Novel Phospholipase A Activity Secreted by Legionella Species (original) (raw)
Abstract
Bacterial phospholipases are regarded as a major virulence factor in infection. In bacteria associated with pneumonia, destruction of lung surfactant and host cell membranes by bacterial phospholipases secreted during infection is thought to contribute to the disease. Phospholipase C (PLC) activity has been described in several Legionella species (W. B. Baine, J. Gen. Microbiol. 134:489–498, 1988; W. B. Baine, J. Gen. Microbiol. 131:1383–1391, 1985). By using detection methods such as thin-layer chromatography and mass spectrometry, PLC activity could not be detected in several strains of Legionella pneumophila. Instead, phospholipid degradation was identified to be caused by a novel PLA activity. We could demonstrate that PLA secretion starts at the mid-exponential-growth phase when bacteria were grown in liquid culture. Several Legionella species secreted different amounts of PLA. Legionella PLA may act as a powerful agent in the mediation of pathogenicity due to destruction of lung surfactant and epithelial cells.
Destruction of phospholipids (PLs) by bacterial phospholipases and the subsequent change of membrane constituents which can lead to cell damage is regarded to be a major virulence mechanism in infection. More than a decade ago, Baine evaluated several species of Legionella for cytolytic activity and elaboration of phospholipase C (PLC) to examine one possible mechanism of damage to leucocytes and tissue cells in legionellosis. PLC activity was detected by release of _p_-nitrophenol (_p_-NP) from _p_-nitrophenylphosphorylcholine (_p_-NPPC) and by release of tritiated phosphorylcholine (3H-PrC) from l-α-dipalmitoyl-[choline-_methyl_-3H]phosphatidylcholine (3H-PC). Legionella pneumophila, L. bozemanii, L. micdadei, L. dumoffii, L. gormanii, L. longbeachae, and L. jordanis all lysed dog red blood cells, which have a high ratio of membrane phosphatidylcholine (PC) to sphingomyelin. The same strains hydrolyzed various amounts of _p_-NPPC; L. bozemanii exhibited the greatest activity. In decreasing quantity, L. pneumophila, L. dumoffii, L. jordanis, L. bozemanii, and L. longbeachae, but not L. micdadei, released a radioactive water-soluble product from 3H-PC which was assumed to be 3H-PrC. Baine concluded that five of the six Legionella species under investigation possessed PLC activity (3).
In 1988, Baine purified PLC from L. pneumophila sg 5 Dallas 1E from the supernatant of a liquid culture in buffered yeast extract (BYE) broth by ion-exchange chromatography, followed by manganous chloride and ammonium sulfate precipitation. Enzyme activity was assayed by hydrolysis of _p_-NPPC and was confirmed by release of radioactivity from 3H-PC. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the purified preparation yielded a single 50- to 54-kDa band upon Coomassie blue staining. In contrast to hemolysis induced by bacteria grown on ABYE blood agar, the purified enzyme did not exhibit hemolytic activity (2). Since alveolar hyaline membrane formation has been reported in legionellosis (39), Baine speculated that the release of PLC by legionellae may cause damage of lung surfactant by hydrolysis of dipalmitoylphosphatidylcholine. Moreover, since PLC catalyzes the hydrolysis of phospholipid phosphodiesters and releases phosphomonoester and 1,2-diacylglycerol (1,2-DG), the latter could play an important role as a second messenger in signal transduction (4). To show destruction of lung surfactant by _Legionella_-PLC (A. Flieger et al., submitted for publication) and to characterize the contribution of this enzyme to intracellular survival of bacteria within monocytic cells (25), we tried to reproduce the previous purification protocol. By using detection methods such as thin-layer chromatography (TLC) and mass spectrometry (MS), which are more appropriate for characterization of phospholipases that are produced in combination with other enzymes (11a), PLC activity could not be detected in several strains of L. pneumophila. As we show here, generation of _p_-NP from _p_-NPPC was caused by Legionella phosphatase, and release of radioactivity from 3H-PC was caused by release of tritiated glycerophosphorylcholine (GPC) instead of 3H-PrC. GPC was formed by a newly described PLA activity which could be detected in several Legionella species.
MATERIALS AND METHODS
Reagents.
BYE supplement for Legionella BYE broth was obtained from Oxoid (Wessel, Germany). Yeast extract for Legionella BYE broth, ingredients for BCYEα agar, sodium azide, Tris-HCl, methanol, _n_-hexane, diethyl ether, glacial acetic acid, manganous chloride, sodium potassium tartrate, silica gel TLC plates, and chloroform were purchased from Merck (Darmstadt, Germany). Alveofact (consisting of 3% cholesterol and cholesterol ester, 0.5% free fatty acids [FFA], 4% triglycerides, 90% PLs [72% PC, 10% phosphatidylglycerol, 8% other PLs], and 1% protein) was kindly provided by Boehringer-Ingelheim-Pharma-KG (Biberach, Germany).
