Temporal and Spatial Arrangement of Lymphocytes within Lung Granulomas Induced by Aerosol Infection with Mycobacterium tuberculosis (original) (raw)
Abstract
The progression of the immune response in the lungs after aerosol infection with Mycobacterium tuberculosis is a complex cellular event dominated by macrophages and lymphocytes. Although the phenotype of lymphocytes participating in this response is becoming increasingly well characterized, the dynamic influx of these cells during the infection and their spatial arrangements within the lung tissue are still poorly understood. This study shows that in the first month after aerosol infection with M. tuberculosis there was a steady increase in the percentages of total CD3+, CD3+ CD4+ and CD3+ CD8+ cells, with consistently larger numbers of CD3+ CD4+ cells than of CD3+ CD8+ cells. As granuloma formation continued, the granuloma was found to consist of macrophages, CD4, and CD8 T cells, as well as a smaller number of B cells. Whereas CD4 T cells formed organized aggregates, CD8 T cells were fewer and more scattered and tended to be more prominent toward the periphery of the granulomas. The possible ramifications of the juxtapositions of these two major T-cell subsets are discussed.
It is estimated that one-third of the population worldwide has been infected with Mycobacterium tuberculosis, the causative agent of tuberculosis (26). Natural infection with M. tuberculosis occurs via the airway, where the bacillus infects the macrophages in the lungs. Vaccines and chemotherapeutic agents against M. tuberculosis infection do exist, but for multiple reasons they are unable to contain the epidemic (18). One of the main reasons for this failure is the lack of understanding of the pathogenesis and immune mechanisms that take place in the lungs during the infection.
Acquired immunity generated against M. tuberculosis infection develops slowly in the lungs (3, 5, 15), with bacterial growth tending to stop as this immunity appears (7, 16). Numerous studies using specific-gene-disrupted mice (4, 25) and immune T-cell transfer (14, 17) have demonstrated that immunity to M. tuberculosis infection is dependent on the emergence of specific subpopulations of T cells. It is well known that CD4 T-cell populations are critical for survival of this infection (4, 20), but it is also becoming apparent that other cell populations such as CD8 T cells (11, 13, 25), γδ T cells (1, 9), and NK cells may also be important, although to date their roles are far less well characterized. What is clear, however, is that the host response to M. tuberculosis infection is dependent on the production of gamma interferonγ by primed T cells (6, 12, 21), which in turn is dependent on interleukin-12 production by antigen-presenting cells (macrophages and dendritic cells) (8).
In the infected lung, these complex interactions take place in the context of a host tissue remodeling response called granuloma formation, and it is the construction of these structures that forms the hallmark of the disease. This process is complex and seems to follow a series of pathologically distinct stages, as we have previously described (22). However, the actual makeup of the granuloma in terms of which T-cell subsets enter and where they are subsequently to be found has not previously been documented.
In the present study, we have used flow cytometry to define the early influx of CD4 and CD8 T cells into the lungs and immunohistochemistry to define their relative distribution. The results obtained indicate that these two major subsets occupy discrete and different patterns within the overall granuloma, which are presumably directly related to their functions during the active and chronic phases of the disease process.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice, 6 to 8 weeks of age, were purchased from Jackson Laboratory, Bar Harbor, Maine. The mice were maintained in a specific-pathogen-free biosafety level-3 facility. All animals had free access to water and standard mouse chow. The pathogen-free nature of mouse colonies was monitored by testing sentinel animals for 12 known mouse pathogens. The mice were negative for all the pathogens.
Bacteria and infection.
