Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells (original) (raw)
Abstract
- We combined patch clamp and fura-2 fluorescence methods to characterize human TRP3 (hTRP3) channels heterologously expressed in cultured bovine pulmonary artery endothelial (CPAE) cells, which do not express the bovine trp3 isoform (btrp3) but express btrp1 and btrp4.
- ATP, bradykinin and intracellular Ins_P_3 activated a non-selective cation current (_I_hTRP3) in _htrp3_-transfected CPAE cells but not in non-transfected wild-type cells. During agonist stimulation, the sustained rise in [Ca2+]i was significantly higher in _htrp3_-transfected cells than in control CPAE cells.
- The permeability for monovalent cations was _P_Na > _P_Cs≈_P_K >> _P_NMDG and the ratio _P_Ca/_P_Na was 1·62 ± 0·27 (_n_= 11). Removal of extracellular Ca2+ enhanced the amplitude of the agonist-activated _I_hTRP3 as well as that of the basal current The trivalent cations La3+ and Gd3+ were potent blockers of _I_hTRP3 (the IC50 for La3+ was 24·4 ± 0·7 μM).
- The single-channel conductance of the channels activated by ATP, assessed by noise analysis, was 23 pS.
- Thapsigargin and 2,5-di-_tert_-butyl-1,4-benzohydroquinone (BHQ), inhibitors of the organellar Ca2+-ATPase, failed to activate _I_hTRP3. U-73122, a phospholipase C blocker, inhibited _I_hTRP3 that had been activated by ATP and bradykinin. Thimerosal, an Ins_P_3 receptor-sensitizing compound, enhanced _I_hTRP3, but calmidazolium, a calmodulin antagonist, did not affect _I_hTRP3.
- It is concluded that hTRP3 forms non-selective plasmalemmal cation channels that function as a pathway for agonist-induced Ca2+ influx.
Cytosolic Ca2+ ([Ca2+]i) plays a crucial role in various cellular functions of virtually all cell types. Agonists that increase [Ca2+]i cause a release of Ca2+ from intracellular stores followed by a transmembrane Ca2+ entry. This Ca2+ influx, which is responsible for the sustained phase of [Ca2+]i increase, is important for various cellular functions, including gene expression, secretion, contraction and cellular metabolism (Clapham, 1995). In endothelial cells, these Ca2+ signals trigger the secretion of various agents such as prostacyclin (PGI2), nitric oxide (NO), tissue plasminogen activator (tPA), platelet activating factor (PAF), von Willebrand factor (vWF) and tissue factor pathway inhibitor (TFPI) (Jacob et al. 1988; Jacob, 1990; Carter & Ogden, 1992; Inagami et al. 1995; Nilius et al. 1997_b_). Some of these processes, such as NO release, depend on a sustained influx of extracellular Ca2+ (Iouzalen et al. 1996; Lantoine et al. 1998). It is widely accepted that this influx is controlled by agonist-activated Ca2+-permeable ion channels and/or by store-controlled mechanisms (capacitative Ca2+ entry, CCE).
CCE, which is regulated by the filling state of the intracellular Ca2+ store, has been detected in a wide variety of cells. The Ca2+ current activated by store depletion was first identified in mast cells using the patch clamp technique and termed as Ca2+ release-activated Ca2+ current (_I_CRAC) (Hoth & Penner, 1992). It is generally accepted that Ca2+-permeable channels activated by store depletion form the major pathway for agonist-induced Ca2+ influx (Berridge, 1995; Parekh & Penner, 1997). However, various other Ca2+ influx pathways have been proposed to exist in non-excitable cells such as endothelial cells (Oike et al. 1994; Clapham, 1995; Fasolato & Nilius, 1998).
The molecular candidates for store-operated channels are the proteins encoded by members of the trp gene family (Bennett et al. 1995; Birnbaumer et al. 1996). So far, seven mammalian homologues of trp genes have been described, which form four main subfamilies (Birnbaumer et al. 1996; Zhu et al. 1996). trp encodes a protein with six putative transmembrane segments that is similar to voltage-gated Ca2+ channels but lacks the positive amino acids responsible for voltage sensing (Montell, 1997). Recently, heterologous expression experiments have provided evidence that proteins encoded by various mammalian trp homologues may form CCE channels (Zitt et al. 1996, 1997; Philipp et al. 1996; Zhu et al. 1996, 1998; Boulay et al. 1997; Okada et al. 1998), and _trp_-encoded proteins (TRPs) have been shown to form store-operated channels (Groschner et al. 1998). However, a comprehensive analysis of the permeation properties of TRP channels has not yet been performed and their gating mechanism is still controversial.
In the present study we characterized human TRP3 (hTRP3) channels in mammalian macrovascular endothelial cells that did not express the endogenous trp3 gene. We show that after transient transfection with htrp3 these cells functionally express non-selective cation channels in the plasma membrane, which serve as an influx pathway for Ca2+ during agonist stimulation.
METHODS
Polymerase chain reaction and cDNA cloning of bovine trp3
Poly(A)+ RNA (200 ng) isolated from bovine pulmonary artery endothelial (CPAE) cells was reverse transcribed into first strand cDNA using oligodeoxynucleotide (oligo (dT)18) primers and SuperScript reverse transcriptase (Life Technologies). For amplification of _trp_-related bovine DNA sequences the following primer pairs were used: 5′-GACAGATGTCAGGTTACC-3′ and 5′-ATTCTTTCAAGGGCTGGC-3′, corresponding to the peptides G337QMSGYR and G417PALERI, respectively, to amplify the 259 bp fragment of the bovine trp1 gene (Chang et al. 1997); 5′-TGGCGTCTCGCTGGTAC-3′ and 5′-AGGACCCACGGTAATATC-3′, corresponding to the peptides L312ASRWY and M407ILPWVL, respectively, to amplify the 304 bp fragment of bCCE1 (also btrp4; Philipp et al. 1996); 5′-CAATCTGGTACGAGAACC-3′ and 5′-CAGTCCAAGTGAACTGTG-3′, corresponding to the peptides T6IWYENL and T106QFTWTE, respectively, to amplify the 318 bp fragment of btrp3 (Fig. 1_A_). The bovine orthologue of trp3 has not been cloned so far and primers were derived from a btrp3 cDNA fragment, which was obtained as follows. A 395 bp cDNA fragment was amplified from reverse-transcribed bovine spleen poly(A)+ RNA using the degenerated primers 5′-AGTTYGTBKCYCARYCYAACTG-3′ and 5′-CCAHATMAWHCCHAKRAYCCA-3′, corresponding to peptides K319FVAHPNC and W444VLGMMW, respectively, of the human trp3 orthologue (Zhu et al. 1996). Amplified cDNAs were subcloned and six independent clones sequenced. The sequence of btrp3 has been deposited in GenBank (accession number AJ006781). Polymerase chain reactions were performed under the following conditions: 94°C, 3 min, followed by addition of 2 U Taq polymerase; 15 cycles (94°C, 30 s; 52°C, 45 s; 72°C, 45 s) followed by 25 cycles (94°C, 30s; 52°C, 45s; 72°C, 45 s plus 2 s increment per cycle) and, finally, 72°C for 3 min. Amplified btrp1, btrp3 and btrp4 cDNAs were analysed on 5 % polyacrylamide gels. Control reactions were performed either by using ∼1 fmol of the corresponding cloned cDNAs or else in the absence of first strand DNA. The identity of the amplified cDNA fragments was verified by restriction analysis. Two independent experiments gave identical results.
Figure 1. Nucleotide and deduced amino acid sequence of the bovine trp3 cDNA fragment aligned to the amino acid sequence of human trp3, and expression of bovine _trp_-related genes in CPAE cells.
