The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 15.
Published in final edited form as: J Leukoc Biol. 2000 Jan;67(1):53–62. doi: 10.1002/jlb.67.1.53
Abstract
Continuous expression of the MGSA/GROα, β, or γ chemokine bestows tumor-forming capacity to the immortalized murine melanocyte cell line, melan-a. The mechanism for this transformation is unclear, although both autocrine and paracrine processes are possible because melan-a cells as well as endothelial cells express a low level of the receptor for this ligand. To further define the role of MGSA/GRO proteins in melanocyte transformation, two types of experiments were designed to neutralize the biological effects of MGSA/GRO in the transfected melan-a clones: (1) the effect of neutralizing antiserum to MGSA/GRO proteins on melan-a tumor growth was assessed; (2) the tumor-forming capacity of melan-a clones expressing ELR motif-mutated forms of MGSA/GRO with compromised receptor affinity was compared to the tumor-forming capacity of clones expressing wild-type MGSA/GRO. These experiments revealed that SCID mice inoculated with MGSA/GROα- or γ-expressing melan-a cells and subsequently treated with antiserum to the respective chemokine exhibited decreased tumor growth. This reduction in tumor growth was accompanied by declining angiogenic activity in MGSA/GROγ-expressing tumors. Moreover, athymic nude mice injected with melan-a cells expressing ELR-mutant forms of MGSA/GROα exhibited markedly impaired tumor-forming capacity compared with those mice injected with melan-a clones expressing wild-type MGSA/GRO. These data suggest that continuous expression of MGSA/GRO proteins may facilitate tumor growth by stimulating the growth of microvessels into the tumor (paracrine) and by affecting melanocyte growth (autocrine).
Keywords: chemokines, angiogenesis
INTRODUCTION
CXC chemokines encoding the ELR motif (ELR+) at the amino terminus have been demonstrated to exhibit angiogenic activity in a variety of contexts [1–7]. In contrast, those CXC chemokines not containing this motif (ELR−) are angiostatic [4, 8, 9]. Angiogenic activity exhibited by ELR+ CXC chemokines can be inhibited with antibodies to these chemokines [1, 3, 5, 10–14]. The angiogenic response to ELR+ CXC chemokines interleukin-8 (IL-8), MGSA/GRO, or ENA-78 appears to be mediated through the binding of these ligands to CXC chemokine receptors [15, 16]. The expression of receptors for ELR+ chemokines on endothelial cells in wounded tissues and in both endothelial cells and tumor cells in melanoma and head and neck squamous cell carcinoma (HNSCC) tumors has been verified [15–17].
We have previously demonstrated that overexpression of MGSA/GRO proteins in immortalized melanocytes is associated with enhanced ability to form large colonies in soft agar and enhanced ability to form tumors in nude mice [18, 19]. To determine whether the tumor formation in stable clones expressing wild-type MGSA/GRO proteins was directly linked to chemokine production and not the result of random clonal selection, we designed a study in SCID mice where it is possible to follow the effects of administration of rabbit antibodies to the human MGSA/GRO proteins on tumor growth, without obtaining an antigenic response to the rabbit antiserum. Mice were inoculated with immortalized melan-a cells expressing MGSA/GRO proteins and either antiserum to MGSA/GRO or normal rabbit serum (control) was administered every-other day into the inoculation site to determine whether direct inhibition of the chemokine would alter tumor growth. In the SCID mouse model antiserum to MGSA/GROγ almost totally blocked tumor formation by MGSA/GROγ-expressing melan-a cells, whereas antiserum to MGSA/GROα only partially retarded the tumor formation by melan-a cells expressing MGSA/GROα. We were better able to demonstrate a direct role for MGSA/GROα in tumor growth by following tumor formation of melan-a clones expressing MGSA/GROα mutated in the ELR motif, a conserved motif required for high-affinity binding to the receptor. In this study we demonstrate that melan-a cells expressing MGSA/GROα mutated in the ELR motif exhibit markedly compromised ability to form tumors. To determine whether the MGSA/GRO antibody treatment of SCID mice reduced tumor growth by blocking the growth of new blood vessels into the tumor, we monitored the expression of the endothelial cell surface marker, CD31, in frozen sections of tumors from SCID mice. In mice where tumors developed, we observed that reduced tumor size correlated with reduced CD31 immunoreactivity. These data suggest that MGSA/GRO proteins affect tumor growth through both autocrine and paracrine mechanisms.
METHODS
Design of expression vector for MGSA/GRO proteins
A portion (550 bp) of the MGSA/GROα cDNA including the entire open reading frame was subcloned into pALTER and mutagenesis was performed according to standard protocols to produce MGSA/GROα with the following mutations: E6A, L7A, R8A, and ELR/AAA. Mutated sequences including this entire open reading frame were subsequently subcloned into the pRC/CMV expression vector and plasmid DNA was transfected into immortalized melan-a cells that were the kind gift of Dorthea Bennett. Stably transfected clones were selected for neomycin resistance and maintained in selective medium, as previously described [18]. Levels of mutated MGSA/GRO released into the culture medium were quantitated by enzyme-linked immunosorbent assay (ELISA) with the use of the Quantikine kit from R & D Systems. Conditioned medium was collected from confluent cultures after 48 h of conditioning in medium containing serum. The level of expression of mutant MGSA/GRO was equivalent to that of the melan-a clone expressing wild-type (WT) MGSA/GROα (WT, 0.78 ng/mL; E6A, 0.89 ng/mL; L7A, 0.70 ng/mL; R8A, 1.01 ng/mL; AAA, 1.11 ng/mL).
Tumorigenesis models
Melan-a clones selected to express MGSA/GROα, MGSA/GROα with mutations in the ELR motif (E6A, L7A, R8A, and ELR/AAA), or the neomycin-selectable marker alone (V1) were injected into female nude mice (5 × 106 cells per mouse) as previously described with an n of 10 mice per cell type [18]. Tumor formation was monitored over a period of 8 months.