PC (P-5394), dipalmitoylphosphatidylcholine (P-6267), lysophosphatidylcholine (L-5254), GPC (G-4007), bovine serum albumin (BSA), EDTA, Triton X-100, _p_-NPPC, _p_-NP, PrC-HCl (P-0378), phosphorylethanolamine (P-0503), potassium-α-ketoglutarate (K-2000), calcium chloride, ferric chloride (F-2877), l-cysteine-HCl (C-1276), ACES _N_-(2-acetamido)-2-aminoethanesulfonic acid; A-9758), 1,2-dipalmitoylglycerol (D-9135), palmitic acid (P-0500), and PC-specific PLC (EC 3.1.4.3) from Clostridium perfringens (P-7633) were purchased from Sigma-Aldrich (Munich, Germany). Source 30Q, l-α-dipalmitoyl-[choline-_methyl_]-3H]phosphatidylcholine (specific activity, 81 Ci/mmol), 12.5% homogenous SDS-PAGE ready gels, SDS-PAGE buffer strips, and a silver staining kit for proteins were obtained from Amersham Pharmacia Biotech (Freiburg, Germany). Low-molecular-weight standards for SDS-PAGE were obtained from Bio-Rad (Munich, Germany). The scintillation solution Ultima Gold was purchased from Packard (Dreieich, Germany). Roti-Nanoquant reagent for protein determination was obtained from Roth (Karlsruhe, Germany).
Bacteria.
The Legionella strains used for this investigation are listed in Table 1.
TABLE 1.
Legionella strains used for detection of phospholipase activitya
| Species | Serogroup (subtype) | Origin (reference) | Source | No. of passages on BCYEα agar | Abbreviation |
|---|---|---|---|---|---|
| L. pneumophila | 1 (Philadelphia-1) | H | ATCC 33152 | U | Lp1-ATCC |
| L. pneumophila | 1 (Philadelphia-1) | H | CDC stock 5 | Many | Lp1-CDC |
| L. pneumophila | 1 (Philadelphia-1) | ATCC 33152 passaged 15 times in Mono Mac 6 cells (25) | <3 after passage in MM6 | Lp1-MM6 | |
| L. pneumophila | 1 (Philadelphia-1) | ATCC 33152 passaged 15 times in MRC cells (40) | <3 after passage in MRC | Lp1-MRC | |
| L. pneumophila | 1 (Pontiac-1) | H | G. Ruckdeschel, University of Munich, Munich, Germany | <3 | Lp1P-c |
| L. pneumophila | 2 (Togus) | H | ATCC 33154 | U | Lp2-ATCC |
| L. pneumophila | 6 (Schwerin) | H | C. Lück, German Legionella Reference Laboratory, University of Dresden, Dresden, Germany | <3 | Lp6-c |
| L. pneumophila | 12 | H | ATCC 43290 | U | Lp12-ATCC |
| L. anisa | E | CDC F1496 | <3 | La-CDC | |
| L. dumoffii | E | CDC F1407 | <3 | Ld-CDC | |
| L. gormanii | E | CDC F462 | <3 | Lg-CDC | |
| L. jordanis | E | CDC F338 | <3 | Lj-CDC | |
| L. longbeachae | H | CDC D4154 | <6 | Ll-CDC | |
| L. micdadei | H (via yolk sac) | ATCC 33218 | U | Lm-ATCC | |
| L. micdadei | E | CDC F976 | <3 | Lm-CDC | |
| L. oakridgensis | H | CDC D4401 | <6 | Lo-CDC | |
| L. parisiensis | E | CDC F242 | >6 | Lpa-CDC | |
| L. steigerwaltii | E | ATCC 35302 | U | Ls-ATCC | |
| L. steigerwaltii | E | CDC 464 | <3 | Ls-CDC |
Preparation of concentrated crude culture supernatant (CCCS) for detection of PLC activity.
Lp6-c, Lp1-MM6, Lp1-MRC, Lp2-ATCC, and Lp12-ATCC were grown on BCYEα agar at 37°C in 3% CO2 for 3 days (29). Colonies were suspended in 500 ml of both BYE broth (2) (0.25% yeast) and low-phosphate BYE broth (−P broth; ferric pyrophosphate was substituted by ferric chloride containing 0.4 mM inorganic phosphate) and adjusted to an optical density at 578 nm (OD578) of 0.2 (Ultrospec 2000; Amersham Pharmacia Biotech). Bacteria were cultured for 17 h at 37°C in 3% CO2 by vigorous shaking. Culture supernatant was obtained by centrifugation at 5,000 × g for 30 min. Then, 3 mM sodium azide was added to prevent contamination. Culture supernatant was concentrated 40-fold by ultrafiltration using a Acryl-Minitan with polyethersulfone membrane (molecular weight cutoff, 30 kDa) purchased from Millipore (Eschborn, Germany).
Partial purification of the 54-kDa protein.