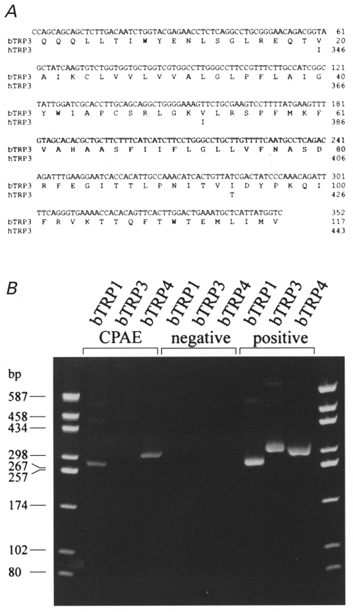
A, only those nucleotides outside the sequence of the degenerated primers used for amplification are shown. The numbering of htrp3 is according to Zhu et al. (1996). Identical amino acids are indicated by blanks. B, RT-PCR analysis using specific primer pairs, showing the expression of btrp1 and btrp4 but not_btrp3_ in CPAE cells. The left and right lanes show the DNA site markers in base pairs as indicated.
Vector construction for green fluorescent protein-TRP3 co-expression
An open reading frame for the human trp3 gene was constructed by in-frame ligation of the hAM1 and hFM1 cDNA clones. First, an _Eco_RI-_Pvu_II fragment of hFM1 containing nucleotides 771-3429 of the open reading frame (nucleotide 1 corresponds to the A of the ATG start codon) and part of the 3′ untranslated region were subcloned in a pCINeo vector (Promega) cut with _Eco_RI and _Sma_I. Second, an _Eco_RI fragment of hAM 1 containing nucleotides 1-770 of the open reading frame and part of the 5′ untranslated region was subcloned in the _Eco_RI site of the pCINeo/hFM1 vector. The entire open reading frame for the human trp3 gene was then isolated as a Xho I-Not I fragment from this vector and subcloned in an _Eco_RI-linearized and blunt-ended pCINeo/IRES-GFP (green fluorescent protein) vector (Trouet et al. 1997), which resulted in the pCINeo/IRES-GFP/hAM1-hFM1 vector.
Cell culture and transfection
Cultured CPAE cells purchased from the American Type Culture Collection (ATCC no. CCL 209) were grown in Dulbecco's modified Eagle's medium containing 10 % human serum, 2 mmol l−1 L-glutamine, 2 U ml−1 penicillin and 2 mg ml−1 streptomycin.
CPAE cells were transiently transfected with the pCINeo/IRES-GFP/hAM1-hFM1 vector using methods identical to those described previously (Kamouchi et al. 1997_b_). In short, cells were incubated with a transfection cocktail containing DNA and polycationic SuperFect Transfection Reagent (Qiagen, Hilden, Germany). Cells were then transferred to gelatin-coated coverslips 24 h after transfection and electrophysiological measurements were made 2-3 days after transfection. Between 1 and 3 % of these cells showed green fluorescence.
Incorporation of htrp3 in the bicistronic unit allows coupled expression of the channels and GFP. Transfected cells were visually identified in the patch clamp set-up. GFP was excited at a wavelength between 450 and 490 nm and the emitted light was passed through a 520 nm long-pass filter.
Electrophysiology
Electrophysiological methods and Ca2+ measurements have been described in detail elsewhere (Nilius et al. 1994). The patch clamp technique was used in the whole-cell configuration and whole-cell currents were measured using ruptured patches. Currents and voltages were monitored in voltage and current clamp modes with an EPC-9 (HEKA Elektronik, Lambrecht, Germany; sampling rate, 1 ms; 8-pole Bessel filter, 2·9 kHz).
We applied a ‘ramp protocol’ every 5 s, consisting of a voltage step to -100 mV for 40 ms followed by a 200 ms linear ramp to +100 mV. For noise analysis, the membrane potential was kept at -50 mV and the membrane current was recorded at 2 kHz. Sweeps of 512 samples were recorded every 2 s.
The current density was calculated from the size of the net Na+ influx current at -50 mV. Unidirectional Na+ influx was estimated by the difference between the currents recorded in external Na+ solution and those recorded in external NMDG+ solution. This procedure was justified as the permeability of the non-selective cation channels was low for NMDG+, as shown below.
The permeability of monovalent cations relative to that of Na+ was estimated from the shift in reversal potential upon replacing external Na+ with these cations, according to eqn (1). Ca2+ permeability was estimated either from the shift in reversal potential upon replacing 150 mM NaCl with 75 mM CaCl2, (_V_Ca - _V_Na; eqn (2)), or from the reversal potential, _V_Ca, in the presence of high external Ca2+ (eqn (3)). Hyperosmolarity in 75 mM CaCl2 was used to prevent the activation of volume-regulated anion channels (VRACs), as described below. The relative permeability to K+ over Na+ was calculated by eqn (4) (for details, see Lewis, 1979).
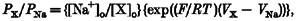 |
(1) |
|---|
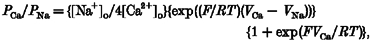 |
(2) |
|---|
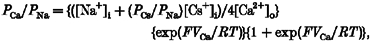 |
(3) |
|---|
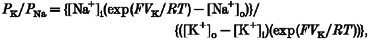 |
(4) |
|---|
where _P_X is the permeability constant, [X]i and [X]o are the internal and external concentrations of the cations and F, R and T have their usual meanings. _V_Ca, _V_Na and _V_K are reversal potentials measured in the presence of the respective cations in the external solution.
The dose-response curve for La3+ block was evaluated by normalizing the net Na+ influx current in the presence of La3+ to that in its absence. The concentration for half-maximal inhibition was obtained from a fit of these data to the Hill equation:
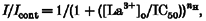 |
(5) |
|---|
where _I_cont and I are the inward Na+ currents through hTRP3 channels before and after application of La3+, respectively, IC50 is the concentration for 50 % block and _n_His the Hill coefficient.
The relationship between the mean current amplitude (I) and the current variance (σ2) is given by the equation (Sigworth, 1981):
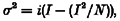 |
(6) |
|---|
where i is the single-channel current amplitude and N is the total number of channels in the membrane.
Solutions
The standard external solution contained (mM): 150 NaCl, 10 Hepes, 10 glucose and 1·5 CaCl2. To eliminate volume-activated Cl− currents, the osmolality of all external solutions was increased to 390 ± 5 mosmol kg−1 by adding mannitol. For studying the relative permeabilities of Cs+ or NMDG+, NaCl was replaced with equimolar amounts of CsCl or NMDGCl, respectively. To determine the Ca2+ permeability, external Na+ was replaced with 75 mM CaCl2. The pH of the extracellular solutions was adjusted to 7·4 with NaOH or CsOH. The internal high-Cs+ pipette solution contained (mM): 145 caesium glutamate, 8 NaCl, 2 MgCl2, 10 Hepes and 1 Na2ATP buffered with CsOH to 7·2. For buffering free Ca2+, Ca2+-free and Ca2+-saturated caesium BAPTA or caesium EGTA were added at concentrations calculated by the CaBuf program (G. Droogmans, KU Leuven). The concentrations of BAPTA and EGTA were 10 and 5 mM, respectively. In the experiments where we did not buffer intracellular Ca2+, we used a pipette solution that contained an EGTA concentration of 0·1 mM.
For simultaneous measurement of membrane current and [Ca2+]i we used an external Krebs solution and an internal high-K+ solution unless mentioned otherwise. The Krebs solution contained (mM): 144 NaCl, 6 KCl, 1·5 CaCl2, 10 Hepes and 10 glucose. The internal high-K+ pipette solution contained (mM): 40 KCl, 100 potassium aspartate, 2 MgCl2, 0·1 EGTA, 10 Hepes and 4 Na2ATP. The pH of the extracellular solutions was adjusted to 7·4 with NaOH and that of the internal solutions was adjusted to 7·2 with KOH.
ATP (Sigma), bradykinin (Sigma) and thimerosal (Acros Organics, New Jersey, USA) were added to the external solutions and Ins_P_3 (Sigma) to the internal solution. U-73122, U-73343 (Calbiochem) and 2,5-di_-tert_-butyl-1,4-benzohydroquinone (BHQ; Sigma) were applied from a stock solution in DMSO. Thapsigargin (Sigma) and calmidazolium (Janssen Pharmaceutica, Belgium) were dissolved in ethanol as a stock solution. The final concentration of DMSO or ethanol was less than 0·05 %.
