Major Carbohydrate Antigen of Echinococcus multilocularis Induces an Immunoglobulin G Response Independent of αβ+ CD4+ T Cells (original) (raw)
Abstract
Echinococcus multilocularis causes alveolar echinococcosis, one of the most lethal helminthic (accidental) infections in humans, as the life cycle predominantly includes wildlife rodents as intermediate hosts. The physical barrier between the proliferating parasitic metacestode and the host tissue is the acellular laminated layer (LL), which is characterized by its rich high-molecular-weight polysaccharide composition. Conversely to a crude protein-rich vesicular fluid antigen, a major carbohydrate antigen of the LL—the Em2(G11) antigen—did not stimulate murine T-cell proliferation in vitro. In fact, the persistent metacestode growth and antigenic stimulation induced a Th2 shift in vivo following conventional infection by intraperitoneal inoculation of 100 metacestode vesicles into C57/BL6 mice. Concurrently, the expression of Th1 cytokines (interleukin-2 and gamma interferon) remained persistently low until the late stage of chronic infection. In comparison to a recombinant proteinic II/3 antigen, the specific immunoglobulin G (IgG) response against the Em2(G11) antigen (including all IgG isotypes) maintained persistently low avidity. Furthermore, the Em2(G11) antigen induced a specific IgM and IgG response in T-cell-deficient athymic nude, TCRβ−/−, major histocompatibility complex class II (MHCII)−/−(CD4-deficient), and CD40−/− mice. The Em2(G11)-specific IgG synthesized in nude TCRβ−/− and MHCII−/− mice was predominantly of the IgG3 and IgG2a isotypes and of the IgG3 and IgG2b isotypes in CD40−/− mice. This finding suggested that in vivo, the IgG response to major carbohydrate antigen Em2(G11) of E. multilocularis could take place independently of αβ+ CD4+ T cells and in the absence of CD40-CD40 ligand interactions; thus, the Em2(G11) antigen of the acellular LL represents a T-cell-independent antigen. Functionally, the encapsulating LL, and especially its major carbohydrate antigen, Em2(G11), seems to be one of the key factors in the parasite's survival strategy and acts by modulating the host immune response by virtue of its T-cell-independent nature.
Alveolar echinococcosis (AE) is a severe hepatic disorder caused by infection with the metacestode stage of a small fox tapeworm, Echinococcus multilocularis (26, 27). Despite the public health importance of AE in areas such as Central Europe, Alaska, China, and others, knowledge of the parasite's survival strategy, parasite-host interactions, and immune control of E. multilocularis infection is still not satisfactory with respect to molecular parasite components, in contrast to the already well-explained imbalanced host immune response (2, 15, 17, 24, 30, 45).
Experimental studies on E. multilocularis infection have been carried out mostly with the laboratory mouse model by intraperitoneal or intrahepatic inoculation of metacestode material (13, 27). The metacestode consists of an inner, germinal layer representing the live parasite tissue and an outer, acellular laminated layer (LL) surrounding the entire metacestode. Previous studies have suggested that the LL plays an important role in protecting metacestodes from the host immune response (25, 27). However, the mechanisms by which the LL modulates and/or evades the host immune response are poorly understood (14).
Infection with E. multilocularis induces both parasite-specific cell-mediated and humoral immune responses (27). Cellular immunity is characterized by the development of an intrahepatic granuloma surrounding the parasite tissue (27). It has been shown that a regressive, as well as a progressive, course of disease in both human patients and rodents correlates with a course-specific granuloma cell composition and the induction of an antigen-specific T-cell response (8, 13, 17). Thus, cell-mediated immunity plays a crucial role in the control of E. multilocularis infections. However, a significant lack of knowledge about the protein-versus-carbohydrate composition and function of E. multilocularis antigens remains, especially in view of their contribution to the induction versus suppression of cell-mediated immune responses. Previous studies of both humans and mice infected with E. multilocularis have demonstrated that humoral immunity may also play a functional role in the control of parasite growth (28, 50). The specific humoral immune response includes an antibody pattern against parasite antigens of different molecular classes (26, 27). A carbohydrate antigen named Em2(G11), localized in the periodic acid-Schiff stain-positive LL of the metacestode (12, 25), has attracted considerable interest, as relatively resistant C57BL/10 mice exhibited a markedly high anti-Em2(G11) IgG3 response during chronic infection (27).
Conventionally, antibody synthesis and isotype switching require a cognate interaction between antigen-specific B cells and major histocompatibility complex class II (MHCII)-restricted αβ+ CD4+ T cells. The ability of proteins or peptides to associate with MHCII molecules allows specific engagement of the T-cell receptor (TCR). This MHC-restricted antigen recognition by T cells is followed by the signals given by T-helper cells to induce B-cell activation. The signal delivered from T cells to B cells by cell contact is mediated mainly by CD40-CD40 ligand (CD40L) interactions. However, there is increasing evidence that B-cell activation and immunoglobulin G (IgG) antibody responses may take place in the absence of T-cell help and do not require CD40-CD40L interactions (39, 40). Antigens that stimulate antibody production in the absence of MHCII-restricted T-cell help are classified as T-cell-independent (TI) antigens (6, 39). They can further be divided into two groups: TI type 1 (TI-1) antigens—such as lipopolysaccharide—which induce polyclonal activation of B cells and TI-2 antigens which cannot be cognately recognized in the context of MHCII restriction elements and are capable of stimulating antibody production in nude mice but not in xid mice. Many TI-2 antigens are high-molecular-weight polysaccharides containing multiple identical antigenic epitopes. They exhibit low in vivo degradability. On the other hand, TI-2 antigens cannot be cognately recognized by the TCR in the context of MHC molecules like peptide or protein antigens, so they represent unconventional immunogenic molecules for T-cell activation and for induction of effective high-affinity IgG synthesis. One of the hallmarks of an immune response to TI-2 antigens is the relative abundance of IgG3 antibodies in mice (39). Because of these characteristics, the poor immune response to polysaccharides and polysaccharide vaccines against encapsulated bacteria, such as Streptococcus pneumoniae, or some helminths is of limited effectiveness (9).
In this study, we analyzed some specific antigenic characteristics of in vitro-cultivated parasites and the respective immune reaction patterns of the host in vivo following conventional inoculation of 100 metacestode vesicles to better understand how the LL contributes to the modulation of the host immune response and thus to the protection of the parasite against host effector mechanisms.
MATERIALS AND METHODS
Mice.
Female 8- to 10-week-old C57BL/6 mice and athymic nude mice (C57BL/6 background) were purchased from Biotechnology & Animal Breeding Division, Füllinsdorf, Switzerland. μMT mice were provided by H. Hengartner (University of Zurich, Zurich, Switzerland). MHCII I-Ab−/− mice selectively deficient for CD4+ T cells, β2-m−/− mice deficient for CD8+ T cells, and TCRβ−/− mice were bred free of specific pathogens in the animal facility unit of the Max Planck Institute for Immunobiology, Freiburg, Germany. All T-cell-deficient and B-cell-deficient mouse strains had been backcrossed to C57BL/6 (H-2b) mice. CD40-deficient mice (129/Sv/Ev) and the wild-type (WT) controls were kindly provided by M. Kopf (Basel Institute for Immunology). In all experiments, animals were matched for age and weight.
