Resolving the “muddle in the middle”: The case for Homo bodoensis sp. nov (original) (raw)
Abstract
Recent developments in the field of palaeoanthropology necessitate the suppression of two hominin taxa and the introduction of a new species of hominins to help resolve the current nebulous state of Middle Pleistocene (Chibanian) hominin taxonomy. In particular, the poorly defined and variably understood hominin taxa Homo heidelbergensis (both sensu stricto and sensu lato) and Homo rhodesiensis need to be abandoned as they fail to reflect the full range of hominin variability in the Middle Pleistocene. Instead, we propose: (1) introduction of a new taxon, Homo bodoensis sp. nov., as an early Middle Pleistocene ancestor of the Homo sapiens lineage, with a pan‐African distribution that extends into the eastern Mediterranean (Southeast Europe and the Levant); (2) that many of the fossils from Western Europe (e.g. Sima de los Huesos) currently assigned to H. heidelbergensis s.s. be reassigned to Homo neanderthalensis to reflect the early appearance of Neanderthal derived traits in the Middle Pleistocene in the region; and (3) that the Middle Pleistocene Asian fossils, particularly from China, likely represent a different lineage altogether.
Keywords: hominin taxonomy, Homo bodoensis, Homo heidelbergensis, Homo rhodesiensis, Middle Pleistocene
1. INTRODUCTION
In 2019, we dedicated an entire American Association of Biological Anthropology (formerly American Association of Physical Anthropology) conference session to defining Homo heidelbergensis. The results of the meeting were: (1) no one was happy with the taxon; (2) different people assigned different meanings to the species and included different fossils in the hypodigm; (3) ignoring this problem will not miraculously lead to a solution; and (4) that in order to better understand Middle Pleistocene hominin systematics, it was critical to clear up this “muddle in the middle.” 1 Here, we propose that H. heidelbergensis should be abandoned altogether, as it has been poorly defined and used inconsistently. Instead, we introduce Homo bodoensis sp. nov. as a largely African—and likely eastern Mediterranean—taxon and argue that the Middle Pleistocene hominin fossils that show any derived Neanderthal traits and are traditionally assigned to H. heidelbergensis s.s., including the Mauer mandible, be reassigned to Homo neanderthalensis and considered as early Neanderthals. Taxonomic classification has a strong impact on conceptual understanding of evolution, and the taxonomic practice of reviving old names due to rules of precedence has sometimes played an important role in obfuscating our understanding of the complexity of Middle Pleistocene hominin evolution; the resurrection of H. heidelbergensis is a case in point. By introducing a new, properly defined species, that recognizes and systematizes some of the observed variation, we hope to contribute a foundational piece from which palaeoanthropologists can build more robust explanatory models that better describe hominin evolution during the Middle Pleistocene.
2. A BRIEF HISTORY OF HOW HOMO HEIDELBERGENSIS MUDDLED THE MIDDLE PLEISTOCENE
The study of human evolution in the Middle and Late Pleistocene (LP) has experienced significant advances in recent decades. We now know that the origin of Homo sapiens was African (possibly pan‐African) 2 and extends further back into the late Middle Pleistocene than previously thought. It is also clear that this taxon was dispersing out of Africa prior to 60 ka, likely in multiple smaller waves, with a major dispersal post‐60 ka. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Further, over the past two decades species assigned to the genus Homo (e.g., Homo floresiensis, 12 H. naledi, 13 and H. luzonensis 14 ) that were contemporary with the H. sapiens lineage but are considered to have played little to no role in the latter's evolution, attest to the complexity of the later Pleistocene human evolutionary record. The Middle Pleistocene is no longer dismissed as the proverbial “muddle in the middle,” 1 but is increasingly recognized as a key time frame that witnessed the appearance, on a global scale, of two critical traits of later human morphology: greater encephalization and smaller teeth, and likely the differentiation of geographic groups. The recent questioning of the validity of H. heidelbergensis exposes a growing malaise in lumping together observable variation that characterizes Middle Pleistocene hominins, which hinders our ability to hypothesize the scenarios for the evolution of the genus Homo into the LP.
The field of palaeoanthropology has matured substantially since H. heidelbergensis was proposed by Otto Schoetensack 15 on the basis of the Mauer mandible. Further important discoveries have been made since the revival of the taxon in the last two decades of the 20th century. 16 , 17 , 18 , 19 , 20 Unfortunately, in 1908, Schoetensack had no notion of the evolutionary synthesis, and cladistic methods had yet to be developed. 21 , 22 Furthermore, the revival of the H. heidelbergensis taxon was rooted in the late 20th century understanding of hominin phylogeny/systematics, particularly as related to debates around the origin of modern humans, 23 , 24 , 25 rather than on any particular set of morphological traits, as required by the rules of zoological nomenclature. 26 To further compound the problem, a mandible, without an associated cranium, was used as the holotype for the taxon, even though this bone is normally considered to be extremely plastic and may or may not reflect associated morphological changes in the crania. 27 Similarities between the Mauer specimen and the Arago mandibles, which were represented by associated cranial fragments, led to the indirect reconstruction of H. heidelbergensis. 19 , 28 , 29 , 30 , 31 The Mauer/Arago group was then linked via Petralona to the African specimens such as Kabwe 1 and Bodo, given morphological similarities in the crania, 30 , 32 thus expanding the proposed geographic and temporal range of H. heidelbergensis. It was later raised as a possibility that the Chinese “archaic H. _sapiens_” fossils could also be included in H. heidelbergensis 33 , 34 , 35 (but see References 36, 37). Unfortunately, revival of taxonomic names rarely produces desirable clarity (e.g., the reintroduction of Australopithecus prometheus Dart 1945 38 by Clarke and Kuman 39 sparked a heated debate 40 , 41 , 42 ); H. heidelbergensis is no exception in this regard.
Multiple, often contradictory views on what constitutes H. heidelbergensis make this taxon particularly misleading. Even to nonspecialists (e.g., biologists working in other realms, Palaeolithic archaeologists, etc.) H. heidelbergensis represents either (and sometimes paradoxically both) the generalized Middle Pleistocene hominin, or a chronospecies of Neanderthals. Within the palaeoanthropological community, the taxon's ambiguity has contributed to complex and sometimes hard‐to‐follow discussions: in a single paper, one can find numerous descriptions of the taxon with incompatible hypodigms. 16 , 19 , 20 , 31 , 33 , 34 , 35 , 37 , 43 , 44 , 45 , 46 More troublingly, newly discovered Middle Pleistocene hominin fossils that cannot easily be assigned to Homo erectus, H. neanderthalensis, or early H. sapiens, still tend to be lumped into this one‐size‐fits‐all taxon, often with a sensu lato qualifier to indicate a nonspecific morphology of a Middle Pleistocene hominin. 35 , 47 , 48 , 49 , 50 Alternatively, they are assigned more general or descriptive names like “archaic H. sapiens,” 51 “mid‐Pleistocene Homo,” 52 or “Homo sp.,” 53 which do little to convey their evolutionary position.
