 (0-1) | ns |
| edema (decreased vascularity) | 1 (1-1) | 1 (0-1) | ns | 1 (1-1) | 0 (0-1) | ns |
| stricture | 0 (0-0) | 0 (0-0) | ns | 0 (0-0) | 0 (0-0) | ns |
| total score | 4 (1-7) | 3 (0-7) | ns | 3 (2-5) | 1 (0-4) | 0.0156** |
| number of esophageal eosinophilia (/HPF)(median, range) | 57.5 (18-118) | 76 (0-110)*** | ns | 54 (33-162) | 10 (0-46)*** | ns |
Figure 2.
Chronological changes in the total endoscopic score between the first and last endoscopy session in asymptomatic patients who had a long-term follow-up of more than three years. After excluding from both groups a total of 6 PPI users at the last follow-up endoscopy session, the decreased total endoscopic score of the last endoscopy remained significant in the localized group but not in the diffuse group (p=0.0313, Wilcoxon’s signed rank test). PPI: proton pump inhibitor
Discussion
In this study of consecutively recruited patients with EE at three hospitals in Japan, we found that one-third of the patients exhibited localized involvement of the lower esophagus. To our knowledge, this is a novel finding. Since asymptomatic EE patients in this studied population were more common, irrespective of endoscopic phenotypes, than symptomatic EE patients, we investigated their clinical features, including the medical treatment and long-term prognosis, according to the presence or absence of esophageal symptoms. We did not detect any pathophysiological differences, including in endoscopic phenotypes, between symptomatic and asymptomatic EE patients; however, we noted that asymptomatic EE patients were unlikely to progress to typical EE, at least during the median follow-up of four or five years in this study.
Furthermore, we found that localized EE may spontaneously remit during its natural course. To our knowledge, this was another novel finding of this study. Patients with EoE diagnosed in Western countries, most of whom are symptomatic and go undiagnosed for a long period of time, experience the complication of esophageal stricture as a consequence of chronic eosinophilic inflammation unless they have undergone appropriate treatment (18-20). Since asymptomatic patients with EE are commonly encountered during screening endoscopy for health checkups in Japan (9), our finding that their conditions do not seem to worsen during the natural course of several years may be useful information in the management of EE in the clinical setting.
Asymptomatic EE is not diagnosed as EoE according to the current diagnostic criteria for EoE (1,4). In Japan, upper gastrointestinal screening by endoscopy has been widely performed in public health care checkup programs and general clinical practice for the early detection of upper gastrointestinal malignancy; it enables the diagnosis of EE in patients with minimal or mild symptoms (9,21). This is concordant with our findings, which revealed that in 80% of the patients with EE, regardless of the endoscopic phenotype, the disease was diagnosed through gastrointestinal screening. Because of the difficulty in adequately assessing subjective symptoms in EoE, it may be expedient in Japanese clinical practice for EE without esophageal symptoms to instead be managed as “asymptomatic EoE” to further clarify the pathogenesis of EoE (22). However, a systematic review of EoE in Asian populations revealed that dysphagia was a major symptom experienced by 40% of affected patients and that food impaction was experienced by only 4% of the patients (23). In our present study, dysphasia was present in 11 (21.2%) patients with diffuse EE and 2 (6.9%) patients with localized EE. Food impaction and esophageal stricture were also found in only 6 (11.5%) and 1 (1.9%) of the 52 patients with diffuse EE, respectively. None of the patients had undergone esophageal dilatation. The similarities in the pathogenic mechanism underlying EoE in Japan and Western countries were shown by a transcriptomic study (24). The cause of milder disease severity observed in Japanese patients than in Western patients, while unclear, might involve environmental factors, such as dietary habits, and early life factors (25).
Given the spatial extent of eosinophilic inflammation in the esophageal lumen, it is logical for dysphagia or food impaction to be milder in the localized type than in the diffuse type. In our present study as well in our previous report, dysphagia and food impaction tended to be less common in patients with localized EE than in those with diffuse EE, but this finding was not statistically significant. Sawada et al. reported that asymptomatic patients were more common in the localized group (42%) than in the diffuse group (7%), with statistical significance (13). Because of the relatively large number of asymptomatic patients included in our diffuse EE group, statistical significance might not have been detected. The significantly lower prevalence of rings and total endoscopic score in localized EE than in diffuse EE may also be due to less spatial extent of eosinophilic inflammation. Ishimura et al. reported that the endoscopic and histological findings were not markedly different between asymptomatic and symptomatic EE, but the localization of endoscopic abnormalities was not described (26). Consequently, we also noted no marked difference in the endoscopic features, except for the special extent of eosinophilic inflammation in the esophageal lumen and the magnitude of esophageal eosinophilia between symptomatic and asymptomatic EE patients.