Melan-a clones previously selected to express MGSA/GROα, β, γ or the neomycin selection marker alone [19] were injected into SCID mice. Cells (5 × 106) from clones γ 3–14 expressing MGSA/GROγ, mel-a-6 expressing MGSA/GROα, or V1 or V4 clones expressing the neomycin selection marker alone, were injected subcutaneously into the interscapular region of 6-week-old female SCID mice, with an n of 12 mice per clone type. At the time of inoculation of the MGSA/GROα or MGSA/GROγ expressing melan-a clones, mice were also injected with decomplemented neutralizing antiserum to MGSA/GROα or MGSA/GROγ, respectively, or NRS. For each group, six of the mice were injected with 500 μL of NRS and the other six mice were injected with 500 μL of antiserum to MGSA/GROα or γ, depending on the form of MGSA/GRO expressed by the transfected clone. Injections were continued every-other day over the course of several weeks, until tumor size reached an approximate diameter of 1–1.5 cm. Mice receiving melan-a cells expressing the neomycin selection marker alone (vector controls) did not receive injections of serum or antiserum. When palpable tumors developed, the tumor volume was estimated by measuring the diameter of the tumors on a weekly basis. When any of the tumors in the group reached a diameter of 2.5 cm3, all the mice in the group were killed, the tumors were resected, and the tumor volumes were measured by water displacement. Tumors were then divided into aliquots for (1) fixation, embedding, sectioning, and histological evaluation; (2) ELISA determination of chemokine expression; and (3) analysis of frozen sections for CD31 immunodetection of endothelial cells.
Western blot analysis of Ras protein in MGSA/GRO-expressing melan-a clones
Western blots were performed on lysates of MGSA/GROα, β, γ expressing melan-a clones to detect the expression of Ras proteins using protocols developed by Santa Cruz. The pan-Ras antibody that detects H-Ras, K-Ras, and N-Ras (Oncogene Research Products) was used at a 1:3000 dilution in TBS-T buffer with 5% dry milk for 12–16 h at 4°C. The membrane was then incubated in a 1:3000 dilution of the anti-mouse antibody conjugated with horseradish peroxidase (Boehringer Mannheim Corp.) in TBS-T buffer with 5% dry milk for 1 h at room temperature. The protein bands were detected with the enhanced chemiluminescence system (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. The density of the bands on the developed blot was quantitated and plotted. The blot was performed three times with similar results each time.
AP-1 transient transfection assays
AP-1 luciferase reporter gene constructs were prepared by inserting two copies of the AP-1 consensus DNA binding sequence into the pGL-3 promoter vector (Promega). Semiconfluent cultures (5 × 105 cells in 30-mm dishes) of mel-a cells expressing MGSA/GROα, β, or γ were transiently co-transfected with 2.5 μg pSV-β-Gal control vector (Promega) and 2.5 μg AP-1 luciferase reporter gene with or without 5 μg of a dominant-negative Ras (DN-Ras) expression construct using the calcium phosphate technique. Three hours later, the cells were glycerol shocked for 3 min. The cells were washed twice with complete medium, and then incubated with complete medium at 37°C/5% CO2. Two days later, the cells were washed with cold phosphate-buffered saline (PBS) and lysed in 1× reporter lysis buffer (Promega) for 15 min at room temperature, and the lysate was cleared by centrifugation. The luciferase and β-Gal activity were measured according to standard protocols (Promega) using a Monolight 2010 luminometer (Analytical Luminescence Laboratory).
Immunodetection of CD31 staining, mCXCR2, and the CD16 natural killer (NK) marker in tumor sections
Paraformaldehyde-fixed tissues were sectioned and processed for staining for mCXCR2 using antibody to mCXCR2 from Santa Cruz Biologicals (1:200 dilution). CD16 antibodies were purchased from PharMingen (1:200 dilution). Frozen sections of tumors were fixed in cold acetone and stained for CD31 using the antibody kindly provided by Alberto Montovani, which is also available through PharMingen [20]. Our standard protocols were used with this rat antibody for indirect immunohistochemical detection using the Vector Laboratories ABC Rat IgG kit. The substrate used for the peroxidase-linked avidin-biotin complex was 3-amino-9-ethylcarbazole (AEC) from Vector Laboratories (Burlingame, CA). Slides were counterstained with Mayer’s hematoxylin, dehydrated, and mounted with aqueous immu-mount (Shandon Inc., Pittsburgh, PA). Quantitation was by morphometric scanning using protocols developed and published previously [21].
Corneal micropocket/angiogenesis assay
The ability of extracts from tumors expressing chemokines to stimulate angiogenesis in the avascular cornea of F344 rat eyes was assayed according to established procedures [1, 22, 23]. Tumor samples were ground and sonicated, and then a 100-μL aliquot was combined from each of six tumors arising in either the NRS-treated or antiserum-treated SCID mice to yield a combined sample of 600 μL from each group. The MGSA/GRO antiserum-treated group of tumors had only two tumors for analysis so 300 μL from each of these tumors was combined to produce the 600-μL sample. Specimens were concentrated by Speed-Vac, reconstituted in 50 μL of sterile water, and total protein was measured. Samples were adjusted for equal amounts of total protein, and pellets were made for analysis of the pooled extract in the corneal micropocket assay.
ELISA assay
Levels of immunoreactive MGSA/GROα and γ were determined using a modified double-ligand ELISA according to procedures previously described [22, 24]. ELISA assays were also performed for human MGSA/GRO α and γ, murine macrophage inflammatory protein-2 (MIP-2), murine KC, murine interferon-γ (IFN-γ), and murine IL-12 and IL-13 with the R & D Systems Quantikine assay. Aliquots of conditioned medium (50 μL) from cultured melan-a cells or aliquots of tumor tissue sonicated in lysis buffer were analyzed in 96-well microtiter plates coated with rabbit anti-chemokine antibodies according to our established procedures. Data from tumors are reported in picograms per milligram protein. Protein determinations were by the Bio-Rad protein assay kit.
MGSA/GRO receptor expression in melan-a cells
Confluent cultures of melan-a cells expressing the neo vector alone, MGSA/GROα (mel-a-6), MGSA/GROβ (β2–19), and MGSA/GROγ (γ3–14) were assayed for 125I-MGSAα binding as previously described. Nonspecific binding was monitored by determining the binding of radiolabeled ligand in the presence of 100-fold excess cold ligand. Receptor expression was also monitored by immunocytochemistry on cultured melan-a clones expressing the various forms of MGSA/GRO and by immunohistochemistry of tissue sections of tumors that arose in nude or SCID mice. The Santa Cruz antibody to CXCR2 was used as the primary antibody in combination with the Vectastain ABC immunostaining protocols and kits.
