Different intrinsic activities of bucindolol, carvedilol and metoprolol in human failing myocardium (original) (raw)
Abstract
- Clinical studies have shown different effects of β-blockers on the β-adrenergic system, tolerability and outcome in patients with heart failure.
- The study examines β-adrenoceptor-G-protein coupling and intrinsic activity of bucindolol, carvedilol and metoprolol in human ventricular myocardium.
- Radioligand binding studies ([125I]-Iodocyanopindolol) were performed in membrane preparations of human failing and nonfailing myocardium. Functional experiments were carried out in isolated muscle preparations of human left ventricular myocardium from failing hearts.
- Bucindolol and carvedilol bound non-selectively to β1- and β2-adrenoceptors and exerted guanine nucleotide modulatable binding. Metoprolol was 35-fold β1-selective and lacked guanine nucleotide modulatable binding.
- All β-blockers antagonized isoprenaline-induced enhancement of contractility.
- In preparations in which the coupling of the stimulatory G-protein to adenylate cyclase was facilitated by forskolin, bucindolol increased force of contraction in three and decreased it in five experiments. Carvedilol increased force in one and decreased it in six experiments. Metoprolol decreased force in all experiments by 89.4±2.2% (P<0.01 metoprolol vs carvedilol and bucindolol). The negative inotropic effect of metoprolol was antagonized by bucindolol.
- It is concluded that differences in intrinsic activity can be detected in human myocardium and have an impact on cardiac contractility. In human ventricular myocardium, bucindolol displays substantially higher intrinsic activity than metoprolol and carvedilol. Bucindolol can behave as partial agonist or partial inverse agonist depending on the examined tissue.
- Differences in intrinsic activity may contribute to differences in β-adrenoceptor regulation and possibly to differences in tolerability and outcomes of patients with heart failure.
Keywords: β-adrenoceptor antagonists, intrinsic activity, inverse agonism, human myocardium, heart failure
Introduction
Clinical studies have demonstrated beneficial effects of β-adrenoceptor antagonist treatment of chronic heart failure (Hash & Prisant, 1997 for review). However, effects of β-adrenoceptor antagonists on survival are different. Carvedilol (Packer et al., 1996), bisoprolol (CIBIS II, 1999) and metoprolol (MERIT-HF Study Group, 1999) were shown to reduce mortality in patients with chronic heart failure. In contrast, xamoterol had adverse effects on survival in patients with heart failure (Nicholas et al., 1990), and bucindolol failed to produce a beneficial effect compared to placebo (BEST, 1995; Bristow, 2000). Thus, differences among β-adrenoceptor antagonists are likely to occur.
Previous data indicate that these agents exert different effects on the β-adrenergic system. Gilbert et al. (1993) reported a restoration of down-regulated right ventricular β-adrenoceptor density after treatment with metoprolol, but not with carvedilol. These in vivo observations were confirmed by in vitro experiments in chick heart cells, where metoprolol, but neither carvedilol nor bucindolol, reversed agonist-induced down-regulation of β1-adrenoceptors (Yoshikawa et al., 1996).
Transgenic mice overexpressing the human β2-adrenoceptor (Bond et al., 1995) or a constitutively active mutant (CAM) adrenoceptor (Samana et al., 1993) have led to the expansion of the classical ternary complex model of receptor action (De Lean et al., 1980). The β-adrenoceptor exists in an equilibrium between an active (R*) and an inactive (R) conformation. When unoccupied, the β-adrenoceptor already exerts basal intrinsic activity. Agonists induce a conformational change towards the R*-state, whereas neutral antagonists cause no change. Some antagonists are able to stabilize the inactive conformation (R), thus decreasing the basal activity of the receptor. These antagonists are termed inverse agonists, as they exert a ‘negative' intrinsic activity, driving the equilibrium to the opposite direction than the agonist (Bond et al., 1995).