A total of 6,000 ml of Lp6-c supernatant (−P broth) was concentrated, and CCCS was directly applied to a 50-ml Source 30Q column preequilibrated in buffer containing 20 mM Tris-HCl (pH 7.5) for anion-exchange chromatography (AEC). AEC was carried out at 4°C, and a gradient of 0 to 1 M sodium chloride in equilibration buffer was used for elution of proteins. Finally, 10-ml fractions were collected.
Determination of protein.
Protein was determined by using Roti-Nanoquant reagent according to the manufacturer's instructions. BSA was used as the standard protein.
SDS-PAGE.
Homogenous 12.5% polyacrylamide-SDS-ready gels and SDS buffer strips were used according to the method of Laemmli (20). The molecular mass of the protein bands was determined by means of a low-molecular-mass calibration kit from Bio-Rad. Proteins were visualized by using a silver-staining kit for proteins (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Hydrolysis of _p_-NPPC.
For detection of _p_-NPPC hydrolysis by AEC fractions, an assay was carried out using 10 mM _p_-NPPC, 3 mM NaN3, 20 mM CaCl2, 20 mM MnCl2, 0.5% Triton X-100 (vol/vol), and 20 mM Tris-HCl (pH 7.2). Samples were incubated at 37°C with continuous agitation. The OD was determined after 20 h at 410 nm.
Hydrolysis of phosphomonoesters.
For the detection of phosphatase activity, phosphomonoesters were incubated with AEC fractions under the following conditions: 5 mg of PrC-HCl per ml, 2.5 mg of phosphorylethanolamine per ml, 3 mM NaN3, 5 mM CaCl2, 0.5% Triton X-100 (vol/vol), and 20 mM Tris-HCl (pH 7.2). Samples were incubated at 37°C with continuous agitation. Inorganic phosphate (Pi) was estimated after 15 h of incubation according to the method of Eibl and Lands (10).
Hydrolysis of PLs.
For detection of PLC activity, PLs were incubated with different samples in a mixture containing 5 mg of Alveofact or 5 mg of PC per ml, 3 mM NaN3, 0.5% Triton X-100, and 20 mM Tris-HCl (pH 7.2) for 16 h at 37°C. Lipids were extracted as described by Bligh and Dyer (5).
TLC.
For detection of polar lipids, silica gel plates were developed in tanks containing the following solvent mixture: chloroform-methanol-water in a ratio of 65:25:4 (vol/vol/vol). For apolar lipids, a mixture of _n_-hexane–diethyl ether–acetic acid in a ratio of 70:30:4 (vol/vol/vol) was used. For visualization, silica plates were then sprayed with copper sulfate phosphoric acid reagent (37).
Hydrolysis of 3H-dipalmitoylphosphatidylcholine.
A total of 250 nCi of l-α-dipalmitoyl-[choline-_methyl_-3H]phosphatidylcholine per ml was dispersed (1 min of vortexing and 1 min of ultrasonication at a power setting of 5 [B-12 Sonifier; Branson Sonic Power Co., Danbury, Conn.], repeated three times) in 14 mM PC in 20 mM Tris-HCl (pH 7.2) containing 1% Triton X-100 (vol/vol) and 6 mM sodium azide. The resulting mixture was incubated with the same volume of CCCS of Lp6-c or with AEC fractions containing the 54-kDa protein, yielding a final volume of 200 μl. Samples were incubated for 5 h at 37°C with continuous agitation. Lipids were separated by single extraction according to the method of Bligh and Dyer (5). For incubation of 3H-PC with CCCS concentrated BYE broth was used as a negative control, and for incubation with AEC fractions 20 mM Tris-HCl (pH 7.2) was used as negative control. An aliquot of the extracted aqueous phases was transferred to vials containing 10 ml of scintillation solution for liquid scintillation counting (Beckman LS 1801 Counter; Beckman, Irvine, Calif.). Substrate hydrolysis was calculated from the difference between counts of samples and negative control.
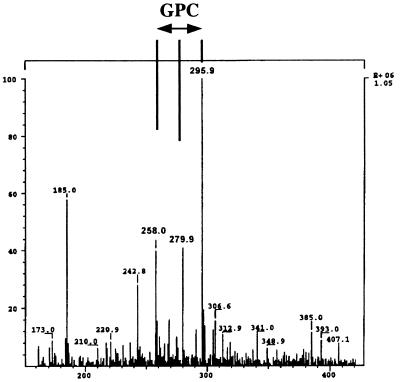
MS.
