Flecainide toxicity associated with the use of goji berries: a case report (original) (raw)
Abstract
Background
Goji berries (GB), usually marketed as a ‘superfruit’, are a widely used herbal supplement. As with other herbal remedies, the use of GB might be associated with herb–drug interactions, increasing plasma levels of other drugs and causing adverse events. Here, we present the case of a patient that developed flecainide toxicity secondary to an herb–drug interaction, associated with the use of GB to prevent COVID-19.
Case summary
A 75-year-old female presented to the emergency department with fainting. She was taking flecainide for the treatment of atrial extrasystoles diagnosed 2 years previously, and she was using a tea of GB for the prevention of COVID-19. The admission electrocardiogram showed a wide complex polymorphic tachycardia that was considered and treated as flecainide toxicity. The patient had a favourable evolution and was discharged 48 h after admission.
Discussion
Flecainide toxicity is uncommon and needs timely recognition and treatment; it is usually secondary to overdose and renal or hepatic failure. In our case, toxicity was associated with GB use, probably by inhibition of CYP2D6 which is the main enzyme associated with the metabolism of flecainide. Clinicians need to be aware of the possible interactions between herbal remedies (in this case used for the prevention of COVID-19) and cardiovascular drugs that are used to treat chronic cardiovascular diseases.
Keywords: COVID-19, SARS-CoV-2, Goji berries, Herb–drug interaction, Flecainide toxicity, Case report
Learning points
- Herbal remedies used for the prevention of COVID-19 have the potential of causing serious adverse effect associated with pharmacological interactions between the herb and drugs used for the treatment of chronic cardiovascular disease.
- Goji berries can cause flecainide and warfarin toxicity by the inhibition of CYP2D6 and CYP2C9 the main enzymes associated with the metabolism of these drugs.
- Flecainide toxicity should be suspected in patients with a history of flecainide consumption and an electrocardiogram showing wide complex tachycardia.
Introduction
COVID-19 is still growing as a global health emergency and with the increase in the number of cases, we are seeing more patients that are using alternative and herbal remedies trying to prevent the infection.1 Alternative and herbal medicines have the potential of causing side effects that can be related to primary toxicity, or to pharmacologic interactions with other drugs that the patients might be taking for different indications.2 This is particularly important in patients with cardiovascular (CV) disease—which are known to have an increased risk of infection and a higher rate of complications—since inhibition of metabolic pathways of CV drugs caused by the use of alternative medicine might enhance or decrease their action, leading to adverse effects.3 Flecainide is a type Ic antiarrhythmic that blocks sodium currents and slows, in a rate-dependent manner the phase 0 of the cardiac action potential. Though effective, flecainide is known to have a narrow therapeutic index (TI) (0.2–1.0 mcg/mL) with the risk of developing toxicity even with mild increases in serum concentrations of the drug.4 Here, we present the case of a patient that developed flecainide toxicity associated with the use of a herbal remedy for the prevention of COVID-19.
Timeline
| Time | Events |
|---|---|
| 3 years before admission | Mitral valve replacement with a bi-leaflet prosthetic valve, anticoagulated with warfarin, with constant international normalized ratio (INR). |
| 2 years before admission | Flecainide started for the treatment of atrial extrasystoles, taking 100 mg twice a day. |
| 2 weeks before admission | Started using a goji berries (GB) tea for the prevention of COVID-19, 1–2 glasses per day. |
| 2 days before admission | Complained of dizziness, nausea and extreme fatigue. |
| Day of admission | She presented with fainting, an electrocardiogram (ECG) showed a wide complex polymorphic tachycardia that was considered due to flecainide toxicity. Treatment with IV bicarbonate, amiodarone and electric cardioversion resulted in an atypical atrial flutter with regain of haemodynamic stability. |
| 48 h after admission | The patient remained stable; a pre-discharge ECG showed a sinus rhythm with atrial extrasystoles and resolution of electrocardiographic abnormalities associated with flecainide toxicity. Discharged with warfarin, amiodarone and told to stop GB use. |
Case presentation
A 75-year-old woman was admitted to the emergency room because she had fainted several times. Her past medical history was relevant for a mitral valve replacement with a bi-leaflet prosthetic valve 3 years previously, that was anticoagulated with warfarin (2.5–3.5 constant international normalized ratio (INR)). She was also using flecainide 100 mg b.i.d. for the treatment of atrial extrasystoles that started 2 years before admission. She referred that for the last 2 weeks, she had been taking a concentrated Chinese herbal tea made of goji berries (GB) for the prevention of COVID-19, drinking 1–2 glasses per day; she was not receiving any other medications.