The activation state of the β-adrenoceptor is of importance for receptor regulation. The intrinsic activity of several partial and full agonists was shown to correlate with the degree of receptor phosphorylation and in turn desensitization and downregulation (Benovic et al., 1988). In this light of receptor action of antagonists, many therapeutically used agents may need reclassification (Bond et al., 1995). In particular, in failing human tissue, direct evaluation of intrinsic activity of relevant compounds has not been performed.
The present study assessed the intrinsic activity of the β-adrenoceptor antagonists bucindolol, carvedilol and metoprolol directly in human left ventricular myocardium from patients with heart failure. By radioligand binding experiments, receptor-G-protein interaction was examined. For evaluating the functional relevance of the biochemical findings, contraction experiments were performed in the presence of forskolin, a diterpene that facilitates the coupling of the α-subunit of the stimulatory G-protein (GSα) with the catalytic unit of adenylate cyclase (Jasper et al., 1988).
Methods
Myocardial tissue
Isolated, electrically stimulated human left ventricular papillary muscle strips or cell membrane preparations from human left ventricular myocardium were used. Tissue was obtained during heart transplantations. Failing hearts were taken from seven patients with end-stage heart failure (New York Heart Association (NYHA) class IV), resulting from either idiopathic (_n_=5) or ischaemic (_n_=2) dilated cardiomyopathy (five male, two female; age, 64±2 years; ejection fraction, 32±1%). Nonfailing hearts were taken from four organ donors whose hearts could not be used for transplantation (three male, one female; age 37±3 years). For the latter group, echocardiography revealed normal left ventricular contractility. All patients gave written informed consent before surgery. Medical therapy of patients with heart failure consisted of diuretics, nitrates, ACE inhibitors and cardiac glycosides. Patients (nonfailing and failing) receiving catecholamines or β-adrenoceptor antagonists were withdrawn from the study. Drugs used for general anaesthesia were flunitrazepam, fentanyl and pancuronium bromide with isoflurane. The tissue was placed immediately in ice-cold cardioplegic solution (containing (in mmol l−1) NaCl 15, KCl 10, MgCl2 4, histidine HCl 180, tryptophane 2, mannitol 30, and potassium dihydrogen oxoglutarate 1), and was delivered to the laboratory within 15 min. For functional experiments, ventricular samples were used immediately. For radioligand experiments, cardiac tissue was frozen in liquid nitrogen immediately after explantation and stored at −70°C. Membrane preparation for binding experiments has been described elsewhere (Böhm et al., 1990b).
Isolated cardiac muscle strip preparation and measurement of force of contraction
Isometric force of contraction was determined on isolated, electrically driven muscle preparations as described previously (Böhm et al., 1990b). Bathing solution was maintained at 37°C, pH 7.4, and aerated with 95% O2 and 5% CO2. Muscles were stretched to the length at which force of contraction was maximal. In experiments with inotropic prestimulation, muscle strips were preexposed to isoprenaline (0.1 μmol l−1, ≈percnt;EC50) for 30 min and to forskolin (0.3 μmol l−1, ≈percnt;EC50) for at least 45 min.
The β-adrenoceptor antagonists bucindolol (0.1–1000 nmol l−1), carvedilol (0.1–1000 nmol l−1) and metoprolol (1–100,000 nmol l−1) were applied cumulatively to the organ bath for 30 min at each concentration. Nevertheless, when concentrations of β-blockers are indicated as Ki or 100×Ki (tables or bar graphs), the results are also derived from these cumulative dose-response curves.
