Shiga Toxin 1 from Escherichia coli Blocks Activation and Proliferation of Bovine Lymphocyte Subpopulations In Vitro (original) (raw)
Abstract
Shiga toxin-producing Escherichia coli (STEC) is widespread in the cattle population, but the clinical significance of Shiga toxins (Stx’s) for the bovine species remains obscure. Since Stx’s exert immunomodulating effects in other species, we examined the effect of purified Stx1 on a bovine B lymphoma cell line (BL-3) and peripheral blood mononuclear cells (PBMC) isolated from adult bovine blood by viability assays and flow cytometry analysis. Stx1 markedly induced apoptosis in stimulated BL-3 cells. The susceptibility of this B-cell-derived cell line was induced only by either lipopolysaccharide (LPS) or pokeweed mitogen, while cultures stimulated with T-cell mitogens were unaffected by the toxin. In contrast, Stx1 did not induce cellular death—neither apoptosis nor necrosis—in primary cultures of PBMC but hindered the mitogen-induced increase in metabolic activity. The influence of Stx1 on single PBMC subpopulations varied with the type of mitogenic stimulus applied. Stimulation with phytohemagglutinin P particularly induced the proliferation of bovine CD8-expressing (BoCD8+) cells, and this proliferative response was blocked by Stx1. On the other hand, Stx1 reduced the portion of viable B cells in the presence of LPS. Modulation of activation marker expression (BoCD25 and BoCD71) by Stx1 indicated that the toxin hindered the proliferation of cells by blocking their activation. In conclusion, we assume that Stx1 contributes to the pathogenesis of STEC-associated diarrhea in calves by suppressing the mucosa-associated immune response. The usefulness of cattle as a model in which to study Stx-induced immunomodulation is discussed.
The family of Shiga toxins (Stx’s) produced by Escherichia coli strains represents potent biological cytotoxins, which enter the cytosol of the target cell, completely truncate protein synthesis, and thereby induce the death of the cell (35). Although cell lines of several mammalian species are susceptible to the Stx’s (9), Stx-producing E. coli (STEC) strains cause diseases only in a limited number of species: hemorrhagic colitis or the hemolytic-uremic syndrome (HUS) in humans (20) and edema disease (ED) in piglets (15). In HUS as well as in ED, Stx-mediated destruction of endothelial cells in venules and arterioles results in a thrombotic microangiopathy, the histological hallmark of both diseases (40, 49). However, there is growing evidence that Stx’s also target immune cells of the host. Human B-cell lines are highly susceptible to the cytotoxic action of Shiga toxin 1 (Stx1) (28). B-cell activation studies have indicated that the vast majority of Stx1-sensitive B cells belong to the immunoglobulin G (IgG) and IgA committed subsets (5). The selective elimination of these cells served as an explanation for the formerly assumed absence of IgG class anti-Stx antibodies in STEC-infected humans, leading to the failure of long-term immunity (5). Similarly, infections with Stx1-producing E. coli (STEC1) strains caused an immunocompromised condition in gnotobiotic pigs (4). However, the hypothesis of a sustained generalized immunosuppression during STEC infections was contradicted by Wieler et al. (51), who demonstrated the appearance of IgG antibodies against Stx2e, the ED principle, following a natural outbreak of the disease. Finally, Reymond et al. (39) confirmed that anti-Stx antibodies are detectable even in humans after subclinical infection. Understanding the discrepancy of an immunosuppressive effect of Stx1 and subsequent antibody titer development in STEC-infected individuals is highly important for devising strategies for vaccines against STEC.
Cattle have been implicated as an important reservoir for STEC (13). Nevertheless, the significance of Stx’s for bovines is obscure. Epidemiological studies (50, 52, 53) and experimental infections (3, 7) have revealed that STEC may cause bloody diarrhea in calves, but pathogenicity was mainly attributed to a different virulence factor of these bacteria: the induction of attaching and effacing mucosal lesions (18, 31, 52, 53). However, Hoffman et al. (14) recently reported a lower lymphocyte proliferative response after infection of calves with STEC strains. Although cattle frequently possess antibody titers against Stx’s (37), these findings point to a possible interaction of Stx’s and the immune system even in the bovine species. Examination of the effect of Stx’s on bovine immune cells would thus not only elucidate the possible role of Stx during STEC pathogenesis in diarrheic calves but also help to explain the discrepancies recently reviewed concerning the interactions of Stx with the immune system in general (24).
In the present study, we examined the effects of purified Stx1 on a bovine lymphoma cell line and freshly isolated bovine peripheral blood mononuclear cells (PBMC) in vitro. Stx1 was found to affect the cellular metabolic activities of BL-3 cells and PBMC profoundly at very low doses. While BL-3 cells were killed by the toxin via the induction of apoptosis, Stx1 reduced the activation and proliferation of PBMC subpopulations without induction of cellular death. The data imply that bovine immune cells do not differ significantly from those of humans and swine in their response to Stx1. Cattle may thus be a useful model for the study of the mechanism of immunomodulation by Stx1, which appears to be a general feature of STEC infections in different species.
(Part of this work was presented at the Sixth European Workshop Conference on Bacterial Protein Toxins, Hindsgavl, Denmark, 1995.)
MATERIALS AND METHODS
Toxin purification.