To analyze water-soluble reaction products, hydrolysis of Alveofact by CCCS of Lp6-c was carried out as described for the hydrolysis of PLs. Lipids were extracted as noted above. Solvents were removed from the water-methanol phase by means of speed vacuum evaporator (Savant, New York, N.Y.) at room temperature. The remaining substances were dissolved in a 1:1 (vol/vol) mixture of water-methanol. After centrifugation (5 min, 2,000 × g), supernatants were introduced into a TSQ700 electrospray ionization (ESI) mass spectrometer (Finnigan, Bremen, Germany) via a syringe pump at a rate of 5 μl/min. The masses of protonated PrC (184.0 ± 0.1) and GPC (258.0 ± 0.1) were detected in addition to the corresponding sodium and potassium adducts. These molecular masses were also observed when authentic substances (Sigma-Aldrich) were analyzed.
Monitoring of PLA formation.
Lp1P-c, Lp1-ATCC, Lp1-CDC, Lp6-c, Lm-ATCC, Lm-CDC, Ls-ATCC, Ls-CDC, Ld-CDC, Lg-CDC, Lj-CDC, Ll-CDC, Lo-CDC, Lpa-CDC, and La-CDC were grown on BCYEα agar at 37°C in 3% CO2 for 3 days. Colonies were suspended in 15 ml of BYE broth containing 1% yeast (2) and adjusted to an OD578 of 0.2 (Ultrospec 2000). Bacteria were cultured in BYE broth at 37°C in 3% CO2 by vigorous shaking. Bacterial supernatants were obtained after 12, 16, 20, 24, and 36 h of incubation by centrifugation at 5,000 × g for 10 min. Then, 3 mM sodium azide was added to prevent contamination. Bacterial supernatants were incubated with Alveofact as mentioned for the hydrolysis of the PLs. BYE broth without bacteria served as a negative control. All incubations were performed at 37°C with continuous agitation. After 24 h, FFA were determined by using the NEFA-C-Kit from Wako Chemicals (Neuss, Germany) according to the instructions of the manufacturer. Data were expressed as the difference between the amount of FFA in samples and in the negative control.
RESULTS AND DISCUSSION
Phospholipase secreted by legionellae can play an important role in pathogenesis. PC is one of the major constituents of cell membranes as well as of lung surfactant. The lung function can be affected seriously when epithelial cells are damaged (28) or when lung surfactant is hydrolyzed by phospholipases (12). We therefore intended to purify Legionella PLC according to the method of Baine (2), with some modifications.
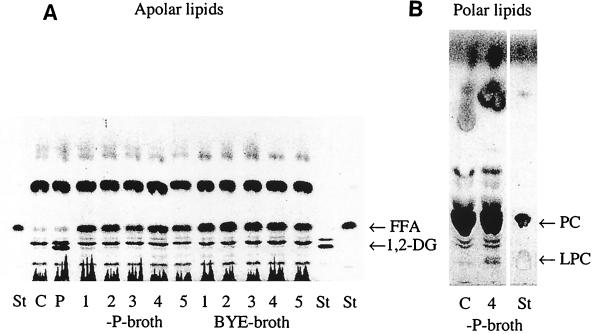
At first, we investigated several strains of L. pneumophila for PLC activity as determined by TLC after hydrolysis of PLs. As shown in Fig. 1A, 1,2-DG could not be detected in the CCCS of any of the five strains. Instead, FFA were generated. Since low Pi concentration of culture media is known to increase PLC secretion by Pseudomonas aeruginosa (33), we used low-phosphate BYE broth (−P broth) for liquid culture of several strains of L. pneumophila. However, no difference in hydrolysis of PC could be found (Fig. 1A). These results could be explained by two possible mechanisms: (i) secretion of PLA instead of PLC or (ii) subsequent cleavage of 1,2-DG by bacterial lipase (36). The latter was regarded as unlikely since CCCS of Lp6-c was not able to release fatty acids from 1,2-DG (data not shown). The assumption that L. pneumophila may secrete PLA was supported by the observation that lysophosphatidylcholine (LPC) was formed after incubation of surfactant with CCCS of Lp6-c (Fig. 1B). Generation of LPC after incubation of surfactant with both whole Lp6-c bacteria and CCCS of Lp6-c could be confirmed by 31P nuclear magnetic resonance spectroscopy (Flieger et al., submitted).
FIG. 1.
Apolar and polar lipids after incubation of PC with CCCS of L. pneumophila. PC was incubated with the CCCS of different L. pneumophila strains for 16 h. Apolar (A) and polar (B) lipids were characterized by TLC. A representative experiment is shown. Abbreviations: St, standard (1A, 1,2-dipalmitoylglycerol, palmitic acid; 1B, LPC, dipalmitoylphosphatidylcholine); C, negative control (concentrated BYE broth or concentrated −P broth, respectively); P, positive control (PLC from C. perfringens); 1, Lp1-MM6; 2, Lp1-MRC; 3, Lp2-ATCC; 4, Lp6-c; 5, Lp12-ATCC.