β-Adrenoceptor binding studies
β-Adrenoceptors in cardiac tissue were investigated using [125I]-iodocyanopindolol (ICYP) as the radiolabelled ligand (specific activity of 2000 Ci mmol−1). For estimation of total β-adrenoceptor density (Bmax) and dissociation constant (Kd), ICYP saturation curves with eight increasing concentrations of ICYP between 3 and 300 pmol l−1 and 3 μmol l−1 of propranolol for determination of nonspecific binding were used. Cold ligand binding affinity was measured by ligand-ICYP competition curves using 25 pmol l−1 of ICYP to maintain the radioligand concentration at approximate Kd. The assay was performed in a total volume of 250 μl. The total amount of protein used per assay was 20–30 μg. Protein concentrations were determined according to the method of Lowry et al. (1951). The incubation at 25°C for 60 min allowed complete equilibration of the β-adrenoceptors with the radioligand. The reaction was terminated by rapid vacuum filtration through Whatman GF/C filters (Whatman Inc., Clifton, NJ, U.S.A.). The filters were washed immediately three times with 6 ml of ice-cold incubation buffer. All experiments were performed in triplicate.
Statistical analysis
Regression analysis was performed with the computer program GraphPadPrism (GraphPad Software, San Diego, Cal., U.S.A.). For determination of Bmax and Kd of radioligand saturation experiments, linear regression according to the method of Scatchard et al. (1949) was performed. Competition curve slope (pseudo-Hill factor, nH), the concentration at which 50% of the effect was achieved (EC50) (for both binding studies and contraction experiments) and the percentage of receptors in a high affinity (%RH) or low affinity (%RL) state were determined by nonlinear regression analysis, comparing the fitting of the curve to either one or two receptor states by _F_-test analysis. Cold ligand dissociation constants for a high affinity (KH) or a low affinity receptor state (KL) were calculated according to the method of Cheng and Prussoff (1973). Ki values were calculated from EC50 values that were determined by fitting the results of competition experiments with a nonlinear regression analysis assuming only one receptor state, regardless if they actually reflect one or two affinity states.
Unless indicated, the data shown are mean±s.e.mean. For multiple comparisons, ANOVA analysis was performed. Otherwise, statistical significance was analysed with the Mann–Whitney or Wilcoxon test. A value of P<0.05 was considered significant.
Materials
Chemicals were from Sigma Chemical Co. ICYP was produced by Amersham-Buchler (Freiburg i.Br., Germany). All other chemicals were of analytic grade or the best commercially available.
Results
Radioligand binding studies
[125I]-Iodocyanopindolol (ICYP) saturation experiments revealed a 4.7-fold lower β-adrenoceptor density in failing (NYHA IV) compared to nonfailing (NF) hearts (NYHA IV, 18.0±3.4 fmol mg protein−1; NF, 84.3±22.3 fmol mg protein−1; P<0.01) with no significant change in Kd (NF, 56.2 (95% confidence Interval: 29.0–83.33) pmol l−1; NYHA IV, 37.6±23.1–52.2) pmol l−1).
Figure 1 shows representative results from competition experiments of the β-adrenoceptor ligands isoprenaline, metoprolol, carvedilol and bucindolol in human myocardium. Figure 1A represents the binding properties of the agonist isoprenaline. It is characterized by a biphasic binding curve with the identification of a high- and a low-affinity binding site. In the presence of guanylylimidodiphosphate (Gpp(NH)p), a non-hydrolyzable guanine nucleotide, binding becomes monophasic with the detection of only one (low-affinity) binding site. As a consequence, the slope factor (nH) steepens and approaches unity (Tables 1 and 2).
Figure 1.