Ca2+ measurement
For [Ca2+]i measurement, cells were loaded with fura-2 (K+ salt) or fura-2 AM (the acetoxylmethyl ester form). Fura-2 (100 μM) was included in the internal solution and applied through the pipette. Fura-2 AM (2 μM) was added to the bath and the cells were incubated for 25 min at 37°C. The loaded cells were illuminated alternatively at wavelengths of 360 and 390 nm through a rotating filter wheel. The fluorescence was measured at 510 nm and autofluorescence was subtracted from the signals. The free Ca2+ concentration was calculated from the ratio of the fluorescence signals produced by excitation at both wavelengths. The calibration procedure was identical to that described previously (Nilius et al. 1994).
All experiments were performed at room temperature (20-22°C). Pooled data are given as means ±s.e.m., n is the number of cells. P < 0·01 is taken as significant.
RESULTS
Cell-specific expression of mammalian trp genes
The expression of mammalian TRP channel homologues was investigated using RT-PCR in bovine pulmonary artery endothelial (CPAE) cells. Using species-specific oligodeoxynucleotide primers, PCR fragments corresponding to bovine trp1 (btrp1) and bovine trp4 (btrp4) but not bovine trp3 (btrp3) were detected in the CPAE cells (Fig. 1). We therefore used CPAE cells to study the functional effects of trp3 gene expression. On the other hand, we have detected by RT-PCR analysis that in another endothelial cell line derived from human umbilical veins, Ea.hy926, the human trp3 (htrp3) gene is present (M. Kamouchi, B. Nilius and V. Flockerzi, unpublished observations).
Functional expression of hTRP3 in CPAE cells
We transfected CPAE cells with a bicistronic vector that co-expresses human TRP3 (hTRP3) and green fluorescent protein (GFP) (for details see Kamouchi et al. 1997_b_;Trouet et al. 1997). CPAE cells are a convenient expression system for trp3 because they do not endogenously express this trp isoform (Fig. 1). The hTRP3-expressing cells were identifiable by their green fluorescence; they were cobblestone-like in appearance, adhered to the coverslips and could not be discriminated morphologically from non-transfected cells.
We compared first the electrical responses and changes in [Ca2+]i during stimulation with ATP in non-transfected and _htrp3_-transfected CPAE cells. In current clamp mode, 1 μM ATP transiently hyperpolarized the membrane in non-transfected CPAE cells if [Ca2+]i was not buffered (Fig. 2_A_, upper trace), which was probably due to the activation of Cl− channels during the peak increase in [Ca2+]i, as shown in the lower trace (Nilius et al. 1997_a_). This is a typical response of depolarized cells (n > 150, see Voets et al. 1996). Stimulation with 3 μM ATP in the absence of extracellular Ca2+ induced a transient peak in [Ca2+]i of 1020 ± 240 nM (_n_= 12), which was not significantly different (P > 0·05) from the peak value in 1·5 mM extracellular Ca2+ of 1203 ± 456 nM (_n_= 9) in non-clamped cells (see also Fig. 3 for clamped cells). Withdrawal of extracellular Ca2+ diminished the plateau level of [Ca2+]i from 179 ± 11 to 42 ± 4 nM (_n_= 5). Thapsigargin (1 μM) or BHQ (20 μM) induced a slow but transient increase in [Ca2+]i in CPAE cells exposed to Ca2+-free solution, reaching a peak value in non-clamped cells of 315 ± 25 nM (_n_= 6). These findings are proof that agonist stimulation also releases Ca2+ from intracellular stores in CPAE cells. During agonist stimulation the sustained rise in [Ca2+]i was small and below threshold for activation of the Ca2+-activated Cl− channels. In _htrp3_-transfected cells, however, ATP caused a sustained depolarization (+2·9 ± 5 mV, _n_= 10) and a much higher sustained rise in [Ca2+]i (Fig. 2_B_). The Ca2+ plateau was abolished if the cells were exposed to Ca2+-free solution, pointing to an influx pathway for Ca2+ activated by the agonist. The depolarization persisted, however, suggesting that the current responsible for the ATP-induced depolarization is probably not activated by changes in intracellular Ca2+. In the lower panels (C and D) we compared the I-V curves measured in both cell types before and during ATP stimulation. In control cells, ATP did not stimulate any additional current at the plateau phase of the [Ca2+]i rise (Fig. 2_C_), while ATP application in the _htrp3_-transfected cell stimulated a huge current (Fig. 2_D_), even at [Ca2+]i levels comparable to or even lower than those in the control cells.
Figure 2. Effects of hTRP3 expression on the ATP-induced electrical response and changes in [Ca2+]i in CPAE cells.
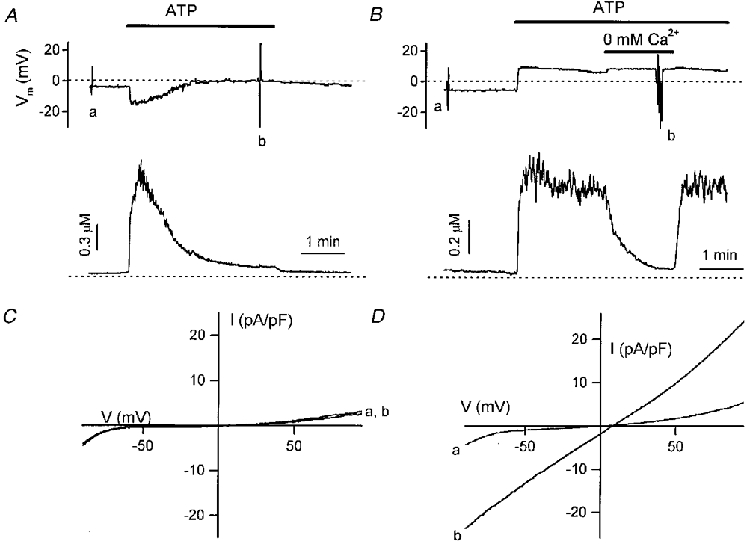
Comparison of the effects of ATP (1 μM) on membrane potential (top trace) and [Ca2+]i (bottom trace) in non-transfected (A) and _htrp3_-transfected CPAE cells (B). Membrane potential was measured using the current clamp mode. The I-V curves in C (non-transfected cells) and _D (htrp3_-transfected cells) measured before (a) and during application of ATP (b) were obtained from a voltage ramp protocol applied in voltage clamp mode at the correspondingly labelled points in A and B. For Ca2+ measurements the dotted line refers to 0 μM [Ca2+]i. Extracellular Ca2+ was 1·5 mM, except for the period marked by the horizontal bar in B (0 mM Ca2+).
Figure 3. Effects of hTRP3 channels on agonist-stimulated Ca2+ transients.
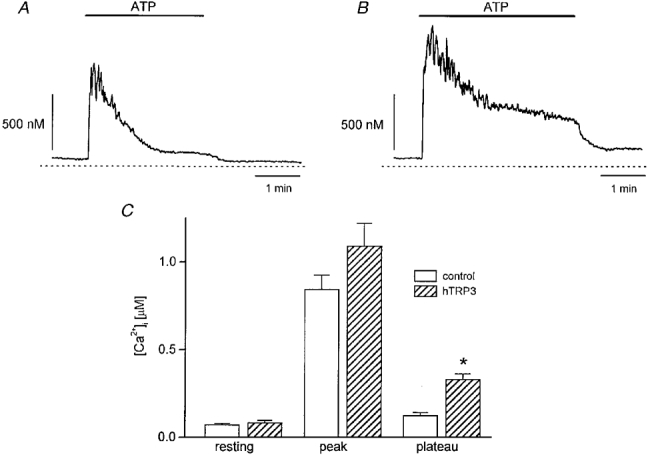
A and B, changes in [Ca2+]i as a result of ATP stimulation of a non-transfected (A) and an _htrp3_-transfected (B) CPAE cell clamped at 0 mV and perfused with Krebs solution. The internal solution was a high-K+ internal solution containing 0·1 mM EGTA. ATP (3 μM) was added to the bath as indicated by the horizontal bars. C, comparison of [Ca2+]i measured at the resting, peak and plateau phases in non-transfected (□) and _htrp3_-transfected ( ) cells. [Ca2+]i was measured before and after application of 3 μM ATP. Plateau [Ca2+]i was measured 3 min after the end of ATP application. *P < 0·01, significantly different from non-transfected cells.