Stx1 was purified from the bovine STEC1 strain 2403 (rough, H−) (50). Bacteria were grown for 12 h at 37°C in 8 liters of minimal essential medium [3.5 g of K2HPO4, 1.5 g of KH2PO4, 0.5 g of sodium citrate dihydrate, 0.1 g of MgSO4 · 7H2O, 1.0 g of (NH4)2SO4, and 2.0 g of glucose (all per 1,000 ml of distilled water)]. Bacteria were harvested by centrifugation and sonicated. Supernatant was clearified by ultracentrifugation (at 100,000 × g for 2.5 h) and diluted 1:1 with 10 mM sodium phosphate buffer (pH 7.4). By use of a fast protein liquid chromatography system (FPLC; Pharmacia, Freiburg, Germany), crude toxin was applied to a column containing 10 ml of Cibacron blue 3G-A linked to agarose beads (HiTrap blue; Pharmacia). The column was washed with 10 mM sodium phosphate buffer (pH 7.4) until the optical density at 280 nm (OD280) reached the baseline. Elution was carried out with a gradient from 0 to 1 M NaCl in 10 mM sodium phosphate buffer (pH 7.4). Fractions with the highest cytotoxicity as determined in the Vero cell cytotoxicity assay were obtained at 0.2 to 0.3 M NaCl and pooled. This partially purified toxin was dialyzed overnight against 250 volumes of 10 mM sodium phosphate buffer (pH 7.4) and used for immunoaffinity chromatography. For this purpose, a column containing 5 ml of protein A/G agarose (Schleicher und Schuell, Dassel, Germany) was prepared by applying 250 ml of cell culture supernatant of the hybridoma cell line 13C4, producing mouse anti-StxB1 monoclonal antibodies (45), to the column. Weakly bound material was eluted with 0.2 M glycine–3 M NaCl (pH 2.15), leaving the antibodies noncovalently linked to the gel matrix. For purification of Stx1, the column was loaded with the partially purified and dialyzed toxin and washed with 10 mM sodium phosphate buffer (pH 7.4) until the OD280 reached the baseline. Elution was carried out stepwise: after a wash with 0.2 M glycine (pH 3.5), the ionic strength was increased gradually from 0 to 0.75 M NaCl in 0.2 M glycine (pH 3.5). Finally, the pH of the flow was decreased from 3.5 to 2.15 while the NaCl concentration was kept constant at 0.75 M. The eluate was collected 2 ml at a time, and 175 μl of 1 M Tris (pH 9.0) was added to each fraction. Fractions with the highest verocytotoxic doses were pooled and dialyzed against 250 volumes of 0.15 M NaCl overnight. Finally, toxin preparations were passed through Detoxi-Gel columns (Pierce, Old-Beijerland, The Netherlands) to remove endotoxin contaminants. The preparations contained <0.01 ng of endotoxin per ml as determined by the Limulus amoebocyte lysate assay. The concentration of Stx1 obtained by this method was 60,000 50% verocytotoxic doses (CD50) per ml, equivalent to about 60 ng of purified Stx1/ml (36). Consequently, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the purified toxin did not show any protein band, at least indicating the absence of major contaminating proteins (data not shown). The preparation was very toxic for Vero and HeLa cells (at dilutions of 1:10,000 and 1:1,000, respectively) but not for MDBK cells (data not shown). However, only those observed effects that could be prevented by preincubation of the preparation with the Stx1-specific monoclonal antibody were related to Stx1 (see below).
Cytotoxicity assay.
Cytotoxic activities of toxin preparations were determined on Vero cells (ATCC CRL 1587) as described previously (10) with minor modifications. Briefly, 50 μl of a 10-fold dilution series of toxin preparations prepared with 0.15 M NaCl was pipetted into microtiter plates in triplicate (Nunc, Wiesbaden, Germany). Fifty microliters of 0.15 M NaCl and 50 μl of 1% SDS in 0.15 M NaCl were used as negative and positive controls, respectively. Next, 50 μl of cell culture medium (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml; Gibco BRL, Berlin, Germany) was added. In neutralization studies, medium was supplemented with purified anti-StxB1 (monoclonal antibody 13C4; 4.5 μg of immunoglobulin per ml). Preceding experiments had revealed that this concentration of antibody is sufficient to completely neutralize the biological activity of at least 200 CD50/ml in the assays used in this study. After incubation (for 30 min at room temperature [RT]) 50 μl of a Vero cell suspension (8 × 105 cells per ml of cell culture medium) was applied to each well and the plates were incubated for 96 h at 37°C in 5% CO2. Cellular metabolic activity was assessed by MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide) reduction assay as described below. The CD50 was calculated from dose-response curves geometrically as the reciprocal of the toxin dilution causing a 50% reduction in cellular metabolic activity.
Cell cultures.
BL-3 cells (ECACC 86062401), a bovine B lymphoma cell line originally isolated from an animal with spontaneous leukosis but secondarily infected with bovine leukemia virus (41) and bovine diarrhea virus, were maintained in a 1:1 mixture of RPMI 1640 and Leibovitz L15 medium supplemented with 20% fetal calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (Gibco BRL). To obtain PBMC, blood samples were taken from healthy, lactating adult cows (holstein X German black pied) from the dairy herd of the Teaching and Research Farm “Oberer Hardthof” of the Justus Liebig University. Samples were diluted 1:1 with Ca2+- and Mg2+-free phosphate-buffered saline (PBS), layered onto Ficoll-Paque (Pharmacia), and centrifuged (at 400 × g and 20°C for 40 min) (2). Cells were recovered from the Ficoll-buffer interface. Contaminating erythrocytes were removed by incubating the cell suspension with lysis buffer (8.26 g of NH4Cl, 1.09 g of NaHCO3, and 0.037 g of Na3EDTA [all per 1,000 ml of distilled water]) for 5 min at RT. Cells were washed twice with PBS and resuspended at 5 × 106 cells/ml in modified cell culture medium (RPMI 1640 supplemented with 10% fetal calf serum and 3 μM 2-mercaptoethanol). PBMC preparations contained about 45% bovine CD4+ (BoCD4+) cells, 15% BoCD8+ cells, 15% B cells, 15% monocytes, and 5% γδT-cell receptor-positive (γδTCR+) T cells, with minor differences between the preparations. Fifty microliters of cell suspension was plated onto 96-well flat-bottom microtiter plates prepared as described above for the cytotoxicity assay. In stimulation assays, medium was additionally supplemented with concanavalin A (ConA; at a final concentration of 5 μg/ml), phytohemagglutinin P (PHA-P; 5 μg/ml), pokeweed mitogen (PWM; 10 μg/ml), or lipopolysaccharide (LPS) from E. coli O111:B4 (25 μg/ml; Sigma, Deisenhofen, Germany). Plates were incubated at 37°C with 5% CO2 for 1 to 8 days.
MTT reduction assay.
Cellular metabolic activity was assayed by measuring the reduction of MTT (Sigma) by mitochondrial enzymes in viable cells (46). Twenty-five microliters of MTT stock solution (5 mg/ml in PBS) was added to each well of the 96-well plates. Upon incubation (at 37°C for 4 h), the reaction was stopped, and dye crystals were dissolved, by adding 100 μl of 10% SDS in distilled water. After overnight incubation, the OD was read on a Titertek Multiscan MCC/340 ELISA plate reader (Flow, Meckenheim, Germany) by using a test wavelength of 540 nm and a reference wavelength of 680 nm. The percent cellular metabolic activity was calculated by the formula [OD (sample) − OD (positive control)]/[OD (negative control) − OD (positive control)] × 100.