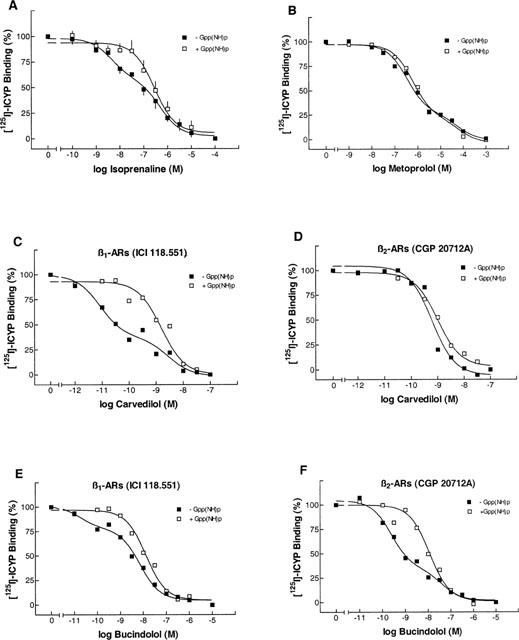
[125I]-ICYP competition curves in human left ventricular myocardium from nonfailing hearts. (A) ICYP-isoprenaline competition in the absence and presence of 100 μmol l−1 of Gpp(NH)p. (B) ICYP-metoprolol competition in the absence and presence of Gpp(NH)p. (C) ICYP-carvedilol competition, both curves in the presence of ICI 118,551 (50 nmol l−1), in the absence and presence of Gpp(NH)p. (D) ICYP-carvedilol competition, both curves in the presence of CGP 20712A (300 nmol l−1), in the absence and presence of Gpp(NH)p. (E, F) ICYP-bucindolol competition, identical conditions like C, D. A, data are the means±s.e.mean from _n_=4 experiments. B–F, data are from one representative experiment. β-ARs, β-adrenoceptors.
Table 1.
Radioligand competition experiments in human nonfailing myocardium
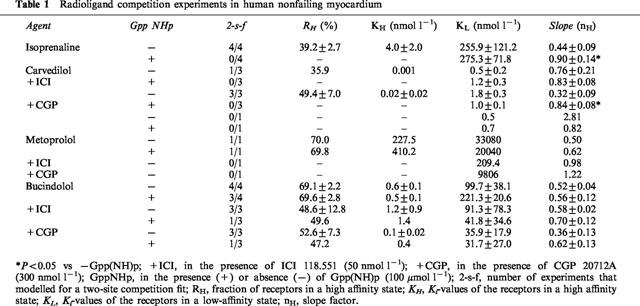
Table 2.
Radioligand competition experiments in human failing myocardium
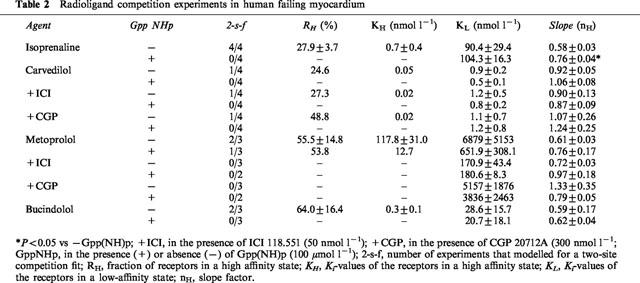
Binding of metoprolol to β-adrenoceptors in human myocardium also revealed two distinct affinity sites (Figure 1B). This biphasic binding is not converted into monophasic binding by the presence of Gpp(NH)p. When adding ICI 118,551 (50 nmol l−1), a highly selective β2-adrenoceptor antagonist, or CGP 20712A (300 nmol l−1), highly selective for β1-adrenoceptors, ligand binding to a homogenous population of β1- or β2-adrenoceptors could be observed, respectively (Tables 1 and 2). The KL values in the presence of ICI 118,551 and CGP 20712A were similar to KH and KL in the absence of these compounds (Tables 1 and 2, Figure 1B). The β1-selectivity of metoprolol was 35-fold.
In two out of seven experiments, carvedilol exerted biphasic binding properties. In both cases, in the presence of Gpp(NH)p the competition curve fitted to a single receptor state (Tables 1 and 2). When looking at β-adrenoceptor subtypes (in the presence of ICI 118,551 or CGP 20712A, respectively), biphasic binding of carvedilol was present in four of seven cases at the β1-adrenoceptor (Figure 1C), but only one of seven cases at the β2-adrenoceptor (1D). In all cases, biphasic binding curves converted into monophasic ones in the presence of Gpp(NH)p. In human myocardium, carvedilol was rather non-selective.