3. HOMININ TAXONOMY AND WHY IT MATTERS
There are manyfold reasons for uncertainties in hominin taxonomy. A significant and obvious hindrance is a sparse fossil record with unequal geographic coverage, which often makes broader regional comparisons difficult. However, the theoretical underpinnings of taxonomy, and hominin taxonomy in particular, is a potentially more serious impediment 54 to understanding human evolution and the place of individual fossils in it. Theoretical and methodological considerations stem from the very history of our science and therefore require a change in perspective rather than a reanalysis of currently available data. 55 Genetics have added additional complexity to the issues of fossil taxonomy as some genetically well‐defined populations (like the Denisovans) are poorly defined skeletally. 5 , 56 , 57
“Species” is the “fundamental unit of classification recognized by the International Commission of Zoological nomenclature” 58 designed within the Linnaean system of binomial taxonomy. As such, it denotes the lowest classification of organisms that form a biologically relevant group. Linnaean taxonomy, as a systematic categorization of living beings, was developed in the 18th century prior to the development of evolutionary theory. Not surprisingly, the history of taxonomical thinking became increasingly complex as both the number of fossils and the range of variation of these fossils increased. The problem is further compounded by the need to use open nomenclature (qualifiers such as cf., aff., s.l., and s.s. 59 , 60 ) in fossil taxonomy. Furthermore, despite “many thousands of pages that have been spent arguing over species concept[s],” 61 the only one that has gained widespread acceptance (at least for the sexually reproducing organisms) is Ernst Mayr's 22 , 62 biological species concept (BSC) that uses reproductive isolation of terminal taxa as its foundation. 63 In defining fossil species, this concept is both implicit and fundamental to cladistic analysis. 61 Unfortunately, several problems are evident in applying BSC to fossil specimens: (1) morphological variation does not necessarily reflect reproductive isolation; (2) reproductive isolation is not absolute even in well‐defined living primate and other mammal species and crossbreeding has been observed even at the genus level, 64 , 65 and (3) as it does not include a temporal framework, BSC is ill‐suited to understanding or examining evolutionary change. 66 The evolutionary species concept (ESC) 67 was proposed as more appropriate for the fossil record, as it requires establishing the ancestor–descendant relationship (e.g., Australopithecus anamensis and Au. afarensis were proposed to represent parts of the same anagenetically evolving lineage 68 ). However, in cases where this relationship is more tenuous, ESC can result in a circular argument. Further, while chronology is important in phylogeny, it cannot be the cornerstone of taxonomic definition, because: (1) the assessed age is subject to change with improved methods; and (2) the parent and daughter species can persist alongside each other for longer in some areas. 65 , 69 , 70
Recently, Silcox 61 proposed a pragmatic, purely morphological, approach to species as a “minimum diagnosable unit,” which in the case of hominins allows us to examine the global distribution of variation and possible ancestor–descendant relationships within a genus based on cladistic analysis, without assuming (or even considering) the question of reproductive isolation. Using the papionins as an analogous model, Jolly 71 suggests that “any hominine species whose ancestries diverged less than 4 Ma previously may well have been able to produce hybrid offspring that could, by backcrossing, introduce alien genes with the potential of spreading if advantageous.” Despite early claims to the contrary, 25 , 72 , 73 , 74 the last 10 years of ancient DNA analyses 3 , 75 , 76 , 77 , 78 , 79 , 80 demonstrated substantial admixture among different hominin lineages. The extent and frequency of interbreeding among hominin terminal branches in the LP has been well established and recent research indicates that interbreeding can be observed in the Middle Pleistocene as well. 79 , 81
Further issues arise from the exceptionalist nature of palaeoanthropologists' approach to human evolution and taxonomy, compared to that of palaeontologists and evolutionary anthropologists. 82 , 83 For instance, when it comes to hominin species, chronology (and therefore ultimately the established scenarios) play an important role in taxonomic determination, which is considered (ideally) irrelevant to the established practice of zoological nomenclature. Ultimately, we do want to understand human evolution as a process, and chronology and phylogeny play essential roles in scenario‐building and determining the appropriateness (or not) of a particular classification. Further, hominins (especially the members of the genus Homo) represent a widely distributed polytypic taxon that displays great behavioral flexibility 84 , 85 , 86 and occupies a “generalist specialist” niche 87 that allows the members to exploit and adapt to different environmental conditions without significant alterations in morphology.
When it comes to Middle Pleistocene hominin evolution, we identify two main options, with the understanding that other possibilities may exist: (1) we could consider entire Pleistocene Homo fossils as a single lineage of H. sapiens with separate subspecies and/or chronospecies, 88 , 89 , 90 or (2) we could consider the observed morphological variation as taxonomically meaningful within the “practical” species concept, 61 without assuming that they were biological species and therefore not interfertile. Given that LP Neanderthals, Denisovans, and modern humans constitute sister taxa, we need to rethink the variability of the Middle Pleistocene hominin record. We find it unlikely that the observed Middle Pleistocene variability can be subsumed under a single taxon such as (nebulously defined) H. heidelbergensis. Middle Pleistocene hominin variation must be organized using better, more precise, and consistent criteria in defining taxa which comply at the same time with the rules of the International Code of Zoological Nomenclature (ICZN), as well as with current developments of our understanding of the process of human evolution.
4. MOVING FORWARD
Using the problematic taxon Homo heidelbergensis will continue to complicate and obfuscate how we think and communicate major issues in later phases of human evolution. To help resolve these issues, we recommend the following: Suppressing the taxa H. heidelbergensis and H. rhodesiensis and introducing a new taxon H. bodoensis.
- The taxon H. heidelbergensis should be suppressed
The taxon H. heidelbergensis sensu stricto should be suppressed altogether and those fossils reassigned to H. neanderthalensis in light of recent genetic and/or morphological data. Supporting this argument is the recent consensus that the Sima de los Huesos hominins should be considered as early members of the Neanderthal lineage. 8 , 27 , 46 , 91 , 92 Dating to at least 430 ka or Marine isotope stage 12, 93 , 94 the Sima hominin fossils already show hyper‐derived dentition, 8 as well as a number of Neanderthal derived traits in cranial and mandibular morphology. 91 The Arago hominins and other Middle Pleistocene Western European hominins show variable but ubiquitous derived Neanderthal traits. 95 As such, there is no need to introduce another species with the same morphology, in turn, making H. heidelbergensis a junior synonym to H. neanderthalensis and therefore redundant. In particular, if Mauer, as is currently considered, displays some derived Neanderthal traits 47 , 96 at 609 ± 40 ka, 97 it could represent an early specimen within the Neanderthal lineage. Recognition of the Western European Middle Pleistocene specimens as H. neanderthalensis, 98 does not preclude, however, the presence of other taxa in Europe (e.g., H. antecessor and possibly others). 46
The assignment of the Asian, particularly Chinese, archaic hominins into H. heidelbergensis should be abandoned (contra tentative suggestions by References 31, 33, 99). A number of researchers familiar with the Chinese record have never felt comfortable assigning the Chinese fossils into H. heidelbergensis. 36 , 37 , 100 For instance, comparisons of maximum and minimum frontal breadths on a range of fossils from Europe, Africa, and China showed that hominins like Petralona, Bodo, and Kabwe cluster relatively close together and well away from the Chinese fossils. 101 In perhaps the most comprehensive comparative study of nonmetric traits, Wu 100 , 102 , 103 (see also References 36, 104) identified the following features that differ between Middle Pleistocene hominins from the western and eastern parts of the Old World: “frontosphenoidal process of the zygomatic bone; upper facial height; maxillary shovel‐shaped incisors; Inca bones; M3 agenesis” and the nasal saddle. 37 For the most part, Chinese mid‐Pleistocene Homo do fall away from their western penecontemporaneous counterparts (e.g., H. bodoensis, H. neanderthalensis). The picture of Middle Pleistocene hominin variability in Asia is much more complex than originally anticipated, with the possibility of multiple lineages being present in the region at the same time, some that may have yet to be identified. 5 , 105 , 106
H. heidelbergensis sensu lato should be abandoned as well since it commonly includes all nonspecific Middle Pleistocene hominins, an approach that is not particularly informative. This taxon was previously considered as the most recent common ancestor (MRCA) of LP hominins, or minimally, the common ancestor of the African and European lineages (i.e., H. sapiens and Neanderthals, respectively). Since the MRCA of the modern human and Neanderthal lineages has been pushed further back in time toward the late Early Pleistocene or very early Middle Pleistocene, 8 the specimens currently assigned to H. heidelbergensis sensu lato cannot be considered representatives of the MRCA. This is a particularly pertinent point given that the split between African and Eurasian hominins has been recently proposed to be earlier than the split between the Denisovan and Neanderthal lineages. 3 As such, H. heidelbergensis sensu lato can no longer be considered the root of all African and European hominin lineages.
