Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: A cross-sectional study (original) (raw)
Dear Editor,
Although most majority of COVID-19 cases are mild as shown in a recent issue of this journal,1 some patients with initial mild to moderate forms of COVID-19, complain of persistent or resurgent symptoms.2 Our aim was to describe the clinical, biological and imaging profile of such patients in order to suggest a classification of the symptoms and raise hypotheses about their pathophysiology.
We established in May 2020, in COCHIN HOTEL DIEU Hospital of Paris, an out-patient clinic for adult patients with persistent and/or recurrent symptoms after a confirmed COVID-19 and performed a cross-sectional monocenter survey on consecutive patients presenting:1 an initial symptomatic COVID-19 infection virologically confirmed by either a positive SARS-CoV-2 RNA RT-PCR or positive SARS-CoV-2 serology,2 who developed prolonged COVID symptoms defined as persistent symptoms (> 2 months after the first day of the initial episode) or resurgent symptoms (at least 3 weeks after the 1st episode). The presence of another obvious cause of symptoms was an exclusion criteria.
Clinical characteristics (previous history, COVID symptoms, physical examination) were collected on a structured questionnaire. A SARS-COV-2 serology was routinely requested. SARS-COV-2 RT-PCR assay on nasopharyngeal swabs and other bioassays were requested based on symptoms. Ethics approval was granted by the local Institutional Review Board of Henri-Mondor Hospital (00011558, Approval number 2020-088). All patients included provided an informed consent. Characteristics of the patients were described by n (%) for categorical data and mean (standard deviation SD) or median (inter-quartile range IQR) for continuous data as appropriate.
Results
Among 70 consecutive patients with a documented SARS-CoV-2 infection, median age was 45 (range 23–75), 78.6% were female.
During the initial episode of COVID-19, the most frequent symptoms were fever, anosmia, headaches and asthenia. Six (8.6%) patients were hospitalized and 2 required oxygen support (Table 1).
Table 1.
Demographic characteristics of the 70 patients presenting with persistent and/or re merging late symptoms of COVID after a documented SARS COV 2 infection.
| Characteristics | Available data | All patients |
|---|---|---|
| Clinical characteristics | ||
| Age, years | 70 | 45 [36–51] |
| Male, No. (%) | 70 | 15 (21.4) |
| Female, No. (%) | 70 | 55 (78.6) |
| Profession | ||
| Healthcare worker, No. (%) | 69 | 19 (27.5) |
| Other, No. (%) | 69 | 50 (72.5) |
| BMI, kg/m² | 53 | 23.0 [21.1–27.2] |
| Allergy | 69 | 34 (49.3) |
| Respiratory allergy, No. (%) | 62 | 12 (19.4) |
| Asthma, No. (%) | 62 | 3 (3.2) |
| Medicinal allergy | 62 | 10 (14.5) |
| Other allergy, No | 62 | 2 (2.9) |
| Autoimmune disease, No. (%) | 70 | 9 (12.9) |
| Personal | 70 | 5 (7.1) |
| Familial | 70 | 4 (5.7) |
| Anxiety or psychiatric history | 70 | 3 (4.3) |
| Smoking, No. (%) | 66 | 2 (3.0) |
| Sport practicing, No. (%) | 51 | 25 (49.0) |
| Virologic confirmation of the 1st SARS COV 2 infection | ||
| Positive SARS-CoV-2 RT-PCR positive in rhino pharyngeal swabs | 47 | 27 (57.4) |
| Positive SARS-CoV-2 IgG positive antibodies | 69 | 64 (92.3) |
| Positive SARS-CoV-2 RT-PCR and serology | 27 | |
| Only a positive SARS-CoV-2 RT-PCR | 6 | |
| Only a positive SARS-CoV-2 serology | 37 |
A symptom-free interval was noted between the first episode and the following episodes in 32/65 cases with a mean interval of 25 days (SD=20). During the prolonged phase, 54.3% patients had symptoms that persisted from the 1st episode, 50% that disappeared and reappeared and 75.7% presented new symptoms that were absent during the 1st episode appeared.