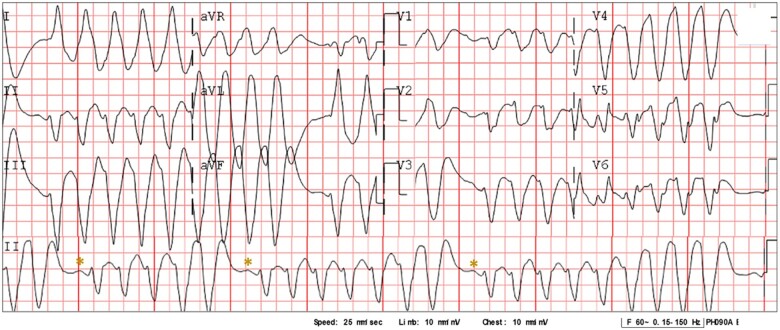
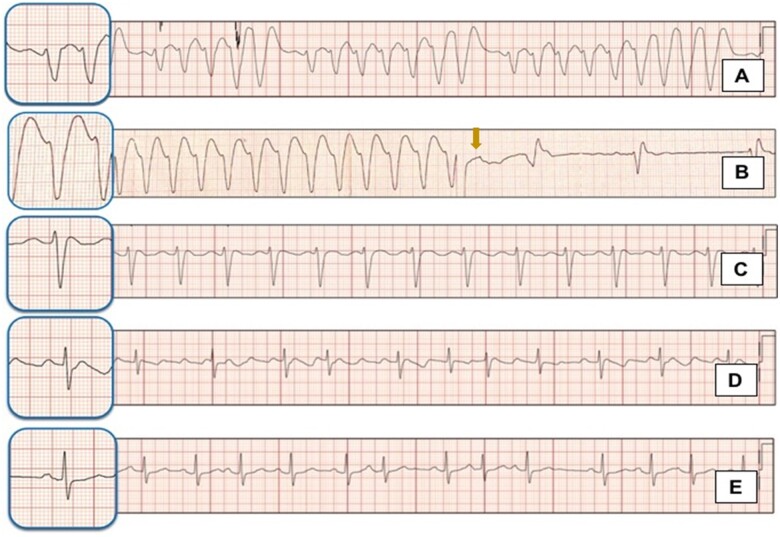
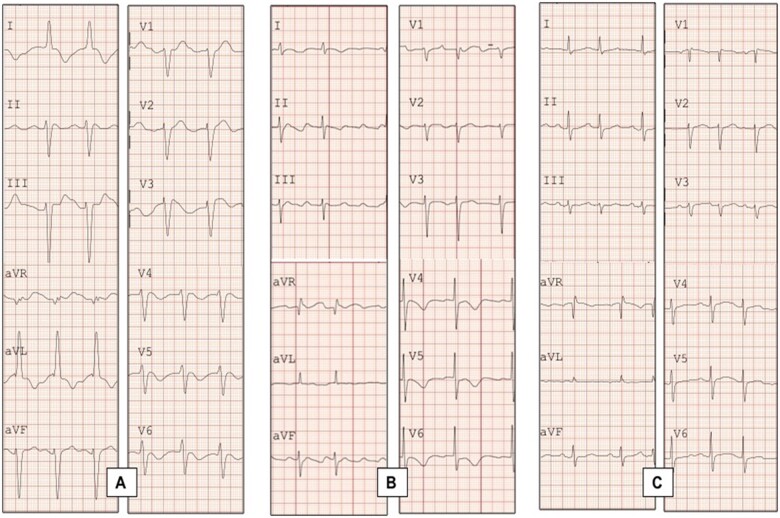
On initial evaluation, she was unresponsive; her blood pressure was 85/60 mmHg, with a respiratory rate of 20 rpm. She had been complaining of dizziness, nausea, and extreme fatigue two days before. Cardiac auscultation revealed a closing click at the apex, there were no audible murmurs, lungs were clear, and there was no oedema or other signs of congestion. There rest of the physical evaluation was within normal. An electrocardiogram (ECG) revealed a very wide QRS complex tachycardia at a rate of 160 b.p.m. with a left bundle branch block (LBBB) morphology and northwest axis deviation, accompanied by periodic bursts and a cyclical increase in the QRS amplitude with repeated sequences of 5, 7, and 9 beats with apparent P-wave-like deflection before each burst (Figures 1 and 2A). Based on the patient’s history and the ECG findings, flecainide toxicity was suspected and 100 mEq of 8.4% sodium bicarbonate, 300 mg of IV amiodarone, and 2 g of magnesium sulfate were administered; this resulted in a 140 b.p.m. regular monomorphic wide QRS tachycardia and no discernible atrial activity (Figure 2B). As the pulse remained feeble, a 200 J electrical cardioversion was delivered and resulted in sinus rhythm (Figure 2B) with immediate onset of an atypical atrial flutter with a very wide P wave, LBBB morphology, and prolonged QTc interval (Figures 2C and 3A) and improvement in consciousness and haemodynamic status. Laboratory results were remarkable: aspartate aminotransferase 152 U/L (10—42 U/L), alanine aminotransferase 92 U/L (10–42 U/L), lactate 797 u/L, troponin 57.7 ng/mL, prothrombin time 77.4 s (9.9–12.3 s), Partial thromboplastin time 46.7 s (25.2–36 s), and INR 7.18, electrolyte levels and renal function were unremarkable. Due to elevated INR, a dose of 10 mg IV vitamin K was administered.