DNA fragmentation assay.
DNA strand breaks in individual apoptotic cells were detected by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) method (11) by use of the fluorescein in situ cell death detection kit (Boehringer, Mannheim, Germany) according to the instructions of the vendor.
Immunophenotyping and flow cytometry analysis.
At the end of the cultivation period, PBMC were thoroughly resuspended and transferred to V-shaped microtiter plates (Greiner, Frickenhausen, Germany). After centrifugation (at 200 × g for 10 min at 4°C), supernatants were removed by flicking of the plate. Pellets were resuspended in 50 μl of cell culture medium as a negative control or with supernatants of hybridoma cell lines (IL-A11 for BoCD11, IL-A105 for BoCD8, IL-A65 for BoCD21, IL-A111 for BoCD25, and IL-A 77 for BoCD71 [33]; all kindly provided by J. Naessens, Nairobi, Kenya). The cells were incubated for 20 min on ice, washed once with PBS, and resuspended with anti-mouse–phycoerythrin conjugate (Sigma) diluted 1:100 in PBS containing 2 μg of propidium iodide (PI) (Sigma)/ml. Following another 20 min on ice, the cells were washed twice and analyzed with an EPICS ELITE Analyzer (Coulter, Krefeld, Germany). Five thousand events were acquired from each sample. Data analysis was performed with the ELITE 3.1 software provided by the manufacturer. Electronic gates were set according to the negative control included in each test series, defining less than 2% of the cells as positive.
Statistical analysis.
Data were analyzed statistically by two-way analysis of variance (ANOVA) using BMDP/Dynamic software (Statistical Software Inc.). Results were evaluated as follows: P ≤ 0.001, highly significant; P ≤ 0.01, significant; P ≤ 0.05, weakly significant; and P > 0.05, not significant.
RESULTS
Effect of Stx1 on BL-3 cells.
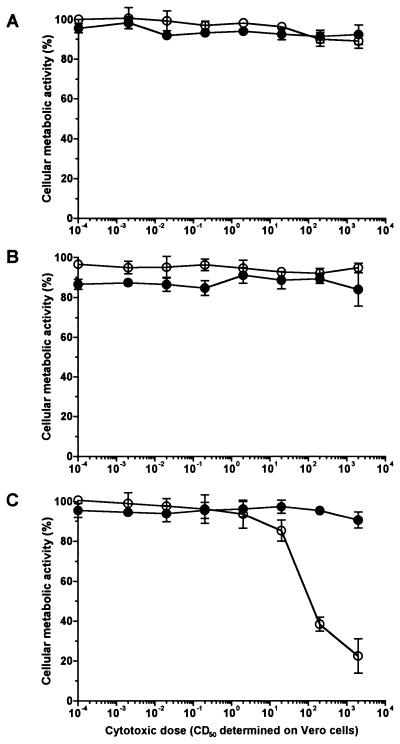
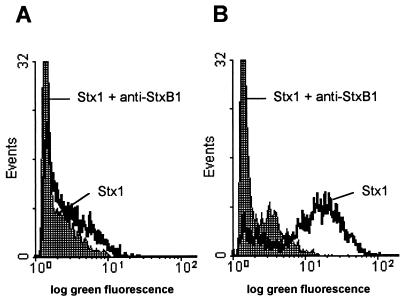
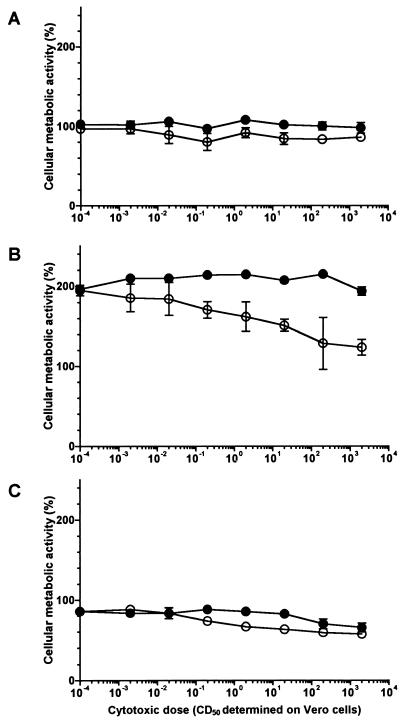
The cellular metabolic activity of the BL-3 cell line as assessed by MTT reduction was not affected by Stx1 in a concentration range from 0.002 to 2,000 CD50/ml even when the cells were incubated with the toxin for 4 days (Fig. 1). Identical results were obtained when the cultures were additionally supplemented with T-cell mitogens (see Fig. 1 for PHA-P; ConA data not shown). However, administration of certain mitogens rendered BL-3 cells highly susceptible to Stx1. When cells of this B-cell-derived cell line were stimulated with B-cell mitogens (see Fig. 1 for LPS; PWM data not shown), even 20 CD50 of Stx1/ml were sufficient to decrease the cellular metabolic activity. Two thousand CD50 per milliliter almost totally abolished the cells’ metabolic activity. This effect was attributable to Stx1, as it was neutralized with the monoclonal anti-StxB1 antibody 13C4 (1.5 μg/ml). Since Stx1 is known to kill the human B lymphoma cell line Daudi by induction of apoptosis (28), BL-3 cells were examined for DNA strand breaks. While 200 CD50 of Stx1/ml did not influence the percentage of BL-3 cells bearing DNA strand breaks in the absence of mitogens, the same dose of Stx1 dramatically increased the percentage of apoptotic cells in LPS-stimulated cultures (Fig. 2). Again, this effect could be neutralized by anti-StxB1 13C4 (1.5 μg/ml).
FIG. 1.
Effect of purified Stx1 on the cellular metabolic activity of BL-3 cells. Cells were incubated with 10-fold dilutions of purified Stx1 (0.002 to 2,000 CD50/ml; quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogen (A) or was supplemented with 5 μg of PHA-P/ml (B) or with 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Cellular metabolic activity was determined by MTT reduction assay. Cells incubated with medium alone were used as a negative control, while cells treated with 1% SDS served as a positive control to calculate percent activity. Data are means ± standard deviations from triplicate determinations. Missing error bars are within symbols. Two-way ANOVA revealed significances only for the curves presented in graph C (P ≤ 0.001 for anti-StxB1; P ≤ 0.001 for the concentration of Stx1).