If the MRCA appears in the late Early Pleistocene or very early Middle Pleistocene, then none of the regional geographic variants (African, European, or Asian) from the Middle Pleistocene can serve as the MRCA of all three. There does exist a likely candidate however, that dates to the late Early Pleistocene. The tantalizing cranial fragments (a partial left parietal MK1 and the right portion of a frontal bone MK2) from Gombore II, Melka Kunture (Ethiopia), dated to ~850 ka, were interpreted by Profico et al. 107 as a possible ancestral form to the African Middle Pleistocene specimens. Given the estimated cranial capacity of 1080 cm3 the MK hominin could represent the MRCA for all Middle Pleistocene lineages that share an enlarged cranial capacity as one of its core traits. The MK cranial remains are generally considered to exhibit an “archaic” morphology. Signs of encephalization—enlarged braincase and more vertical parietal walls—coupled with a primitive morphology, are also observed in older East African specimens such as Daka and Buia. 107 Based on the current fossil record, this suggests East Africa around 1 Ma as the most likely region for the appearance of the MRCA of later Middle Pleistocene and LP hominins. - The taxon H. rhodesiensis should be suppressed
H. rhodesiensis Woodward 1921 108 never gained a wide usage in palaeoanthropology. Indeed, a quick search on the Web of Science provides 274 direct mentions of H. heidelbergensis while only 17 hits for H. rhodesiensis. In our opinion, there are two primary reasons for this: (1) the taxon is poorly defined and variably understood and used; and (2) the taxon name is associated with sociopolitical baggage that our scientific community is trying to dissociate itself from. We elaborate further below.
H. rhodesiensis has come to carry very different meanings. For instance, some see it as an African Middle Pleistocene taxon that parallels H. heidelbergensis sensu stricto in Europe, and that eventually gave rise to H. sapiens in Africa. 16 , 109 Alternatively, it has, at times, been considered as the MRCA to all LP hominin lineages, ancestral to both H. sapiens and Neanderthals. 27 , 35 , 110 It may be argued that if this taxon was considered as a Middle Pleistocene ancestor to the H. sapiens lineage exclusively, then we only need to redefine its hypodigm according to our current understanding. However, because this taxon has been defined in multiple ways it is impossible to dissociate it from these various definitions; thus, continuing to use H. rhodesiensis creates unnecessary confusion. It may be argued that Arthur Smith Woodward's morphological description of H. rhodesiensis, 108 which centered on its differences from Neanderthals complied with the nomenclature practice for pre‐1931 taxonomic names. However, the later resurrection of the taxon was based on similarities of the holotype Kabwe 1 with Petralona, first noted by Stringer, 111 and more recently by Friess. 112 Including Kabwe and Petralona in the same hypodigm resulted in an Afro‐European taxon. Paralleling the usage of H. heidelbergenis sensu lato for an Afro‐European MRCA, this lumping together of Middle Pleistocene specimens is contradicted by observed Neanderthal traits in Petralona and the early appearance of the Eurasian dental pattern. 113
At least part of the reason why H. rhodesiensis never became widely used by palaeoanthropologists stems from its pernicious political baggage. The name is associated with Cecil Rhodes and English mining colonialism and its abhorrent practices used by this self‐proclaimed owner of “Rhodesia” on local indigenous populations. 114 While these considerations are not at the root of our rejection of the name, they are not minor and should not be ignored. Discussions of hominin taxonomy cannot operate in a social void. 115 It requires a judicious evaluation of the social message that names are sending, as they have implications for our understanding of the process in the evolution of our own species. Decolonizing palaeoanthropology is an important task 116 that needs to take precedence over rigid taxonomic rules. The unfortunate reticence of ICZN to allow for a name change is best exemplified by Anophthalmus hitleri Scheibel 1937 117 —a carabid beetle found only in five caves in Slovenia—named as a dedication to Adolf Hitler. This was an honor that was not lost on either the infamous German Chancellor or the collectors of his memorabilia, 118 who have pushed the beetle A. hitleri to the brink of extinction by illegal collecting. 119 Despite this, its taxonomic name remains valid under the rules of ICZN. 120 There are growing criticisms of this traditional rigidness of naming rules in biology, 121 as they are (nor they should be) neither neutral nor absolute. - Introducing a new hominin taxon
We propose that, in addition to suppressing these two taxa, we need to add a new hominin taxon that is clearly defined following ICZN rules and does not carry any social‐political baggage. This taxon would have originated from the MRCA of European, Asian, and African Middle Pleistocene taxa sometime before the split of Eurasian taxa into Neanderthals and Denisovans and would represent the Middle Pleistocene ancestor of H. sapiens.
Here we introduce a new Middle Pleistocene (i.e., Chibanian Age/Stage, 774–129 ka 122 ) hominin species that represents the direct ancestor of H. sapiens (Figure 1). We propose that this new species be based on the Bodo skull and thus be named Homo bodoensis.
FIGURE 1.
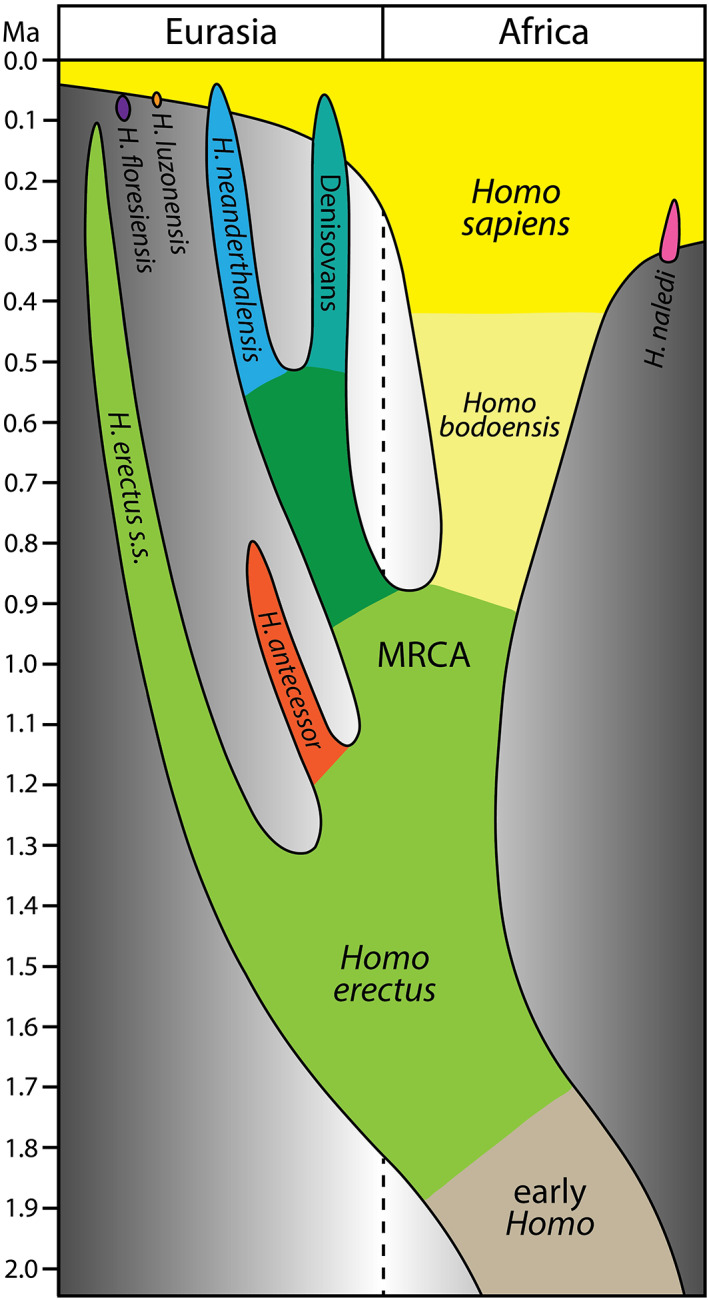
A simplified model for the evolution of the genus Homo over the last 2 million years, with Homo bodoensis sp. nov. positioned as the ancestral (mostly African) form of Homo sapiens
Order Primates Linnaeus 1758.
Suborder Anthropoidea Mivart 1864.
Superfamily Hominoidea Gray 1825.
Family Hominidae Gray 1825.
Tribe Hominini Gray 1825.
Genus Homo Linnaeus 1758.
Homo bodoensis sp. nov.