Parasites and parasite antigen preparations.
The parasite used in this study was the cloned E. multilocularis isolate KF5 (11, 25). E. multilocularis vesicle fluid (VF antigen) was obtained by aspiration of fluid from in vitro-cultivated vesicular cysts (31). The LL was purified from in vitro-generated metacestode vesicles and subsequently solubilized as described by Ingold et al. (32). The carbohydrate E. multilocularis Em2(G11) antigen was purified by monoclonal antibody (MAb) G11-affinity chromatography (12). The recombinant E. multilocularis protein antigen II/3 was expressed in Escherichia coli and subsequently affinity purified from bacterial extracts to be used for enzyme-linked immunosorbent assay (ELISA) (20). All antigens were assessed for protein and carbohydrate concentrations. Protein contents were determined by using the Bio-Rad Bradford protein assay kit (Bio-Rad AG, Glattbrugg, Switzerland) with bovine plasma gamma globulin as the standard. Carbohydrate concentrations were estimated by using the orcinol-sulfuric acid assay with Dextran T-2000 as the standard (38).
Infection of mice.
T-cell-deficient or CD40-deficient (Sv129) mice and corresponding WT control mice were injected intraperitoneally with 100 freshly prepared metacestode vesicles (normal dose) suspended in 100 μl of RPMI 1640 medium. Control mice received an appropriate volume of RPMI 1640 medium.
Histology.
Infected mice were euthanatized with CO2. Livers containing metacestode tissue were removed by dissection and fixed in 4% buffered formaldehyde solution. Paraffin-embedded tissue sections were stained with hematoxylin-eosin.
TEM.
In vitro-generated E. multilocularis metacestodes were fixed for transmission electron microscopy (TEM) in 100 mM sodium phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde and 0.25% tannic acid, followed by postfixation in 2% OsO4 in phosphate buffer. The fixed material was embedded in Epon 812 resin, and sections were cut with an ultramicrotome. Sections were loaded onto 200-mesh nickel grids and stained with uranyl acetate and lead citrate as previously described (31).
SDS-PAGE, Western blotting, carbohydrate staining, and immunostaining.
The soluble LL fraction, the Em2(G11) antigen, and the VF antigen were supplemented with 200 μl of 5×-concentrated sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE) using 7.5% gels. SDS-PAGE-resolved antigens were electrophoretically transferred onto nitrocellulose by using an inverted procedure at pH 2.0 (transfer from the cathodic to the anodic site). This allowed high-_M_r and carbohydrate-rich molecules to be efficiently transferred for subsequent immunodetection (29, 33). In order to identify carbohydrates on Western-blots, the GlycoTrackCarbohydrate Detection Kit (Oxford GlycoSystems, Oxford, England) was employed in accordance with the procedures recommended by the manufacturer. Blots were immunolabeled with Em2-specific MAb G11 as previously described (12). An irrelevant MAb of the same isotype (MAb PU) was used as a negative control.
Immunofluorescence.
Peritoneal cells were allowed to adhere to poly-l-lysine-coated coverslips and were stimulated with VF antigen or Em2(G11) antigen for 16 h. They were then fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min, followed by permeabilization in methanol (−20°C) for 10 min. Samples were rehydrated in PBS, and nonspecific binding sites were blocked in PBS containing 1% bovine serum albumin and 50 mM glycine for 1 h. The primary antibody—a protein A-purified anti-E. multilocularis rabbit antiserum—was applied at a dilution of 1:100 in PBS–0.1% bovine serum albumin for 45 min, followed by several buffer rinses. The secondary antibody (fluorescein isothiocyanate-conjugated anti-rabbit; Sigma) was applied for 30 min. To visualize the entire cells, a MAb directed against α-tubulin (clone B-5-1-2; Sigma) was applied, followed by an anti-mouse-tetramethyl rhodamine isocyanate conjugate. Samples were extensively washed in PBS and embedded as previously described (33).
Cell cultures and lymphocyte proliferation assays.
Spleen cell suspensions were prepared from infected or noninfected mice (11). Spleen cell suspensions were prepared in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (Gibco, Basel, Switzerland), 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 μg of streptomycin (Gibco) per ml. Spleen cells were cultured in 96-well round-bottom plates at 2 × 105/well. Cells were stimulated with crude parasite VF antigen (10 μg of protein and 2.1 μg of carbohydrate per ml) or Em2(G11) antigen (2.8 μg of carbohydrate per ml; no detectable proteins) for 96 h or were left unstimulated as negative controls. Cells were pulsed with 1 μCi of [3H]thymidine (New England Nuclear, Boston, Mass.) per well and harvested 16 to 18 h later. Results were expressed as geometric mean counts per minute minus the background, the background being represented by the counts per minute of wells containing pulsed but unstimulated cells. Assays were validated when the background counts per minute were <10% of the values obtained with concanavalin A-stimulated cells. All tests were performed in quadruplicate.
Quantification of cytokine transcripts by competitive reverse transcription-PCR.
Total cellular RNA was isolated from splenocytes ex vivo (no in vitro stimulation) by the single-step guanidinium isothiocyanate procedure using TRIZOL Reagent (Gibco). The cDNA was synthesized for 90 min at 37°C in 50 μl containing 16 U of Moloney murine leukemia virus reverse transcriptase (Promega, Heidelberg, Germany) per ml and 1.2 ng of random hexamers (New England Biolabs).
Competitive PCR was performed as described previously (10, 48). Briefly, constant amounts (40 ng) of cDNA were coamplified in the presence of appropriate amounts of competitor plasmid DNAs (pNIL and pMUS) with the different cytokine PCR primers. The plasmid was diluted fourfold in nine dilution steps (point 1 to point 9) from a stock concentration of 3.73 ng/ml (106 molecules per μl). Thus, point 1 represented 5 × 106 molecules, point 8 represented about 60 molecules, and point 9 represented about 15 molecules. Relative quantification of cDNA was done by calculating how much of the competitor was required to achieve equal amounts of two products. The cDNA was first standardized to equal concentrations of the housekeeping gene (the gene for β2-microglobulin). PCRs were performed in 50 μl containing 0.25 mM each deoxynucleoside triphosphate, 0.25 μM 5′ and 3′ primers, 10 mM Tris/HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% (wt/vol) gelatin, and 1.25 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Rotkreuz, Switzerland) for 35 cycles (20 s at 94°C, 20 s at 56°C, and 30 s at 72°C).
ELISA for detection of Em2(G11) antigen or of antibodies and respective isotype avidity.
Em2(G11) antigen was detected by sandwich ELISA with MAb G11 as described previously (12). Within this assay, deglycosylation of Em2(G11) antigen was performed by treating the antigen with 50 mM NaIO4 in 100 mM Na acetate-EDTA (pH 5.5) for 1 h at 37°C. To disrupt protein epitopes, the antigen was treated with 50 μg of proteinase K per ml for 1 h at 37°C.