FIG. 2.
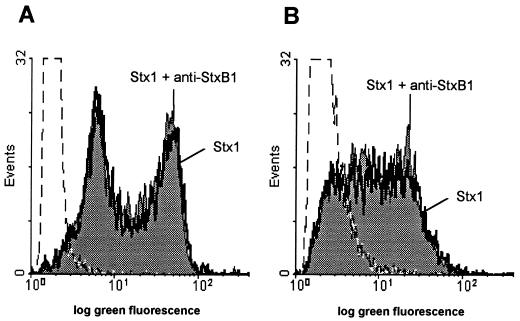
Representative flow cytometric histograms illustrating the induction of DNA strand breaks in BL-3 cells by Stx1. Cells were treated with 200 CD50/ml (quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 25 μg of LPS/ml (B). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence or presence of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. After incubation, DNA strand breaks were labeled by the TUNEL method. Stx1 was able to induce DNA strand breaks only in LPS-treated BL-3 cells. Histograms are from one representative of two experiments.
Cellular metabolic activity and membrane integrity in Stx1-treated and untreated PBMC cultures.
Stx1 also affected the cellular metabolic activity of bovine PBMC in vitro in a dose-dependent manner. As with BL-3 cells, mitogenic stimulation of the cells was a prerequisite for this effect of the toxin (Fig. 3). In unstimulated as well as in LPS-stimulated cultures, Stx1, even at high concentrations, caused only a slight reduction of cellular metabolic activity. In contrast, bovine PBMC highly stimulated with PHA-P (the cellular metabolic activity in PHA-P-stimulated controls was about 200% of the activity in unstimulated controls) were very sensitive to Stx1. Even 0.02 CD50 of Stx1/ml partially blocked the mitogen-induced enhancement in cellular metabolic activity. By increasing the Stx1 concentration up to 2,000 CD50/ml, the stimulating effect of the mitogen was nearly abolished. This metabolism-depressing effect of Stx1 on bovine PBMC could be neutralized by the addition of anti-StxB1 (1.5 μg/ml). Similar results were obtained with ConA and PWM (data not shown). However, Stx1 did not suppress the metabolic activity of the cells completely, since a remaining activity of at least 50% compared to those of untreated and unstimulated controls was detectable with all Stx1 concentrations and mitogens tested. The depressive effect of Stx1 was prominent at day 4 of cultivation, as shown in Fig. 3. The differences in cellular metabolic activity between Stx1-treated and untreated PBMC were almost negligible 2 and 6 days after initiation of the cultures (data not shown).
FIG. 3.
Effect of purified Stx1 on the cellular metabolic activity of bovine PBMC. Cells were incubated with 10-fold dilutions of purified Stx1 (0.002 to 2,000 CD50/ml; quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Cellular metabolic activity was determined by MTT reduction assay. Cells incubated with medium alone were used as a negative control, while cells treated with 1% SDS served as a positive control to calculate percent activity. Data are means ± standard deviations of triplicate determinations from one representative of six independent experiments. Missing error bars are within symbols. Two-way ANOVA revealed significances for all curves in all panels (P ≤ 0.001 for anti-StxB1 and P ≤ 0.001 for the concentration of Stx1).
To determine whether the depressive metabolic effect of Stx1 on PBMC was caused by the induction of cellular death, PBMC incubated with 200 CD50/ml were analyzed flow cytometrically for a loss of membrane integrity, indicated by an uptake of PI. Although the percentage of dead cells constantly increased from the day of preparation to the end of the incubation period, no indication of a cytotoxic effect of Stx1 on bovine PBMC was found. The percentage of PI-positive PBMC did not differ between cultures supplemented with Stx1 only and those with both Stx1 and anti-StxB1 (1.5 μg/ml) for as many as 8 days of incubation (data not shown).
Quantitation of DNA fragmentation in Stx1-treated and untreated PBMC cultures.
Since cells dying from apoptosis lose their membrane integrity at a very late stage of the cell death process, bovine PBMC were additionally examined for DNA strand breaks at day 4 of cultivation. As illustrated in Fig. 4, the percentages of apoptotic cells in cultures of unstimulated PBMC incubated with Stx1 (200 CD50/ml) in the absence or presence of anti-StxB1 (1.5 μg/ml) were 46.85% ± 3.88% and 50.38% ± 3.53%, respectively (means ± standard deviations of six determinations). In cultures stimulated with PHA-P, 35.17% ± 8.85% of the cells treated with Stx1 alone and 40.15% ± 6.22% of the cells incubated with both Stx1 and anti-StxB1 showed DNA strand breaks. These data confirm the observations made with PI uptake and show that Stx1 does not induce apoptosis in bovine PBMC cultures.
FIG. 4.
Representative flow cytometric histograms illustrating DNA strand breaks in bovine PBMC. Cells were treated with 200 CD50/ml (quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence or presence of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. After incubation DNA strand breaks were labeled by the TUNEL method. Stx1 was not able to induce DNA strand breaks either in unstimulated or in PHA-P-stimulated PBMC. Histograms are from one representative of six experiments.
Effect of Stx1 on lymphoblast transformation in bovine PBMC cultures.