) cells. [Ca2+]i was measured before and after application of 3 μM ATP. Plateau [Ca2+]i was measured 3 min after the end of ATP application. *P < 0·01, significantly different from non-transfected cells.
The resting membrane potentials in non-transfected (between -66 and +1 mV, mean -21 ± 9 mV, _n_= 25) and transfected non-stimulated (between -32 and +5 mV, mean -16 ± 6 mV, _n_= 6) CPAE cells were not significantly different.
We also compared the [Ca2+]i responses to ATP stimulation in control and in _htrp3_-transfected cells voltage clamped at 0 mV (Fig. 3_A_ and B). The resting [Ca2+]i level was not significantly different in control (73 ± 7 nM, _n_= 6) and hTRP3-expressing cells (83 ± 13 nM, _n_= 10). Application of ATP (3 μM) rapidly increased [Ca2+]i to a peak, which then decayed to a plateau in both cell types. The Ca2+ peaks during ATP stimulation were not significantly different in both cell types (control, 850 ± 80 nM, _n_= 6; _htrp3_-transfected cells, 1090 ± 130 nM, _n_= 10). On the other hand, the Ca2+ plateau was significantly higher in _htrp3_-transfected cells (control, 131 ± 21 nM, _n_= 6; _htrp3_-transfected cells, 334 ± 30 nM, _n_= 10; Fig. 3_C_). Removing extracellular Ca2+ abolished the agonist-induced Ca2+ plateau, while readmission of Ca2+ restored it. This rise in [Ca2+]i was blocked by La3+ at the same concentration that blocked _I_hTRP3 (data not shown). These results further support the role of hTRP3 channels as a Ca2+ influx pathway during agonist stimulation.
Induction of a non-selective cation channel by expression of hTRP3
The current activated by vasoactive agonists such as ATP and bradykinin in _htrp3_-transfected cells will be referred to as _I_hTRP3. To dissect it out from other current components, in the following experiments we blocked the three major endogenous currents in CPAE cells in the following ways: (i) The volume-regulated anion current (Voets et al. 1996) was eliminated by increasing the external osmolarity by 30 %. (ii) The K+ current through Kir2.1 channels (Kamouchi et al. 1997_a_) was abolished by replacing intra- and extracellular K+ with Cs+. (iii) Ca2+-activated Cl− currents (Nilius et al. 1997_a_) were inhibited by buffering [Ca2+]i at subthreshold concentrations of 100 and 200 nM. (iv) A non-selective background current (Voets et al. 1996), not activated by agonists such as ATP, was eliminated by replacing external Na+ with NMDG+ (Fig. 4_A_). The density of this current at -50 mV in Ca2+-free extracellular solution was only -2·1 ± 0·5 pA pF−1 in non-transfected resting CPAE cells (_n_= 9) and -2·4 ± 0·5 pA pF−1 in the presence of 3 μM ATP (_n_= 4). Its contribution to the current in hTRP3-expressing cells is therefore negligible.
Figure 4. Non-selective cation currents in _htrp3_-transfected cells.
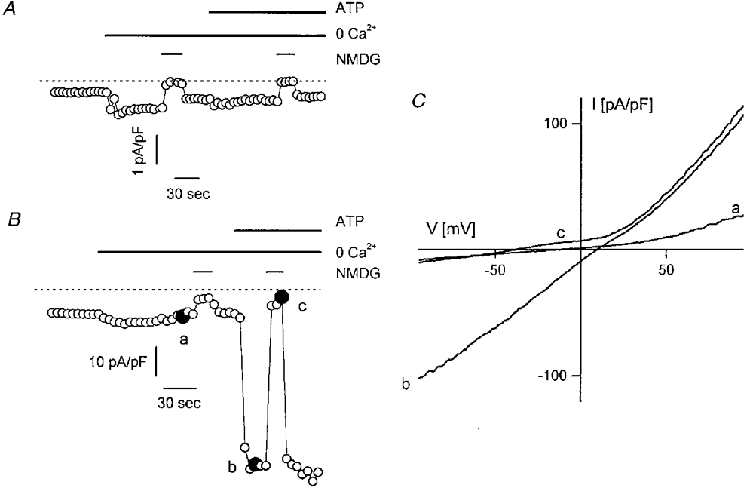
A and B, membrane currents at -50 mV in control (A) and _htrp3_-transfected (B) CPAE cells before and during stimulation with 3 μM ATP. The internal Cs+-containing pipette solution was buffered at 100 nM Ca2+. Bath solution was a 1·5 mM Ca2+-containing Krebs solution, which was replaced by a Ca2+-free Krebs solution (middle bar) before stimulating the cells with ATP, as indicated by the upper bar. The lower bar indicates the replacement of extracellular Na+ with NMDG+. Note the presence in both cell types of an inward current component under resting conditions, which is enhanced in Ca2+-free solution and eliminated by replacing extracellular Na+ with NMDG+. ATP did not induce any additional current in the control cell, but induced a huge current in the transfected cell, which again was abolished in Na+-free solution. C, I-V relationships of the _htrp3_-transfected cells obtained from voltage ramps applied at the time points marked in B.
The basal current at -50 mV in resting _htrp3_-transfected cells was consistently larger than in non-transfected cells. Moreover, this inward current was enhanced by removing extracellular Ca2+ from the extracellular solution, and largely abolished if extracellular Na+ was replaced by NMDG+ (Fig. 4_B_). In addition, ATP stimulation in Ca2+-free medium strongly enhanced this current without affecting its reversal potential. These observations indicate that _htrp3_-transfected cells express non-selective cation channels that are activated by agonist stimulation. Figure 4_C_ shows the I-V curves in resting and ATP-stimulated _htrp3_-transfected cells recorded in Ca2+-free Na+-containing and Na+-free solutions, measured at the points marked in Fig. 4_B_. Both control and ATP-stimulated currents reversed at about the same positive potential, between 10 and 20 mV, indicating that they probably occur through the same non-selective cation channels. Since Cs+ is the main intracellular cation, this finding also implies that the channel is more permeable to Na+ than Cs+.
Application of ATP (3 μM) to cells with [Ca2+]i buffered at 100 nM increased the current density in Ca2+-free solution at -50 mV from -2·7 ± 0·4 to -26·9 ± 4·8 pA pF−1 (_n_= 20 cells). Bradykinin (100 nM) increased _I_hTRP3 density from -3·7 ± 0·9 to -15·8 ± 4·6 pA pF−1 (_n_= 5 cells). Ins_P_3 (10 μM) applied via the patch pipette also activated this current. During agonist activation, _I_hTRP3 was maintained for several minutes in most cells. However, in a fraction of the cells it was transient and these cells were desensitized to ATP. Figure 5 summarizes these results as well as those from cells with [Ca2+]i buffered at 1 μM or not buffered at all (0·1 mM EGTA).
Figure 5. Comparison of hTRP3 currents under various experimental conditions.