RESULTS
Effect of antiserum to MGSA/GRO on tumor growth of MGSA/GROα- or γ-expressing clones
Control SCID mice inoculated with melan-a clones expressing MGSA/GROα or γ and injected with NRS developed palpable tumors between 44 and 59 days after injection of cells. MGSA/GROγ-expressing tumors grew rapidly from a mean tumor diameter of 0.65 cm on day 59 to 1.67 cm by day 77 (tumor volume measurement by fluid displacement, 0.62 cm3). In contrast, only three mice in the group injected in the same manner with antiserum to MGSA/GROγ developed tumors and those three tumors remained very small, with a mean volume for the three tumors of 0.15 cm3 (0.04 cm3 by micrometer calculation) and a mean diameter of 0.1 cm (Fig. 1). Tumor sizes were significantly different (P < 0.05) using a two-sample t test assuming unequal variances. When antiserum injections were stopped, these SCID mice developed tumors that grew rapidly, with a growth rate comparable to that of the mice receiving normal rabbit serum (NRS) injections (data not shown).
Fig. 1.

Effect of antiserum to MGSA/GROα or MGSA/GROγ on the growth of melan-a tumors in SCID mice. Melan-a cells (5 × 106) expressing MGSA/GROγ (A) or MGSA/GROα (B) were injected into 12 young female SCID mice. (A) Mice from each group (expressing MGSA/GROα or γ) were injected every-other day with decomplemented blocking antiserum to these respective chemokines or with heat-inactivated normal rabbit serum. We compared the mean tumor diameter as determined by micrometer measurement in the group injected with antibodies (Ab) to MGSA/GRO or to that of the group injected with normal rabbit serum (NRS). Three mice of the six treated with the antiserum to the MGSA/GROγ slowly developed very small tumors (over 2.5 months; final mean tumor diameter ranged from 0.7 to 0.2 cm for the three out of six mice that developed tumors), whereas those six mice treated with NRS quickly developed very large tumors by 8 weeks after the initial injection of melan-a cells expressing MGSA/GROα (final tumor diameters ranged from 2.3 to 1 cm). (B) For 8.5 weeks antiserum to MGSA/GROα (0.5 mL) or NRS (0.5 mL) was injected every-other day into SCID mice inoculated with MGSA/GROα-expressing melan-a cells (clone mel-a-6). The diameter of the tumors was monitored weekly by micrometer measurement. The MGSA/GROα-expressing cells made tumors in five out of six mice injected with antibody to MGSA/GROα (final tumor diameters ranged from 2.0 to 0.8 cm for the five out of six mice developing tumors) while tumors arose in all six of the mice injected with NRS (final tumor diameters ranged from 2.5 to 1.0 cm).
Mice injected with melan-a cells producing MGSA/GROα developed palpable tumors by day 45. All six of the mice receiving injections of NRS every-other day developed tumors and these tumors grew to a mean tumor diameter of 1.58 cm, or a tumor volume as measured by fluid displacement of 1.1 cm3 by 59 days. For the SCID mice inoculated with MGSA/GROα-expressing melan-a cells and injected with antiserum to MGSA/GROα, five of the six mice developed tumors and these tumors grew more slowly than in the mice injected every-other day with NRS. The antibody-treated tumors reached a mean diameter of only 1.1 cm, or a volume of 0.75 cm3 based on fluid displacement (1.05 cm3 by micrometer calculation), by 59 days after the initial inoculation into the mice (Fig. 1). The P value for differences in mean tumor size between NRS and antibody treatment for MGSA/GROα-expressing tumors did not achieve statistical significance (P = 0.103).
Effect of mutation of the ELR motif on MGSA/GRO induction of tumor formation
Because the growth of MGSA/GROα-expressing tumors was not fully inhibited by antibody to this chemokine, a second strategy was developed to neutralize the activity of this ligand. We produced ELR motif mutations in MGSA/GROα to compromise the high-affinity binding of this chemokine to its receptor, CXCR2. Stable melan-a clones expressing four different mutations in the ELR motif were established: E6A, L7A, R8A, and ELR/AAA. Mice injected with melan-a cells expressing mutant (ELR−) ELR-MGSA/GROα exhibited a marked reduction in tumor formation compared with those expressing wild-type (ELR+) MGSA/GROα (Table 1). No tumors formed in the nude mice injected with the E6A mutant clone. Approximately 5.5 months after injection, only 2–4 of the 10 mice injected with cells expressing the L7A or R8A mutations, respectively, formed tumors. Much later (7.5–8 months after cell inoculation), small nodules (2–10 mm) had begun to form on mice injected with the clones expressing these ELR− mutant forms of MGSA/GROα. In general, the tumors that formed in mice expressing ELR motif mutant forms of MGSA/GROα did not develop until 5–8 months after injection of cells, whereas mice injected with melan-a cells expressing wild-type MGSA/GROα formed tumors within 2.5 months after injection (see Table 1). The incidence of tumor formation for the melan-a cells expressing ELR/AAA mutant MGSA/GROα was similar to that of melan-a cells expressing the neomycin-selectable marker alone (V1). These data directly link the requirement for MGSA/GROα activation of its receptor through an intact ELR motif with induction of tumorigenesis.
TABLE 1.
Effect of Mutation of the ELR Motif of the MGSA/GRO Proteins on Tumor Formation
| Tumor latency period | Total period of tumor growth | Volume of tumor at removal | Diameter of small tumors |
|---|---|---|---|
| MUTANT L7A | |||
| Mouse 1 | 3.5 | 5 months | 1.0 ml |
| Mouse 2 | 5.5 | 7.5 months | 2.5 ml |
| Mouse 3 | 5.5 | 8 months | 3.5 ml |
| Mouse 4 | 5.5 | 8 months | 3.0 ml |
| Mouse 5 | 6.5 | 10 mm | |
| Mouse 6 | 7.5 | 6–7 mm | |
| Mouse 7 | 8 | 4–5 mm | |
| Mouse 8 | 8.5 | 2–3 mm | |
| Mouse 9 | 8.5 | 2–3 mm | |
| Mouse 10 | 8.5 | 2–3 mm | |
| MUTANT R8A | |||
| Mouse 1 | 4.5 | 6 months | 1.3 ml |
| Mouse 2 | 5 | 7.5 months | 2.5 ml |
| Mouse 3 | 5.5 | 8 months | 2.0 ml |
| Mouse 4 | 7.5 | 6–8 mm | |
| Mouse 5 | 7.5 | 6–8 mm | |
| Mouse 6 | 7.5 | 6–8 mm | |
| Mouse 7 | 7.5 | 6–8 mm | |
| Mouse 8 | No tumor formed | ||
| Mouse 9 | No tumor formed | ||
| Mouse 10 | No tumor formed | ||
| MUTANT AAA | |||
| Mouse 1 | 5.5 | 7.5 months | 3.0 ml |
| Mouse 2 | 5.5 | 7.5 months | 2.5 ml |
| Mouse 3 | 8.5 | 2–3 mm | |
| Mouse 4 | No tumor formed | ||
| Mouse 5 | No tumor formed | ||
| Mouse 6 | No tumor formed | ||
| Mouse 7 | No tumor formed | ||
| Mouse 8 | No tumor formed | ||
| Mouse 9 | No tumor formed | ||
| Mouse 10 | No tumor formed | ||
| WT MGSA | |||
| Mouse 1 | 1.5 | 2.5 months | 5.0 ml |
| Mouse 2 | 1.5 | 2.5 months | 2.5 ml |
| Mouse 3 | 1.5 | 2.5 months | 2.0 ml |
| Mouse 4 | 2 | 3 months | 2.5 ml |
| Mouse 5 | 2 | 3 months | 2.5 ml |
| Mouse 6 | 2 | 3 months | 2.0 ml |
| Mouse 7 | 2 | 3 months | 2.2 ml |
| Mouse 8 | 2.5 | 3 months | 1.5 ml |
| Mouse 9 | 8 mm | ||
| Mouse 10 | 4 mm | ||
| Mouse 11 | 5 mm | ||
| Mouse 12 | 2–3 mm | ||
| E6A Mutant and V1 Control | No tumors formed |
Receptor expression in melan-a cells and in tumors arising in mice injected with MGSA/GRO-expressing melan-a cells
Ligand binding assays on melan-a cells expressing MGSA/GROα, β, γ, or neo vector controls using 125I-MGSA revealed comparable specific binding for V1 control, MGSA/GROα, β, and γ expressing clones (1477, 1575, 1717, 1369 cpm, respectively). Specific binding was determined in triplicate after subtracting the counts per minute bound in the presence of 100-fold excess cold ligand. We estimate that this represents approximately 10,000 binding sites per cell.