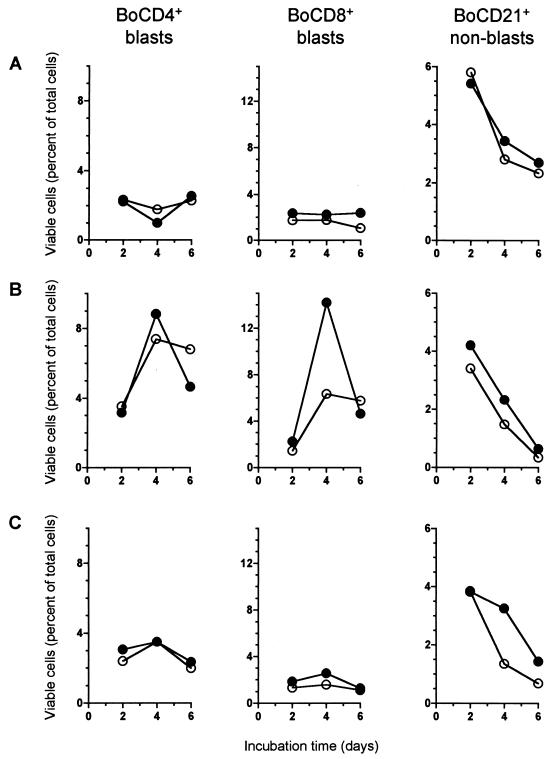
During the cultivation and mitogenic stimulation of PBMC, transformation of activated cells leads to an enlargement in cell size, while the loss of viability results in a reduction in cell size and a subsequent increase in granularity. By using flow cytometry, the populations of vital blast cells, vital non-blast cells, and subvital cells were monitored at days 2, 4, and 6 after initiation of the cultures. In accordance with the finding that Stx1 did not induce cellular death in bovine PBMC cultures, the kinetics of vital and subvital cell populations in PBMC cultures treated with Stx1 (200 CD50/ml) were almost identical regardless of the presence of anti-StxB1 (1.5 μg/ml) (data not shown). However, monitoring of lymphocyte transformation and proliferation in vitro with respect to morphological parameters only may obscure the possible effects of Stx1 on lymphocyte subpopulations. Hence, by immunophenotyping of different subpopulations, we examined the effect of Stx1 on bovine PBMC in more detail. It turned out that Stx1 (200 CD50/ml) did not affect the transformation and proliferation of all subpopulations in a similar way; rather, the effects varied with the type of mitogenic stimulus applied. BoCD4+ T cells exhibited almost identical morphological patterns under all the conditions tested (with or without mitogen, with or without Stx1) (Fig. 5, left column). In contrast, the proliferation of BoCD8+ T cells was dramatically depressed by Stx1 when the cultures were stimulated by the T-cell mitogen PHA-P (Fig. 5B). On day 4, when the percent viable BoCD8+ blast cells was highest, Stx1 reduced this level by nearly 50%. A similar effect was observed with BoCD8+ non-blast cells in cultures stimulated with PHA-P and in cultures stimulated with the T-cell mitogen ConA (data not shown). An effect of Stx1 on BoCD8+ lymphocytes was much less striking in unstimulated or LPS-stimulated PBMC cultures (Fig. 5A and C). In accordance with the effect observed with BoCD8+ T cells under T-cell stimulation, the fate of the BoCD21+ B cells in vitro was affected by Stx1 only when PBMC were stimulated with the B-cell mitogen LPS. Although LPS did not induce measurable B-cell proliferation in cultures of peripheral blood lymphocytes, over time Stx1 forced a decline in the percentage of vital non-blast B cells in LPS-treated PBMC cultures.
FIG. 5.
Effect of purified Stx1 on transformation and proliferation of PBMC subpopulations. Cells were incubated with purified Stx1 (200 CD50/ml; quantified on Vero cells as described in Materials and Methods) at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Lymphocyte subpopulations were identified by immunophenotyping at the time points indicated and quantified by flow cytometry acquiring 5,000 events. Data analysis was performed by using the software of the instrument to calculate the percentages of viable (PI-negative), immunolabeled events belonging to the blast cell or non-blast cell population. Data are single determinations from one representative of five independent experiments.
Effect of Stx1 on activation marker expression by bovine lymphocytes.
Before transformation and proliferation in vitro, lymphocytes have to undergo several activation steps induced by mitogen binding. Thus, we tested whether the proliferation-inhibiting effect of Stx1 on bovine lymphocytes was due to the blockade of the cell cycle and/or to a blockade of one of the previous activation steps. Analyzing activation marker expression on bovine lymphocytes (Fig. 6), we determined that BoCD25 (bovine interleukin-2 [boIL-2] receptor) was expressed on a higher percentage of PHA-P induced blast cells in PBMC cultures treated with Stx1 (200 CD50/ml) alone than in cultures that were additionally supplemented with anti-StxB1 (1.5 μg/ml). In contrast, the percentage of BoCD71 (transferrin receptor)-expressing cells was reduced by Stx1 on blast cells despite the application of mitogenic stimuli.
FIG. 6.
Effect of purified Stx1 on expression of activation markers by bovine PBMC in vitro. Cells were incubated with purified Stx1 (200 CD50/ml; quantified on Vero cells as described in Materials and Methods) at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Lymphocyte subpopulations were identified by immunophenotyping at the time points indicated and quantified by flow cytometry acquiring 5,000 events. Data analysis was performed by using the software of the instrument to calculate the percentage of viable (PI-negative), immunolabeled events belonging to the blast cell population.
DISCUSSION
Experimental infections (3, 7) and epidemiological studies (50) have revealed that STEC strains are a cause of diarrhea in calves. While strains secreting Stx2 are less frequently found in these animals, those secreting Stx1 represent the majority of STEC strains in calves (52, 53), and only the latter strains have been linked to disease (50). However, no direct evidence for the involvement of Stx1 in the pathogenesis of calves’ diarrhea is available to date. Confirming that primary cultures of bovine cells are susceptible to Stx1, this report provides the first direct evidence of a significance of this toxin for bovines. In calves suffering from STEC-induced diarrhea, the ileal and colonic mucosae are colonized with STEC (7) and some viable bacteria are even translocated to the mesenteric lymph nodes (6). Since we showed here that activated bovine lymphocytes are susceptible to Stx1 in very low doses—1 CD50 was calculated to be equivalent to 0.4 to 0.8 pg of purified Stx/ml (36)—we assume that Stx1 produced in the proximity of intraepithelial and lymph node lymphocytes affects the mucosal immune response against STEC, at least in the acute phase of the enteric infection. This hypothesis is supported by the observation of a marked lymphodepletion in the gut-associated lymphatic tissues in STEC-infected diarrheic calves (43). The reduced mitogenic response of peripheral blood lymphocytes after STEC infections in calves reported by Hoffman et al. (14) may therefore be just indicative of severe alterations in function of the gut-associated lymphatic tissues.