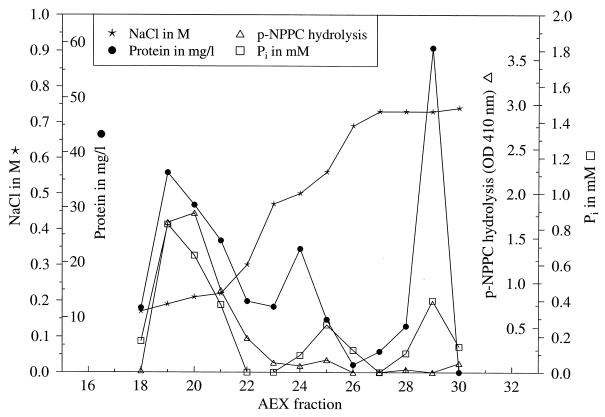
FIG. 2.
Characterization of AEC fractions. CCCS of Lp6-c was applied onto an AEC column. Fractions eluted by 0.1 to 0.7 M sodium chloride were investigated for protein content, hydrolysis of _p_-NPPC (OD410), and hydrolysis of phosphomonoesters (Pi content). A representative experiment is shown.
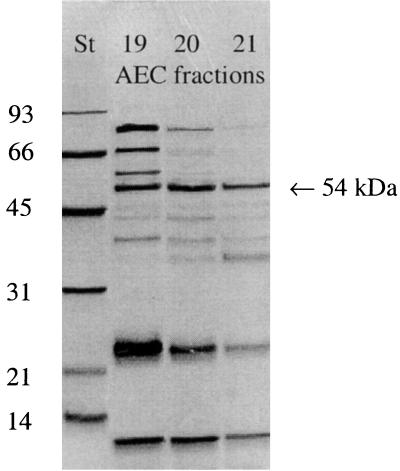
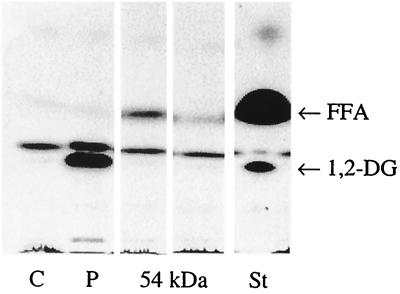
Since we could not find characteristic cleavage products of PLC, we intended to purify the enzyme as described by Baine, with some modifications (2) to exclude further hydrolysis of the cleavage products by other enzymes that are concomitantly secreted by legionellae, such as phosphatases (15, 31) or lipases (36). Fractions after AEC of CCCS (Lp6-c) were tested for their ability to hydrolyze _p_-NPPC. According to Baine (2), fractions eluted at 0.1 to 0.2 M sodium chloride showed hydrolysis toward _p_-NPPC (Fig. 2). Analysis of these fractions by SDS-PAGE revealed the enrichment of a 54-kDa protein among others (Fig. 3). However, when these fractions were examined for PLC activity, no 1,2-DG and a minimal amount of released FFA was found by TLC after incubation with Alveofact (Fig. 4). Release of 1,2-DG could not be stimulated by the addition of manganese ions, calcium ions, or both; by the addition of zinc ions, magnesium ions, ferric ions, BSA, and sorbitol; or by variation of pH (5.5 and 9.5, respectively) (data not shown).
FIG. 3.
SDS-PAGE of AEC fractions causing hydrolysis of _p_-NPPC. St, molecular weight standard in kilodaltons; 19–21, AEC fractions. The 54-kDa protein band is designated by the arrow. A representative experiment is shown.
FIG. 4.
Apolar lipids after incubation of Alveofact with AEC fractions containing the 54-kDa protein. Alveofact was incubated with AEC fractions containing the 54-kDa protein for 16 h. Apolar lipids were characterized by TLC. A representative experiment is shown. Abbreviations: C, negative control (20 mM Tris-HCl, pH 7.2); P, positive control (PLC from C. perfringens); St, standard (1,2-dipalmitoylglycerol, palmitic acid).
The hydrolysis of _p_-NPPC by other enzymes affecting phosphate esters is well established (11a, 34, 35). Therefore, we examined 54-kDa-protein AEC fractions showing hydrolysis of _p_-NPPC for phosphatase activity. Figure 2 shows that 54-kDa-protein AEC fractions were also able to hydrolyze phosphomonoesters. This reaction could be inhibited by the addition of 10 mM sodium potassium tartrate (data not shown), which is a known inhibitor for acid phosphatase (41). We concluded that hydrolysis of _p_-NPPC by the 54-kDa AEC fractions was caused by phosphatase activity present in these fractions. The existence of both acid and alkaline phosphatases is known in legionellae (15, 31).
Next, we examined the hydrolysis of 3H-PC by CCCS and by the 54-kDa AEC fraction of Lp6-c as described by Baine (2, 3) at final concentrations of 20 and 18 μg of protein/ml, respectively. The 54-kDa AEC fraction did not show hydrolysis of 3H-PC (0.2% hydrolyzed) in contrast to CCCS of Lp6-c, 12.3% hydrolyzed again suggesting that phosphatase was responsible for the hydrolysis of _p_-NPPC (11a). Additionally, the phospholipase activity present in the CCCS of L. pneumophila showed a reaction pattern completely different from a well-characterized PLC: in the presence of detergent, PLC from C. perfringens was inhibited from hydrolyzing 3H-PC, whereas destruction of 3H-PC by CCCS of Lp6-c was enhanced (data not shown).