Immunostaining of the cells in the growing tumor revealed that a subpopulation of cells within the tumor stained positively with antibody to CXCR2 (Fig. 2 A–C). However, the majority of the cells within the tumors did not exhibit mCXCR2 immunoreactivity. Moreover, blood vessels within the tumor reacted strongly with mCXCR2 antibody, suggesting that the secreted MGSA/GRO available from the melan-a cells could cause these endothelial cells to induce an angiogenic response (Fig. 2D). These data suggest that as the tumor forms there may be both autocrine and paracrine roles for MGSA/GRO stimulation of tumor growth.
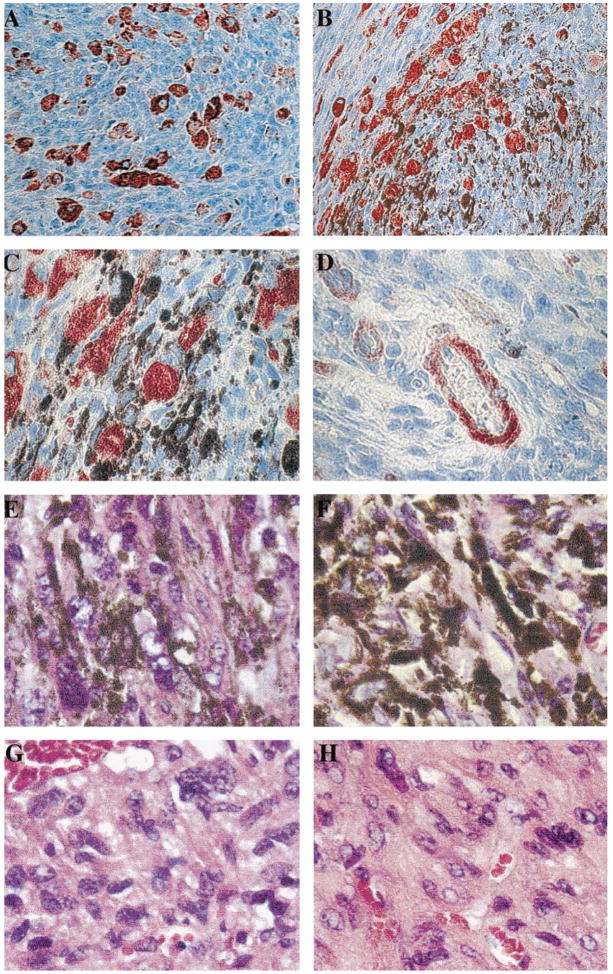
Fig. 2.
Immunolocalization of mCXCR2 and histology of the melan-a tumors. Tumors that arose in SCID mice injected with melan-a cells expressing MGSA/GROγ were fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with antibody to mCXCR2 (Santa Cruz Biotechnology, Inc.) using the ABC Elite kit from Vector Laboratories, with a substrate of aminoethyl carbazole for the peroxidase, which produces a red staining indicative of the presence of the antigen, mCXCR2. (A) Note the presence of a subset of non-melanotic tumor cells that stain strongly for immunoreactive mCXCR2 (original magnification, ×200). (B) The presence of imunoreactive mCXCR2 tumor cells interspersed among highly pigmented tumor cells is shown (original magnification ×200). (C) At higher power (original magnification ×1000) the presence of CXCR2 immunoreactive spindle-shaped tumor cells in the area of a number of highly pigmented tumor cells is shown. (D) The presence of immunoreactive mCXCR2 in the endothelial cells of a blood vessel in the tumor that formed after injection of melan-a cells expressing MGSA/GROγ (original magnification ×1000). Additional capillaries in cross section also exhibit immunoreactivity for the mCXCR2. (E, F) The H & E staining of the MGSA/GROγ-expressing tumors treated with NRS (E) or antiserum to MGSA/GROγ is shown (F) (original magnification ×1000). (G, H) The H & E staining of the MGSA/GROα-expressing tumors treated with NRS (G) or antiserum to MGSA/GROα is shown (H) (original magnification ×1000). Note the typical lack of leukocytic infiltration in these tumors.
Histology of tumors and detection of inflammatory infiltrate or NK cells
To determine whether the antibody treatment of the tumors resulted in binding of antibody to NK cells through the Fc receptor, and potential activation of these cells to enhance cell killing, we examined the histology of the H & E-stained tumors and attempted to localize NK cells within the tumors through the use of specific markers for NK cells (CD16). Histological examination of the tumors that arose in the MGSA/GROα or γ antibody-treated group or the NRS-treated group did not reveal the presence of inflammatory infiltrate or NK cells within the tumors (Fig. 2, E–H).