Etymology: The name bodoensis refers to the site of Bodo D'ar where the fossil specimen Bodo 1 was discovered. 123
Holotype: Bodo 1, a partial cranium of an adult (presumably male) individual, preserving the face and the anterior braincase, found in autumn 1976 by Alemayehu Asfaw, Paul Whitehead and other members of the Rift Valley Research Mission in Ethiopia headed by Jon Kalb. 123 , 124 The specimen is currently curated in the National Museum of Ethiopia in Addis Ababa, Ethiopia. H. bodoensis has been deposited in the ZooBank database (http://zoobank.org/) with Life Science Identifier urn:lsid:zoobank.org:act:50AC3EA4‐82E0‐4AAD‐BCDA‐6DE6055888A7.
Description (modified from References 20, 30, 35, 123, 125): Bodo 1 comprises a damaged facial skeleton, partial neurocranium, and basicranium anterior to the basion of a single individual, reconstructed from dozens of individual bone fragments (Figure 2). Aside from the fact that the lateral portion of the right maxilla, the right zygomatic bone, and the left temporal process are missing, the face is generally well preserved. The palate is missing the portion posterior to the P4, and except for some small fragments of the right molar roots, the teeth are not preserved, and the alveolar processes show damage. The neurocranium preserves an almost complete frontal bone, the sphenoid, parts of the left temporal and both parietals, and the right portion of the occipital bone. The basicranial portion includes the partially preserved left mandibular fossa and articular eminence, the basioccipital, and the petrous portion of the temporal bone. The face is strikingly massive, with large rectangular orbits and a very broad interorbital region, a wide nasal root and aperture, a deep and robust left zygomatic, and a broad and deep palate. Though projecting and heavily built, the supraorbital tori are arched, segmented (i.e., divided into medial and lateral segments), and attenuated laterally; they do not form a continuous bony shelf but are rather separated by a prominent glabellar region, behind which is a flattened plane (rather than a sulcus). There is a distinct sagittal keeling in the frontal view, especially in the bregmatic region of the vault. The maxillary sinus is expanded, and there is no canine fossa. The frontal sinuses are also extensive and asymmetrical (the right sinus is larger). In lateral view, the skull is long and low, and the frontal presents a low and flattened profile. There is a prominent parietal angular torus, and the temporal squama is high and arched. The anterior nasal aperture is almost vertical in the lateral projection. In the superior view, the skull presents a piriform shape, broadening posteriorly from the noticeable postorbital constriction. In the inferior view, the large incisive foramen is placed anterior on the hard palate, the mandibular fossa is shallow, and the preserved part of the articular eminence is flat; the petrous portion of the temporal is placed in such a way that the foramen lacerum displays a crevice‐like configuration. The endocranial capacity was estimated to ∼1250 cm3 (i.e., between ∼1200 and 1325 cm3). 125 The series of cut marks situated on the facial and posterior parietal regions were interpreted as intentional postmortem defleshing. 126
FIGURE 2.
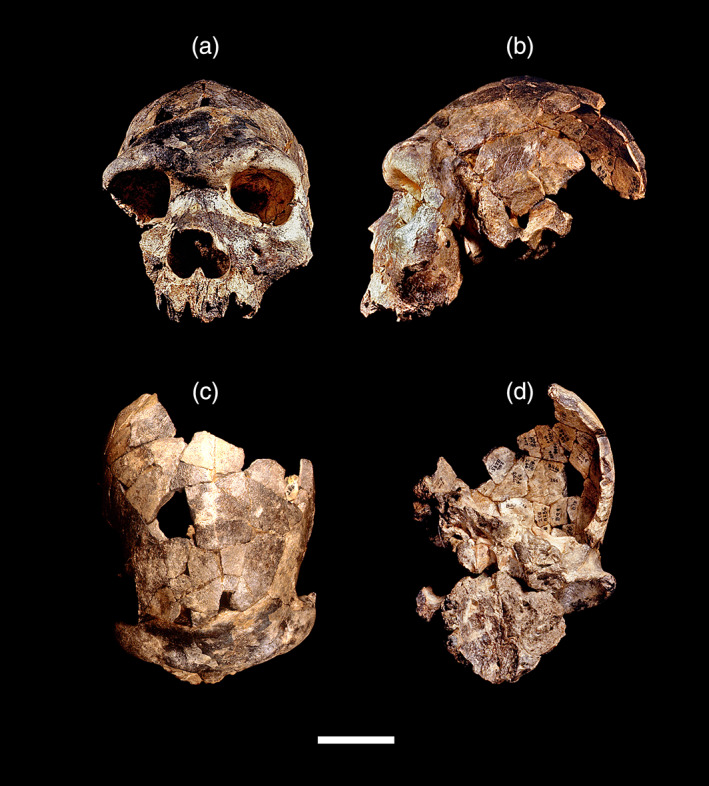
Homo bodoensis sp. nov. holotype partial cranium Bodo 1 (Middle Awash, Ethiopia). Frontal (a), left lateral (b), superior (c) inferior (d) views. Scale bar: 5 cm. _Source: Original photos Copyright © Jeffrey H. Schwartz
Type locality: Bodo D'ar, the Middle Awash research area, Afar Depression, the northwestern part of the former Hararghe Province, Ethiopia.
Geological age and stratigraphic position: Upper Bodo Sand Unit. 123 Dated to ca. 600 ka by laser‐fusion 40Ar/39Ar technique (0.64 ± 0.03 Ma), biostratigraphy and tephrochronology. 127
Archaeological context: The specimen is associated with an Acheulean stone tool assemblage. 123 , 125
Species diagnosis: The species is diagnosed by a unique combination of cranial traits. The Bodo specimen has already been described as showing a mix of H. _erectus_‐like and H. _sapiens_‐like features. 20 , 35 , 123 The species is similar to H. erectus in having: a robustly built midface; total facial prognathism 128 ; projecting tori and a flattened low frontal squama; sagittal keeling; a low vault profile; a prominent parietal angular torus; thick vault bones; no foramen lacerum is observable—it is presented as a narrow crevice. 20 , 128 These traits can be linked to the retention of the general cranial structure from H. erectus. Traits similar to other Middle Pleistocene and later hominin taxa include: increased cranial capacity and associated traits (broader frontal and mid‐vault, reduced postorbital constriction, signs of parietal bossing, high and arched temporal squama), a vertical (rather than forward sloping) nasal margin, and the position of the incisive canal in front of the hard palate. 20 , 99 , 128 Excessively thick and projecting, but segmented brow ridges, with the incipient division of the brow at mid‐orbit and attenuated laterally may be considered a distinctive trait of the species.
Comparisons: In comparison to H. _erectu_s, H. bodoensis differs by the increased cranial capacity (intermediate between H. erectus and H. sapiens) and a suite of associated derived traits: the curvature of the temporal squama; broader mid‐vault; signs of parietal bossing; and relatively broad frontal bone where the maximal cranial breadth lies above the lower third of the skull in posterior view, with more vertical parietal walls.
Increased cranial capacity is shared among most of the Middle Pleistocene hominins (excluding H. naledi and island isolates of Southeast Asia such as H. floresiensis). This trait is presumably already under selection in the MRCA in the latter portion of the Early Pleistocene. 107 , 129 Other features are not shared with Middle Pleistocene hominins such as H. neanderthalensis, late H. erectus, and potentially other Asian groups yet to be systematized. The species differs from H. neanderthalensis as it does not show any of the Neanderthal‐specific morphology associated with midfacial prognathism and neurocranial shape. It also differs in the particular form of the brow ridges, which are smoothly continuous and double‐arched in H. neanderthalensis. 32
H. bodoensis lacks a number of the H. _sapien_s specific features—warranting a separate species designation. This is contrary to what is observed in H. neanderthalensis where the autapomorphies emerge early in the Middle Pleistocene. However, all of the later H. sapiens specific features can be derived from traits present in H. bodoensis, including the massive but segmented (divided into lateral and medial parts) browridges. 20 , 34
Hypodigm: In addition to the holotype Bodo 1, the hypodigm is based on the sufficiently preserved cranial specimens with the exclusion of isolated mandibles and includes at a minimum: Kabwe 1 (Broken Hill), Ndutu, Saldanha (Elandsfontein), Ngaloba (LH 18), and potentially Salé in Africa. 30 , 108 , 130 , 131 , 132 , 133 , 134 , 135 Kabwe 1 could represent a late survivor of the taxon. 136 Some Middle Pleistocene specimens from Europe (e.g., Ceprano calvarium 137 , 138 ), could be included in this group as well. Locations, dating, previous taxonomic designations, and references for the included specimens are provided in the Table S1.