It is known that PLC, PLD, the combined action of PLA1 and PLA2, or the collaboration of PLA and acyltransferase are each able to release tritiated water-soluble components such as 3H-PrC, 3H-choline, and 3H-GPC from 3H-PC, respectively. Since we could demonstrate that Legionella possesses PLA (Fig. 1A and B), two possible mechanisms could be responsible for the appearance of radioactivity in the aqueous phase after incubation of CCCS with 3H-PC followed by lipid extraction. First, tritiated LPC produced by PLA is water soluble and can migrate into the aqueous phase during lipid extraction, but we could demonstrate that LPC, even at higher concentrations, is sufficiently extracted with the chloroform-methanol phase (data not shown). Second, water-soluble GPC may be generated from PC by PLA in case both fatty acids are released from the PLs.
Therefore, we determined the release of water-soluble products from Alveofact by CCCS of Lp6-c by using ESI-MS. PrC (signals at 184.0 [PrC-H], 205.9 [PrC-Na], and 221.9 [PrC-K]) could be found after incubation of PLC from C. perfringens with surfactant (data not shown). After incubation of CCCS of Lp6-c with Alveofact, only GPC (signals at 258.0 [GPC-H], 279.9 [GPC-Na], and 295.9 [GPC-K]) but not PrC could be detected (Fig. 5). Since the concentration of inorganic phosphate within the aqueous phase of lipid extraction did not increase after incubation of PLs with CCCS of Lp6-c (data not shown), a possible cleavage of PrC by phosphatases could be excluded. These results further support the absence of PLC secretion in Lp6-c. Instead, PLA activity could be detected by formation of FFA, LPC, and GPC from the PLs. Due to the generation of GPC by Lp6-c, we assume that both PLA1 and PLA2 are secreted by Lp6-c. However, the presence of a single PLA activity plus additional acyltransferase activity which can cause transition of fatty acid chains within the lipid molecule must be taken into account.
FIG. 5.
ESI-MS. CCCS of Lp6-c was incubated with Alveofact for 16 h. Lipids were separated from the water-soluble substances by lipid extraction. Water-soluble substances were analyzed by ESI-MS. A representative experiment is shown.
We can conclude that the previously described phospholipase activity in L. pneumophila (2, 3) may have been misinterpreted as PLC due to the hydrolysis of _p_-NPPC by Legionella phosphatase and the generation of 3H-GPC (which was formerly suspected to be 3H-PrC) as a water-soluble product released from 3H-PC by PLA.
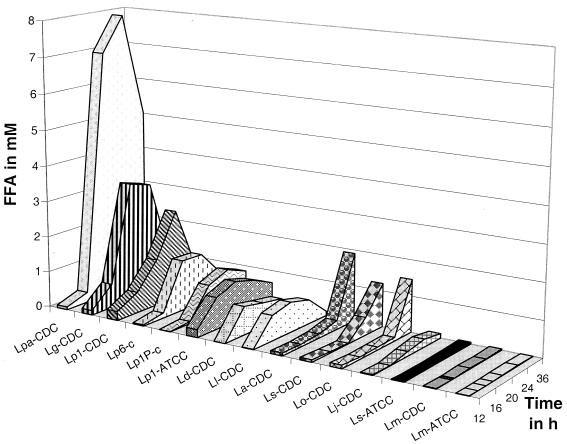
Finally, we examined the distribution of PLA secretion among different Legionella species. We could show that, except Lm-ATCC, Lm-CDC, and Ls-ATCC, all species under investigation were able to secrete PLA activity (Fig. 6). Baine reported that, with the exception of L. micdadei, all species showed hydrolysis of 3H-PC (3). This finding further supports the assumption of misinterpretation of 3H-PC hydrolysis by Legionella phospholipase at that time.
FIG. 6.
Kinetics of PLA secretion in different Legionella species. Legionellae were grown in BYE broth. PLA activity was determined after incubation of the culture supernatant with Alveofact for 24 h by detection of FFA release. Data are mean values. For a better survey, standard deviations which were maximally ±0.1 mM were not added to the diagram.
We found that PLA secretion started with the mid-exponential-growth phase and reached maximal extent with entry into stationary-growth phase. With the exception of La-CDC, Ls-CDC, and Lo-CDC which reached an OD of about 2 in 24 to 36 h, all other strains achieved an OD of about 2 in 16 h. A delayed growth in BYE broth was accompanied by delayed PLA secretion (data not shown). A considerable difference in PLA secretion between Ls-ATCC (strain with unknown passage history, with no PLA) and Ls-CDC (isolate passaged fewer than three times on BCYEα agar, with high PLA) could be detected (Fig. 6). A prolonged passage history of the ATCC strain can be responsible for agar adaptation and a concomitant loss of PLA as a putative virulence factor of legionellae.