M. tuberculosis strain Erdman was grown to mid-log phase from low-passage seed lots in Proskauer-Beck liquid medium containing 0.02% Tween 80, aliquoted, and frozen at −70°C until use. Mice were infected via the aerosol route with a low dose of bacteria. Briefly, the nebulizer compartment of a Middlebrook airborne-infection apparatus (Glas-Col, Terre Haute, Ind.) was filled with a suspension of bacteria, resulting in the delivery of approximately 100 bacteria per lung during 30 min of exposure. The data are representative of two independent experiments, with 12 mice per time point (4 mice were used for the viable-bacterium count; 6 mice, 4 infected and 2 uninfected, were used for flow cytrometric analysis; and 2 mice were used for immunohistochemistry analysis).
The number of viable bacteria in the lungs of the mice was determined by plating serial dilutions of individual whole homogenized lungs onto nutrient Middlebrook 7H11 agar and counting bacterial colony formation after 3 weeks of incubation at 37°C in humidified air. The data were expressed as the log10 mean number of colonies counted (n = 4 animals).
Isolation of cells from infected lungs.
Briefly, mice were euthanized and the pulmonary cavity was opened. The blood circulatory system in the lungs was cleared by perfusion through the pulmonary artery with 3 ml of saline containing 50 U of heparin per ml (Sigma-Aldrich, St. Louis, Mo.). The lungs were aseptically removed and cut into small pieces in cold RPMI 1640 medium. The dissected tissue was then incubated for 30 to 45 minutes at 37°C in RPMI medium containing collagenase XI (0.7 mg/ml; Sigma-Aldrich) and type IV bovine pancreatic DNase (30 μg/ml; Sigma-Aldrich). The action of the enzymes was stopped by adding 10 ml of RPMI 1640 medium, and the digested lungs were further disrupted by gently pushing the tissue through a nylon screen. The single-cell suspension was then washed and centrifuged at 200 × g for 5 min. To lyse the remaining contaminating red blood cells, the cell pellet was incubated for 5 min at room temperature with 5 ml of Gey's solution (NH4Cl plus KHCO3). Cells were resuspended in deficient RPMI medium (Irvine Scientific, Santa Ana, Calif.) which was supplemented with 1% l-glutamine, 1% HEPES, 0.1% N3Na, and 2% fetal bovine serum for flow cytometric studies.
Flow cytometry.
Monoclonal antibodies specific for mouse CD3 (145-2C11, hamster immunoglobulin G1 [IgG1]; or 17A2, rat IgG2b), CD4 (L3T4 clone GK1.5, rat IgG2b), CD8 (53-6.7, rat IgG2a), or CD45R/B220 (RA3-6B2, rat IgG2a) or rat IgG1 (R3-34), rat IgG2a (R35-95), rat IgG2b (A95-1), or hamster IgG (Ha4/8) were purchased from PharMingen (San Diego, Calif.) as direct conjugates to fluorescein isothiocyanate, or peridinin chlorophyll a protein (PerCP) or in a purified form. Cell suspensions for each individual mouse were stained with specific monoclonal antibody against murine CD3 and either CD4, CD8, or CD45R/B220.
Lung cells were washed in dRPMI medium, stained for 30 min on ice with direct conjugated antibodies, and washed twice with dRPMI. Acquisition was performed on a FACscalibur instrument (Becton Dickinson, Mountain View, Calif.), and data were analyzed using CellQuest software (Becton Dickinson). Cells were gated for lymphocytes by their characteristic forward- and side-scatter profile, and 30,000 events in the lymphocyte gate per sample were counted. Due to cell aggregation and excessive clumping of lung cell suspensions after 50 days postinfection, we were unable to collect data from later time points of infection.
Immunohistochemistry.