Sera were analyzed for parasite-specific antibodies by ELISA basically as described elsewhere (28). The following E. multilocularis antigen fractions were used to coat ELISA plates: recombinant protein antigen II/3 (0.5 μg of protein per ml), VF antigen (5 μg of protein per ml), and MAb G11 affinity-purified Em2(G11) antigen (0.57 μg of carbohydrate per ml). These coating concentrations had been previously optimized by serial-dilution experiments using positive sera obtained from another experiment. Sera were diluted at 1:100, and the conjugates used were goat anti-mouse IgM or IgG isotypes linked to alkaline phosphatase (all from Southern Biotechnology Associates. Inc., Birmingham. Ala.). The absorbance values used to discriminate between seropositivity and seronegativity were determined by the mean value plus 2 standard deviations of 20 sera obtained from noninfected mice of the identical genotype.
IgG avidity tests were performed by ELISA as described by Jenum et al. (34). Briefly, each serum sample was analyzed in two fourfold titration rows, with one row (row A) starting at a dilution of 1:50 and the other row (row B) starting at a dilution of 1:200. After serum incubation at 4°C overnight, row A was washed three time with 250 μl of PBS containing 6 M urea and 0.05% Tween 20, which resulted in the removal of low-avidity antibodies from their binding sites. Row B was washed three times with conventional washing buffer (0.05% Tween 20–PBS). All subsequent steps and secondary antibodies were used as described above. For each serum sample, two endpoint titers, one after washing with urea (row A) and one control (row B), were calculated with the following formula: titer = dilution_x_−1 × 10_a_, where dilution_x_ is the highest dilution giving an _A_405 of >0.1 and a is equal to log4 × (_A_405 x − 0.1)/(_A_405 x − _A_405 y), where 4 is the dilution factor, _A_405 x is A_405 at dilution_x, and _A_405 y is the A_405 at the next higher dilution from dilution_x. Percent IgG avidity was calculated with the following formula: (titerrow A/titerrow B) × 100.
Statistical methods.
Comparative analyses were done with the Student t test using the Microsoft Excel (Microsoft Office 98) software. Significance was defined as a P < 0.05.
RESULTS
Analysis of the LL antigen.
Previous studies have suggested that the LL may play an important role in parasite survival (1, 5). For the present study, it was necessary to assess some further chemical parameters of the LL and related or comparative antigens. Thus, different parasite antigens, including crude VF antigen, soluble LL antigen, and affinity-purified Em2(G11) antigen, were assessed for their protein and carbohydrate concentrations. The VF antigen contained 2.0 mg of protein and 430 μg of carbohydrate per ml (protein/carbohydrate ratio = 4.65:1). The crude LL antigen concentrations were 150 μg of protein and 800 μg of carbohydrate per ml, respectively (protein/carbohydrate ratio = 1:5.33). The purified Em2(G11) antigen contained 28.5 μg of carbohydrate per ml and no detectable proteins. The recombinant II/3 antigen concentration was 4.3 mg of protein per ml with no detectable carbohydrates.
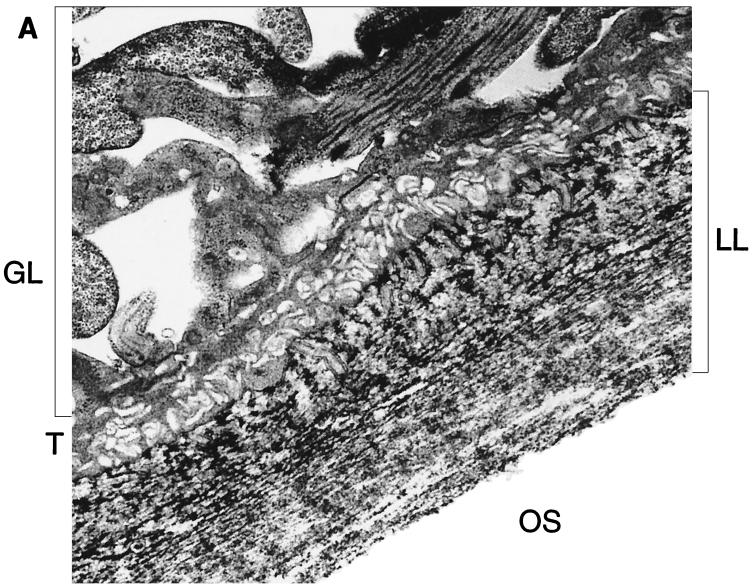
Histological analysis of in vitro-cultivated vesicles or of vesicles obtained from infected livers had shown that the LL is strongly periodic acid-Schiff stain positive (data not shown). In vitro-generated vesicles were fixed in the presence of tannic acid for transmission electron microscopy, which resulted in increased ultrastructural preservation of carbohydrate-based structures (31). By using this staining protocol, the microfibrillar carbohydrate-rich content of the LL could be confirmed (Fig. 1A).
FIG. 1.
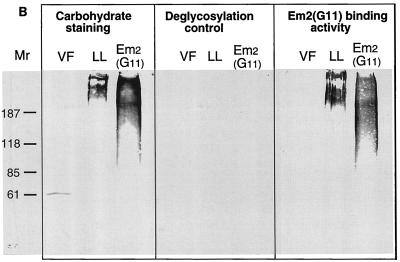
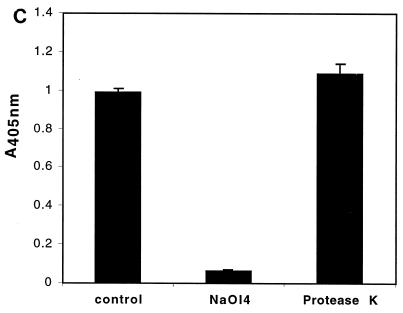
The LL is composed largely of carbohydrate-based structures. (A) Transmission electron micrograph of a section the E. multilocularis metacestode tissue. The tissue was fixed in the presence of tannic acid for increased preservation and contrast of carbohydrate-based structures such as the LL. GL, germinal layer; T, tegument; OS, outer surface. Microtriches extend well into the LL. Bar = 1 μm. (B) Carbohydrate staining and immunoblot analysis of VF antigen, soluble LL antigen, and affinity-purified Em2(G11) antigen. The VF, LL, and Em2(G11) antigens were separated by SDS–7.5% PAGE and transferred onto nitrocellulose. This was either biochemically labeled with the GlycoTrackCarbohydrate Detection Kit or immunologically stained with Em2-specific MAb G11 _M_rs in thousands are shown on the left. (C) Demonstration of the specific carbohydrate-binding epitope of the Em2(G11) antigen. ELISA plates were coated with Em2-specific MAb G11 for subsequent sandwich ELISA. The Em2(G11) antigen was either deglycosylated with NaOI4 or treated with protease K. MAb-antigen complexes were visualized with alkaline phosphatase-conjugated MAb G11.
VF, soluble LL, and affinity-purified Em2(G11) antigens were further separated by SDS-PAGE and stained for carbohydrates by the GlycoTrackCarbohydrate Detection Kit (Fig. 1B). Both LL and Em2(G11) were characterized by a predominantly high-molecular-mass (>200 kDa) carbohydrate content. The immunological identification of Em2-related epitopes by immunoblotting revealed that the high-molecular-mass LL carbohydrates and the Em2(G11) carbohydrates exhibited MAb G11-binding activity, whereas no MAb G11 binding could be detected within the VF antigen (Fig. 1B). The banding patterns of the LL were nearly identical between carbohydrate staining and Em2(G11) immunostaining. These data thus indicated that the high-molecular-mass carbohydrates of Em2(G11) (detectable by MAb G11) also constitute the major carbohydrate antigens of the LL.