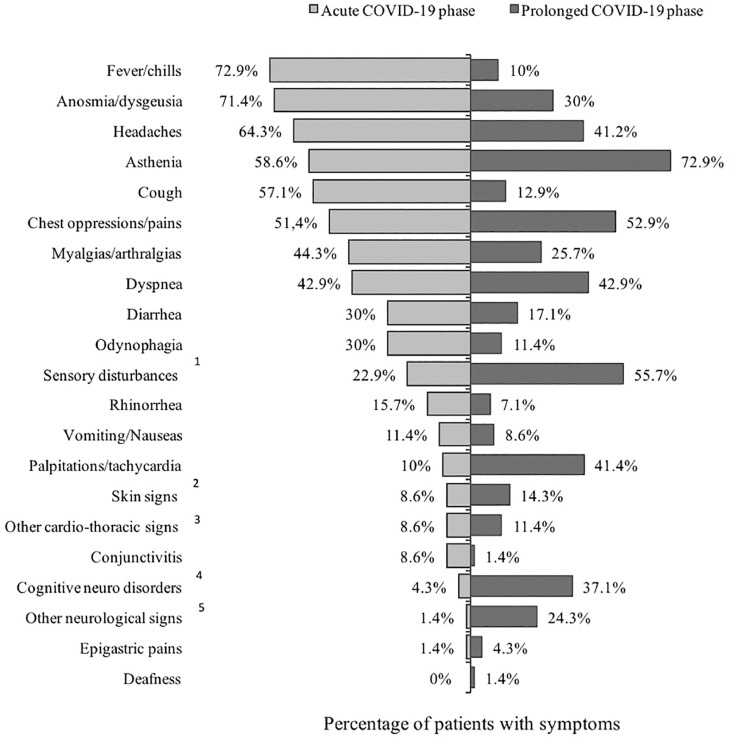
Characteristics of late symptoms could be classified in 7 main categories (Fig. 1):
-
Major fatigue or exhaustion for 51 patients (72.9%) -
Neurological symptoms, in 54 (77.1%). Those were divided into neuro-cognitive disorders (such as memory, mood or attention disorders), headaches, sensory disturbances (such as balance disorders, tingling, burning sensations and neurogenic pains), or others (swallowing or speech disorders, thermoregulation disorders). -
Cardiothoracic symptoms in 50 patients (71.4%): chest pain and tightness, palpitations, cough, dyspnea. -
Muscular or/and articular pains for 20 (25.7%). -
ENT symptoms: persistent or recurrent anosmia, hyposmia and/or dysgeusia for 21 (30%). -
Gastro-intestinal symptoms for 17 (24.3%): diarrhea, nausea/vomiting, epigastric or abdominal pain. -
Skin and vascular symptoms in 10 (14.4%).
Fig. 1.
Distribution of the symptoms of the acute COVID-19 phase versus those of the prolonged -COVID phase in 70 patients presenting with persistent and/or remerging late symptoms of COVID after a confirmed SARS-CoV-2 infection. Legend of the figure: 1. Sensory disturbances include balance disorders, tingling, burning sensations and neurogenic pains. 2. Mucco cutaneo signs include acrocyanosis, rash, flash face, eczema, tongue swelling, pruritus, vascular inflammation, spontaneous ecchymosis. 3. Other cardiological signs include sudden malaise, desaturation. 4. Cognitive psycho disorders include memory, humor or attention disturbances and disorientation. 5. Other neurological signs include or swallowing disorders, thermal dysregulation and speech.
Other symptoms included odynophagia, low-grade fever, rhinorrhea, conjunctivitis suppress. The majority of the patients (n = 63, 90%) had more than three categories of symptoms. The course of symptoms was intermittent in 42.9% of the cases, alternating symptoms-free intervals of a few days or hours with sudden relapses, often worsening after physical or intellectual exercise.
During the prolonged COVID-19 phase, the SARS-CoV-2 RT-PCR was still positive in rhino pharyngeal swabs in 11/43 patients and remained positive more than 3 months for 3 subjects (Supplementary Fig. S1). SARS-CoV-2 serology was positive in most of the cases (n = 64/69, 92.8%).
Inflammatory proteins (C reactive protein, ferritin, IL-6 levels) were in the normal range or slightly elevated in 10% of the patients. Autoimmune antibodies were absent in 90% patients (data not shown). Cardiac echography and/or MRI, led to the diagnosis of pericarditis and/or myopericarditis in 9 cases. Cerebral CT scan and/or MRI were normal, except in 4 cases. An inflammation of olfactive bulbs was occasionally found in case of persistent or recurrent anosmia.
Discussion
It was striking that these patients had essentially made a benign form of COVID-19 and consisted mainly of young women. The phenotype was dominated by a major fatigue, associated with neurological and cardiovascular symptoms. If some cases may have been related to the involvement of an organ (pericarditis, myocarditis), some symptom groupings in a same patient were suggestive of an autonomic dysfunction. Another striking finding was that the RT-PCR was occasionally detected in rhino pharyngeal swabs during the prolonged episodes although no patient was immunosuppressed and the majority had developed antibodies against SARS-CoV-2. Unfortunately we were not able to perform viral culture (supplementary Fig. S1).