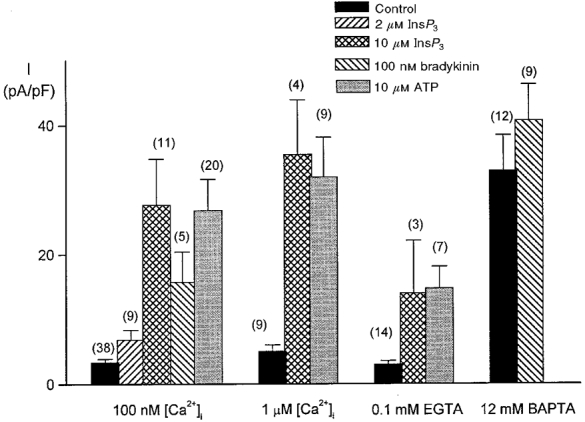
Comparison of the densities of Na+ influx currents in _htrp3_-transfected CPAE cells after the addition of 2 or 10 μM Ins_P_3, 100 nM bradykinin or 10 μM ATP. The currents were measured at -50 mV in cells dialysed with the internal solutions, which were buffered with 5 mM EGTA at either 100 nM [Ca2+]i or 1 μM [Ca2+]i, not buffered at all (0·1 mM EGTA) or buffered with 12 mM BAPTA in the patch pipette. Numbers in parentheses, number of cells.
When [Ca2+]i was buffered at extremely low values using the high concentration of 12 mM BAPTA, the background current at -50 mV in _htrp3_-transfected cells was increased significantly (-33 ± 5·5 pA pF−1, _n_= 12) in comparison to cells buffered at 100 nM [Ca2+]i (-7·4 ± 0·4 pA pF−1, _n_= 38) and non-buffered cells perfused with 0·1 mM EGTA (-3·1 ± 0·48 pA pF−1, _n_= 14). However, this current did not increase significantly in the presence of 10 μM Ins_P_3 (-40·8 ± 5·5 pA pF−1, _n_= 9). Buffering [Ca2+]i at 100 nM or 1 μM in non-stimulated cells did not activate any current (Fig. 5), suggesting that activation of _I_hTRP3 is not due to agonist-induced changes in [Ca2+]i.
Calcium permeation and block by trivalent cations
Permeability ratios for various cations were estimated from shifts in the reversal potentials induced by cation substitution (eqn (1)). Na+ appeared to be slightly more permeant than Cs+, with a _P_Cs/_P_Na value of 0·82 ± 0·03 (_n_= 21). It was not possible to use the same procedure to estimate the relative permeability for K+ because of the existence of a large inwardly rectifying K+ current in CPAE cells. The permeability ratio _P_K/_P_Na calculated from the reversal potential measured with a K+-containing internal solution was 0·68 ± 0·05 (eqn (4), _n_= 10). The amplitude of the inward current component attributed to _I_hTRP3 was significantly reduced if external monovalent cations were replaced with Ca2+. The permeability ratio of Ca2+ over Na+ calculated from the shift in the reversal potential was 1·13 ± 0·15 (_n_= 11). Its value calculated from eqn (3), taking into account the calculated _P_Cs/_P_Na ratio, was 1·62 ± 0·27 (_n_= 11).
A constant finding was that although the hTRP3 channels are apparently permeable to Ca2+, increasing [Ca2+] from 0 to 1·5 mM significantly reduced the amplitude of _I_hTRP3 at all potentials. At -50 mV, 1·5 mM Ca2+ reduced the current to 30 ± 1 % (_n_= 5) of its value in Ca2+-free solution. Mg2+ (1·5 mM) and Ba2+ (1·5 mM) reduced _I_hTRP3 to, respectively, 50 ± 6 % (_n_= 5) and 33 ± 3 % (_n_= 5) of its control value. The trivalent cations La3+ and Gd3+ blocked the hTRP3 channels (Fig. 6_A_ and B). La3+ (100 μM) blocked _I_hTRP3 dose dependently with an IC50 of 24·4 ± 0·7 μM (Fig. 6_C_). Gd3+ at 100 μM significantly reduced the current to 31 ± 6 % of control values (_n_= 3, Fig. 6_C_).
Figure 6. Block of _I_hTRP3 by the trivalent cations La3+ and Gd3+.
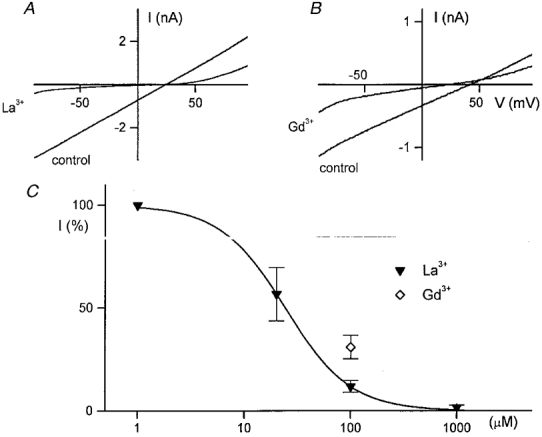
A and B, comparison of the I-V curves of the current activated by 3 μM ATP before and after application of 100 μM La3+ or 100 μM Gd3+. C, concentration-response curve for the La3+ block. The ordinate represents the percentage of the control current at -50 mV that is not affected by La3+ or Gd3. The continuous line represents the best fit of the La3+ data to eqn (5) with an IC50 of 24·4 μM and _n_H= 1·42.
Single-channel conductance
We have tried extensively to record single hTRP3 channel activity in cell-attached mode and also in outside-out patches. Incidentally, we noticed very short and high-conductance channel openings similar to those reported recently by Kiselyov et al. (1998), but were not able to analyse these data (see also Discussion). We have therefore tried to assess the single-channel conductance of hTRP3 channels from analysis of the macroscopic current fluctuations in the presence of ATP (Fig. 7_A_ and B). From the parabolic fit of the relationship between current variance and mean current amplitude (eqn (6) and Sigworth, 1981), we obtained values for the single-channel conductance ranging between 9·9 and 36·6 pS with a mean value of 22·6 ± 2·4 pS (_n_= 14).
Figure 7. Relationship between current fluctuations and current amplitude.
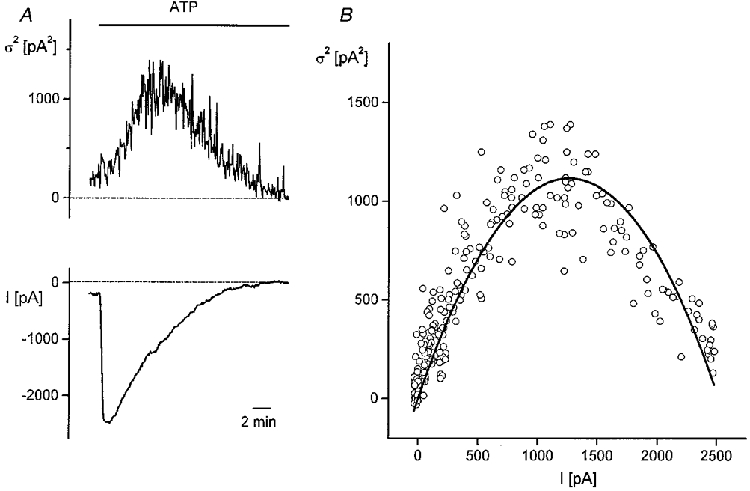
A, time course of current variance (upper trace) and current amplitude (lower trace) at a holding potential of -50 mV during application of 3 μM ATP. [Ca2+]i was buffered at 100 nM. B, current variance (σ2) as a function of current amplitude from the data in A. Parameters from the fit of the data to eqn (6) (continuous line) were _i_= 1·8 pA, _N_= 1420.
Is _I_hTRP3 store operated?
It has been proposed that _trp_-encoded channels might be activated by a store-dependent mechanism. We have therefore investigated whether the Ca2+-ATPase inhibitors BHQ (20 μM) and thapsigargin (2 μM) also activate _I_hTRP3. Neither affected _I_hTRP3 in cells with [Ca2+]i buffered at 100 nM (Fig. 8_A_ and B). In five cells, the basal current density at -50 mV was -6·6 ± 2·6 pA pF−1 before and -6·5 ± 2·5 pA pF−1 after application of 20 μM BHQ. In seven other cells, the current densities before and during application of 2 μM thapsigargin were -9·3 ± 1·8 pA pF−1 and -8·2 ± 2·0 pA pF−1, respectively. BHQ also did not significantly activate _I_hTRP3 if [Ca2+]i was not buffered (control, 3·3 ± 2·8 pA pF−1; after application of 20 μM BHQ, -2·5 ± 2·1 pA pF−1, _n_= 3). However, stimulation with ATP could still activate _I_hTRP3 in the BHQ- or thapsigargin-treated cells (Fig. 8).