The fact that the MAb G11-reactive epitope of Em2(G11) is of carbohydrate nature was further confirmed by MAb G11 sandwich ELISA (Fig. 1C). Em2(G11) lost its ability to interact with MAb G11 after deglycosylation treatment with NaOI4, while treatment with protease K had no relevant influence on its Em2(G11)-binding activity (Fig. 1C). As a control, the same procedure completely abrogated the protein-binding activity detected in a corresponding interleukin-2 (IL-2) and gamma interferon (IFN-γ) sandwich ELISA (data not shown).
Immunogenic characteristics of the Em2(G11) antigen in vitro.
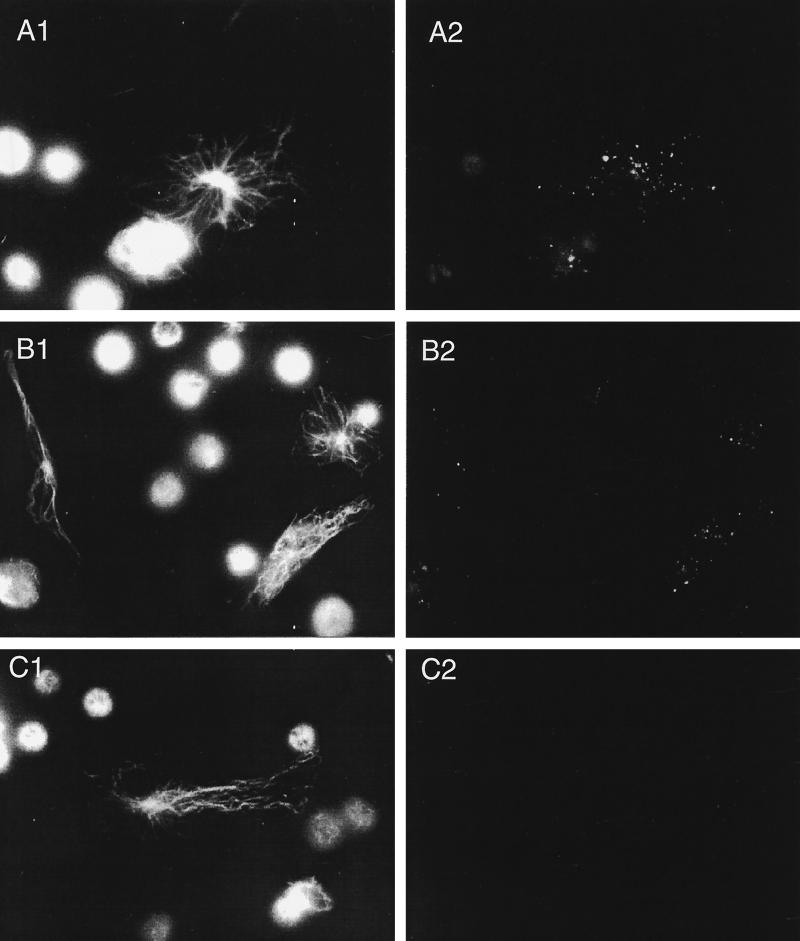
We further tested whether the Em2(G11) antigen, as a high-molecular-weight carbohydrate, would be taken up by macrophages and whether the same antigen could stimulate lymphocyte activation in vitro. Peritoneal cells from normal mice were incubated with protein-rich VF antigen or the carbohydrate Em2(G11) antigen and were subsequently stained by intracellular immunofluorescence. Results showed that both types of antigen had been taken up by the resident macrophages (Fig. 2), a prerequisite for the further antigen processing and presentation events.
FIG. 2.
Demonstration of antigen uptake by resident peritoneal macrophages. Peritoneal cells from uninfected, normal mice (4 × 105/ml) were incubated with VF (A) or Em2(G11) antigen (B) or medium alone (negative control [C]) for 16 h. The free soluble antigens were removed by washing the cells three times with PBS. The endocytosed antigens were subsequently visualized by intracellular immunofluorescence using a polyclonal anti-E. multilocularis antibody (right column). The corresponding preimmune serum (data not shown) exhibited no antibody staining. The control antitubulin immunostaining characterized the entire cell structures present on the slides (left column).
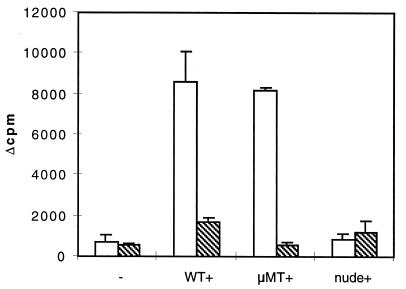
With regard to lymphoproliferative induction potential, however, only the VF antigen was able to induce splenocyte proliferation in vitro, as the Em2(G11) antigen exhibited a random, nonsignificant stimulatory effect on the proliferative response in spleen cells from C57BL/6 mice infected with 100 parasite vesicles (Fig. 3). By using spleen cells from B-cell-deficient μMT mice (rich in T lymphocytes) that had been infected for 1 month or from athymic nude mice (rich in B lymphocytes), it could be demonstrated that VF antigen induced predominantly T-cell proliferation in μMT mice. In response to Em2(G11) antigen stimulation, the proliferation values of spleen cells from infected μMT mice were comparable to those of spleen cells from control mice (Fig. 3). Consequently, in contrast to protein-rich VF antigen, the purified carbohydrate Em2(G11) antigen induced no specific T-lymphocyte proliferation in vitro.
FIG. 3.
Lymphocyte proliferation after antigen stimulation in vitro. C57BL/6 WT, antibody KO (μMT), and athymic nude mice of the same genetic background (five animals per group) were infected i.p. with 100 E. multilocularis metacestode vesicles. Splenocytes were isolated either from WT control mice (−) or from mice infected for 1 month (+) (WT+, μMT+, and nude+). The splenic proliferative responses to stimulation with the predominantly proteinic VF antigen (open bars) and with the carbohydrate Em2(G11) antigen (stippled bars) were determined in two independent experiments that produced similar results.
T-cell activation and polarization in vivo
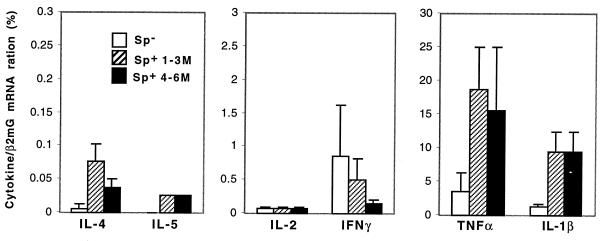
Next, we addressed the question of whether T cells are highly activated and polarized in vivo by the persistently proliferating larval parasite. The pattern of cytokine expression in the spleen was analyzed ex vivo by quantitative reverse transcription-PCR. Five mice were infected with 100 metacestode vesicles, and splenocytes were isolated at 3 and 6 months postinfection (p.i.), respectively. As shown in Fig. 4, expression of the Th1 cytokines IFN-γ and IL-2 was constitutive (3 months p.i.) or reduced (6 months p.i.) after chronic infection, compared to that in the spleens of control mice. The Th2 cytokines IL-4 and IL-5 (also IL-13; data not shown) were slightly increased, indicating a Th2 shift during chronic E. multilocularis infection. Overall, the T-cell cytokine expression remained at a low level in vivo during the whole course of chronic infection (<50 molecules), especially in comparison to the increased inflammatory cytokines (>3,000 molecules; Fig. 4).