Tumor vascularity assessment
To determine whether antiserum inhibition of tumor growth was due in part to inhibition of angiogenesis, we examined the vascularity of antiserum-treated tumors with the use of an immunohistochemical marker for endothelial cells, CD31 [25] (Fig. 3). Although there are visual differences in the extent of vascularity as assessed by CD31 immunostaining, quantitation of the total immunoreactivity using 15 high-magnification microscrope fields revealed little difference in the CD31 immunodetection in the antibody-treated group of tumors compared with the NRS-treated tumors (Table 2). The MGSA/GROγ tumors treated with NRS had a mean staining volume over the tumor of 2208 ± 1506, whereas the antiserum-treated group from the two small tumors that arose from the six mice treated gave a mean staining volume of 1345 (range 979–1710) over the tumors (P = 0.28). Similarly, for the tumors expressing MGSA/GROα, the mean CD31 staining volume over the tumors was 4462 ± 955 from the NRS-treated serum compared with 6168 ± 1361 (P = 0.05) for the group of tumors arising in the MGSA/GROα antiserum-treated group. Although the raw data indicated that the fields examined from the MGSA/GROγ antiserum-treated group had highly similar areas of CD31 staining, closer inspection by multiplying the total tumor volume by the area of CD31-positive vessels within tumors revealed a trend toward reduced vascularity per tumor in the antiserum-treated group compared with the NRS treatment group (vascularity index: NRS-treated MGSA/GROγ tumors, 1542 ± 1558; antibody-treated MGSA/GROγ tumors, 183 ± 12, P = 0.08). A difference was also observed in the vascularity index for the MGSA/GROα-expressing tumors: NRS-treated tumors, 6016 ± 4827; anti-serum-treated tumors, 3922 ± 4360. However, differences in these means did not show statistical significance based on the two-sample t test. For tumors that arose from mel-a cells expressing both MGSA/GROα and MGSA/GROγ, there were no significant differences in the distribution of the blood vessels with NRS or chemokine antiserum treatment.
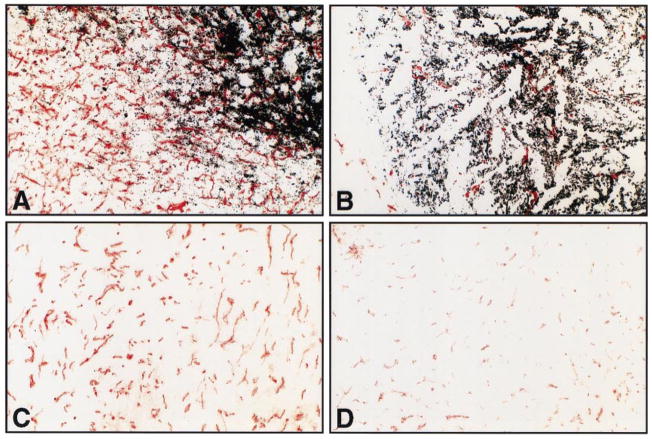
Fig. 3.
Immunohistochemical staining of tumor-associated blood vessels as visualized by CD31 antisera. (A, B) Heavily pigmented MGSA/GRO tumors: A, received treatment with normal rabbit serum and shows robust in-growth of blood vessels; B, received treatment with MGSA/GRO antisera and shows scant numbers of blood vessels. (C, D) Non-pigmented MGSA/GRO tumors: panel C shows moderate neovascularization after treatment with normal rabbit serum; panel D shows little evidence of neovascularization after treatment with MGSA/GRO antisera.
TABLE 2.
CD31 Immunostaining of Tumors from SCID Mice Treated with NRS or Antiserum to MGSA/GRO
| Relative CD31 staining units | |||||
|---|---|---|---|---|---|
| Tumor | Fat | Total dermis | Tumor volume | Staining × Tumor Vol | |
| MGSA/GROγ tumors with NRS | |||||
| 1 | 4222 | 6465 | 10,687 | 1.0 | 4222 |
| 2 | 3633 | 1984 | 7823 | 0.3 | 1089 |
| 3 | 1670 | 4285 | 5955 | 0.3 | 501 |
| 4 | 2312 | 2117 | 4229 | 1.1 | 2543 |
| 5 | 230 | 1396 | 1628 | 0.3 | 69 |
| 6 | 1186 | 1987 | 3173 | 0.7 | 830 |
| Mean | 2208 ± 1506 | 1542 ± 1558 | |||
| MGSA/GROγ Tumors with Ab | |||||
| 1 | 979 | 3335 | 4314 | 0.2 | 196 |
| 2 | 1710 | 1569 | 3279 | ~0.1 | 171 |
| Mean | 1345 | 183 | |||
| MGSA/GROα tumors with NRS | |||||
| 1 | 5218 | 2154 | 7372 | 2.5 | 13045 |
| 2 | 2768 | 2114 | 4882 | 0.1 | 277 |
| 3 | 5160 | 2542 | 7702 | 0.7 | 3612 |
| 4 | 5126 | 1096 | 6222 | 1.5 | 9333 |
| 5 | 4483 | 984 | 5467 | 1.4 | 7654 |
| 6 | 4019 | 1416 | 5435 | 0.4 | 2174 |
| Mean | 4462 ± 955 | 6016 ± 4827 | |||
| MGSA/GROα tumors with AB | |||||
| 1 | 5456 | 2805 | 8261 | 1.0 | 5456 |
| 2 | 4955 | 2050 | 7005 | 2.2 | 10901 |
| 3 | 8480 | 1836 | 10,316 | ~.1 | 848 |
| 4 | 5908 | 2649 | 8557 | ~.1 | 591 |
| 5 | 6043 | 2248 | 8291 | 0.3 | 1812 |
| Mean | 6168 ± 1361 | 3922 ± 4360 |
Angiogenic response to tumor extracts from mice treated with NRS versus antiserum to MGSA/GRO
When extracts of the tumor lysates were analyzed for angiogenic activity in the corneal micropocket/angiogenesis assay, we observed that the MGSA/GROα and MGSA/GROγ tumors arising in the NRS-treated group were all able to produce a strong angiogenic response, 4/4 positive for the MGSA/GROα tumor extracts and 3/3 positive for the MGSA/GROγ tumors (Table 3). In contrast, the tumors arising in the antiserum-treated animals did not yield a positive angiogenic response (3/3 negative for the MGSA/GROγ tumors and 4/4 negative for the MGSA/GROα-expressing tumors). Thus, the antibody treatment neutralized the tumor angiogenesis.
TABLE 3.