Figure 8. Effect of Ca2+-ATPase inhibitors on the membrane current of _htrp3_-transfected cells.
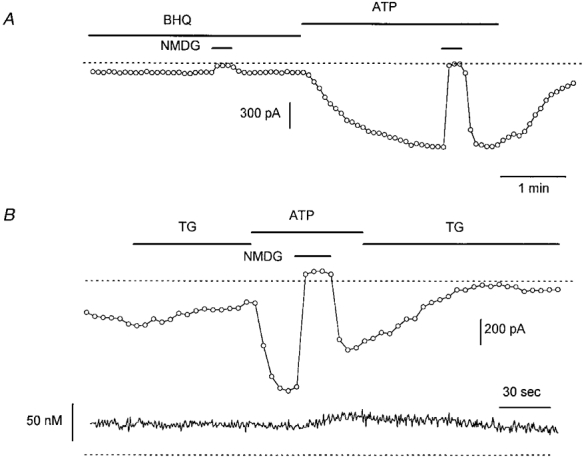
A, effects of 20 μM BHQ (A) on a cell clamped at -50 mV using a pipette solution with Ca2+ buffered at 100 nM in a Na+-containing, Ca2+-free bath solution. B, same protocol but for 2 μM thapsigargin (TG). Note that 1 μM ATP could activate _I_hTRP3 in both cells whereas both Ca2+-ATPase inhibitors had no effect. The lower trace in B shows the intracellular Ca2+ concentration during this experiment.
Modulation by pharmacological tools
The phospholipase C (PLC) inhibitor U-73122 (2 μM) rapidly decreased the current activated by bradykinin (Fig. 9_A_;_n_= 3) and ATP (Fig. 9_B_;_n_= 6). The inactive analogue U-73343 was ineffective (data not shown), suggesting that there was no specific inhibition of _I_hTRP3 by the PLC blocker itself. Exposure of non-stimulated cells to 2 μM U-73122 did not affect either the membrane currents or [Ca2+]i (_n_= 4). Thimerosal (2 μM), which sensitizes the Ins_P_3 receptor to its endogenous agonist, augmented the basal _I_hTRP3 in four out of six cells ([Ca2+]i buffered at 100 nM; Fig. 9_C_). The activated current has similar properties to those reported above with respect to reversal potential and NMDG effect. Thimerosal exerted a similar stimulating effect on the current activated by 2 μM Ins_P_3 in the patch pipette (6 out of 8 cells).
Figure 9. Effects of U-73122, a PLC inhibitor, and thimerosal, a sensitizer of the Ins_P_3 receptor, on agonist-activated current in transfected cells.
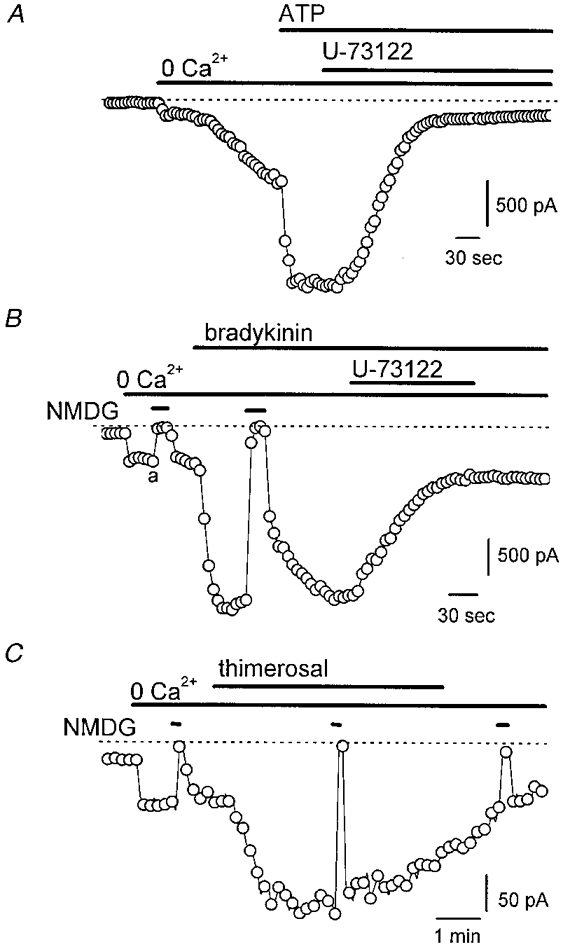
A and B, effects of 2 μM U-73122 on _I_hTRP3 activated by either 100 nM bradykinin or 3 μM ATP. The current was recorded at -50 mV; [Ca2+]i was buffered at 100 nM [Ca2+]i. The external bath solution contained Na+ as the major cation. C, effects of 5 μM thimerosal applied to the cell in a Ca2+-free solution. The membrane current was recorded at -50 mV with an internal high-Cs+ solution containing 0·1 mM EGTA and an external Na+ solution. Na+ was briefly replaced by NMDG+ to demonstrate the cationic nature of the current.
Because _trp_-encoded proteins contain calmodulin binding sites (for review, see Montell, 1998), we have evaluated possible calmodulin-mediated effects on _I_hTRP3. The calmodulin antagonist calmidazolium (1 μM) did not, however, affect _I_hTRP3 activated by 3 μM ATP (_n_= 8) or 10 μM Ins_P_3 (_n_= 3 for 1 μM [Ca2+]i and _n_= 6 for 200 nM [Ca2+]i; data not shown).
DISCUSSION
The dissection of different Ca2+ entry pathways and their molecular analysis are still a matter of discussion. It has been suggested that the trp gene family may comprise Ca2+ channels that are involved in Ca2+ signalling in non-excitable cells (Birnbaumer et al. 1996; Montell, 1997, 1998). In the present study, we have characterized the channel properties of a candidate Ca2+ entry channel, hTRP3.
Properties of hTRP3 channels expressed in endothelial cells
CPAE cells, which do not endogenously express the btrp3 gene, were transiently transfected with htrp3. Heterologous expression of hTRP3 induced a non-selective cation current that was clearly different from the two types of endogenous Ca2+-permeable channels in CPAE cells, i.e. the background non-selective cation (bNSC) current and _I_CRAC (Voets et al. 1996; Nilius et al. 1997_a_;Fasolato & Nilius, 1998).
The biophysical properties and pharmacological profiles of the hTRP3 currents are distinct from those of the CRAC currents in that: (i) their single-channel conductance is higher than that of CRAC channels, (ii) they have a lower Ca2+ selectivity, and (iii) they are less sensitive to trivalent cations than _I_CRAC (Hoth & Penner, 1992; Parekh & Penner, 1997).
It has been reported that the htrp3 gene expressed in human embryonic kidney (HEK) cells induced a non-selective cation channel with a conductance of approximately 60 pS (Zitt et al. 1997; Hurst et al. 1997; Kiselyov et al. 1998), but values of 17 pS have also been observed (Kiselyov et al. 1998). All our efforts to record single hTRP3 channel events failed. We occasionally observed very short openings similar to those reported by Kiselyov et al. (1998), but were not able to analyse these very short-lasting events reliably. A possible reason for this failure might be that the channels are clustered in compartments such as the caveolae, which are very abundant in endothelial cells. From the noise analysis of the macroscopic currents we obtained consistently smaller values for the single-channel conductance than those obtained from the single-channel data in HEK cells. This divergence could be explained by the formation of heteromultimeric channels consisting of hTRP3 and endogenous TRPs.
The activation mechanisms of hTRP3 and CRAC channels might also be different, since Ca2+-ATPase inhibitors, which have been reported to activate _I_CRAC, do not activate _I_hTRP3. Moreover, _I_CRAC can only be detected when [Ca2+]i is strongly buffered (Fasolato & Nilius, 1998), and it is inactivated by [Ca2+]i (Zweifach & Lewis, 1995), which is obviously not the case for _I_hTRP3.