FIG. 4.
Cytokine mRNA expression in the spleen after chronic E. multilocularis infection. Five C57BL/6 mice were infected i.p. with 100 E. multilocularis metacestode vesicles. Splenocytes (Sp) from control mice (open bars), mice infected for 3 months (black bars), and mice infected for 6 months (cross-hatched bars) were harvested and pooled in each group. Cytokine transcripts were standardized to the levels of β2-microglobulin transcripts and quantitated by using fourfold dilutions of the competitive plasmid pMus. The number of molecules of each cytokine was based on the point where PCR products were equivalent to the products of pMus, where the pMus value is a definite numbers of molecules (see Materials and Methods). The results were calculated as the mean number of cytokine molecules in two independent experiments.
Specific IgG avidity in sera of infected C57BL/6 mice.
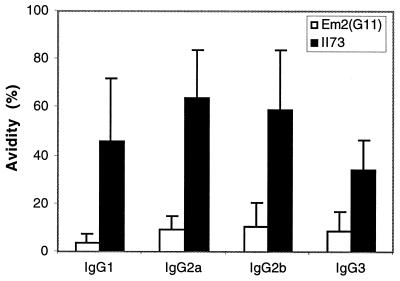
In general, antibody responses to typical carbohydrate antigens have been shown to be different from those developed against conventional protein antigens. Furthermore, the opsonophagocytosis and/or complement-mediated lysis is dependent upon the binding strength of the IgGs involved. Thus, we determined the avidity of specific anti-Em2(G11) IgG and anti-recombinant II/3 IgG by ELISA. For this, 10 different sera obtained at 3 months p.i. and 10 sera obtained at 6 months p.i. were preselected upon triple seropositivity to the Em2(G11), VF, and II/3 antigens. As shown in Fig. 5, the avidity of all IgG isotypes against recombinant protein II/3 antigen was high but remained significantly lower for the Em2(G11) antigen. These data demonstrated the inability of the carbohydrate Em2(G11) antigen to induce IgG avidity maturation.
FIG. 5.
Comparison of IgG isotype avidity values against the carbohydrate antigen Em2(G11) and the recombinant protein antigen II/3. Relative avidity values of the anti-Em2(G11)-specific IgG isotype (white bars) and relative avidity values of the anti-II/3-specific IgG isotype (black bars) were tested by ELISA using sera from C57BL/6 WT mice infected for 3 months. The results are expressed as the mean plus the standard error of the mean (_A_405). Similar results were obtained with sera from mice infected for 6 months (data not shown).
Anti-Em2(G11) antibody production and isotype switching in T-cell-deficient mice.
Because the major LL Em2(G11) antigen contains predominantly high-molecular-weight carbohydrates and induces no T-cell proliferation in vitro and low-avidity IgG isotypes in immunocompetent mice in vivo, we used T-cell-deficient mice to further test if this antigen is a TI antigen, which may help to explain poor immune responsiveness and effectiveness in AE.
Anti-Em2(G11) antibody production and respective isotype switching in infected athymic nude mice, TCRβ−/−, MHCII−/− mice, and MHCI−/− mice were tested by ELISA (Table 1). Besides the immunocompetent C57BL/6 WT mice, which exhibited a specific anti-Em2(G11) antibody response including all IgG isotypes, the MHCI−/− mice produced comparable IgM concentrations and a respective IgG switch, suggesting that CD8+ T cells play a minor role in antibody production. Nude TCRβ−/− and MHCII−/− mice infected for 1 month also produced IgM and a switch to IgG2a and IgG3 but not to IgG1. The Em2(G11)-specific IgG1 concentration was consistently lower than the level of detection in nude TCRβ−/− and MHCII−/− mice, suggesting that the synthesis of IgG1 is dependent on αβ+ CD4+ T-cell help. The IgG2a isotypes in the nude TCRβ−/− and MHCII−/− mice were significantly lower in concentration than those in infected immunocompetent C57BL/6 mice, thus indicating that the help of αβ+ CD4+ T cells is crucial for optimal antibody production. However, anti-Em2(G11) IgG3 concentrations in nude and TCRβ− knockout (KO) mice were even comparable to those in immunocompetent C57BL/6 WT mice, and in MHCII−/− (CD4-deficient) mice, such IgG3 was also clearly detectable. These data confirmed an alternative and T-cell (αβ+ CD4+ T-cell)-independent pathway of IgG3 switching during E. multilocularis infection. The Em2(G11) antigen, as a TI antigen, induced qualitatively distinct antibody production and IgG2a and IgG3 isotype switching in the absence of the help of αβ+ CD4+ T cells.
TABLE 1.
Anti-Em2(G11) antibody synthesis and switching in T-cell-deficient mice infected for 1 montha
| Mice | Mean _A_405 ± SE of serum | ||||
|---|---|---|---|---|---|
| IgM | IgG1 | IgG2a | IgG2b | IgG3 | |
| C57BL/6 control | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.15 ± 0.02 | 0.16 ± 0.01 | 0.18 ± 0.01 |
| C57BL/6 WT | 1.35 ± 0.04b | 0.86 ± 0.12b | 1.20 ± 0.08b | 1.38 ± 0.12b | 0.76 ± 0.12b |
| Nude | 0.65 ± 0.13b | 0.045 ± 0.01 | 0.65 ± 0.23b | 0.08 ± 0.03 | 0.68 ± 0.27b |
| TCRβ KO | 0.49 ± 0.14b | 0.27 ± 0.10 | 0.32 ± 0.04b | 0.21 ± 0.05c | 0.85 ± 0.36b |
| MHCII KO | 0.64 ± 0.18b | 0.22 ± 0.06 | 0.23 ± 0.04b | 0.18 ± 0.03 | 0.38 ± 0.16c |
| MHCI KO | 1.14 ± 0.11b | 0.59 ± 0.12b | 1.25 ± 0.21b | 1.39 ± 0.17b | 0.60 ± 0.22b |
Anti-Em2 antibody production and isotype switching in CD40-deficient mice.