Lungs from C57BL/6 mice were infused with 30% OCT (Tissue-Tek, Inc., Torrance, Calif.) in phosphate-buffered saline (PBS) through the trachea. After the lungs were removed from the pulmonary cavity, they were embedded in OCT, frozen in a bath of liquid nitrogen for a few seconds, and then stored at −70°C. Serial sections, 5 to 7 μm thick, from each lung were cut on a cryostat (Leica CM 1850), employing the Instrumedics Inc. (Hackensack, N.J.) tape transfer system, fixed in cold acetone for 10 min, and air dried. After the endogenous peroxidase was blocked by incubating for 5 to 10 min at room temperature with peroxidase block (Innogenex, San Ramon, Calif.), the sections were washed and incubated for 30 min with power block reagent (Innogenex) and then washed in PBS for another 5 min. Purified primary antibodies, at the appropriate concentration, were incubated overnight at 4°C. Other sections were incubated with the isotype control IgG2a or IgG2b. All sections were washed three times in PBS and incubated with the secondary detection antibody goat F(ab′)2 anti-rat Ig conjugated to horseradish peroxidase (Biosource International, Camarillo, Calif.). Finally, the reaction was developed using aminoethylcarbazole (Innogenex) or diaminobenzine (Ventana Medical Systems, Tucson, Ariz.) as substrate. Sections were counterstained with Meyer's hematoxylin and covered with crystal/mount (Biomeda corp, Foster City, Calif.). Immunohistochemical testing of lung tissue was performed in independent studies by two scientists using similar standard methods. Both produced very similar and reproducible results.
RESULTS
Course of M. tuberculosis infection in the lungs.
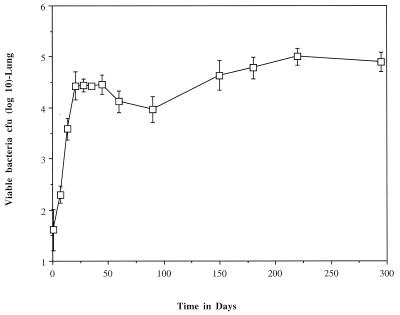
The course of the infection is shown in Fig. 1. After an initial phase of growth, a chronic disease ensued with little change in bacterial numbers.
FIG. 1.
Course of infection in the lungs of C57BL/6 mice following low-dose aerosol exposure to M. tuberculosis Erdman. Data are representative of three experiments and are expressed as the mean number of viable bacteria at 0, 7, 14, 21, 28, 35, 45, 60, 90, 150, 180, 220, and 295 days p.i. Standard deviations are indicated by vertical bars. Four mice were used at each time point.
Specific T-cell populations in lungs during early infection.
The total number of cells obtained by lung digestion on different days after aerosol exposure increased with time. The total number of cells 0, 7, 14, 28, and 35 days after aerosol exposure to infection (p.i.) was 2.8 ± 0.7, 3.6 ± 0.3, 4.1 ± 0.3, 6.8 ± 1.9, and 11.6 ± 1.7, respectively (mean ± standard deviation).
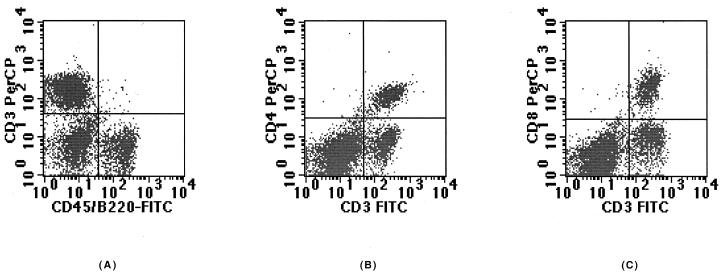
The lymphocyte populations in the lungs of both uninfected and _M. tuberculosis_-infected mice were composed of two major distinct classes: T cells (CD3+ cells) and B cells (CD45R/B220+ cells) (Fig. 2A). In addition, all CD4+ cells (Fig. 2B) and CD8+ cells (Fig. 2C) expressed the CD3 molecule; this expression is therefore characteristic of CD4 and CD8 T cells.
FIG. 2.
Flow cytometric dot plot analysis for CD3+, CD4+, CD8+, and CD45/RB220+ positive lymphocytes obtained after counting 30,000 events. Data are from one mouse infected for 35 days and are representative of the experimental group (eight mice from two independent experiments).