Figure 1.
Twelve-lead electrocardiogram on admission. A 12-lead electrocardiogram obtained during admission shows a wide complex tachycardia with a heart rate of 160 b.p.m., with a polymorphic QRS complex and progressive widening of QRS in cycles of 5, 7, and 9 beats with a p-wave like deflection (*) between each cycle.
Figure 2.
Rhythm strips showing electrocardiographic evolution. (A) Shows the rhythm strip of the electrocardiogram during admission. (B) A wide complex, monomorphic tachycardia that appeared after treatment with 8.4% bicarbonate infusion, the arrow indicates the moment when synchronized cardioversion was administrated because of haemodynamic instability. (C) An atypical atrial flutter appearing after electric cardioversion. (D) A repeated electrocardiogram showing persistence of the atypical flutter with a narrow QRS complex and prolonged QTc interval. (E) A pre-discharge electrocardiogram obtained 48 h. after admission shows a sinus rhythm with atrial extrasystoles and resolution of abnormalities associated with flecainide toxicity.
Figure 3.
Twelve-lead electrocardiogram showing electrocardiographic evolution. (A) Twelve-lead electrocardiogram showing an atypical atrial flutter with wide complex QRS appearing immediately after synchronized cardioversion. (B) A repeated 12-lead electrocardiogram showing persistence of atypical atrial flutter now with narrow QRS complex. (C) A pre-discharge 12 electrocardiogram showing resolution of the electrocardiogram abnormalities, with a sinus rhythm and atrial extrasystoles.
An echocardiogram revealed normal left ventricular systolic function without segmental wall motion abnormalities, mild pulmonary hypertension, and left ventricular hypertrophy; mechanical valve showed no abnormalities. After 8 h, her mental status improved; control laboratories showed an INR of two. A repeated ECG showed an atypical flutter with narrow QRS associated with slow repolarization, QTc 530 ms and asymmetric T-wave inversion in the anterior wall (Figures 2D and 3B). The patient was started on enoxaparin and was later bridged to warfarin. She was discharged home 48 h after admission with oral amiodarone 200 mg qd, warfarin dose was the same as before the Lycium barbarum (Goji) intake. A pre-discharge ECG showed sinus rhythm at a rate of 87 b.p.m., atrial extrasystoles, and resolution of all morphological patterns associated with flecainide toxicity (Figures 2E and 3C); the patient was cited to the outpatient cardiology clinic 1 week after discharge from the hospital with a new INR determination for monitoring and adjustment of warfarin dose.
Discussion
This case reports a drug–herb interaction that caused both warfarin overdose and a life-threatening pleomorphic arrhythmia associated with flecainide toxicity. To the best of our knowledge, this is the first report of a patient developing flecainide toxicity associated with the use of GB, which in this specific patient was used for the prevention of COVID-19. Our case highlights another challenge clinicians face during this pandemic, the recognition and management of patients that develop medication toxicity secondary to pharmacologic interactions with herbal and alternative remedies used to prevent SARS-CoV-2 infection.