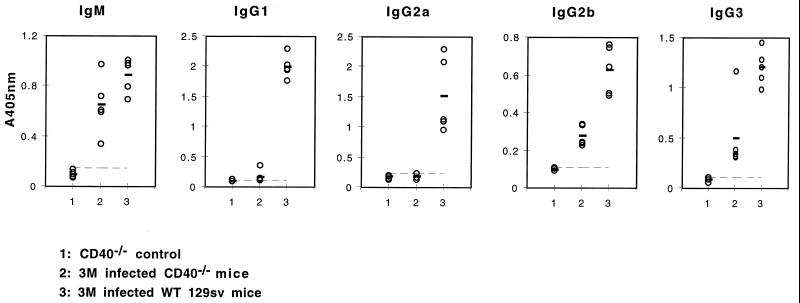
Unlike antibody responses to protein antigens, IgM and IgG responses to TI antigens do not require CD40-CD40L interactions. To further confirm the TI antibody responses during E. multilocularis infection and the TI nature of the Em2(G11) antigen, parasite-specific anti-Em2(G11) antibody production and respective isotype switching were further determined by ELISA in infected CD40−/− mice (Fig. 6). At 2 (data not shown) or 3 months p.i., CD40−/− mice had developed anti-Em2(G11)-specific IgM. The IgG isotype switching included predominantly the IgG3 and IgG2b isotypes. These data suggested that during E. multilocularis infection, the carbohydrate Em2(G11) antigen induced antibody production and IgG2b and IgG3 switching in the absence of CD40 molecules. Quantitatively, however, a marked reduction in antigen-specific IgG2b and IgG3 concentrations was seen in CD40−/− mice, relative to that in infected WT controls, which may point out again that the help signal from T cells mediated by CD40 molecules is important for an optimal antibody response in vivo. This, however, does not call into question the fact that the Em2(G11) antigen acts a TI antigen.
FIG. 6.
Anti-Em2(G11) antibody production in infected CD40−/− mice. Specific anti-Em2(G11) antibody production in infected CD40−/− mice was further determined by ELISA. CD40−/− mice were infected with 100 metacestode vesicles for 3 months. IgM and IgG isotype antibody concentrations were determined in individual mice by ELISA. Each symbol represents an individual mouse, and the bold bar indicates the mean of each group. The broken line indicates the mean plus 2 standard deviations of the group of control mice. This is one representative of two independent experiments with identical results. Similar results were obtained with mice infected for 2 months.
DISCUSSION
The parasite LL is composed mainly of high-molecular-weight carbohydrates. A major aim of this report was to demonstrate that the Em2(G11) antigen, a major lectin-binding carbohydrate antigen localized in the LL (25), did not stimulate a lymphoproliferative response in vitro, while the crude, predominantly protein VF antigen induced significant T-cell proliferation. In vivo, the progressive growth of the metacestode and the related continuous antigenic stimulation resulted in a Th2 shift with a persistently low-level expression of respective cytokines. In contrast to the antibody response against the recombinant protein II/3 antigen, the Em2(G11) antigen induced the synthesis of low-avidity IgG only, including all isotypes. Subsequent experiments with T-cell-deficient mice demonstrated that the carbohydrate Em2(G11) antigen acts as a TI antigen, similar to antigens which have been found in some encapsulated bacteria, fungi, or viruses (9, 18, 19, 23, 37, 41, 46). It appears that the glycocalyx of the E. multilocularis metacestode—the LL—protects the parasite against host effector mechanisms. This protection is putatively attributed to the high-molecular-weight carbohydrate content of the LL and its low immunogenicity as a TI antigen.
AE in human patients requires continuous chemotherapy for a nearly unlimited time if complete surgical resection is not possible (3). Obviously, humoral and cellular immune responses in human (as well as murine) AE are not sufficient to control parasite proliferation. Thus, experimental infection, even with a single metacestode vesicle, was enough to allow parasite survival and subsequent metastasis formation, despite the development of a specific immune response (unpublished data). The restricted effectiveness in controlling the infection may be correlated with the immune status observed in AE. In many parasitic diseases, especially protozoan diseases, a chronically persisting parasite infection is associated with continuous antigenic stimulation and normally results in a highly polarized immune response pattern. This polarization becomes particularly evident in the profile of CD4+ T-cell-associated cytokine expressions. In helminthic infections (21), and also in human AE (45), the persistent parasite or E. multilocularis metacestode proliferation and antigenic stimulation, respectively, yields a Th2 cytokine profile. Our present data confirm this peculiarity also in the experimental murine model. It is known that Th2 cytokines provide more potent helper functions for antibody, especially IgE or IgG1, production and also support eosinophil-mediated and mast cell-mediated reactions, while Th1 cytokines relate to macrophage activation, antibody-dependent cytotoxicity, delayed-type hypersensitivity, and inflammation, thus focusing on cellular immunity (1). In contrast to allergy or those helminthic infections which induce prominent Th2 cytokine response, AE is not characterized by high IgE production, mucosal mastocytosis, and eosinophilia, and the reasons remain unknown. Thus, the slight increase in Th2 cytokine expression following experimental E. multilocularis infection seems not to be enough for the induction of a powerful Th2 cell-mediated effect reaction. The low level of Th1 cell cytokine expression during chronic infection in C57BL/6 mice in vivo indicated a generally depressed Th1 cell-mediated immune response. Furthermore, chronically infected mice maintained a markedly low-avidity IgG response to the LL antigen Em2(G11). A similar phenomenon had been observed in infections with the closely related parasite E. granulosus (43).
One of the possible regulatory mechanisms responsible for the restricted effectiveness of the immune response following E. multilocularis infection was proposed to be based upon an immunosuppression phenomenon (11). We had shown that high nitric oxide production by macrophages could suppress the immunoproliferative response during AE (11). An additional possibility may be provided by alternative antigenic stimulation. Being a highly organized metazoan pathogen, E. multilocularis may challenge the host immune system by expressing a highly complex antigen pattern. The site of constant interaction between the metacestode and the host and its immune system is the LL (26). Previous studies and our results showed that the LL is rich in carbohydrates and that high-molecular-weight glycans are major structural elements of the LL (33). One of its major components is a lectin-binding carbohydrate antigen called Em2(G11) (12, 25). The LL and the Em2(G11) antigen remain within the infected host tissue, even following dying out of the metacestodes (42). This indicates poor in vivo degradability of the LL and its associated antigens. We showed in this paper that—despite endocytosis of both the carbohydrate Em2(G11) antigen and the predominantly protein VF antigen by macrophages—the Em2(G11) antigen, in contrast to the VF antigen, exhibited no antigenic stimulation of T-cell proliferation in vitro. In addition, the Em2(G11) antigen induced the production of only low-avidity IgG in vivo in C57BL/6 mice. The fact that antibody synthesis and the subsequent switch to IgG3 and IgG2a took place in C57BL/6 athymic nude mice and other T-cell-deficient mice further underlined the TI nature of this parasite antigen. Our results are consistent with data obtained by others with regard to murine E. granulosus infections in which mice were treated with anti-CD4 antibodies prior to infection (7).