Distribution: The species had a pan‐African distribution with the peripheral range extending into the eastern Mediterranean (Southeast Europe and the Levant) from which it could have contributed to the repopulation of European (and possibly Central and East Asian) demographic sinks after the glaciations.
5. DISCUSSION AND CONCLUSIONS
Here, we present H. bodoensis as a new species and suggest that it is ancestral to H. sapiens. However, our new species is not to be considered the MRCA of Eurasian (Neanderthals, Denisovans) and African (H. sapiens) hominins. As schematically presented in Figure 1, H. bodoensis separated from the Eurasian groups before the split of the Eurasian forms into Neanderthals, Denisovans, and possibly other groups. While essentially an African species, H. bodoensis may have played a role in the evolutionary history of the Levant and Europe. In particular, Middle Pleistocene specimens from the two regions (mostly concentrated in the eastern Mediterranean), which do not demonstrate any Neanderthal traits, such as Mala Balanica (Serbia) and some specimens from the Levant such as Hazorea and Nadaouiyeh Aïn Askar (for review see Reference 46) could be considered as H. bodoensis. We did not include them in the H. bodoensis hypodigm at this stage, because these fossils are too fragmentary. However, the species was potentially present in Europe during the Middle Pleistocene (as evidenced by the Ceprano specimen) and may have contributed to a mixed morphology seen in Arago, Petralona, and possibly other fossils in Western Europe.
The newly defined species H. _bodoensi_s, described on the basis of the Bodo 1 specimen has clear advantages: (1) it recognizes the variability and geographic distribution of Middle Pleistocene hominins; and (2) it describes the unique morphology of the African Middle Pleistocene hominins that extends into the eastern Mediterranean that is distinct from H. neanderthalensis and predates the appearance of H. sapiens. While not a true species in the strict biological sense (since there is strong and growing evidence of migrations as well as gene flow between these diverged groups) this newly defined taxon cuts through the obfuscating and inconsistent use of improperly named and defined Middle Pleistocene hominins in Europe and Africa and should facilitate more consistent and meaningful discussions around these various topics presented here.
Supporting information
Table S1. Homo bodoensis sp. nov. proposed hypodigm, with locations (south to north), dating, and previous taxonomic designations for the included specimens.
ACKNOWLEDGMENTS
The authors wish to thank Jeffrey Schwartz and the National Museum of Ethiopia, Addis Ababa for the photographs of the Bodo 1 specimen. We thank Jason Kamilar for the invitation to contribute this Issues piece and to Joshua Lindal, Marta Mirazón Lahr and three anonymous reviewers for useful feedback on an earlier draft of this manuscript. We take full responsibility for any errors that may appear. Funding was provided by the Natural Science and Engineering Research Council of Canada NSERC RGPIN‐ 2017‐04702 and RGPIN‐2019‐04113 to MR and PR and the Chinese Academy of Sciences XDB26000000 to XW.
Biographies
Mirjana Roksandic is a Professor at the University of Winnipeg (Canada), whose main research topics include Pleistocene hominin evolution in the Balkans and the early peopling of the Caribbean, with field projects in Serbia and Cuba/Nicaragua. She is currently the president of the Palaeoanthropological Society of Canada (PASC/SCPA).
Predrag Radović, MSc is a PhD candidate at the Faculty of Mining and Geology and an Assistant at the Department of Archaeology, Faculty of Philosophy at the University of Belgrade (Serbia). He researches a variety of topics in vertebrate palaeontology and palaeoanthropology, including fossil mammals from the Paleogene and Neogene of Serbia, with a focus on primate evolution and the Middle to Late Pleistocene hominins from the Balkans.
Xiu‐Jie Wu is a Professor of Paleoanthropology at the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences. Her research area is Pleistocene hominin evolution, with a focus on cranial and endocranial studies.
Christopher J. Bae is a Professor of Anthropology at the University of Hawai'i at Manoa. He is a paleoanthropologist who has worked on a wide array of field and laboratory research projects in many different areas of eastern Asia, particularly related to Out of Africa 1 and modern human origins.
Roksandic M, Radović P, Wu X‐J, Bae CJ. Resolving the “muddle in the middle”: The case for Homo bodoensis sp. nov. Evolutionary Anthropology. 2022;31:20–29. 10.1002/evan.21929
Mirjana Roksandic and Predrag Radović should be considered joint first author.
Funding information Chinese Academy of Sciences, Grant/Award Number: XDB26000000; Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: RGPIN‐2019‐04113
Contributor Information
Mirjana Roksandic, Email: m.roksandic@uwinnipeg.ca.
Christopher J. Bae, Email: cjbae@hawaii.edu.
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- 1.Isaac GLL. Sorting out the muddle in the middle: an Anthropologist's post‐conference appraisal. In: Butzer KW, Isaac GLL, eds. After the Australopithecines ‐ Stratigraphy, Ecology and Culture Change in the Middle Pleistocene. Mouton; 1975:875‐887. [Google Scholar]
- 2.Hublin J‐J, Ben‐Ncer A, Bailey SE, et al. New fossils from Jebel Irhoud, Morocco and the pan‐African origin of Homo sapiens. Nature. 2017;546:289‐292. [DOI] [PubMed] [Google Scholar]
- 3.Prüfer K, Racimo F, Patterson N, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae CJ, Douka K, Petraglia MD. On the origin of modern humans: Asian perspectives. Science. 2017;358:eaai9067. [DOI] [PubMed] [Google Scholar]
- 5.Bae CJ, Douka K, Petraglia MD. Human colonization of Asia in the Late Pleistocene: an introduction to supplement 17. Curr Anthropol. 2017;58:S373‐S382. [Google Scholar]
- 6.Schlebusch C‐M, Malmstrom H, Gunther T, et al. Southern African ancient genomes estimate modern human divergence to 350,000 to 260,000 years ago. Science. 2017;358:652‐655. [DOI] [PubMed] [Google Scholar]
- 7.Hershkovitz I, Weber GW, Quam R, et al. The earliest modern humans outside Africa. Science. 2018;359:456‐459. [DOI] [PubMed] [Google Scholar]
- 8.Gómez‐Robles A. Dental evolutionary rates and its implications for the Neanderthal–modern human divergence. Sci Adv. 2019;5:eaaw1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvati K, Röding C, Bosman AM, et al. Apidima Cave fossils provide earliest evidence of Homo sapiens in Eurasia. Nature. 2019;571:500‐504. [DOI] [PubMed] [Google Scholar]
- 10.Bergström A, Stringer C, Hajdinjak M, Scerri EML, Skoglund P. Origins of modern human ancestry. Nature. 2021;590:229‐237. [DOI] [PubMed] [Google Scholar]
- 11.Norton CJ, Jin J. The evolution of modern humans in East Asia: behavioral perspectives. Evol Anthropol. 2009;18:247‐260. [Google Scholar]
- 12.Brown P. A new small‐bodied hominin from the late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055‐1061. [DOI] [PubMed] [Google Scholar]