There have been few studies reporting on the medication for localized EoE. Sawada et al. showed that the rate of response to PPI therapy was significantly higher in patients with localized EE (100%) than in those with diffuse EE (63%), suggesting that localized EE has a stronger acid-related cause than diffuse EE (13). Although the available information on medical treatment was largely limited in the present study because of the retrospective nature of the medical chart review, PPI therapy was necessary in only 10% of the patients with localized EE.
In our study, it was difficult to assess the difference in PPI responsiveness between diffuse and localized EE because of the small number of treated patients; however, two-thirds of patients in each group experienced improvement in symptoms after PPI therapy. Acid reflux is commonly observed in patients with typical EoE (27,28). The acid reflux profile of patients with localized EE, however, remains to be investigated. A previous study demonstrated abnormal acid exposure immediately above the esophagogastric junction as a common finding in patients who had no manifestations of gastroesophageal reflux disease (29). Thus, minor gastric acid exposure within the physiological range might also be associated with the pathogenesis of localized EE.
Although EoE is known to be common in young to middle-aged men, not only in white populations but also in Japanese populations (1,25), we found a marked male predominance in localized EE compared with diffuse EE in this study. This trend was also shown by another report (13). Erosive esophagitis, especially mild type, such as grade A or B according to the Los Angeles classification, is significantly more prevalent in young to middle-aged men than in women in Japan (30,31). Mild acidic damage of the lower esophagus may trigger localized EE and has been proposed as an underlying mechanism in typical EoE (32). As shown in Table 1, concomitant erosive esophagitis was found only in one with grade A of 29 patients with localized EE in this study. However, when certain causal antigens come into contact with reflux-damaged mucosa of the lower esophagus, marked eosinophilic inflammation with endoscopic abnormalities can occur locally; consequently, endoscopic manifestation of erosive esophagitis may be masked by the characteristic findings of EoE, such as furrows, rings, exudate, or edema.
Several limitations associated with the present study warrant mention. First, it was retrospective, so the endoscopic diagnosis of localized or diffuse EE might have been uncertain. Indeed, inter-observer reliability in the endoscopic diagnosis of EoE has been reported to be insufficient (33,34). In this study, endoscopic examinations in each hospital were principally performed only by board-certified endoscopists who were familiar with EoE. In addition, as mentioned in the methods, all endoscopic images were reviewed in detail by two endoscopists with a consensus achieved through discussion. Second, most of the patients with localized EE underwent only one biopsy in which samples were taken from normal-appearing mucosa for the histological confirmation of nonsignificant eosinophilic infiltration. The number of biopsies might have been insufficient for the judgement of localized EE because the distribution of infiltrating eosinophils in EoE is reported to be considerably heterogeneous (35). However, this issue would not be resolved completely even if multiple biopsies was performed. Although other histological findings, such as eosinophilic microabscess basal cell hyperplasia or subepithelial fibrosis, may also differ between the localized and diffuse types, we were unable to explore those issue. Third, the retrospective assessment of the symptoms was based on the medical charts; therefore, the indications for medical treatment were not uniformly determined, and certain conclusions on the treatment outcome cannot be drawn based on the results of this study. However, at least according to our review of medical charts, aggressive medical treatment seems to be unnecessary for localized EE in clinical practice, as the localized inflammation induces fewer symptoms (13). Finally, our findings that significant symptoms and endoscopic progression were less likely to occur in asymptomatic EE patients for at least several years were based on a retrospective observation of a small number of patients. This may imply transient eosinophilic inflammation in localized EE. A large prospective study is necessary to determine whether localized or asymptomatic EE patients progress to typical EoE.