Flecainide is mostly eliminated in the urine, 30% as an unchanged drug and other part as metabolites.4,5 Two major metabolites are produced by hepatic oxidation via cytochrome CYP2D6 and CYP1A2, the active meta-O-dealkylated flecainide and its inactive lactam.5 Toxicity occurs most commonly associated with accidental overdosing or with intentional ingestion;6 it should be used with caution in patients with renal failure, hepatic insufficiency, and electrolyte derangements.4 Although hepatic clearance of the drug seems to be minimal, it has been demonstrated that impaired CYP2D6 activity is associated with a 21% reduction in flecainide clearance in intermediate metabolizers and a 41% reduction in poor metabolizers; this, associated with the narrow TI of flecainide increases the chance of toxicity associated with cytochrome inhibition.7
Goji berries are obtained from the plants Lycium chinense and L. barbarum; they have been marketed in the western world as a ‘superfruit’.8 They are rich in macro- and micro-nutrients and contain carotenoids, phenolic acids, and flavonoids such as caffeic acid, caffeoylquinic acid, chlorogenic acid, p-coumaric acid, quercetin-diglucoside, kaempferol-3-_O_-rutinoside, and rutin, all with high antioxidant capacity.8 Whether the use of GB for the prevention of COVID-19 is widespread is not clear, since we could not find other reports on the literature of its use, however, our patient referred that she was using the remedy specifically for COVID-19 prevention. Recently, it has been found that some compounds like kaempferol (present in GB) have the potential to inhibit SARS-CoV-2 main protease in vitro, suggesting that there might be a benefit for their use as preventive or therapeutic options.9 However, there is no evidence of their effectiveness in vivo, or in properly conducted clinical trials.
It has been demonstrated that GB have the potential to cause strong inhibition (over 75%) of most of the major CYP450 enzymes.10 The GB juices have shown at least a moderate inhibitory effect of the CYP2D6, with a 50–60% of enzyme activity inhibition.10 Since our patient had no evidence of accidental or intentional flecainide overdosing, and there was no evidence of renal or hepatic impairment, we suggest that toxicity developed secondary to CYP2D6 inhibition by GB with the consequent increase in serum levels of flecainide. At least four case reports have described the development of warfarin toxicity associated with Goji consumption, in all of them, toxicity may have developed at least in part from the inhibition of the CYP2C9 by GB, an enzyme associated with warfarin metabolism.11–13 The increase in INR seen at admission in our patients is also associated with inhibition of the CYP2C9 by GB.
Timely recognition of flecainide toxicity is of major relevance, severe intoxication with Class Ic antiarrhythmics, including flecainide has a reported mortality of 22.5%.6 Flecainide slows conduction in cardiac fibres and increases conduction times in the atria, atrioventricular node, His–Purkinje system, and ventricles. In the ECG, this can manifest as a prolongation of the PR and QRS intervals.4,14,15 In flecainide toxicity, supraventricular rhythms can present with bizarre, wide QRS complexes with either right or left bundle branch block and varying frontal axes, which can easily be mistaken for ventricular tachycardia (VT).14 The challenge in differentiating flecainide toxicity from VT on the ECG relies on the fact that the attempts to restore sinus rhythm and haemodynamic stability with electric cardioversion and antiarrhythmics like in VT might prove futile in the setting of flecainide toxicity since the mechanisms of the electrocardiographic abnormalities are different.15 Recently, Valentino et al.14 described two morphological patterns that may emerge during intoxication. According to their findings, flecainide toxicity can present with QRS duration of ≥200 ms, or ≤200 ms. As in our case, those patients with a QRS duration of ≥200 ms are more likely to present with an LBBB morphology, while those patients with a QRS duration ≤200 ms might present a right bundle branch block (RBBB) morphology.14 In addition, patients with a wider QRS seem to have an increased need for mechanical circulatory support and increased mortality.14 In their report, they suggest that flecainide toxicity should be suspected in patients with: (i) access to flecainide or history of use and (ii) an LBBB morphology with QRS ≥ 200 ms or RBBB with QRS ≤ 200 ms.14 The use of 8.4% sodium bicarbonate has been a mainstay in the treatment of toxicity; sodium loading displaces flecainide from its receptor, while alkalinization reduces sodium channel blockage by flecainide.14,15
Conclusion
The GB extract shows an important inhibitory effect on CYP isoenzymes that are associated with the metabolism of warfarin and flecainide; since both of these drugs have a narrow TI, interaction with herbal remedies can lead to adverse events. Flecainide toxicity is uncommon and needs timely recognition and treatment; physicians need to be aware of the possible interactions between drugs used to treat chronic diseases and herbal remedies, especially those with an effect on CYP isoenzymes. The target drug may need to be substituted or the dose adjusted, to account for a potential decrease or increase in metabolism.