- 13.Berger LR, Hawks J, de Ruiter DJ, et al. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. Elife. 2015;4:e09560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Détroit F, Mijares AS, Corny J, et al. A new species of Homo from the Late Pleistocene of The Philippines. Nature. 2019;568:181‐186. [DOI] [PubMed] [Google Scholar]
- 15.Schoetensack O. Der Unterkiefer des Homo heidelbergensis aus dem Sanden von Mauer bei Heidelberg: ein Beitrag zur Paläontologie des Menschen. Wilhelm Engelmann; 1908. [Google Scholar]
- 16.Stringer CB. Some further notes on the morphology and dating of the Petralona hominid. J Hum Evol. 1983;12:731‐742. [Google Scholar]
- 17.Rightmire GP. Homo erectus and later Middle Pleistocene humans. Ann Rev Anthropol. 1988;17:239‐259. [Google Scholar]
- 18.Adam KD. The chronological and systematic position of the Steinheim skull. In: Delson E, ed. Ancestors: The Hard Evidence. Alan R. Liss; 1985:272‐276. [Google Scholar]
- 19.Tattersall I. Species recognition in human paleontology. J Hum Evol. 1986;15:165‐175. [Google Scholar]
- 20.Rightmire GP. The human cranium from Bodo, Ethiopia: evidence for speciation in the Middle Pleistocene? J Hum Evol. 1996;31:21‐39. [Google Scholar]
- 21.Hennig W. Phylogenetic Systematics. University of Illinois Press; 1966. [Google Scholar]
- 22.Mayr E. The Growth of Biological Thought: Diversity, Evolution and Inheritance. Harvard University Press; 1982. [Google Scholar]
- 23.Wolpoff MH, Xinzhi W, Thorne AG. Modern Homo sapiens origins: a general theory of hominid evolution involving the fossil evidence from East Asia. In: Smith FH, Spencer F, eds. The Origins of Modern Humans: A World Survey of the Fossil Evidence. Liss; 1984:411‐483. [Google Scholar]
- 24.Cann R, Stoneking M, Wilson A. Mitochondrial DNA and human evolution. Nature. 1987;325:31‐36. [DOI] [PubMed] [Google Scholar]
- 25.Stringer CB, Andrews P. Genetic and fossil evidence for the origin of modern humans. Science. 1988;239:1263‐1268. [DOI] [PubMed] [Google Scholar]
- 26.International Code of Zoological Nomenclature. International Trust for Zoological Nomenclature; 1999. [Google Scholar]
- 27.Buck LT, Stringer CB. Homo heidelbergensis. Curr Biol. 2014;24:R214‐215. [DOI] [PubMed] [Google Scholar]
- 28.Rosas A. Two new mandibular fragments from Atapuerca/Ibeas (SH site). A reassessment of the affinities of the Ibeas mandibles sample. J Hum Evol. 1987;16:417‐427. [Google Scholar]
- 29.Howell FC. Paleo‐demes, species clades, and extinctions in the Pleistocene Hominin record. J Anthropol Res. 1999;55:191‐243. [Google Scholar]
- 30.Schwartz JH, Tattersall I. The human fossil record. Craniodental Morphology of Genus Homo (Africa and Asia). Vol 2. Wiley‐Liss; 2003. [Google Scholar]
- 31.Stringer C. The status of Homo heidelbergensis (Schoetensack 1908). Evol Anthropol. 2012;21:101‐107. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz JH, Tattersall I. Fossil evidence for the origin of Homo sapiens. Am J Phys Anthropol. 2010;143:94‐121. [DOI] [PubMed] [Google Scholar]
- 33.Groves CP, Lahr MM. A bush not a ladder: speciation and replacement in human evolution. Perspect Hum Biol. 1994;4:1‐11. [Google Scholar]
- 34.Rightmire GP. Human evolution in the Middle Pleistocene: the role of Homo heidelbergensis. Evol Anthropol. 1998;6:218‐227. [Google Scholar]
- 35.Rightmire GP. Homo in the Middle Pleistocene: hypodigms, variation, and species recognition. Evol Anthropol. 2008;17:8‐21. [Google Scholar]
- 36.Pope GG. Craniofacial evidence for the origin of modern humans in China. Yearb Phys Anthropol. 1992;35:243‐298. [Google Scholar]
- 37.Bae CJ. The late Middle Pleistocene hominin fossil record of eastern Asia: synthesis and review. Yearb Phys Anthropol. 2010;53:75‐93. [DOI] [PubMed] [Google Scholar]
- 38.Dart RA. The Makapansgat proto‐human Australopithecus prometheus. Am J Phys Anthropol. 1948;6:259‐284. [DOI] [PubMed] [Google Scholar]
- 39.Clarke RJ, Kuman K. The skull of StW 573, a 3.67 Ma Australopithecus prometheus skeleton from Sterkfontein Caves, South Africa. J Hum Evol. 2019;134:102634. [DOI] [PubMed] [Google Scholar]
- 40.Berger LR, Hawks J. Australopithecus prometheus is a nomen nudum. Am J Phys Anthropol. 2019;168:383‐387. [DOI] [PubMed] [Google Scholar]
- 41.Clarke RJ. Australopithecus prometheus was validly named on MLD 1. Am J Phys Anthropol. 2019;170:479‐481. [DOI] [PubMed] [Google Scholar]
- 42.Hawks J, Berger LR. Reply to Clarke, "Australopithecus prometheus was validly named on MLD 1". Am J Phys Anthropol. 2019;170:482‐483. [DOI] [PubMed] [Google Scholar]
- 43.Rightmire GP. The Evolution of Homo erectus. Comparative Anatomical Studies of an Extinct Human Species. Cambridge University Press; 1990. [Google Scholar]
- 44.Lahr MM, Foley RA. Towards a theory of modern human origins: geography, demography, and diversity in recent human evolution. Yearb Phys Anthropol. 1998;41:137‐176. [DOI] [PubMed] [Google Scholar]
- 45.Tattersall I, Schwartz JH. The morphological distinctiveness of Homo sapiens and its recognition in the fossil record: clarifying the problem. Evol Anthropol. 2008;17:49‐54. [Google Scholar]
- 46.Roksandic M, Radović P, Lindal J. Revising the hypodigm of Homo heidelbergensis: a view from the Eastern Mediterranean. Quat Int. 2018;466:66‐81. [Google Scholar]
- 47.Mounier A, Marchal F, Condemi S. Is Homo heidelbergensis a distinct species? New insight on the Mauer mandible. J Hum Evol. 2009;56:219‐246. [DOI] [PubMed] [Google Scholar]
- 48.Manzi G. Humans of the Middle Pleistocene: the controversial calvarium from Ceprano (Italy) and its significance for the origin and variability of Homo heidelbergensis. Quat Int. 2016;411:254‐261. [Google Scholar]
- 49.Mounier A, Lahr MM. Virtual ancestor reconstruction: revealing the ancestor of modern humans and Neandertals. J Hum Evol. 2016;91:57‐72. [DOI] [PubMed] [Google Scholar]
- 50.Skinner MM, de Vries D, Gunz P, et al. A dental perspective on the taxonomic affinity of the Balanica mandible (BH‐1). J Hum Evol. 2016;93:63‐81. [DOI] [PubMed] [Google Scholar]
- 51.Athreya S. Was Homo heidelbergensis in South Asia? A test using the Narmada fossil from Central India. In: Petraglia MD, Allchin B, eds. The Evolution and History of Human Populations in South Asia. Springer Press; 2007:137‐170. [Google Scholar]
- 52.Xiao D, Bae CJ, Shen G, et al. Metric and geometric morphometric analysis of new hominin fossils from Maba (Guangdong, China). J Hum Evol. 2014;74:1‐20. [DOI] [PubMed] [Google Scholar]
- 53.Roksandic M, Mihailović D, Mercier N, et al. A human mandible (BH‐1) from the Pleistocene deposits of Mala Balanica cave (Sićevo Gorge, Niš, Serbia). J Hum Evol. 2011;61:186‐196. [DOI] [PubMed] [Google Scholar]
- 54.Dubois A. The international code of zoological nomenclature must be drastically improved before it is too late. Bionomina. 2011;2:1‐1004. [Google Scholar]
- 55.Willermet CM, Clark GA. Paradigm crisis in modern human origins research. J Hum Evol. 1995;29:487‐490. [Google Scholar]
- 56.Bailey SE, Hublin J‐J, Antón CS. Rare dental trait provides morphological evidence of archaic introgression in Asian fossil record. PNAS. 2019;116:14806‐14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen F, Welker F, Shen C‐C, et al. A late Middle Pleistocene Denisovan mandible from the Tibetan Plateau. Nature. 2019;569:409‐412. [DOI] [PubMed] [Google Scholar]