The pathophysiology of the persistence of symptoms is currently unknown and probably non univocal. Those observations made us raise several hypotheses.
The first one is a viral persistence in the host. The virus could continue to replicate in usual sites, as shown in our study and a few previous series.3 , 4 It might also have spread elsewhere, via neurological or vascular invasion, in cells reported to express ACE2 receptors such as endothelial cells.5
A second hypothesis is that of a reinfection. Although a rare event, several cases evidenced by a phylogenetically distinct strain of SARS COV-2, have now been demonstrated.6, 7, 8 These reinfections have been shown to occur several months after the 1st episode. Knowing that our population consisted of 27% of health care workers, the hypothesis of a reinfection cannot be excluded. However, the difference in clinical profile between the first infection and the prolonged symptoms does not favor of this hypothesis.
A third hypothesis could be that of an inadequate immune response leading to an auto inflammatory chronic condition in genetically predisposed individual.
A last hypothesis is that of a condition similar to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). After SARS epidemic in Hong Kong, a follow-up study of 233 patients infected with SARS showed that 27% met the diagnostic criteria for ME/CSF.9 A subset of patients with prolonged COVID-19 symptoms have symptoms that overlap with ME/CFS.
Finally, we cannot exclude at this stage the possibility of a post-traumatic stress induced by the COVID-19 pandemic and/or exacerbated by these symptoms themselves.
One strength of this study it that it focused on patients tested positive for SARS-COV-2 infection, in order to reduce the risk of attributing to COVID 19 symptoms that could be of psycho somatic origin. All our patients filled the same questionnaire and were explored similarly in case of neurological symptoms with a cerebral CT scan and/or MRI and a neurologist advice, and in case cardiothoracic symptoms with an echography and/or a cardiac MRI if needed.
The limitations are linked to its small sample. Our description could be possibly the tip of the iceberg, only the most affected patients being inclined to visit the clinic. Moreover, our design did not allow us to determine the prevalence of prolonged symptoms. A few recent data establish that more than 20% of patients will present with prolonged symptoms at least for three months among which the fatigue is the predominant symptom although none of them has described precisely the profile of clinical symptoms.2 , 10
In conclusion, although prolonged manifestations of COVID-19 are mostly subjective, the repetition of similar neurological and cardiothoracic complaints and the fact that we observed objective cases of myopericarditis suggests that there is a real prolonged COVID-19 entity. We believe that is crucial to promptly report our results to the scientific community as these patients have an urgent need to be taken into account and supported. While waiting to find a virological, immune or inflammatory and/or genetic signature that could explain the symptoms, it is difficult to recommend a treatment.
Standardized and multidisciplinary investigations and a cohort follow-up are urgently needed in order to precisely assess the prevalence of such symptoms after COVID-19, better understand their pathophysiology and natural evolution and evaluate therapeutic approaches.
Declaration of Competing Interest
All the authors have read and agreed with the paper's content. No authors have financial or personal conflicts. Neither the work nor any part of its essential substance, tables or figures have been or will be published or submitted to another scientific journal or are being considered for publication elsewhere.
Acknowledgments
Acknowledgments
The authors would like to thank Mrs Yannie CUVILLIER for technical assistance. We thank all patients who participated in the study. We thank Nicolas DUMESGES for linguistic revision.
Funding
This study was not sponsored by any external financial support
Footnotes
Appendix. Supplementary materials
References
- 1.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. Epub 2020 Mar 19PMID: 32171869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group. Carfì A. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. PMID: 32644129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang H., Wang Y., Tong Z., Liu X. Retest positive for SARS-CoV-2 RNA of “Recovered” patients with COVID-19: persistence, sampling issues, or re-infection? JMV. 2020 doi: 10.1002/jmv.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goussef M., Penot P., Gallay L., Batisse D., Bouiller K., Collarino R. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020 doi: 10.1016/j.jinf.2020.06.073. S0163-4453(20)30454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K., Hung I.F., Ip J.D., Chu A.W., Chan W.M., Tam A.R., Fong C.H., et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis.;ciaa1275. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed]
- 7.Van Elslande J., Vermeersch P., Vandervoort K., Wawina-Bokalanga K., Vanmechelen B., Wollants E., et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain, Clin Infect Dis, ciaa1330, 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed]
- 8.Tillett R., Sevinsky J., Hartley P., Kerwin H., Crawford N., Gorzalski A., et al. Genomic evidence for a case of reinfection with SARS-CoV-2 (2020). Available at SSRN: https://ssrn.com/abstract=3680955 or 10.2139/ssrn.3680955. [DOI] [PMC free article] [PubMed]
- 9.Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 10.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C. Duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. PMID: 32730238; PMCID: PMC7392393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.