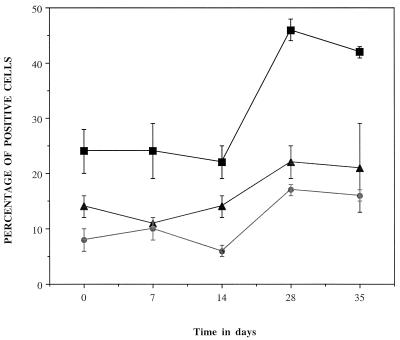
The percentages of T- and B-cell populations in the lungs of mice infected with M. tuberculosis in different days after aerosol exposure are shown in Fig. 3. The total number of CD3+T lymphocytes in the lungs of uninfected (0 days p.i.) mice accounted for all the CD3+ CD4+ plus CD3+ CD8+ cells. After infection, the percentage of CD3+T lymphocytes in the lungs increased, peaking at 28 days p.i. (46% ± 2% of the total number of lymphocytes). This was a twofold increase compared with the percentage in uninfected mouse lungs (24% ± 4% of the total number of lymphocytes). In the same way, the percentage of CD3+ CD4+ and CD3+ CD8+T lymphocytes also peaked on day 28 p.i. These showed an approximate 1.5- and 2-fold increase (22% ± 3% and 17% ± 1%, respectively, of the total number of lymphocytes) compared with the values on day 0 p.i. (14% ± 2% and 8% ± 2%, respectively, of the total number of lymphocytes).
FIG. 3.
Flow cytometric assessment of the percentages of CD3+ (■), CD3+ CD4+ (▴), and CD3+ CD8+ (●) cells in the lungs during the first 35 days of infection. Cells were gated for lymphocytes by their characteristic forward- and side-scatter profile, and 30,000 events in the lymphocyte gate per sample were counted. Data are expressed as the mean percentage of positive cells from four individual mice. Standard deviations are indicated by the vertical bars. Data are representative of two independent experiments.
CD45/RB220+ cell lymphocytes constituted 20 to 30% of the total number of lymphocytes in the lungs of uninfected mice. During infection, the total percentage of B cells was between 25 and 30% at all subsequent time points (data not shown).
CD4- and CD8-positive lymphocytes are found at distinct locations within the lymphoid aggregates of developing pulmonary granulomatous lesions.
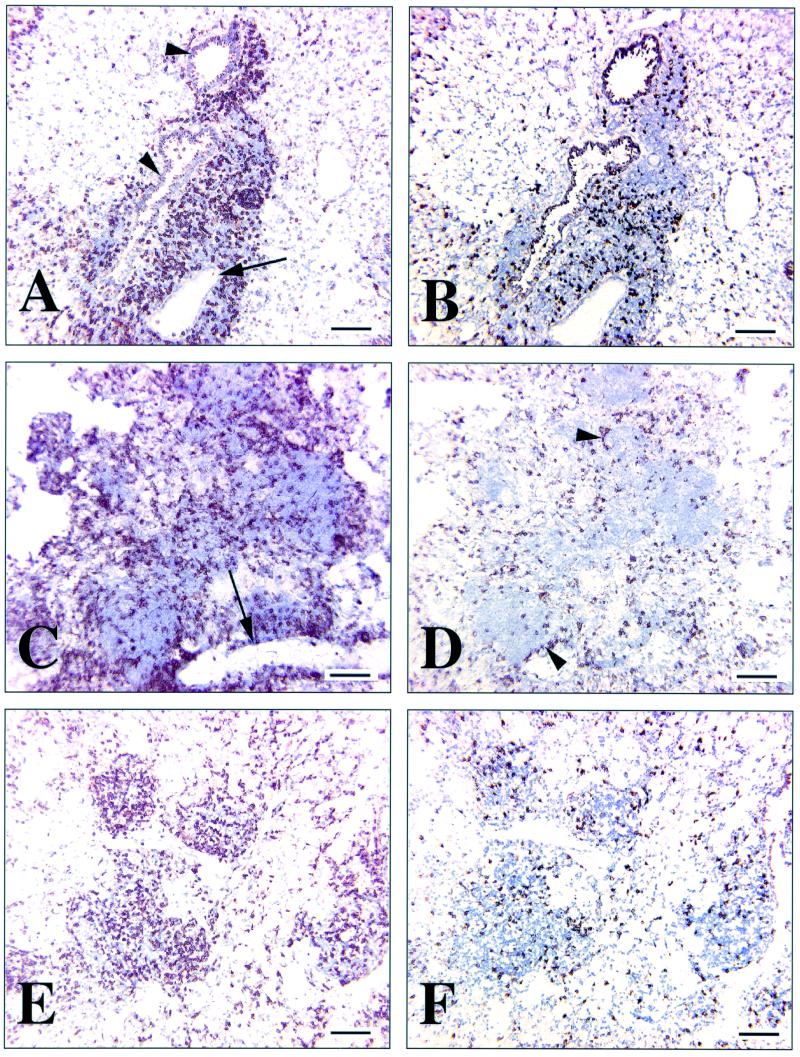
At 30 days p.i., multiple blood vessels and associated airways were surrounded by dense lymphocytic cuffs. Immunohistochemical staining for CD4 and CD8 cells demonstrated that the majority of these cells were CD4+ (Fig. 4A). CD8+ cells were less abundant, and although positive cells were seen at all levels of the cellular infiltration, they were