Lead author biography

Carlos E. Guzman, MD, FACC is a Mexican cardiologist and electrophysiologist. He attended a 4-year cardiology residency at the Instituto Nacional de Cardiología ‘Ignacio Chavez’ in México City. Subsequently, made a master’s degree in Medical Sciences at the Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’. He also performed a fellowship in Invasive Electrophysiology at Center for Cardiac Stimulation and Rhythmology. ‘Jean Rostand Hospital’, (Paris France). His research interests include electrophysiology and three‐dimensional mapping systems for tachycardia. He is currently an attending electrophysiologist and professor at the Hospital Christus Muguerza in Monterrey, México.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
ytab204_Supplementary_Data
References
- 1.Huang J, Tao G, Liu J, Cai J, Huang Z, Chen J-X.. Current prevention of COVID-19: natural products and herbal medicine. Front Pharmacol 2020;11:588508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K, Gao Q, Zhang T, Rao J, Ding L, Qiu F.. Inhibition of CYP2C9 by natural products: insight into the potential risk of herb-drug interactions. Drug Metab Rev 2020;52:235–257. [DOI] [PubMed] [Google Scholar]
- 3.Liperoti R, Vetrano DL, Bernabei R, Onder G.. Herbal medications in cardiovascular medicine. J Am Coll Cardiol 2017;69:1188–1199. [DOI] [PubMed] [Google Scholar]
- 4.Andrikopoulos GK, Pastromas S, Tzeis S.. Flecainide: current status and perspectives in arrhythmia management. World J Cardiol 2015;7:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conard GJ, Ober RE.. Metabolism of flecainide. Am J Cardiol 1984;53:41b–51b. [DOI] [PubMed] [Google Scholar]
- 6.Köppel C, Oberdisse U, Heinemeyer G.. Clinical course and outcome in class IC antiarrhythmic overdose. J Toxicol Clin Toxicol 1990;28:433–444. [DOI] [PubMed] [Google Scholar]
- 7.Doki K, Homma M, Kuga K, Aonuma K, Kohda Y.. Effects of CYP2D6 genotypes on age-related change of flecainide metabolism: involvement of CYP1A2-mediated metabolism. Br J Clin Pharmacol 2009;68:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma ZF, Zhang H, Teh SS, Wang CW, Zhang Y, Hayford F. et al. Goji berries as a potential natural antioxidant medicine: an insight into their molecular mechanisms of action. Oxid Med Cell Longev 2019;2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential Inhibitor of COVID-19 Main Protease (Mpro) from Several Medicinal Plant Compounds by Molecular Docking Study. 2020; 2020030226. Preprints doi: 10.20944/preprints202003.0226.v1.
- 10.Liu R, Tam TW, Mao J, Salem A, Arnason JT, Krantis A. et al. In vitro activity of Lycium barbarum (Goji) against major human phase I metabolism enzymes. J Complement Integr Med 2016;13:257–265. [DOI] [PubMed] [Google Scholar]
- 11.Rivera CA, Ferro CL, Bursua AJ, Gerber BS.. Probable interaction between Lycium barbarum (goji) and warfarin. Pharmacotherapy 2012;32:e50–3. [DOI] [PubMed] [Google Scholar]
- 12.Leung H, Hung A, Hui AC, Chan TY.. Warfarin overdose due to the possible effects of Lycium barbarum L. Food Chem Toxicol 2008;46:1860–1862. [DOI] [PubMed] [Google Scholar]
- 13.Lam AY, Elmer GW, Mohutsky MA.. Possible interaction between warfarin and Lycium barbarum L. Ann Pharmacother 2001;35:1199–1201. [DOI] [PubMed] [Google Scholar]
- 14.Valentino MA, Panakos A, Ragupathi L, Williams J, Pavri BB.. Flecainide toxicity: a case report and systematic review of its electrocardiographic patterns and management. Cardiovasc Toxicol 2017;17:260–266. [DOI] [PubMed] [Google Scholar]
- 15.Ghataoura R, Patil S.. Flecainide toxicity: a presentation to the emergency department with literature review. BMJ Case Rep 2020;13:e232691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ytab204_Supplementary_Data