It has been shown that mammalian homologues of trp also form Ca2+-permeable channels (Zitt et al. 1996, 1997; Philipp et al. 1996; Boulay et al. 1997; Preuss et al. 1997; Okada et al. 1998). Some of these channels have a much higher relative permeability for Ca2+ than the hTRP3 channels described in this paper (Philipp et al. 1996; Okada et al. 1998), whereas others barely discriminate between Na+ and Ca2+ (Zitt et al. 1996). However, the permeability sequence _P_Na > _P_Cs in hTRP3 channels was also observed in bTRP4 channels (Philipp et al. 1996) and mouse TRP5 (mTRP5) channels (Okada et al. 1998), suggesting that this characteristic may be conserved in the TRP channel family.
These diverse properties of TRP channels in different expression systems might be explained by the formation of heteromultimers between expressed and endogenous TRP isoforms. Heteromultimeric interactions between TRPC1 and TRPC3 have been described recently (Zhu et al. 1996), and it has also been shown that Drosophila TRP and TRPL co-assemble to form functional channel complexes (Gillo et al. 1996; Xu et al. 1997). Since CPAE cells endogenously express bTRP1 and bTRP4 proteins (Fig. 1), the possibility cannot be excluded that the heterologously expressed hTRP3 proteins form heteromultimeric complexes with the endogenous bTRP1 and/or bTRP4.
Comparison with agonist-activated non-selective cation channel in human umbilical vein-derived endothelial cells
We have recently described a 25 pS non-selective cation channel in human umbilical vein-derived endothelial cells (Ea.hy926) which express hTRP3 (Kamouchi et al. 1999). The non-selective cation current (_I_NSC) though this channel is activated by agonist stimulation. Importantly, the plateau phase of the agonist-induced Ca2+ response in _htrp3_-transfected cells, which is much higher than in non-transfected cells, is similar to that observed in Ea.hy926 cells. Moreover, the biophysical and gating properties of _I_hTRP3 in this study and _I_NSC in Ea.hy926 cells are quite similar: (i) the kinetics of both currents are voltage and time independent; (ii) the permeability sequence for monovalent cations (Na+ > Cs+ > NMDG+) is similar; (iii) their Ca2+ permeability compared with monovalent cations is rather low; (iv) both are inhibited by extracellular La3+ and Gd3+; (v) both are activated by agonist stimulation or intracellular perfusion of Ins_P_3, whereas inhibitors of PLC abolish this activation, suggesting that PLC activation and the subsequent production of Ins_P_3 are required for channel activation; (vi) both can be activated in the presence of intracellular Ca2+; and (vii) the filling state of the store is not a major regulator. We therefore conclude that TRP3 forms agonist-activated, non-selective Ca2+-permeable channels similar to the endogenous NSC channel in Ea.hy926. This view is supported by the finding that expression of an N-terminal fragment of htrp3 downregulates _I_NSC in human vascular endothelial cells (HUVEC; Groschner et al. 1998). In spite of the above similarities, _I_hTRP3 and _I_NSC display some differences: (i) _P_Ca/_P_Na is higher for _I_hTRP3 than for _I_NSC; (ii) the inhibitory effect of external Ca2+ is more pronounced for _I_hTRP3; (iii) the activation time course of _I_NSC and _I_hTRP3 is different, as _I_NSC activates slowly and with a long delay, whereas _I_hTRP3 develops rapidly after the application of agonists. Because of the possible heteromultimeric composition of these channels, these differences do not necessarily contradict our conclusion that TRP3 forms NSC-type channels. It is not inconceivable that overexpression of hTRP3 in CPAE cells results in channel complexes with a different subunit composition and/or stoichiometry compared with the endogenous NSC in Ea.hy926 cells. Overexpression may also alter the coupling of the channel with the signal transduction complex responsible for channel activation. It has recently been reported for the Drosophila photoreceptor that INAD, a PDZ domain protein, is required for anchoring the TRP channel to a signalling complex consisting of a heterotrimeric Gq protein and PLCβ (NorpA) (Huber et al. 1996; Chevesich et al. 1997; Tsunoda et al. 1997; Philipp & Flockerzi, 1997). By analogy, similar transduction complexes may be required for activation of NSC channels in endothelial cells, and the molecular interactions between these signalling complexes and overexpressed hTRP3 may differ slightly from those of the endogenous NSC channel.
Effects of hTRP3 expression on Ca2+ signalling in endothelial cells
As a result of fura-2 fluorescence experiments using intact cells (Zhu et al. 1996, 1998; Boulay et al. 1997; Zitt et al. 1997), it has been suggested that the expression of TRP channels increases agonist-activated capacitative Ca2+ entry. The Ca2+ plateau during agonist stimulation of CPAE cells has been shown to be due exclusively to Ca2+ influx (Kamouchi et al. 1997_b_;Madge et al. 1997). The observation that the ATP-induced Ca2+ plateau in _htrp3_-transfected cells was much higher than in non-transfected CPAE cells points to a functional role for hTRP3 channels as a Ca2+ influx pathway during agonist stimulation.
Gating mechanisms of hTRP3 channels
The gating mechanisms of TRP channels are still controversial. A possible confounding factor might be that TRP channels are just a part of multiprotein signalling complexes containing the G-protein-coupled serpent receptors INAD, PLC and protein kinase C (Montell, 1997, 1998). Thus, it is possible that overexpression of only one component of such a complex will not restore the physiologically relevant properties of endogenous TRP channels. Electrophysiological studies have shown that Drosophila TRP (Vaca et al. 1994; Sinkins et al. 1996; Xu et al. 1997), a splice variant of hTRP1 (Zitt et al. 1996), bTRP4 (Philipp et al. 1996) and bTRP5 (Philipp et al. 1998) were activated by application of thapsigargin. However, recent studies indicate that other TRP channels are insensitive to store depletion induced by thapsigargin (hTRP3, Zhu et al. (1998), mTRP5, Okada et al. (1998) and mTRP6, Boulay et al. (1997). Moreover, it has been reported (Zitt et al. 1996, 1997) that hTRP3 was constitutively active and that this activity was not related to store depletion but activated by an increase in [Ca2+]i. Thapsigargin and BHQ have been shown to deplete endothelial Ins_P_3-sensitive stores (Gericke et al. 1993; Nilius et al. 1997_b_). Since these tools do not activate hTRP3 channels, it is unlikely that store depletion is the physiological trigger for hTRP3 activation.
In the present experiments we were able to activate _I_hTRP3 by external application of ATP or bradykinin and internal perfusion with Ins_P_3. The inhibition of _I_hTRP3 by a PLC inhibitor indicates that factors downstream of PLC activate hTRP3 channels during agonist stimulation. It has recently been shown that hTRP3 can be directly activated by diacylglycerol (Hofmann et al. 1999). This could also explain the effect of PLC inhibitors and the lack of effect of Ca2+-ATPase inhibitors in our experiments.
Our results do not disprove the possibility that Ins_P_3 may activate _I_hTRP3 directly. Such a mechanism has been described in detail in endothelial cells (Vaca & Kunze, 1995). Viewed from this perspective, the effect of thimerosal could also be interpreted as the sensitization of a direct Ins_P_3 effect on hTRP3 channels.
TRP channel proteins contain calmodulin binding sites, implying that [Ca2+]i might be involved in channel regulation (Montell, 1997). This is supported by the recent finding that hTRP3 channels are activated by an increase in [Ca2+]i (Zitt et al. 1997). In the present experiments, however, buffering of [Ca2+]i at concentrations of 100 nM or 1 μM did not activate hTRP3 channels. Neither was the activation of these channels affected by calmodulin inhibitors. In the presence of 12 mM BAPTA, Ins_P_3 did not significantly activate a current. These data argue against Ca2+ being the activator of hTRP3. However, the possibility that Ca2+ plays a permissive role in channel activation cannot be excluded.