The discovery of a novel PLA in legionellae leads to speculation about (i) its function in the destruction of lung surfactant and the subsequent induction of acute respiratory distress syndrome during Legionnaires' disease (1, 7, 19, 23, 30, 39; Flieger et al., submitted), (ii) its capacity to elicit inflammation (18, 24), (iii) its influence on signal transduction in host cells (4, 8, 9, 21, 29), (iv) its contribution to uptake and intracellular multiplication (pore formation, inhibition of phagosome-lysosome fusion by induction of phospholipid rearrangement) in amoebae and alveolar macrophages (1, 13, 14, 16, 17, 22, 26, 32, 38), (v) its significance for the escape of bacteria from the phagosome after intracellular replication (6), and (vi) its ability to acquire nutritional substances (11).
Thus, PLA activity within the genus Legionella may contribute to many different steps during pathogenesis. Detailed investigations are now necessary to determine the key mechanisms which are associated with PLA secretion.
ACKNOWLEDGMENTS
We acknowledge Hinnak Northoff, Elvira Fehrenbach, Friedrich Goetz, Andreas Peschel, and Markus Koerber for helpful suggestions. We thank Martha Szamel and Wilhelm Stoffel, who helped to promote this investigation by their scientific experiences. We thank Eckhart Schmidt for support with the ESI-MS. We acknowledge Josef Mussotter and Eberhardt Weller for supportive discussions about PL biochemistry. We thank Barry Fields, Christian Lück, and Gotthardt Ruckdeschel for providing Legionella strains.
Antje Flieger was supported by grants of Boehringer-Ingelheim-Pharma-KG (Biberach, Germany), the Boehringer-Ingelheim-Foundation (Mainz, Germany), and the Fortuene Fund (179-1 and 179-2; University Hospital of Tubingen, Tubingen, Germany). Shimei Gong was supported by the Fortuene Fund (305-1 and 305-2; University Hospital of Tubingen, Tubingen, Germany).
REFERENCES
- 1.Aronson J F, Johns L W. Injury of lung alveolar cells by lysolecithin. Exp Mol Pathol. 1977;27:35–42. doi: 10.1016/0014-4800(77)90017-x. [DOI] [PubMed] [Google Scholar]
- 2.Baine W B. A phospholipase C from the Dallas 1E strain of Legionella pneumophila serogroup 5: purification and characterization of conditions of optimal activity with an artificial substrate. J Gen Microbiol. 1988;134:489–498. doi: 10.1099/00221287-134-2-489. [DOI] [PubMed] [Google Scholar]
- 3.Baine W B. Cytolytic and phospholipase C activity in Legionella species. J Gen Microbiol. 1985;13:1383–1391. doi: 10.1099/00221287-131-6-1383. [DOI] [PubMed] [Google Scholar]
- 4.Belyi Y F. Intracellular parasitism of Legionella and signaling in eukaryotic cells. FASEB J. 1993;7:1011–1005. doi: 10.1096/fasebj.7.11.8370469. [DOI] [PubMed] [Google Scholar]
- 5.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan J W, Williams A, Ashworth L A. In vivo production of a tissue-destructive protease by Legionella pneumophila in the lungs of experimentally infected guinea pigs. J Gen Microbiol. 1988;134:143–149. doi: 10.1099/00221287-134-1-143. [DOI] [PubMed] [Google Scholar]
- 8.Coxon P Y, Summersgill J T, Ramirez J A, Miller R D. Signal transduction during Legionella pneumophila entry into human monocytes. Infect Immun. 1998;66:2905–2913. doi: 10.1128/iai.66.6.2905-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis E A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 10.Eibl H, Lands W E. A new, sensitive determination of phosphate. Anal Biochem. 1969;30:51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- 11.Finnerty W R, Makula R A, Feeley J C. Cellular lipids of the Legionnaires' disease bacterium. Ann Intern Med. 1979;90:631–634. doi: 10.7326/0003-4819-90-4-631. [DOI] [PubMed] [Google Scholar]
- 11a.Flieger, A., S. Gong, M. Faigle, and B. Neumeister. Critical evaluation of p-nitrophenylphosphorylcholine (p-NPPC) as artificial substrate for the detection of phospholipase C. Enzyme Microb. Technol., in press. [DOI] [PubMed]
- 12.Holm B A, Keicher L, Liu M Y, Sokolowski J, Enhorning G. Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol. 1991;71:317–321. doi: 10.1152/jappl.1991.71.1.317. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M J, Rogers J H, Hurley M C, Engleberg N C. Phosphatase-negative mutants of Legionella pneumophila and their behavior in mammalian cell infection. Microb Pathog. 1994;17:51–62. doi: 10.1006/mpat.1994.1051. [DOI] [PubMed] [Google Scholar]
- 16.Kirby J E, Isberg R R. Legionnaires' disease: the pore macrophage and the legion of terror within. Trends Microbiol. 1998;6:256–258. doi: 10.1016/s0966-842x(98)01310-9. [DOI] [PubMed] [Google Scholar]