The present study revealed that the effect of Stx1 on the proliferation of bovine lymphocyte subpopulations is restricted to mitogen-stimulated cultures. The effect was most prominent for the BoCD8+ population when they were stimulated with the presumptive T-cell mitogen PHA-P. Nevertheless, bovine B cells stimulated with the B-cell mitogen LPS were also slightly affected by the toxin, resulting in an accelerated decline of living B cells in these cultures (Fig. 5). It has been shown for some species other than mice that peripheral blood B cells respond only poorly to LPS (4). As depicted in Fig. 3, LPS did not induce an enhancement in cellular metabolic activity in our PBMC cultures. The slight effect of Stx1 on LPS-stimulated B cells presumably just reflects the weak stimulation of this cell population by LPS. On the other hand, PHA-P also stimulates bovine B cells slightly, and consequently the decline in the level of living B cells in PHA-P-stimulated cultures was marginally accelerated by Stx1 (Fig. 5). We therefore assume that the effect of Stx1 on the transformation of bovine lymphocytes is not restricted to a particular subpopulation or type of stimulant but rather extends to activated cells in general. Previous studies have revealed a correlation of Stx receptor (Gb3/CD77) expression with the state of cellular activation (29, 38). Induction of Gb3/CD77 expression on bovine lymphocytes by mitogens may thus easily explain the activation-dependent effect of Stx1. Studies on this topic are currently in progress in our laboratory.
In order to understand the immunomodulating effects of Stx’s in their entirety, the events following toxin receptor interactions also need to be elucidated. Apoptosis of lymphoid cells is a naturally occurring process by which an organism removes damaged or unnecessary cells, but it may also be triggered by bacterial products such as butyric acid, Pasteurella haemolytica leukotoxin, or Stx1 (23, 28, 44). Vero cells treated with Stx1 show DNA strand breaks by 8 h after the onset of exposure to Stx1 (16). However, the effect of Stx1 on the proliferation of bovine lymphocytes was most prominent 72 to 120 h after initiation of the cultures tested by us. This was apparently due to the activation requirement and the maximum of proliferation that occurred at that time. Consequently, we examined cells for DNA strand breaks 96 h after initiation of the cultures. Surprisingly, although Stx1 clearly induced apoptosis in a bovine lymphoma cell line, there was no evidence of increased apoptosis in primary cultures of bovine lymphocytes exposed to Stx1. The failure to detect DNA strand breaks is in accordance with other examples in which apoptosis induced by Stx1 did not result in DNA fragmentation (42). However, staining with PI, commonly used to detect both necrotic and late-apoptotic cells (27), did not reveal an increase in dead cells due to Stx1 during the entire observation period of 8 days. These results are in contrast to those obtained from the bovine B lymphoma cell line BL-3. Like primary isolated lymphocytes, BL-3 cells had to be activated to render them susceptible to Stx1. However, BL-3 cells underwent apoptosis, but no induction of apoptosis could be observed in PBMC cultures treated with the same dose of Stx1. The human B lymphoma cell line Daudi has been extensively used as a model for the study of the effect of Stx1 on lymphocytes (5, 26, 28), and, like BL-3 cells, these cells are sensitive to the apoptotic effect of Stx1 in the nanogram range (28). Accordingly, the Stx receptor Gb3/CD77 is present on human germinal center B cells entering apoptosis (29), but in the light of the present study, confirming striking differences between B lymphoma cells and lymphocytes isolated from the same species, it remains to be determined whether the effect of Stx1 on primary cultures of human tonsillar B cells (5) also involves the induction of apoptosis.
As with bovine PBMC cultures, some effects of Stx’s reported so far do not involve the induction of cellular death. For example, mouse macrophages responded to Stx’s solely by an increased release of monokines (47). Human endothelial cells, recognized as the main targets of Stx cytotoxicity during STEC infections in vivo (49), may also be driven by Stx’s to an altered release of prostaglandins or clumping factors (19, 25). While primary cultures of human enterocytes were susceptible to the cytotoxic activity of Stx1 (32), polarized CaCo2 and T84 cell lines retained electrical resistance under treatment with Stx1 and translocated the toxin (1). The pathways involved in such modulations of physiological functions by Stx’s have been minimally examined to date. What is known is that Daudi cells become apoptotic when treated with the Stx1 holotoxin as well as with the isolated receptor binding B-subunit of the toxin (28) and that the cytotoxic effect of Stx1 on Vero cells can be modulated by inhibitors of protein kinases (54), indicating that there are signal cascades involved in the biological effect of Stx’s apart from the classical endocytosis pathway.
Although the intracellular pathways leading to the blockade of bovine lymphocyte proliferation by Stx1 remain to be elucidated, it has to be taken into account that this inhibiting effect may additionally involve intercellular events. There are other examples of inhibition of lymphocyte proliferation by bacterial products without direct binding of the product to the affected cells and induction of cellular death, i.e., depletion of medium supplements (8) or perturbation of cytokine networks (55). With respect to the importance of cytokines for STEC pathogenesis in general (48), cytokines may also be involved in the effect of Stx1 on bovine PBMC. The treatment of bovine lymphocytes with Stx1 led to a higher percentage of cells expressing BoCD25 (boIL-2 receptor), while the percentage of BoCD71 (transferrin receptor)-expressing cells was reduced. Expression of both receptors represents early events during the activation of lymphocytes (17, 34). The boIL-2 receptor is downregulated when the cells bind sufficient amounts of boIL-2 that are produced by the PBMC themselves in a later stage of activation. A sustained high expression of boIL-2 receptor paralleled by a reduced expression of BoCD71 points to an arrest of cellular activation in a stage before boIL-2 is produced. Since proliferation of BoCD8+ cells, which was predominantly affected by Stx1, depends on boIL-2 to a higher extent than BoCD4+ cell proliferation (30, 56), Stx1 may have blocked cellular activation by impairing paracrine boIL-2 release. Recently, Malstrom and James (27) reported the inhibition of mitogen-stimulated IL-2, IL-4, and gamma interferon production by splenic and mucosal lymphocytes. This inhibitory effect was attributed to a protease-sensitive, heat-labile factor of <6 to 8 kDa, present in lysates of enteropathogenic E. coli strains as well as in the lysates of some related bacteria, including STEC strain EDL 933 (22). The Stx1 preparation used in our study was dialyzed with a 26,000- to 28,000-molecular-weight cutoff membrane, and the effects could be clearly ascribed to the toxin by neutralization with monoclonal anti-Stx1. Hence, we speculate that inhibition of lymphocyte functions by Stx1 via perturbation of cytokine profiles represents an additional and unique mechanism of STEC to control the host response.