It was shown that TI carbohydrate antigens can modulate the immune response in different ways (36). Our working hypothesis focused on the existence of TI immunogenic carbohydrates in the LL of E. multilocularis, which may be involved in the parasite's evasion of host immunity. Like some other pathogens, E. multilocularis synthesizes particulate antigens chemically composed of a complex mixture of T-cell-dependent (TD) antigens (peptides and proteins) and TI antigens (glycolipids and complex polysaccharides) (32, 33). The carbohydrate-rich LL encapsulates the entire parasite and thus may restrict or inhibit the physical exposure of somatic or metabolic proteins of the germinal layer to the host immune system. Further studies, e.g., competitive macrophage uptake assays, may be required to experimentally support this hypothesis. Different TI carbohydrates are implicated in suppression of the antigen presentation of defined TD antigens to T cells (36, 47). For example, they can inhibit protein antigen uptake and processing by macrophages (39). Furthermore, polysaccharides of encapsulated Cryptococcus neoformans (49) inhibit the expression of costimulatory molecules and thus influence T-cell activation. Most importantly, beside putative inhibition of exposure and presentation of TD antigen by LL-associated glycans or carbohydrates, the Em2(G11) antigen, being a TI antigen, would not be recognized by the TCR in the context of MHC molecules. Signaling by the TCR is important for T-cell activation and also for providing help in antibody synthesis. In fact, Em2(G11) antigen did not stimulate T-cell proliferation in vitro and induced an IgG response lacking subsequent avidity maturation. Thus, the restricted specific T-cell activation by the LL Em2(G11) antigen in vitro and by the proliferating parasite in vivo, as well as a low-avidity anti-Em2(G11) antibody response, may be one of the many factors contributing to the lack of protection against proliferating metacestodes.
Like many other carbohydrate antigens (39, 44, 47), Em2(G11) induced marked antibody synthesis and isotype switching in C57BL/6 athymic nude TCRβ−/− mice and MHCII−/− mice (Table 1). These results suggested that the specific anti-Em2(G11) antibody production and isotype switching could take place independently of αβ+ and CD4+ T cells. The Em2(G11)-specific IgG synthesized in nude TCRβ−/− and MHCII−/− mice were predominantly of the IgG3 and IgG2a isotypes. Nevertheless, in C57BL/6 WT mice, the IgM and IgG2a antibody levels were significantly higher than those in T-cell-deficient mice. This points out the importance of CD4+ αβ+ T cells in the achievement of optimal antibody production. However, we documented detectable amounts of IgG2a and IgG3 in nude TCRβ−/− and MHCII−/− mice. The IgG3 concentrations in nude and TCRβ−/− mice were even comparable to those of WT mice. The detailed TI pathways of antibody synthesis and isotype switching at the in vivo operative level, however, are not known (39, 44). Relatively higher concentrations of IgG2a and IgG3 were found in nude and TCRβ−/− mice than in MHCII−/− mice. These data indicated that the few resident T cells (fluorescence-activated cell sorter analysis showed 1% CD4+ T cells) in nude mice, especially γδ+ T cells in TCRβ−/− mice, may play an important role in the IgG2a and IgG3 switch after infection, in the absence of αβ+ T-cell help. It has been shown that the switching to isotype IgG2a in TCRα−/− mice infected with vesicular stomatitis virus was supported mainly by γδ+ T cells (37). It will be of interest to assess antibody synthesis and isotype switching in αβ and γδ double-KO mice in further experiments. In addition, cytokines such as IFN-γ, transforming growth factor β, and local cell contact-delivered signals may contribute to the induction of a TI antibody response (5, 44). The signal delivered by cell contact from T to B cells is mediated mainly via the CD40-CD40L interaction. CD40 and CD40L have been shown to be essential for humoral immune responses to TD antigens but play no role in determining the intensity and isotype distribution of humoral immune responses to most purified TI antigens (22, 35). E. multilocularis infection induced a specific anti-Em2(G11) IgM production and a subsequent IgG2b and IgG3 switch in CD40−/− mice. However, conversely to those of other purified TI antigens, concentrations of the different anti-Em2(G11) IgG isotypes were significantly lower than those in WT mice. A possible reason for this is that the immune system of an infected mouse encounters the Em2(G11) antigen in vivo not as an isolated epitope but in the context of a complex mixture of parasite products. In addition, TD B-cell responses are usually associated with germinal center (GC) formation, a specialized microenvironment for somatic mutation and affinity maturation (4). Respectively, CD40-CD40L interactions are crucial for GC formation and maintenance. In our currently ongoing and future experiments, it will be interesting to investigate putative GC formation in T-cell-deficient or CD40-deficient mice following E. multilocularis infection and to study the possible factors which could contribute to the IgG2a and IgG3 switch in the absence of T-cell help.
In summary, our results show that the E. multilocularis has chosen to shield itself with a layer composed mainly of an inefficient TI antigen and therefore avoids an immune attack. Our results contribute to a better understanding of the parasite's survival strategy and will help in the design of potential immunotherapeutic or vaccination tools. For example, conjugation of polysaccharide antigens to TD protein antigens or adjuvants such as IL-12 (9) or anti-CD40 antibodies (16)—in order to recruit T-cell help—may become attractive. Finally, our results also strongly support the idea that there is an alternative pathway of B-cell activation in AE that is independent of αβ+ or CD4+ T-cell help and may not require CD40-CD40L interactions.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (grant 31-45575.95) and the Interreg II Project (BWA 30.027).
We thank R. Zinkernagel (Institute of Experimental Immunology, Department of Pathology, University of Zurich, Zurich, Switzerland), T. Jungi and G. Bertoni (Institute of Veterinary Virology, University of Berne, Berne, Switzerland), and M. Kopf (Basel Institute of Immunology, Basel, Switzerland) for helpful discussion and criticism of the manuscript.
REFERENCES
- 1.Abbas A, Murphy M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Amiot F, Vuong P, Defontaines M, Pater C, Dautry F, Liance M. Secondary alveolar echinococcosis in lymphotoxin-alpha and tumour necrosis factor-alpha deficient mice: exacerbation of Echinococcus multilocularis larval growth is associated with cellular changes in the periparasitic granuloma. Parasite Immunol. 1999;21:475–483. doi: 10.1046/j.1365-3024.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Ammann R W, Ilitsch N, Marincek B, Freiburghaus A U. Effect of chemotherapy on the larval mass and the long-term course of alveolar echinococcosis. Hepatology. 1994;19:735–742. doi: 10.1002/hep.1840190328. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann M. The role of germinal centers for antiviral B cell responses. Immunol Res. 1998;17:329–344. doi: 10.1007/BF02786455. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann M, Zinkernagel R, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161:5791–5794. [PubMed] [Google Scholar]
- 6.Bachmann M F, Zinkernagel R. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 7.Baz A, Richieri A, Puglia A, Nieto A, Dematteis S. Antibody response in CD4-depleted mice after immunization or during early infection with Echinococcus granulosus. Parasite Immunol. 1999;21:141–150. doi: 10.1046/j.1365-3024.1999.00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Bresson-Hadni S, Liance M, Meyer J P, Houin R, Bresson J L, Vuitton D A. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:378–383. doi: 10.1111/j.1365-2249.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan R, Arulanandam B, Metzger D. IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–5533. [PubMed] [Google Scholar]