conspicuous at the margins (Fig. 4B). By day 100 p.i. (Fig. 4C), distinct granulomas were present within the pulmonary parenchyma. There were now distinct lymphocytic aggregates (away from the perivascular and peribronchial cuffs) within sheets of epithelioid macrophages. These aggregates were also composed predominantly of CD4+ cells (Fig. 4C), with fewer CD8+ cells. Most of these CD8+ cells were present at the margins of the aggregates (Fig. 4D). At 250 days p.i., the granulomas had enlarged and the lymphocytic aggregates had increased in number. Once again, they were composed mainly of CD4+ cells (Fig. 4E), with fewer CD8+ cells (Fig. 4F). In this instance, however, the CD8+ cells were more evenly spread throughout all levels of the aggregates (Fig. 4F).
FIG. 4.
Immunohistochemical staining of CD4+ and CD8+ lymphocytes in frozen sections of lung tissue from C57BL/6 mice at sequential time points after low-dose aerosol infection with M. tuberculosis. Each field is representative of the pulmonary lymphocytic accumulation at the indicated time point. (A) CD4+ staining of lymphocytes after 30 days. Note the presence of a dense accumulation of positive cells around airways (arrowheads) and a vein (arrow). There were a few scattered positive cells in the surrounding parenchyma. (B) CD8+ staining of lymphocytes after 30 days. Note the sparse distribution of positive cells within the same peribronchiolar and perivascular area. There were a few positive cells in the surrounding parenchyma. (C) CD4+ staining of lymphocytes after 100 days. Note the dense accumulation of positive cells throughout the lesion, which is associated with a large vein (arrow). (D) CD8+ staining of lymphocytes after 100 days. Note the sparse distribution of positive cells throughout the lesion, with increased numbers and aggregation of these cells at the periphery of the lesion (arrowheads). (E) CD4+ staining of cells lymphocytes after 250 days. Note the dense accumulation of positive cells throughout the lesion. (F) CD8+ staining of lymphocytes after 250 days. Note the sparse distribution of CD8+ cells throughout the lesion. These are serial sections with 5 to 10 μm separating each couplet and are representative of three experiments. Ba, 100 μm.