Angiogenic Activity in Tumor Lysates From SCID Mice Treated With NRS or MGSA/GRO Antiserum
| MGSA/GROγ tumors with NRS | 3/3 eyes positive |
|---|---|
| MGSA/GROγ tumors with MGSA/GRO antibody | 3/3 eyes negative |
| MGSA/GROα tumors with NRS | 4/4 eyes positive |
| MGSA/GROα tumors with MGSA/GRO antibody | 4/4 eyes negative |
ELISA determination of ELR+ versus ELR− chemokines: quantitation of the levels of MGSA/GRO proteins expressed in the tumors that formed
To determine whether the growth of the MGSA/GROα- and MGSA/GROγ-expressing tumors was differentially inhibited by antiserum to the respective chemokine because the α-expressing tumors produced higher levels of ELR+ chemokine, we allowed tumors to develop in SCID mice without antiserum injections, removed the tumors at the same time, then monitored the tumor levels of MGSA/GROα, MGSA/GROγ, KC, and MIP-2 by ELISA. The results from this experiment revealed considerable variation in the level of expression of chemokine from tumor to tumor (Table 4). The MGSA/GROα tumors produced a mean MGSA/GROα of 432 pg/mg protein (range 20–1120); the MGSA/GROγ tumors produced a mean of only 30 pg of MGSA/GRO/mg of protein (range 9–116; P = 0.001). There was a trend toward higher expression of KC and MIP-2 in the MGSA/GROα-expressing tumors, compared with MGSA/GROγ-expressing tumors: MGSA/GROγ tumor MIP-2 levels were 45 pg/mg protein (range 4–210); KC levels were 20 pg/mg protein (range 3–117); for MGSA/GROα tumors the MIP-2 levels were 197 pg/mg protein (range 24–321), and KC levels were 59 pg/mg protein (range 9–148). It has been demonstrated previously that MGSA/GRO can augment the expression of its own mRNA in cultured endothelial cells [34]. We postulate that expression of MGSA/GRO by the injected melan-a cells can also activate expression of KC and MIP-2 in the endothelial cells of the tumors and thus enhance chemokine levels. When the total mean expression of MGSA/GRO, MIP-2, and KC are totaled for the α- and γ-expressing tumors from the experiment using five mice to calculate the mean chemokine expression, there is a trend toward higher expression of these CXC chemokines in the MGSA/GROα-expressing tumors compared with the MGSA/GROγ-expressing tumors, although the ranges of expression overlap between these two groups: MGSA/GROα-expressing clone, 95 pg/mg protein; MGSA/GROγ-expressing clone, 688 pg/mg protein, P < 0.001 (Table 3). A similar trend was observed using the R & D Quantikine ELISA kit. Because MGSA/GROα, MGSA/GROγ, MIP-2, and KC all bind and activate the same receptor, these total differences in ligands for CXCR2 may have a significant biological impact on the growth of tumors in the mice.
TABLE 4.
Quantitation of MGSA/GRO, KC, and MIP-2 Levels in Tumors that Arose on the SCID Mice Innoculated with MGSA/GROα or MGSA/GROγ Expressing Melan-a Cells
| Tumor line | MGSA/GROα, pg/mg protein | MGSA/GROγ, pg/mg protein | KC, pg/mg protein | MIP-2, pg/mg protein | Total |
|---|---|---|---|---|---|
| MGSA/GROγ | 30 (9–116) | 20 (3–117) | 45 (4–210) | 95 | |
| MGSA/GROα | 432 (20–1120) | 59 (9–148) | 197 (24–321) | 688 |
To determine whether the differences in the tumor growth and antibody inhibition between the MGSA/GROα and γ-expressing clones were due to variation in the production of cytokines that might inhibit tumor growth or alter the recruitment of NK cells or other leukocytes, we performed ELISA assays for key cytokines on conditioned medium from these clones. The levels of IFN-γ, IL-12, and IL-13 production by the MGSA/GROα, γ, and vector control melan-a clones were examined. There was no detectable release of these cytokines into the culture medium in any of the three melan-a clones (data not shown). Thus differences in tumor growth between MGSA/GROα- or γ-expressing clones are not due to differential production of these cytokines.
Differential Ras expression and AP-1 activity in MGSA/GROα-expressing Mel-a-6 cells compared with melan-a clones expressing MGSA/GROβ or γ
We postulated that the differences in tumor growth of the MGSA/GROα-expressing clones could be the result of the ability of MGSA/GROα acting through CXCR2 on the melan-a cells to induce a secondary transforming event, which would not be sensitive to inhibition by antibody to MGSA/GROα. Because ras is often involved in melanoma tumorigenesis, we examined the expression of Ras proteins in melan-a clones expressing the various forms of MGSA/GRO compared with vector controls using Western blot analysis as described in Materials and Methods. We observed that the MGSA/GROα-expressing clone gave a stronger expression of Ras protein, using an antibody that would detect H, N, and K-Ras (Table 5). This enhanced expression of Ras protein in the MGSA/GROα-expressing clone was associated with increased AP-1 transcriptional activity using an assay that involved transient transfection with an AP-1-luciferase reporter construct, using co-transfection with β-Gal to normalize for transfection efficiency (Table 5). We examined AP-1 activity in three separate clones expressing MGSA/GROα, three clones expressing MGSA/GROβ, and three clones expressing MGSA/GROγ (data not shown). Consistently, the MGSA/GROα-expressing clones exhibited elevated AP-1 transactivation compared with the MGSA/GROβ- and γ-expressing clones. These data argue that the AP-1 activation occurs in response to MGSA/GROα expression and is not the result of clonal variation. Moreover, transfection of a dominant-negative Ras construct along with the AP-1 reporter demonstrated that elimination of the endogenous Ras activity blocked the elevation in endogenous AP-1 activity in the MGSA/GROα-expressing mel-a-6 clone (Table 5). These data suggest that constitutive expression of MGSA/GROα can have secondary effects on gene expression that contribute to the stronger transforming effect of MGSA/GROα compared with MGSA/GROβ or γ.
TABLE 5.