The susceptibility of bovine B and T lymphocytes to Stx1 suggests that the bovine immune system is affected more profoundly by the toxin than is the human immune system. In humans, the effect of Stx1 is believed to be restricted to B cells only, since Stx1 elaborates a suppressive effect on tonsillar B cells but not on tonsillar T cells and thymocytes (5). Contrarily, Keusch et al. (21) reported inhibition of B-cell as well as T-cell mitogenic responses of human PBMC by a crude preparation of Stx from Shigella shigae. In vivo administration of STEC1 to gnotobiotic pigs and of STEC2 to calves also resulted in decreased mitogenic responses of B cells as well as T cells in vitro compared to those of PBMC prepared from animals inoculated with Stx-negative strains (4, 14). Thus, despite the conflicting data reported for humans, an immunomodulating effect of Stx’s appears to be widespread among the species that are naturally infected with STEC. However, its importance during natural STEC infections is still a matter of speculation (24) because Stx-induced immunosuppression does not totally prevent a humoral response against Stx’s and STEC-associated O antigens (12, 14, 37, 39, 51). Since bovine lymphocytes resemble those of humans and swine with respect to their response to Stx1, but cattle >3 weeks of age do not suffer from clinical manifestations of STEC infections (6), this species should be a useful model for the elucidation of the interactions of Stx’s with the immune system.
ACKNOWLEDGMENTS
We thank J. Naessens at ILRI, Nairobi, Kenya, for generously supplying hybridoma cell lines producing antibodies to bovine leukocyte antigens. We also thank Burkhard Schütz, Institut für Mikroökologie, Herborn, Germany, for performing the LAL test, and Klaus Failing, biomathematics work group, Faculty of Veterinary Medicine of the Justus-Liebig University, for statistical analysis.
C. Menge was supported by a predoctoral fellowship of the Hessische Graduiertenförderung.
REFERENCES
- 1.Acheson D W K, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch G J. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol Suppl. 1976;5:9–15. [PubMed] [Google Scholar]
- 3.Chanter N, Hall G A, Bland A P, Hayle A J, Parsons K R. Dysentery in calves caused by an atypical strain of Escherichia coli (S102-9) Vet Microbiol. 1986;12:241–253. doi: 10.1016/0378-1135(86)90053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopher-Hennings J, Willgohs J A, Francis D H, Raman U A K, Moxley R A, Hurley D J. Immunocompromise in gnotobiotic pigs induced by verotoxin-producing Escherichia coli (O111:NM) Infect Immun. 1993;61:2304–2308. doi: 10.1128/iai.61.6.2304-2308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A, Madrid-Marina V, Estrov Z, Freedman M H, Lingwood C A, Dosch H M. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int Immunol. 1990;2:1–8. doi: 10.1093/intimm/2.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Cray W C, Moon H W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:1586–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom E A, Bosworth B T, Cray W C, Moon H W. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect Immun. 1997;65:1842–1848. doi: 10.1128/iai.65.5.1842-1848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degnan B A, Palmer J M, Robson T, Jones C E D, Fischer M, Glanville M, Mellor G D, Diamond A G, Kehoe M A, Goodacre J A. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect Immun. 1998;66:3050–3058. doi: 10.1128/iai.66.7.3050-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiklid K, Olsnes S. Interaction of Shigella shigae cytotoxin with receptors on sensitive and insensitive cells. J Recept Res. 1980;1:199–213. doi: 10.3109/10799898009044098. [DOI] [PubMed] [Google Scholar]
- 10.Gentry M K, Dalrymple J M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 12.Greatorex J S, Thorne G M. Humoral immune response to Shiga-like toxins and Escherichia coli O157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J Clin Microbiol. 1994;32:1172–1178. doi: 10.1128/jcm.32.5.1172-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuvelink A E, van den Biggelaar F L A M, de Boer E, Herbes R G, Melchers W J G, Huis in’t Veld J H J, Monnens L A H. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 strains from Dutch cattle and sheep. J Clin Microbiol. 1998;36:878–882. doi: 10.1128/jcm.36.4.878-882.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman M, Casey T, Bosworth B. Abstracts of the 3rd International Symposium and Workshop on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections 1997. 1997. Bovine immune response to Escherichia coli O157, abstr. V67/VIII; p. 117. [Google Scholar]
- 15.Imberechts H, De Greve H, Lintermans P. The pathogenesis of edema disease in pigs. A review. Vet Microbiol. 1992;31:221–233. doi: 10.1016/0378-1135(92)90080-d. [DOI] [PubMed] [Google Scholar]
- 16.Inward C D, Williams J, Chant I, Crocker J, Milford D V, Rose P E, Taylor C M. Verocytotoxin induces apoptosis in Vero cells. J Infect. 1995;30:213–218. doi: 10.1016/s0163-4453(95)90693-2. [DOI] [PubMed] [Google Scholar]
- 17.Janeway C A, Travers P. Immunologie. Heidelberg, Germany: Spektrum Akademischer Verlag GmbH; 1995. p. 299. [Google Scholar]
- 18.Janke B H, Francis D H, Collins J E, Libal M C, Zeman D H, Johnson D D, Neiger R D. Attaching and effacing Escherichia coli infection as a cause of diarrhea in young calves. J Am Vet Med Assoc. 1990;196:897–901. [PubMed] [Google Scholar]
- 19.Karch H, Bitzan M, Pietsch R, Stenger K-O, Von Wulffen H, Heesemann J, Düsing R. Purified verotoxins of E. coli O157:H7 decrease prostacyclin synthesis by endothelial cells. Microb Pathog. 1988;5:215–221. doi: 10.1016/0882-4010(88)90024-1. [DOI] [PubMed] [Google Scholar]
- 20.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keusch G T, Papenhausen P R, Jacewicz M, Hirschhorn K. Comparison of Shigella (S) and cholera (C) toxin effects using lymphocytes as target cells. Clin Res. 1976;24:287A. [Google Scholar]
- 22.Klapproth J-M, Donnenberg M S, Abraham J M, Mobley H L T, James S P. Products of enteropathogenic Escherichia coli inhibit lymphocyte activation and lymphokine production. Infect Immun. 1995;63:2248–2254. doi: 10.1128/iai.63.6.2248-2254.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korita-Ochiai T, Fukushima K, Ochiai K. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect Immun. 1997;65:35–41. doi: 10.1128/iai.65.1.35-41.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingwood C A. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 25.Louise C B, Obrig T G. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic-uremic syndrome as related to fibrinolysis factor expression, in vitro. Microvasc Res. 1994;47:377–387. doi: 10.1006/mvre.1994.1030. [DOI] [PubMed] [Google Scholar]