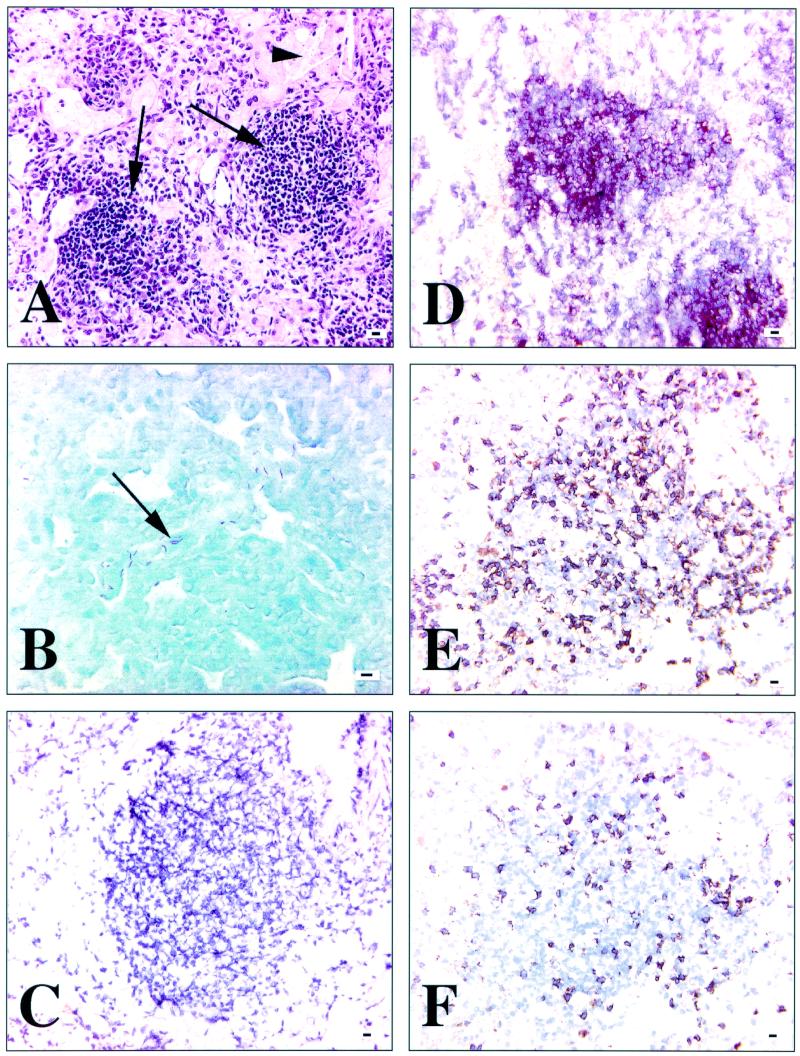
Specific lymphocyte populations are found at distinct locations within the chronic pulmonary granuloma.
Mice infected with M. tuberculosis for 220 days or longer typically had large granulomas, characterized by distinct aggregates of lymphocytes within sheets of epithelioid macrophages. These were selected for further immunohistochemical analysis (Fig. 5A). To confirm the presence of bacilli, staining with Kinyoun's acid-fast stain was performed (Fig. 5B). In each experiment, an isotype control staining procedure, consisting of either IgG2a or IgG2b, was performed (Fig. 5C). CD3+ cells were present throughout the lymphoid aggregates; however, they did not account for all of the cells (data not shown). This led us to believe that some of the lymphocytes within the granuloma could also be B lymphocytes. Staining of tissue sections with monoclonal antibody CD45R/B220, which recognizes all stages of B-cell differentiation, revealed that there were indeed CD45R/B220+ cells within the granuloma (Fig. 5D). The highest concentration of CD45R/B220+ cells was present within the central portion of the lymphoid aggregates and along the perivascular lymphoid cuffs, often “overlying” CD3+ lymphocytes (data not shown). CD4+ and CD8+ cells were distributed as described previously, with both cell types being evenly spread throughout the lymphoid aggregates and with the majority being CD4+ (Fig. 5E and F).