- 17.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 18.Kume N, Cybulsky M I, Gimbrone M A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Investig. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwaik Y A. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–695. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth J D. Receptor-regulated phospholipases and their generation of lipid mediators, which activate protein kinase C. In: Kuo J F, editor. Protein kinase C 1994. New York, N.Y: Oxford University Press; 1997. pp. 121–170. [Google Scholar]
- 22.Lindahl M, Hede A R, Tagesson C. Lysophosphatidylcholine increases airway and capillary permeability in the isolated perfused rat lung. Exp Lung Res. 1986;11:1–12. doi: 10.3109/01902148609062823. [DOI] [PubMed] [Google Scholar]
- 23.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumeister B, Kleihauer A, Rossmann V, Fehrenbach E, Faigle M, Baumbach S, Eichner M, Northoff H. Induction of cytokines and expression of macrophage surface receptors in Mono Mac 6 cells after infection with different Legionella species. APMIS. 1998;106:319–333. [PubMed] [Google Scholar]
- 25.Neumeister B, Schoniger S, Faigle M, Eichner M, Dietz K. Multiplication of different Legionella species in Mono Mac 6 cells and in Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:1219–1224. doi: 10.1128/aem.63.4.1219-1224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewoehner D E, Rice K, Sinha A A, Wangensteen D. Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J Appl Physiol. 1987;63:1979–1986. doi: 10.1152/jappl.1987.63.5.1979. [DOI] [PubMed] [Google Scholar]
- 27.Pasculle A W, Feeley J C, Gibson R J, Cordes L G, Myerowitz R L, Patton C M, Gorman G W, Carmack C L, Ezzell J W, Dowling J N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980;141:727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- 28.Petty T L. Acute respiratory distress syndrome (ARDS) Dis Mon. 1990;36:1–58. [PubMed] [Google Scholar]
- 29.Prokazova N V, Zvezdina N D, Korotaeva A A. Effect of lyso-phosphatidylcholine on transmembrane signal transduction. Biochem Mosc. 1998;63:31–37. [PubMed] [Google Scholar]
- 30.Rice K L, Duane P G, Niewoehner D E. Lysophosphatidylcholine augments elastase-induced alveolar epithelial permeability and emphysema in the hamster. Am Rev Respir Dis. 1987;136:941–946. doi: 10.1164/ajrccm/136.4.941. [DOI] [PubMed] [Google Scholar]
- 31.Saha A K, Dowling J N, LaMarco K L, Das S, Remaley A T, Olomu N, Pope M T, Glew R H. Properties of an acid phosphatase from Legionella micdadei which blocks superoxide anion production by human neutrophils. Arch Biochem Biophys. 1985;243:150–160. doi: 10.1016/0003-9861(85)90783-0. [DOI] [PubMed] [Google Scholar]
- 32.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–255. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 33.Shortridge V D, Lazdunski A, Vasil M L. Osmoprotectants and phosphate regulate expression of phospholipase C in Pseudomonas aeruginosa. Mol Microbiol. 1992;6:863–871. doi: 10.1111/j.1365-2958.1992.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 34.Sok D E, Kim M R. A spectrophotometric assay of Zn2+-glycerolphosphorylcholine phosphocholine phosphodiesterase using p-nitrophenylphosphorylcholine. Anal Biochem. 1992;203:201–205. doi: 10.1016/0003-2697(92)90303-o. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava P N, Brewer J M, White R A., Jr Hydrolysis of p-nitrophenylphosphorylcholine by alkaline phosphatase and phospholipase C from rabbit sperm-acrosome. Biochem Biophys Res Commun. 1982;108:1120–1125. doi: 10.1016/0006-291x(82)92116-7. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe T C, Miller R D. Extracellular enzymes of Legionella pneumophila. Infect Immun. 1981;33:632–635. doi: 10.1128/iai.33.2.632-635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touchstone J C, Levin S S, Dobbins M F, Matthews L, Beers P C, Gabbe S G. (3-sn-Phosphatidyl)cholines (lecithins) in amniotic fluid. Clin Chem. 1983;29:1951–1954. [PubMed] [Google Scholar]
- 38.Weltzien H U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979;559:259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 39.Winn W C, Jr, Myerowitz R L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981;12:401–222. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- 40.Wong M C, Peacock W L, McKinney R M, Wong K H. Legionella pneumophila: avirulent to virulent conversion through passage in cultured human embryonic lung fibroblasts. Curr Microbiol. 1981;5:31–34. [Google Scholar]
- 41.Zollner H. Handbook of enzyme inhibitors. Weinheim, Germany: VCH; 1993. [Google Scholar]