- 10.Dai W J, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 11.Dai W J, Gottstein B. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology. 1999;97:107–116. doi: 10.1046/j.1365-2567.1999.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deplazes P, Gottstein B. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology. 1991;103:41–49. doi: 10.1017/s0031182000059278. [DOI] [PubMed] [Google Scholar]
- 13.Devouge M, Ali-Khan Z. Intraperitoneal murine alveolar hydatidosis: relationship between the size of the larval cyst mass, immigrant inflammatory cells, splenomegaly and thymus involution. Tropenmed Parasitol. 1983;34:15–20. [PubMed] [Google Scholar]
- 14.Diaz A, Ferreira A, Sim R. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J Immunol. 1997;158:3779–3786. [PubMed] [Google Scholar]
- 15.Dreweck C M, Soboslay P T, Schulz-Key H, Gottstein B, Kern P. Cytokine and chemokine secretion by human peripheral blood cells in response to viable Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 1999;21:433–438. doi: 10.1046/j.1365-3024.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 16.Dullforce P, Sutton D, Heath A. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 17.Emery I, Liance M, Deriaud E, Vuitton D, Houin R, Leclerc C. Characterization of T-cell immune response to Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:463. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 18.Fehr T, Bachmann M, Bluethmann H, Kikutani H, Hengartner H, Zinkernagel R. T-independent activation of B cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- 19.Fehr T, Naim H, Bachmann M, Ochsenbein A, Spielhofer P, Bucher E, Hengartner H, Billeter M, Zinkernagel R. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat Med. 1998;4:945–948. doi: 10.1038/nm0898-945. [DOI] [PubMed] [Google Scholar]
- 20.Felleisen R, Gottstein B. Comparative analysis of full-length antigen II/3 from Echinococcus multilocularis and E. granulosus. Parasitology. 1994;109:223–232. doi: 10.1017/s0031182000076344. [DOI] [PubMed] [Google Scholar]
- 21.Finkelman F, Pearce E J, Urban J P, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991;12:346–348. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- 22.Foy T, Aruffo A, Bajorath J, Buhlmann J, Noelle R. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 23.Franco M, Greenberg H. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 24.Godot V, Harraga S, Beurton I, Descheaux M, Sarciron E, Gottstein B, Vuitton D A. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparison of patients with progressive and abortive lesions. Clin Exp Immunol. 2000;121:484–490. doi: 10.1046/j.1365-2249.2000.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottstein B, Deplazes P, Aubert M. Echinococcus multilocularis: immunological study on the “Em2-positive” laminated layer during in vitro and in vivo post-oncospheral and larval development. Parasitol Res. 1992;78:291–297. doi: 10.1007/BF00937086. [DOI] [PubMed] [Google Scholar]
- 26.Gottstein B, Felleisen R. Protective immune mechanisms against the metacestode of Echinococcus multilocularis. Parasitol Today. 1995;11:320–324. doi: 10.1016/0169-4758(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 27.Gottstein B, Hemphill A. Immunopathology of echinococcosis. Chem Immunol. 1997;66:177–208. doi: 10.1159/000058670. [DOI] [PubMed] [Google Scholar]
- 28.Gottstein B, Wunderlin E, Tanner I. Echinococcus multilocularis: parasite-specific humoral and cellular immune response subsets in mouse strains susceptible (AKR, C57B1/6J) or ‘resistant’ (C57B1/10) to secondary alveolar echinococcosis. Clin Exp Immunol. 1994;96:245–252. doi: 10.1111/j.1365-2249.1994.tb06549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hames B D. One dimensional polyacrylamide gel electrophoresis. In: Hames B D, Rickwood D E, editors. Gel electrophoresis of proteins. A practical approach. Oxford, United Kingdom: IRL Press; 1990. pp. 173–181. [Google Scholar]
- 30.Harraga S, Godot V, Bresson-Hadni S, Pater C, Beurton I, Bartholomot B, Vuitton D A. Clinical efficacy of and switch from T helper 2 to T helper 1 cytokine profile after interferon alpha2a monotherapy for human echinococcosis. Clin Infect Dis. 1999;29:205–206. doi: 10.1086/520157. [DOI] [PubMed] [Google Scholar]
- 31.Hemphill A, Gottstein B. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol Res. 1995;81:605–614. doi: 10.1007/BF00932028. [DOI] [PubMed] [Google Scholar]
- 32.Ingold K, Gottstein B, Hemphill A. High molecular mass glycans are major structural elements associated with the laminated layer of in vitro cultivated Echinococcus multilocularis metacestodes. Int J Parasitol. 1999;30:207–214. doi: 10.1016/s0020-7519(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 33.Ingold K, Gottstein B, Hemphill A. Identification of a laminated layer-associated protein in Echinococcus multilocularis metacestodes. Parasitology. 1998;116:363–372. doi: 10.1017/s0031182098002406. [DOI] [PubMed] [Google Scholar]
- 34.Jenum P A, Stray-Pedersen B, Gundersen A G. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol. 1997;35:1972–1977. doi: 10.1128/jcm.35.8.1972-1977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 36.Leyva-Cobian F, Outschoorn I M, Carrasco-Marin E, Alvarez-Dominguez C. The consequences of the intracellular retention of pathogen-derived T-cell-independent antigens on protein presentation to T cells. Clin Immunol Immunopathol. 1997;85:1–15. doi: 10.1006/clin.1997.4426. [DOI] [PubMed] [Google Scholar]
- 37.Maloy K, Odermatt B, Hengartner H, Zinkernagel R. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci USA. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miguez M, Baz A, Nieto A. Carbohydrates on the surface of Echinococcus granulosus protoscoleces are immunodominant in mice. Parasite Immunol. 1996;18:559–569. doi: 10.1046/j.1365-3024.1996.d01-30.x. [DOI] [PubMed] [Google Scholar]
- 39.Mond J, Lees A, Snapper C. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 40.Mond J, Vos Q, Lees A, Snapper C. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 41.Nosanchuk J, Rosas A, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 42.Rausch R L, Wilson J F, Schantz P M, McMahon B J. Spontaneous death of Echinococcus multilocularis: cases diagnosed serologically (by Em2 ELISA) and clinical significance. Am J Trop Med Hyg. 1987;36:576–585. doi: 10.4269/ajtmh.1987.36.576. [DOI] [PubMed] [Google Scholar]
- 43.Severi M A, Ferragut G, Nieto A. Antibody response of Echinococcus granulosus infected mice: protoscolex specific response during infection is associated with decreasing specific IgG1/IgG3 ratio as well as decreasing avidity. Parasite Immunol. 1997;19:545–552. doi: 10.1046/j.1365-3024.1997.d01-172.x. [DOI] [PubMed] [Google Scholar]
- 44.Snapper C M, Mond J J. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 45.Sturm D, Menzel J, Gottstein B, Kern P. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995;63:1688–1697. doi: 10.1128/iai.63.5.1688-1697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szomolanyi-Tsuda E, Welsh R. T cell-independent antibody-mediated clearance of polyomavirus in T cell-deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szomolanyi-Tsuda E, Welsh R. T-cell-independent antiviral antibody responses. Cur Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 48.Trauth-Noben N, Paul W E, Sacks D L. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 49.Vecchiarelli A, Monari C, Retini C, Pietrella D, Palazzetti B, Pitzurra L, Casadevall A. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86) expression on human monocytes. Eur J Immunol. 1998;28:114–121. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 50.Vuitton D A, Lassègue A, Miguet J P, Hervé P, Barale T, Seillés E, Capron A. Humoral and cellular immunity in patients with hepatic alveolar echinococcosis. A 2 year follow-up with and without Flabendazole treatment. Parasite Immunol. 1984;6:329–340. doi: 10.1111/j.1365-3024.1984.tb00805.x. [DOI] [PubMed] [Google Scholar]