- 58.Gittleman JL. Species; 2019. https://www.britannica.com/science/species-taxon
- 59.Bengtson P. Open nomenclature. Palaeontology. 1988;31:223‐227. [Google Scholar]
- 60.Sigovini M, Keppel E, Tagliapietra D. Open nomenclature in the biodiversity era. Methods Ecol Evol. 2016;7:1217‐1225. [Google Scholar]
- 61.Silcox MT. A pragmatic approach to the species problem from a paleontological perspective. Evol Anthropol. 2014;23:24‐26. [DOI] [PubMed] [Google Scholar]
- 62.Mayr E. Systematics and the Origin of Species. Columbia University Press; 1942. [Google Scholar]
- 63.Kimbel WH, Martin LB. Species, Species Concepts, and Primate Evolution. Plenum; 1993. [Google Scholar]
- 64.Ackermann RR, Arnold ML, Baiz MD, et al. Hybridization in human evolution: insights from other organisms. Evol Anthropol. 2019;28:189‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holliday T. Neanderthals and modern humans: an example of a mammalian syngameon? In: Bailey SE, Hublin J‐J, eds. Neanderthals Revisited: New Approaches and Perspectives. Springer; 2006:281‐297. [Google Scholar]
- 66.Eldredge N. What, if anything, is a species? In: Kimbel WH, Martin LB, eds. Species, Species Concepts, and Primate Evolution. Plenum; 1993:3‐20. [Google Scholar]
- 67.Simpson GG. Principles of Animal Taxonomy. Columbia University Press; 1961. [DOI] [PubMed] [Google Scholar]
- 68.Kimbel WH, Lockwood CA, Ward CV, et al. Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. J Hum Evol. 2006;51:134‐152. [DOI] [PubMed] [Google Scholar]
- 69.Hull DL. The limits of Cladism. Syst Biol. 1979;28:416‐440. [Google Scholar]
- 70.Curnoe D. Problems with the use of cladistic analysis in palaeoanthropology. Homo. 2003;53:225‐234. [DOI] [PubMed] [Google Scholar]
- 71.Jolly CJ. A proper study for mankind: analogies from the Papionin monkeys and their implications for human evolution. Am J Phys Anthropol. 2001;116:177‐204. [DOI] [PubMed] [Google Scholar]
- 72.Currat M, Excoffier L. Modern humans did not admix with Neanderthals during their range expansion into Europe. PLoS Biol. 2004;2:e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serre D, Langaney A, Chech M, et al. No evidence of Neandertal mtDNA contribution to early modern humans. PLoS Biol. 2004;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bräuer G, Broeg H, Stringer C. Earliest upper Paleolithic crania from Mladeč, Czech Republic and the question of Neanderthal–modern continuity: metrical evidence from the Fronto‐facial region. In: Harvati K, Harrison T, eds. Neanderthals Revisited: New Approaches and Perspectives. Springer; 2006:269‐279. [Google Scholar]
- 75.Green RE, Krause J, Briggs AW, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer M, Kircher M, Gansauge M‐T, et al. A high‐coverage genome sequence from an archaic Denisovan individual. Science. 2012;338:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prüfer K, de Filippo C, Grote S, et al. A high‐coverage Neandertal genome from Vindija cave in Croatia. Science. 2017;358:655‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pääbo S. The diverse origins of the human gene pool. Nat Rev Genet. 2015;16:313‐314. [DOI] [PubMed] [Google Scholar]
- 79.Posth C, Wißing C, Kitagawa K, et al. Deeply divergent archaic mitochondrial genome provides lower time boundary for African gene flow into Neanderthals. Nat Commun. 2017;8:16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villanea FA, Schraiber JG. Multiple episodes of interbreeding between Neanderthal and modern humans. Nat Ecol Evol. 2019;3:39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petr M, Hajdinjak M, Fu Q, et al. The evolutionary history of Neanderthal and Denisovan Y chromosomes. Science. 2020;369:1653‐1656. [DOI] [PubMed] [Google Scholar]
- 82.Tattersall I, Schwartz JH. Is paleoanthropology science? Naming new fossils and control of access to them. Anat Rec. 2002;269:239‐241. [DOI] [PubMed] [Google Scholar]
- 83.De Vos J, Reumer JWF. Human and mammalian evolution: is there a difference? In: Schwartz JH, ed. Rethinking Human Evolution. The MIT Press; 2018:53‐59. [Google Scholar]
- 84.Potts R. Variability selection in hominid evolution. Evol Anthropol. 1998;7:81‐96. [Google Scholar]
- 85.Foley RA, Lahr MM. On stony ground: lithic technology, human evolution, and the emergence of culture. Evol Anthropol. 2003;12:109‐122. [Google Scholar]
- 86.Plummer T. Flaked stones and old bones: biological and cultural evolution at the dawn of technology. Am J Phys Anthropol. 2004;125:118‐164. [DOI] [PubMed] [Google Scholar]
- 87.Roberts P, Stewart BA. Defining the 'generalist specialist' niche for Pleistocene Homo sapiens. Nat Hum Behav. 2018;2:542‐550. [DOI] [PubMed] [Google Scholar]
- 88.Wolpoff MH, Thorne AG, Jelinek J, Zhang Y. The case for sinking Homo erectus. 100 years of pithecanthropus is enough! Cour Forsch‐Inst Senckenberg. 1994;171:341‐361. [Google Scholar]
- 89.Wolpoff M. Paleoanthropology. 2nd ed. McGraw‐Hill; 1999. [Google Scholar]
- 90.Hawks J. Significance of Neanderthal and Denisovan genomes in human evolution. Ann Rev Anthropol. 2013;42:433‐449. [Google Scholar]
- 91.Arsuaga JL, Martínez I, Arnold LJ, et al. Neandertal roots: cranial and chronological evidence from Sima de los Huesos. Science. 2014;344:1358‐1363. [DOI] [PubMed] [Google Scholar]
- 92.Poza‐Rey EM, Gómez‐Robles A, Arsuaga JL. Brain size and organization in the Middle Pleistocene hominins from Sima de los Huesos. Inferences from endocranial variation. J Hum Evol. 2019;129:67‐90. [DOI] [PubMed] [Google Scholar]
- 93.Arnold LJ, Demuro M, Parés JM, et al. Luminescence dating and palaeomagnetic age constraint on hominins from Sima de los Huesos, Atapuerca, Spain. J Hum Evol. 2014;67:85‐107. [DOI] [PubMed] [Google Scholar]
- 94.Demuro M, Arnold LJ, Aranburu A, Sala N, Arsuaga JL. New bracketing luminescence ages constrain the Sima de los Huesos hominin fossils (Atapuerca, Spain) to MIS 12. J Hum Evol. 2019;131:76‐95. [DOI] [PubMed] [Google Scholar]
- 95.Bermúdez de Castro JM, Martinón‐Torres M, Martínez de Pinillos M, et al. Metric and morphological comparison between the Arago (France) and Atapuerca‐Sima de los Huesos (Spain) dental samples, and the origin of Neanderthals. Quat Sci Rev. 2019;217:45‐61. [Google Scholar]
- 96.Rosas A, Bermúdez de Castro JM. The Mauer mandible and the evolutionary significance of Homo heidelbergensis. Geobios. 1998;31:687‐697. [Google Scholar]
- 97.Wagner GA, Krbetschek M, Degering D, et al. Radiometric dating of the type‐site for Homo heidelbergensis at Mauer, Germany. PNAS. 2010;107:19726‐19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hublin J‐J. Climatic changes, paleogeography, and the evolution of the Neandertals. In: Akazawa T, Aoki K, Bar‐Yosef O, eds. Neandertals and Modern Humans in Western Asia. Plenum Press; 1998:295‐310. [Google Scholar]
- 99.Rightmire GP. Later Middle Pleistocene Homo. In: Henke W, Tattersall I, eds. Handbook of Paleoanthropology. 2nd ed. Springer; 2015:2221‐2242. [Google Scholar]