Levels of Ras Protein and AP-1 Transactivating Activity
| Clone | Vector | MGSA/GROβ | MGSA/GROγ | MGSA/GROα |
|---|---|---|---|---|
| Ras protein expression level (fold) | 1 | 4.15 ± 0.65 | 3.25 ± 0.05 | 7.00 ± 0.90 |
| Basal AP-1 transactivation (fold) | 1 | 1.12 ± 0.32 | 2.10 ± 0.18 | 3.16 ± 0.45 |
| Dominant-negative Ras inhibits AP-1 transactivation | 0.54 ± 0.10 | 0.48 ± 0.01 | 0.43 ± 0.12 |
DISCUSSION
The role of CXC chemokines in angiogenesis [1, 7, 23] and wound healing [26] has been recently clarified. Squamous cell carcinoma and non-small-cell lung tumors express ELR+ chemokines and the angiogenic activity in these tumors can be inhibited with antibodies to ELR+ chemokines such as IL-8 [5, 11, 27, 28]. The elaboration of chemokines by tumor cells has been clearly established, and it has been postulated that release of chemokines from tumor cells expressing receptors for the ELR motif positive chemokines might also facilitate tumor growth through an autocrine mechanism [4, 5, 9, 11, 27, 29–36]. In work reported here, we demonstrate that immortalized melanocytes expressing MGSA/GROα mutated in the ELR motif are compromised with regard to tumor-forming capacity. Moreover, we demonstrate that stable clones of melanocytes releasing the transgene product MGSA/GROα or MGSA/GROγ form tumors in SCID mice and that treatment of the mice with antiserum to MGSA/GRO from the time of the initial inoculation with melanocytes expressing these chemokines results in a significant reduction in tumor growth. The murine CXCR2 receptor [37] is clearly visible on a population of the tumor cells and in the blood vessels within the tumor, being displayed prominently in the endothelial cells, pericytes, and smooth muscle cells of the vessels. Thus, it is conceivable that MGSA/GRO activation of melan-a cells and blood vessel growth could involve CXCR2. Analysis of the ability of the tumor extracts from the antibody-treated mice revealed little angiogenic activity in these tumors in comparison to the NRS-treated mice where a full angiogenic response was observed [19]. Because the tumors showed prominent vascularity, it is apparent that some tumor-associated angiogenesis did take place during the development of the tumor, although this process had apparently been suppressed by the time the tumor was extracted and the CMA was performed. Although the ELR+ murine CXC chemokines, KC and MIP-2, were present in the tumor extracts, the angiogenic potential of these ligands must have been blocked by angiostatic substances within the tumor, based on the 100% inhibition of the angiogenic response in the presence of antibodies to MGSA/GROα or γ. A recent report from Bergers et al. shows that CD31 staining/cm3 of tumor remains equivalent in tumors treated with endostatin and angiostatin, whereas tumor volume shrinks due to apoptosis of endothelial cells [39].
The antiserum to MGSA/GROγ almost totally blocked tumor growth in vivo, based on reduced tumor formation and reduced tumor volume. These antibodies also inhibited angiogenesis by 73 or 89%, based on CD31 staining. We did not observe the same quantitative antibody inhibition for clones expressing MGSA/GROα compared with MGSA/GROγ. We postulate three possible reasons for these differences. (1) Based on the ELISA results summarized in Table 4, there may be a contribution from the somewhat higher combined level of expression of MGSA/GRO, MIP-2, and KC in the MGSA/GROα-expressing cells (688 pg/mg protein) compared with the MGSA/GROγ-expressing cells (95 pg/mg protein). However, the in vitro expression of chemokines was similar for the α- and γ-expressing cells [19]. Because the angiogenic activity of the tumor lysates was totally eliminated in the antiserum-treated tumor group (Table 3), these data suggest that the antiserum to MGSA/GROα was present at sufficiently high levels to neutralize the angiogenic activity of this chemokine, even if it were expressed at higher levels. Specific ELISAs for murine mig and IP-10 are not available, so we were unable to accurately access the levels of these angiostatic chemokines in the tumors that developed. (2) Alternatively, there may also be innate differences in the biological activity of MGSA/GROα versus MGSA/GROγ. Here we show that melan-a cells expressing MGSA/GROα with mutation in the ELR motif formed tumors much more slowly than melan-a cells expressing wild-type MGSA/GRO. These data demonstrate that compromising the biological activity of the MGSA/GROα ligand by altering the affinity for the receptor [39] markedly reduces tumor growth. Although it has been previously demonstrated that ELR mutant forms of CXC chemokines exhibit loss of angiogenic activity [22], there has been no prior demonstration that these mutations affect tumor growth. (3) Finally, there may be quantitative differences in the cellular response to MGSA/GROα compared with γ, which affects gene expression, and this may render the cells less responsive to antibody inhibition. We show here that there is enhanced expression of Ras protein and enhanced AP-1 transcriptional activity. These events could contribute to secondary effects on gene transcription, which could conceivably contribute to the increased transforming activity of MGSA/GROα or to the partial resistance of melan-a tumors formed by clones expressing MGSA/GROα to growth inhibition using antibody to the chemokine transgene product. Moreover, we have examined differences in gene expression of the α- and γ-expressing clones using the differential display technique to determine whether other factors might be contributing to differences in the antibody inhibition of tumor growth between these two MGSA/GROα- and γ-expressing clones. Initial results suggest that expression of MGSA/GROα is a stronger inducer of expression of several genes, including Ras, than is MGSA/GROγ [Wang et al., unpublished results]. Moreover, the differences in the _Ras_-dependent constitutive AP-1 transcriptional activity in MGSA/GROα-expressing clones compared with clones expressing MGSA/GROβ or γ or the ELR/AAA mutant form of MGSA/GROα could lead to altered transcription of other genes involved in melanocyte transformation. These differences in gene expression could also contribute to the transformed phenotype of melan-a cells and to the resistance to antiserum inhibition of growth of the MGSA/GROα-expressing clones.
One potential nonspecific effect of antibody injection into SCID mice could include the activation of NK cells through the binding of the Fc portion of the antibody to the Fc receptor on the NK cells. Several lines of evidence suggest that this is not the case in the model tested here. First, we did not observe leukocytic infiltration into the developing tumors with or without antibody injection. Second, differential activation of NK cells could not explain the differences observed with the treatment of developing tumors with antibody to MGSA/GROα versus MGSA/GROγ because we injected the same amount of antiserum into mice inoculated with cells expressing both MGSA/GROα and MGSA/GROγ, yet the antiserum to MGSA/GROγ produced a greater inhibition of tumor growth.
A second potential problem with this model might be differential expression of an inhibitory substance such as IFN-γ, IL-13, or IL-12, which could differentially affect tumor growth in the various clones. We did not observe differences in IFN-γ expression in the conditioned medium of these cultures (data not shown). The markedly reduced tumor burden in mice expressing ELR mutant forms of MGSA/GROα also argues against the possibility that differential induction of cytokine expression by transfected bacterially produced DNA might be responsible for the differences in tumor growth observed between the α and γ expressors. Thus, we believe that the tumor growth is occurring in response to continuous expression of chemokines.
In summary, we have shown here that continuous MGSA/GRO expression may lead to enhanced melanocyte tumor formation through autocrine stimulation of melanocyte growth, enhancement of the growth of blood vessels to the tumor, and induction of changes in gene expression. Continuous expression of the α, β, or γ forms of MGSA/GRO in melanocytes results in transformation, but the effects on melanocyte growth and gene expression may vary with the form of MGSA/GRO expressed.