- 26.Maloney M D, Lingwood C A. CD19 has a potential CD77 (globotriaosylceramide)-binding site with sequence similarity to verotoxin B-subunits: implications of molecular mimicry for B cell adhesion and enterohemorrhagic Escherichia coli pathogenesis. J Exp Med. 1994;180:191–201. doi: 10.1084/jem.180.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malstrom C, James S. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect Immun. 1998;66:3120–3127. doi: 10.1128/iai.66.7.3120-3127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangeney M, Lingwood C A, Taga S, Caillou B, Tursz T, Wiels J. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993;53:5314–5319. [PubMed] [Google Scholar]
- 29.Mangeney M, Richard Y, Coulaud D, Tursz T, Wiels J. CD77: an antigen of germinal center B cells entering apoptosis. Eur J Immunol. 1991;21:1131–1140. doi: 10.1002/eji.1830210507. [DOI] [PubMed] [Google Scholar]
- 30.Morrison W L, Baldwin C L, MacHugh N O, Teale A J, Goddeeris B M, Ellis J. Phenotypic and functional characterization of bovine lymphocytes. Prog Vet Microbiol Immunol. 1988;4:134–164. [PubMed] [Google Scholar]
- 31.Moxley R A, Francis D H. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect Immun. 1986;53:339–346. doi: 10.1128/iai.53.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyer M P, Dixon P S, Rothman S, Brown J E. Cytotoxicity of Shiga toxin for primary cultures of human colonic and ileal epithelial cells. Infect Immun. 1987;55:1533–1535. doi: 10.1128/iai.55.6.1533-1535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naessens J, Howard C J, Hopkins J. Nomenclature and characteristics of leukocyte differentiation antigens in ruminants. Immunol Today. 1997;18:365–368. doi: 10.1016/s0167-5699(97)81055-9. [DOI] [PubMed] [Google Scholar]
- 34.Naessens J, Grab D J, Fritsch G. Characterisation of bovine transferrin receptor on normal activated and Theileria parva-transformed lymphocytes by a new monoclonal antibody. Vet Immunol Immunopathol. 1996;52:65–76. doi: 10.1016/0165-2427(95)05537-1. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsnes S, Reisbig R, Eiklid K. Subunit structure of Shigella cytotoxin. J Biol Chem. 1981;256:8732–8738. [PubMed] [Google Scholar]
- 37.Pirro F, Wieler L H, Failing K, Bauerfeind R, Baljer G. Neutralizing antibodies against Shiga-like toxins from Escherichia coli in colostra and sera of cattle. Vet Microbiol. 1995;43:131–141. doi: 10.1016/0378-1135(94)00089-f. [DOI] [PubMed] [Google Scholar]
- 38.Ramegowda B, Tesh V L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect Immun. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reymond D, Johnson R P, Karmali M A, Petric M, Winkler M, Johnson S, Rahn K, Renwick S, Wilson J, Clarke R C, Spika J. Neutralizing antibodies to Escherichia coli Vero cytotoxin 1 and antibodies to O157 lipopolysaccharide in healthy farm family members and urban residents. J Clin Microbiol. 1996;34:2053–2057. doi: 10.1128/jcm.34.9.2053-2057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 41.Romano M J, Stewart J A, Lewin H A. Phenotypic characterization of bovine lymphoblastoid cell lines. Vet Immunol Immunopathol. 1989;23:293–307. doi: 10.1016/0165-2427(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 42.Sandvig K, van Deurs B. Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp Cell Res. 1992;200:253–262. doi: 10.1016/0014-4827(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 43.Schoonderwoerd M, Clarke R, van Dreumel A A, Rawluk S A. Colitis in calves: natural and experimental infection with a verotoxin-producing strain of Escherichia coli O111:NM. Can J Vet Res. 1988;52:484–487. [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens P K, Czuprynski C. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strockbine N A, Marques L R M, Holmes R K, O’Brien A D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985;50:695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- 47.Tesh V L, Ramegowda B, Samuel J E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesh V L. Virulence of enterohemorrhagic Escherichia coli: role of molecular crosstalk. Trends Microbiol. 1998;6:228–233. doi: 10.1016/s0966-842x(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 49.van de Kar N C, Monnens L A H, Karmali M A, van Hinsbergh V W M. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic-uremic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- 50.Wieler L H, Bauerfeind R, Baljer G. Characterization of Shiga-like toxin-producing Escherichia coli (SLTEC) isolated from calves with and without diarrhoea. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:243–253. doi: 10.1016/s0934-8840(11)80011-3. [DOI] [PubMed] [Google Scholar]
- 51.Wieler L H, Franke S, Menge C, Rose M, Bauerfeind R, Karch H, Baljer G. Investigations on the immunoresponse during edema disease of piglets after weaning by using a recombinant B subunit of Shiga-like-toxin IIe. DTW (Dtsch Tieraerztl Wochenschr) 1995;102:40–43. [PubMed] [Google Scholar]
- 52.Wieler L H, Vieler E, Erpenstein C, Schlapp T, Steinrück H, Bauerfeind R, Byomi A, Baljer G. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J Clin Microbiol. 1996;34:2980–2984. doi: 10.1128/jcm.34.12.2980-2984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieler L H, Schwanitz A, Vieler E, Busse B, Steinrück H, Kaper J B, Baljer G. Virulence properties of Shiga toxin-producing Escherichia coli (STEC) strains of serogroup O118, a major group of STEC pathogens in calves. J Clin Microbiol. 1998;36:1604–1607. doi: 10.1128/jcm.36.6.1604-1607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams J M, Milford D V, Taylor C M. Abstracts of the 7th European Workshop Conference on Bacterial Protein Toxins 1995. 1995. Intracellular signalling pathways involved in verocytotoxin-induced apoptosis in Vero cells, abstr. 53. [Google Scholar]
- 55.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winkelstein A, Weaver L D, Salva N, Machen C L. Interleukin-2-induced lymphoproliferative responses. Cancer Immunol Immunother. 1990;32:110–116. doi: 10.1007/BF01754207. [DOI] [PMC free article] [PubMed] [Google Scholar]