FIG. 5.
Immunohistochemical staining of frozen sections of lungs from C57BL/6 mice with chronic M. tuberculosis infection (220 to 280 days p.i.). Panels A and B are paraffin sections, and panels C to F are frozen sections. (A) Hematoxylin and eosin stain after 250 days. Islands of lymphocytes (arrows) surrounded by epithelioid macrophages. Cholesterol clefts (arrowhead) are indicative of chronic disease. (B) Kinyoun's acid-fast stain after 250 days. Note the multiple red, rod-shaped bacilli. (C) Isotype control after 280 days. Note no positive staining. (D) CD45/RB220+ staining of lymphocytes using AEC chromogen after 280 days. Specific positive staining is concentrated in the central portion of the lymphoid aggregate. (E) CD4+ staining of lymphocytes using DAB chromogen after 250 days. There is abundant positive staining throughout the lymphoid aggregate. (F) CD8+ staining of lymphocytes using DAB chromogen after 250 days. There is sparse positive staining throughout the same lymphoid aggregate as represented in panel E. All images are representative of three experiments. Bars, 10 μm.
DISCUSSION
The results of this study show that over the first month following exposure of mice to pulmonary tuberculosis, there is an influx of CD4 and CD8 T cells into the lungs. However, as the granuloma continues to grow and become more organized, the primary aggregates of T cells that are characteristic of this structure in the mouse model (22) were predominantly of the CD4 type, whereas CD8 T cells tended to be more scattered. In addition, interestingly, many of the aggregates contained a significant number of B cells.
Histologic examination of the lung early during the course of the infection suggested that both CD4 and CD8 T cells accumulated in a perivascular and peribronchiolar fashion as initial interstitial pneumonia began to develop into the beginnings of the granulomatous response, suggesting that both subsets were receiving inflammatory signals and possessed the correct expression of adhesion markers to allow them to cross these inflamed vessels. However, as the granuloma developed and the disease became chronic, it was clear that the great majority of the T cells within the lymphocyte aggregates were CD4 T cells. We have hypothesized elsewhere (19) that T cells in the mouse response infiltrate into the epithelioid macrophage field and form these structures, but it remains unknown whether this is because of a constant stream of such cells migrating from the regional arterioles or whether these aggregates arise from lymphocyte proliferation once they have arrived.
In contrast, the pattern of distribution of the CD8 cells suggest that their migration into the granulomas is much slower and that fewer cells are involved. Because of this much slower influx, the CD8 cells seemed to be more closely associated with the periphery of the granuloma. These kinetics of accumulation of the CD8 T-cell subset are in keeping with our observations (25) that mice in which the CD8 gene has been disrupted control and contain a pulmonary infection in a normal manner but the bacterial load in the lungs gradually increases as the chronic stage of the disease process ensues. As such, therefore, these data point to an important role for CD8 T cells in perpetuating the chronic disease state. This may take the form of immunosurveillance, designed to detect any possible dissemination of bacteria into macrophages away from the centers of the granuloma, which would be in keeping with the more peripheral distribution of this T-cell subset.
Assuming this hypothesis is correct, the way these cells accomplish this remains a matter of debate. Some recent studies indicate that the CD8 cells in the lungs are cytotoxic (23), but our own recent data suggest that perforin-deficient mice maintain a chronic disease state and instead favor the possibility that destruction of infected cells by CD8 T cells occurs is via an apoptotic mechanism (25). This mechanism is attractive, because it avoids cell necrosis and local tissue damage, which could otherwise be lethal.