- 100.Wu X, Poirier FE. Human Evolution in China: a Metric Description of the Fossils and a Review of the Sites. Oxford University Press; 1995. [Google Scholar]
- 101.Liu W, Wu XJ, Zhang YY. The comparisons between the Middle Pleistocene human cranium from Bodo, Ethiopia and the Homo erectus of Zhoukoudian (in Chinese with English abstract). Acta Anthropol Sin. 2004;23:119‐129. [Google Scholar]
- 102.Wu XZ. Comparative study of early Homo sapiens from China and Europe. Acta Anthropol Sin. 1988;7:292‐299. [Google Scholar]
- 103.Wu X, Brauer G. Morphological comparison of archaic Homo sapiens crania from China and Africa. Z Morphol Anthropol. 1993;79:241‐259. [PubMed] [Google Scholar]
- 104.Bailey SE, Liu W. A comparative dental metrical and morphological analysis of a Middle Pleistocene hominin maxilla from Chaoxian (Chaohu), China. Quat Int. 2010;211:14‐23. [Google Scholar]
- 105.Xing S, Martinón‐Torres M, Bermúdez de Castro JM, et al. Middle Pleistocene Hominin teeth from Longtan Cave, Hexian, China. PLoS One. 2014;9:e114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaifu Y. Archaic Hominin populations in Asia before the arrival of modern humans: their phylogeny and implications for the “Southern Denisovans”. Curr Anthropol. 2017;58:S418‐S433. [Google Scholar]
- 107.Profico A, di Vincenzo F, Gagliardi L, Piperno M, Manzi G. Filling the gap. Human cranial remains from Gombore II (Melka Kunture, Ethiopia; ca. 850 ka) and the origin of Homo heidelbergensis. J Anthropol Sci. 2016;94:41‐63. [DOI] [PubMed] [Google Scholar]
- 108.Woodward AS. A new cave man from Rhodesia, South Africa. Nature. 1921;108:371‐372. [Google Scholar]
- 109.Bermúdez de Castro JM, Martinón‐Torres M, Sarmiento S, Lozano M. Gran Dolina‐TD6 versus Sima de los Huesos dental samples from Atapuerca: evidence of discontinuity in the European Pleistocene population? J Archaeol Sci. 2003;30:1421‐1428. [Google Scholar]
- 110.Hublin J‐J. The origin of Neandertals. Proc Natl Acad Sci U S A. 2009;106:16022‐16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stringer CB. A multivariate study of the Petralona skull. J Hum Evol. 1974;3:397‐404. [Google Scholar]
- 112.Friess M. Calvarial shape variation among middle Pleistocene hominins: an application of surface scanning in palaeoanthropology. C R Palevol. 2010;9:435‐443. [Google Scholar]
- 113.Martinón‐Torres M, Bermúdez de Castro JM, Gómez‐Robles A, et al. Dental evidence on the hominin dispersals during the Pleistocene. PNAS. 2007;104:13279‐13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mlambo AS. Racism in colonial Zimbabwe. In: Ratuva S, ed. The Palgrave Handbook of Ethnicity. Palgrave Macmillan; 2019:1‐17. [Google Scholar]
- 115.Ackermann RR, Athreya S, Black W, et al.. Upholding “good Science” in Human Origins Research: A Response to Chan et al (2019); 2019. 10.31730/osf.io/10.31730/osf.io/qtjfp [DOI]
- 116.Schroeder L. Revolutionary fossils, ancient biomolecules, and reflections in ethics and decolonization: paleoanthropology in 2019. Am Anthropol. 2020;122:306‐320. [Google Scholar]
- 117.Scheibel O. Ein neuer Anophthalmus aus Jugoslawien. Entomologische Blätter. 1937;33:438‐440. [Google Scholar]
- 118.Berenbaum M. ICE breakers. Am Entomol. 2010;56:132‐185. [Google Scholar]
- 119.Jóźwiak P, Rewicz T, Pabis K. Taxonomic etymology – in search of inspiration. ZooKeys. 2015;513:143‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heard S, Damastra E. The name of evil. In: Heard S, ed. Charles Darwin's Barnacle and David Bowie's Spider: How Scientific Names Celebrate Adventurers, Heroes, and Even a Few Scoundrels. Yale University Press; 2020:64‐72. [Google Scholar]
- 121.Athreya S, Hopkins A. Conceptual issues in hominin taxonomy: Homo heidelbergensis and an ethnobiological reframing of species. Yearb Phys Anthropol. 2021;2021:1‐23. doi: 10.1002/10.1002/ajpa.24330 [DOI] [PubMed] [Google Scholar]
- 122.Cohen KM, Harper DAT, Gibbard PL. ICS international Chronostratigraphic chart 2021/05. International Commission on Stratigraphy, IUGS; 2021. https://stratigraphy.org/
- 123.Conroy GC, Jolly CJ, Cramer D, Kalb JE. Newly discovered fossil hominid skull from the Afar depression, Ethiopia. Nature. 1978;276:67‐70. [DOI] [PubMed] [Google Scholar]
- 124.Kalb J. Adventures in the Bone Trade: The Race to Discover Human Ancestors in Ethiopia's Afar Depression. Copernicus; 2001. [Google Scholar]
- 125.Conroy GC, Weber GW, Seidler H, Recheis W, Zur Nedden D, Mariam JH. Endocranial capacity of the Bodo cranium determined from three‐dimensional computed tomography. Am J Phys Anthropol. 2000;113:111‐118. [DOI] [PubMed] [Google Scholar]
- 126.White TD. Cut marks on the Bodo cranium: a case of prehistoric defleshing. Am J Phys Anthropol. 1986;69:503‐509. [DOI] [PubMed] [Google Scholar]
- 127.Clark JD, de Heinzelin J, Schick KD, et al. African Homo erectus: old radiometric ages and young Oldowan assemblages in the middle Awash Valley, Ethiopia. Science. 1994;264:1907‐1910. [DOI] [PubMed] [Google Scholar]
- 128.Cartmill M, Smith FH. The Human Lineage. John Wiley & Sons; 2009. [Google Scholar]
- 129.Gómez‐Robles A, Smaers JB, Holloway RL, Polly PD, Wood BA. Brain enlargement and dental reduction were not linked in hominin evolution. PNAS. 2017;114:468‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clarke RJ. New cranium of Homo erectus from Lake Ndutu, Tanzania. Nature. 1976;262:485‐487. [DOI] [PubMed] [Google Scholar]
- 131.Rightmire GP. The Lake Ndutu cranium and early Homo sapiens in Africa. Am J Phys Anthropol. 1983;6:245‐254. [DOI] [PubMed] [Google Scholar]
- 132.Drennan M. The Saldanha skull and its associations. Nature. 1953;172:791‐793. [PubMed] [Google Scholar]
- 133.Day MH, Leakey MD, Magori C. A new hominid fossil skull (L.H.18) from the Ngaloba Beds, Laetoli, northern Tanzania. Nature. 1980;284:55‐56. [DOI] [PubMed] [Google Scholar]
- 134.Jaeger J‐J. The mammalian faunas and hominid fossils of the Middle Pleistocene of the Maghreb. In: Butzer KW, Isaac GLL, eds. After the Australopithecines ‐ Stratigraphy, Ecology and Culture Change in the Middle Pleistocene. Mouton; 1975:399‐418. [Google Scholar]
- 135.Hublin J‐J. Northwestern African Middle Pleistocene hominids and their bearing on the emergence of Homo sapiens. In: Barnham L, Robson‐Brown K, eds. Human Roots: Africa and Asia in the Middle Pleistocene. Western Academic and Specialist Press Limited; 2001:99‐121. [Google Scholar]
- 136.Grün R, Pike A, McDermott F, et al. Dating the skull from Broken Hill, Zambia, and its position in human evolution. Nature. 2020;580:372‐375. [DOI] [PubMed] [Google Scholar]
- 137.Ascenzi A, Biddittu I, Cassoli PF, Segre AG, Segre‐Naldini E. A calvarium of late Homo erectus from Ceprano, Italy. J Hum Evol. 1996;31:409‐423. [Google Scholar]
- 138.Manzi G, Magri D, Milli S, et al. The new chronology of the Ceprano calvarium (Italy). J Hum Evol. 2010;59:580‐585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Homo bodoensis sp. nov. proposed hypodigm, with locations (south to north), dating, and previous taxonomic designations for the included specimens.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.