Acknowledgments
This work was funded by Associate Career Scientist and Merit Awards from the Department of Veterans Affairs (A. R.) and by National Cancer Institute Grants CA34590 (A. R.), CA66180 (R. M. S.), 5P30AR4194 (S. D. R. C.), and 3P30CA68485 (Vanderbilt Ingram Cancer Center). We are indebted to James Owen for developing the γ3–14 melan-a clone, and to Eddy Balentien for developing the clone expressing MGSA/GROα. We thank Logan Quian for assisting with the ELISA assays, and Amy Pruitt, Ben Johnston, Alli Byrum, Neepa Ray, and Angela Pan for technical support.
References
- 1.Koch AE, Polverin PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 2.Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284. [PMC free article] [PubMed] [Google Scholar]
- 3.Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiol. 1994;62:134–139. doi: 10.1159/000163891. [DOI] [PubMed] [Google Scholar]
- 4.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 5.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norrby K. Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif. 1996;29:315–323. doi: 10.1111/j.1365-2184.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 8.Streiter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 9.Keane MP, Arenberg DA, Lynch JP, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, Digiovine B, Kunkel SL, Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 10.Hu BR, Hori Y, Presta M, Gresham GA, Fan TP. Inhibition of angiogenesis in rats by IL-1 receptor antagonist and selected cytokine antibodies. Inflammation. 1994;18:45–58. doi: 10.1007/BF01534597. [DOI] [PubMed] [Google Scholar]
- 11.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–1415. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi Y, Shono T, Isono M, Hori S, Matsushima K, Ono M, Kuwano M. Dual pathways of tubular morphogenesis of vascular endothelial cells by human glioma cells: vascular endothelial growth factor/basic fibroblast growth factor and interleukin-8. Jpn J Cancer Res. 1995;86:1189–1197. doi: 10.1111/j.1349-7006.1995.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, Kohno K, Kuwano M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yatsunami J, Tsuruta N, Ogata K, Wakamatsu K, Takayama K, Kawasaki M, Nakanishi Y, Hara N, Hayashi S. Interleukin-8 participates in angiogenesis in non-small cell, but not small cell carcinoma of the lung. Cancer Lett. 1998;120:101–108. doi: 10.1016/s0304-3835(97)00296-6. [DOI] [PubMed] [Google Scholar]
- 15.Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Co-expression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997;174:507–512. doi: 10.1016/s0002-9610(97)00165-7. [DOI] [PubMed] [Google Scholar]
- 16.Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity or growth-regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol. 1995;147:1248–1260. [PMC free article] [PubMed] [Google Scholar]
- 17.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 18.Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GRO on melanocyte transformation. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- 19.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuckbrandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- 21.Roesel JF, Nanney LB. Assessment of differential cytokine effects on angiogenesis using an in vivo model of cutaneous wound repair. J Surg Res. 1995;58:449–459. doi: 10.1006/jsre.1995.1071. [DOI] [PubMed] [Google Scholar]
- 22.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Roczniak S, Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 23.Arenberg DA, Polverini PJ, Kunkel RG, Shanafelt A, Strieter RM. In vitro and in vivo systems to assess role of C-X-C chemokines in regulation of angiogenesis. In: Abelson JN, Simon MI, editors. Methods in Enzymology. New York: Academic Press; 1997. pp. 190–220. [DOI] [PubMed] [Google Scholar]
- 24.Strieter RM, Kunkel SL, Burdick MD, Lincoln PM, Walz A. The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Invest. 1992;21:589–596. doi: 10.3109/08820139209069393. [DOI] [PubMed] [Google Scholar]
- 25.Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, Delaney J, Zukowski M, Yao Z. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:23668–23674. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 26.Rennekampff HO, Hansbrough JF, Woods V, Jr, Dore C, Kiessig V, Schroder JM. Role of melanoma growth stimulatory activity (MGSA/GRO) on keratinocyte function in wound healing. Arch Dermatol Res. 1997;289:204–212. doi: 10.1007/s004030050181. [DOI] [PubMed] [Google Scholar]
- 27.Arenberg DA, Polverini PJ, Kunkel SL, Shanafelt A, Hesselgesser J, Horuk R, Strieter RM. Angiogenesis in non-small cell lung cancer. J Leukoc Biol. 1997;62:554–562. doi: 10.1002/jlb.62.5.554. [DOI] [PubMed] [Google Scholar]
- 28.Koch AE, Kronfeld-Harrington LB, Szekanecz Z, Cho MM, Haines GK, Harlow LA, Strieter RM, Kunkel SL, Massa MC, Barr WG, Jimenez SA. In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiol. 1993;61:239–246. doi: 10.1159/000163802. [DOI] [PubMed] [Google Scholar]
- 29.Stoeckle MY, Hanafusa H. Processing of 9E3 mRNA and regulation of its stability in normal and rous sarcoma virus-transformed cells. Mol Cell Biol. 1989;9:4738–4745. doi: 10.1128/mcb.9.11.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–2675. [published erratum appears in J. Immunol. (1994) 153, 3360] [PubMed] [Google Scholar]
- 31.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin-8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 32.Luca M, Huang SY, Gershenwald JE, Singh RK, Reich R, Bareli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 33.Singh RK, Gutman A, Reich R, Bar-Eli M. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin-8. Cancer Res. 1995;55:3669–3674. [PubMed] [Google Scholar]
- 34.Wen DZ, Rowland A, Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989;8:1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abruzzo LV, Thornton AJ, Liebert M, Grossman HB, Evanoff H, Westwick J, Strieter RM, Kunkel SL. Cytokine-induced gene expression of interleukin-8 in human transitional cell carcinomas and renal cell carcinomas. Am J Pathol. 1992;140:365–373. [PMC free article] [PubMed] [Google Scholar]
- 36.Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996;156:1132–1137. [PubMed] [Google Scholar]
- 37.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 38.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistate carcinogenesis in mice. Science. 1999;284:808–811. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 39.Hesselgesser J, Chitnis CE, Miller LH, Yansura DG, Simmons LC, Fairbrother WJ, Kotts C, Wirth C, Gillece-Castro BL, Horuk R. A mutant of melanoma growth stimulatory activity does not activate neutrophils but blocks erythrocyte invasion by malaria. J Biol Chem. 1995;270:11472–11476. doi: 10.1074/jbc.270.19.11472. [DOI] [PubMed] [Google Scholar]