It was also evident from these studies that the granulomas contained an appreciable number of B cells. This explains some recent observations (2; J. Turner, A. A. Frank, J. V. Brooks, M. Gonzalez-Juarrero, and I. M. Orme, submitted for publication) that granulomas in chronically infected B-cell-gene-disrupted mice are much smaller than in controls, but it casts no light on the reason why these cells are present in the first place. On one hand, it is possible that these cells are the source of antibodies to mycobacterial antigens, and indeed it has been suggested that these may even contribute to protection (24). On the other hand, their arrival may be purely accidental, driven by the sustained expression of adhesion molecules in the chronically inflamed lung (10; J. Turner, M. Gonzalez-Juarrero, B. M. Saunders, J. V. Brooks., P. Marietta, D. L. Ellis, A. A. Frank, A. M. Cooper, and I. M. Orme, submitted for publication) that could attract an influx of B cells equally as well as it could attract T cells.
In summary, to our knowledge this study provides the first description of the spatial and temporal distribution of the two major T-cell subsets within the developing lung granuloma. These data, combined with recent data from the mouse gene knockout models, seem to indicate clear distinctions between the roles of the CD4 and CD8 subsets, with the former playing an important role in the expression of acquired resistance and the latter playing another important role in maintenance of the integrity of the granuloma once a stable form of chronic disease has become established. Isolation of these lung CD8 T cells and determination of their antigen recognition may provide important information for rational vaccine design and could be used specifically to prevent reactivation disease arising from the chronic/latent state.
ACKNOWLEDGMENT
This work was supported by NIH grant AI-44072.
REFERENCES
- 1.Boom W H, Chervenak K A, Mincek M A, Ellner J J. Role of the mononuclear phagocyte as an antigen-presenting cell for human γδ T cells activated by live Mycobacterium tuberculosis. Infect Immun. 1992;60:3480–3488. doi: 10.1128/iai.60.9.3480-3488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosio C, Gardner D, Elkins K L. Infection of B cell-deficient mice with CDC1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164:6417–6425. doi: 10.4049/jimmunol.164.12.6417. [DOI] [PubMed] [Google Scholar]
- 3.Cardona P J, Cooper A M, Luquin M, Ariza A, Filipo F, Orme I M, Ausina V. The intravenous model of murine tuberculosis is less pathogenic than the aerosol model owing to a more rapid induction of systemic immunity. Scand J Immunol. 1999;49:362–366. doi: 10.1046/j.1365-3083.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Caruso A M, Serbina N, Klein E, Triebold K, Bloom B R, Flynn J L. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 5.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A M, Flynn J L. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 8.Cooper A M, Magram J, Ferrante J, Orme I M. IL-12 is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–46. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza C D, Cooper A M, Frank A A, Mazzaccaro R J, Bloom B R, Orme I M. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 10.Feng C G, Britton W J, Palendira U, Groat N L, Briscoe H, Bean A G D. Up-regulation of VCAM-1 and differential expansion of beta integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J Immunol. 2000;164:4853–4860. doi: 10.4049/jimmunol.164.9.4853. [DOI] [PubMed] [Google Scholar]
- 11.Feng C G, Bean A D, Hool H, Briscoe H, Britton W J. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in the lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67:3242–3247. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefford M J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975;11:1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North J N. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than the intravenous route. J Infect Dis. 1995;172:1550–1553. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]
- 16.Orme I, Andersen P, Boom W. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 17.Orme I, Collins F. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme I M. Beyond BCG: the potential for a more effective TB vaccine. Mol Med Today. 1999;5:487–492. doi: 10.1016/s1357-4310(99)01594-4. [DOI] [PubMed] [Google Scholar]
- 19.Orme I M. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 20.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 21.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 22.Rhoades E, Frank A, Orme I. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 23.Serbina N V, Liu Chau-Ching, Scanga Charles A, Flynn J L. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins J B, Unanue E, Casadevall A, Bloom B R. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner, J., C. D. D'Souza, J. E. Pearl, P. Marietta, M. Noel, A. A. Frank, R. Appelberg, I. M. Orme, and A. M. Cooper. CD8− and CD95/95L dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. in press. for publication. [DOI] [PubMed]
- 26.World Health Organization. The world health report 1999. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]