In summary, we found that one-third of the patients with EE displayed localized involvement of the lower esophagus; patients with localized EE were predominantly men, and localized EE necessitated less active medical treatment than did diffuse EE. Localized EE might not worsen progressively, at least during the short-term period of a few years.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Liacouras CA, Furta GT, Hirano I, et al. . Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 128: 3-20, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Prasad GA, Alexander JA, Schleck CD, et al. . Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 7: 1055-1061, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rhijn BD, Verheij J, Smout AJPM, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil 25: 47-53, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Lucendo AJ, Molina-Infante J, Arias Á, et al. . Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J 5: 335-358, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta K, Adachi K, Kowari K, et al. . A Japanese case of eosinophilic esophagitis. J Gastroenterol 41: 706-710, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Abe Y, Iijima K, Ohara S, et al. . A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol 46: 25-30, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara Y, Sugawa T, Tanaka F, et al. . A multicenter study on the prevalence of eosinophilic esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern Med 51: 3235-3239, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Shimura S, Ishimura N, Tanimura T, et al. . Reliability of symptoms and endoscopic findings for diagnosis of esophageal eosinophilia in a Japanese population. Digestion 90: 49-57, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Suitable biopsy site for detection of esophageal eosinophilia in eosinophilic esophagitis suspected cases. Dig Endosc 28: 139-144, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 62: 489-495, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of Endoscopic Features of Eosinophilic Esophagitis: A Meta-analysis. Clin Gastroenterol Hepatol 10: 988-996, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe Y, Iijima K, Ohara S, et al. . Localized esophageal eosinophilia: Is it an early manifestation of eosinophilic esophagitis or a subtype of gastroesophageal reflux disease?. Dig Endosc 26: 337-343, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Sawada A, Hashimoto A, Uemura R, et al. . Association between endoscopic findings of eosinophilic esophagitis and responsiveness to proton pump inhibitors. Endosc Int Open 7: E433-E439, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusano M, Kouzu T, Kawano T, Ohara S. The prevalence of hiatus hernia in the Japanese. Gastroenterol Endosc 47: 962-973, 2005. [Google Scholar]
- 15.Hill LD, Kozarek RA, Kraemer SJ, et al. . The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 44: 541-547, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Molina-Infante J, Bredenoord AJ, Cheng E, et al. . Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 65: 524-531, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashida K, Sakurai Y, Hori T, et al. . Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 43: 240-251, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singla MB, Chehade M, Brizuela D, et al. . Early comparison of inflammatory vs. fibrostenotic phenotype in eosinophilic esophagitis in a multicenter longitudinal study. Clin Transl Gastroenterol 6: e132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 50: 134-140, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Koutlas NT, Dellon ES. Progression from an inflammatory to a fibrostenotic phenotype in eosinophilic esophagitis. Case Rep Gastroenterol 11: 382-388, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato H, Honma T, Nozawa Y, et al. . Eosinophilic esophagitis in Japanese patients: a mild and slow-progressing disorder. PLoS One 13: e0206621, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Matary W. Natural history of eosinophilic esophagitis in asymptomatic patients. Gastroenterology 146: 1426, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita Y. Systematic review: eosinophilic esophagitis in Asian countries. World J Gastroenterol 21: 8433-8440, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoda T, Morita H, Nomura I, et al. . Comparison of gene expression profiles in eosinophilic esophagitis (EoE) between Japan and Western countries. Allergol Int 64: 260-265, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Ishimura N, Shimura S, Jiao D, et al. . Clinical features of eosinophilic esophagitis: differences between Asian and Western populations. J Gastroenterol Hepatol 30: 71-77, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Ishimura N, Sumi S, Okada M, et al. . Is asymptomatic esophageal eosinophilia the same disease entity as eosinophilic esophagitis? Clin Gastroenterol Hepatol 17: 1405-1407, 2019. [DOI] [PubMed] [Google Scholar]
- 27.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 63: 3-12, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. . Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 9: 110-117, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher J, Wirz A, Henry E, McColl KEL. Studies of acid exposure immediately above the gastro-oesophageal squamocolumnar junction: evidence of short segment reflux. Gut 53: 168-173, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa N, Iwakiri R, Koyama T, et al. . Proportion of reflux esophagitis in 6010 Japanese adults: prospective evaluation by endoscopy. J Gastroenterol 34: 441-444, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Shimazu T, Matsui T, Furukawa K, et al. . A prospective study of the prevalence of gastroesophageal reflux disease and confounding factors. J Gastroenterol 40: 866-872, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 102: 1301-1306, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Izumi D, Ishimura N, Okada M, et al. . Poor inter-observer agreement on the endoscopic diagnosis of eosinophilic esophagitis among Japanese endoscopists. Esophagus 14: 309-316, 2017. [Google Scholar]
- 34.Peery AF, Cao H, Dominik R, et al. . Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin Gastroenterol Hepatol 9: 475-480, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiwara Y, Hashimoto A, Umemura R, et al. . Optimal biopsy protocol to evaluate histological effectiveness of proton pump inhibitor therapy in patients with eosinophilic esophagitis. Digestion 100: 64-71, 2019. [DOI] [PubMed] [Google Scholar]