In conclusion, hTRP3 forms a functional, non-selective, Ca2+-permeable cation channel when expressed in bovine endothelium. This channel is activated by agonists, via a PLC-dependent mechanism, and by Ins_P_3. A direct activation by Ca2+ is unlikely. Calmodulin does not modulate its activity. Functionally, this channel may be involved in agonist-stimulated Ca2+ influx.
Acknowledgments
We thank Dr X. Zhu (UCLA, Los Angeles, USA) for the kind gift of the htrp3 clone. M. Kamouchi was supported by a fellowship from the Onderzoeksfonds KU Leuven and the FWO, Belgium. A. Mamin was supported by INTAS. J. Eggermont is a Research Associate of the Flemish Fund for Scientific Research (FWO-Vlaanderen). We thank J. Prenen and D. Hermans for the skilful technical assistance during the experiments and A. Florizone and M. Crabbé for their help with the cell cultures. We are grateful to Dr C. Fasolato for her helpful suggestions. This work was supported by the Federal Belgian State (Interuniversity Poles of Attraction Programme, Prime Ministers’ Office IUAP No. 3P4/23), the Flemish Government (F.W.O. G.0237.95, C.O.F./96/22-A0659), the European Commission (BMH4-CT96-0602) and INTAS -94-0241.
References
- Bennett DL, Petersen CC, Cheek TR. Calcium signalling. Cracking ICRAC in the eye. Current Biology. 1995;5:1225–1228. doi: 10.1016/s0960-9822(95)00243-0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Capacitive calcium entry. Biochemical Journal. 1995;312:1–1. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitive calcium entry: Role for Trp proteins. Proceedings of the National Academy of Sciences of the USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. Journal of Biological Chemistry. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Carter TD, Ogden D. Kinetics of intracellular calcium release by inositol 1,4,5-trisphosphate and extracellular ATP in porcine cultured aortic endothelial cells. Proceedings of the Royal Society. 1992;B 250:235–241. doi: 10.1098/rspb.1992.0154. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Garcia RL, Schilling WP. Concomitant and hormonally regulated expression of trp genes in bovine aortic endothelial cells. FEBS Letters. 1997;415:335–340. doi: 10.1016/s0014-5793(97)01155-1. [DOI] [PubMed] [Google Scholar]
- Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Nilius B. Store depletion triggers the calcium release-activated calcium current (ICRAC) in macrovascular endothelial cells: a comparison with Jurkat and embryonic kidney cell lines. Pflügers Archiv. 1998;436:69–74. doi: 10.1007/s004240050605. [DOI] [PubMed] [Google Scholar]
- Gericke M, Droogmans G, Nilius B. Thapsigargin discharges intracellular calcium stores and induces transmembrane currents in human endothelial cells. Pflügers Archiv. 1993;422:552–557. doi: 10.1007/BF00374001. [DOI] [PubMed] [Google Scholar]
- Gillo B, Chorna I, Cohen H, Cook B, Manistersky I, Chorev M, Arnon A, Pollock JA, Selinger Z, Minke B. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitive Ca2+ entry. Proceedings of the National Academy of Sciences of the USA. 1996;93:14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, Schreibmayer W. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Letters. 1998;437:101–106. doi: 10.1016/s0014-5793(98)01212-5. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Huber A, Sander P, Gobert A, Bähner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO Journal. 1996;15:7036–7045. [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Zhu X, Boulay G, Birnbaumer L, Stefani E. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Letters. 1997;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annual Review of Physiology. 1995;57:171–189. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- Iouzalen L, Lantoine F, Pernollet MG, Millanvoye-Van-Brussel E, Devynck MA, David-Dufilho M. SK&F96365 inhibits intracellular Ca2+ pumps and raises cytosolic Ca2+ concentration without production of nitric oxide and von Willebrand factor. Cell Calcium. 1996;20:501–508. doi: 10.1016/s0143-4160(96)90092-5. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. The Journal of Physiology. 1990;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Merritt JE, Hallam TJ, Rink TJ. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Bremt KVD, Eggermont J, Droogmans G, Nilius B. Modulation of inwardly rectifying potassium channels in cultured bovine pulmonary artery endothelial cells. The Journal of Physiology. 1997a;504:545–556. doi: 10.1111/j.1469-7793.1997.545bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamouchi M, Mamin A, Droogmans G, Nilius B. Non-selective cation channels in endothelial cells derived from human umbilical vein. Journal of Membrane Biology. 1999;169:29–38. doi: 10.1007/pl00005898. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Trouet D, Greef CD, Droogmans G, Eggermont J, Nilius B. Functional effects of expression of hslo Ca2+ activated K+ channels in cultured macrovascular endothelial cells. Cell Calcium. 1997b;22:497–506. doi: 10.1016/s0143-4160(97)90077-4. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Lantoine F, Iouzalen L, Devynck MA, Millanvoye-Van-Brussel E, David-Dufilho M. Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochemical Journal. 1998;330:695–699. doi: 10.1042/bj3300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. The Journal of Physiology. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge L, Marshall ICB, Taylor CW. Delayed autoregulation of the Ca2+ signals resulting from capacitive Ca2+ entry in bovine pulmonary artery endothelial cells. The Journal of Physiology. 1997;498:351–369. doi: 10.1113/jphysiol.1997.sp021863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. New light on TRP and TRPL. Molecular Pharmacology. 1997;52:755–763. doi: 10.1124/mol.52.5.755. [DOI] [PubMed] [Google Scholar]
- Montell C. TRP trapped in fly signaling web. Current Opinion in Neurobiology. 1998;8:389–397. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- Nilius B, Oike M, Zahradnik I, Droogmans G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. Journal of General Physiology. 1994;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Szücs G, Wei L, Tanzi F, Voets T, Droogmans G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. The Journal of Physiology. 1997a;498:381–396. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annual Review of Physiology. 1997b;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- Oike M, Gericke M, Droogmans G, Nilius B. Calcium entry activated by store depletion in human umbilical vein endothelial cells. Cell Calcium. 1994;16:367–376. doi: 10.1016/0143-4160(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. Journal of Biological Chemistry. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitive calcium entry channel homologous to Drosophila TRP and TRPL. EMBO Journal. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Flockerzi V. Molecular characterization of a novel human PDZ domain protein with homology to INAD from Drosophila melanogaster. FEBS Letters. 1997;413:243–248. doi: 10.1016/s0014-5793(97)00877-6. [DOI] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalié A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO Journal. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss KD, Noller JK, Krause E, Gobel A, Schulz I. Expression and characterization of a trpl homolog from rat. Biochemical and Biophysical Research Communications. 1997;240:167–172. doi: 10.1006/bbrc.1997.7528. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. Interpreting power spectra from nonstationary membrane current fluctuations. Biophysical Journal. 1981;35:289–300. doi: 10.1016/S0006-3495(81)84790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins WG, Vaca L, Hu Y, Kunze DL, Shilling WP. The COOH− terminal domain of Drosophila TRP channels confers thapsigargin sensitivity. Journal of Biological Chemistry. 1996;271:2955–2960. doi: 10.1074/jbc.271.6.2955. [DOI] [PubMed] [Google Scholar]
- Trouet D, Nilius B, Voets T, Droogmans G, Eggermont J. Use of a biscistronic GFP-expression vector to characterise ion channels after transfection in mammalian cells. Pflügers Archiv. 1997;434:632–638. doi: 10.1007/s004240050445. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Vaca L, Kunze DL. IP3-activated Ca2+ channels in the plasma membrane of cultured vascular endothelial cells. American Journal of Physiology. 1995;269:C733–738. doi: 10.1152/ajpcell.1995.269.3.C733. [DOI] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. American Journal of Physiology. 1994;267:C1501–1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Nilius B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. The Journal of Physiology. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-Z S, Li H-S, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human trp3 stably expressed in human embryonic kidney (HEK)293 cells. Journal of Biological Chemistry. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitive Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strübing C, Zobel A, Kalkbrenner F, Lückhoff A, Shultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. Journal of Cell Biology. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. Journal of General Physiology. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]