Bucindolol exerted biphasic binding in the absence of Gpp(NH)p, with slope factors substantially less than unity (Tables 1 and 2). In only 50% of the experiments, the computer modeled two-affinity state fit could be converted into a one-affinity state fit by the presence of Gpp(NH)p. Bucindolol identified a rather high percentage of high-affinity binding states (Tables 1 and 2). In the presence of ICI 118,551 and CGP 20712A (Figure 1E,F, Table 1), respectively, these agonist-like binding properties could be observed at β1- as well as at β2-adrenoceptors. The slope factors in the presence of Gpp(NH)p (Tables 1 and 2) indicate that the guanine nucleotides do not sufficiently resolve agonist-like binding properties of bucindolol. In human myocardium, bucindolol was non-selective.
Functional studies
In order to estimate the implications of different β-adrenoceptor binding properties of β-adrenoceptor antagonists on myocardial contractile function, experiments in left ventricular papillary muscle strips from patients with terminal heart failure due to idiopathic or ischaemic dilated cardiomyopathy were performed.
The application of 0.1 μmol l−1 of isoprenaline enhanced basal force of contraction by 101±24% (n_=18). After equilibration, cumulative concentrations of the β-adrenoceptor antagonists bucindolol, carvedilol and metoprolol were added to the organ bath. There was no significant difference among the three groups in basal as well as in isoprenaline-enhanced force of contraction (Table 3). All β-adrenoceptor antagonists significantly antagonized isoprenaline enhanced force of contraction (Figure 2, Table 3). The extent to which force of contraction was reduced significantly differed among the groups. Whereas bucindolol and carvedilol at 100×_Ki (reflecting approximately 100% receptor occupation) reduced force of contraction close to basal values (Figure 2B,C), the negative inotropic effect of metoprolol was substantially more pronounced (P<0.01 vs carvedilol and bucindolol, respectively; Figure 2A).
Table 3.
Effects of β-blockers on cardiac contractility after prestimulation
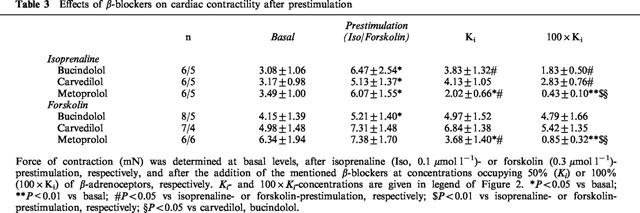
Figure 2.
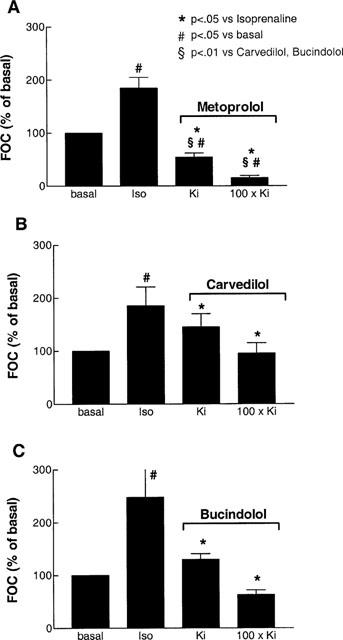
The effects of metoprolol (_n_=6 experiments from n_=5 hearts, A), carvedilol (n_=6/5, B) and bucindolol (n_=6/5, C) at Ki and 100×_Ki on isoprenaline (Iso, 0.1 μmol l−1) enhanced force of contraction (FOC) in human left ventricular myocardium from patients with heart failure. Results are from cumulative dose-response experiments, but only Ki_- and 100×_Ki_-values are indicated as bar graphs. Ki_-values are derived from radioligand binding experiments. The concentrations used in the experiment are 0.01 (Ki) and 1 μmol l−1 (100×_Ki) for bucindolol, 0.001 (Ki) and 0.1 μmol l−1 (100×_Ki) for carvedilol and 1 (Ki) and 100 μmol l−1 (100×_Ki) for metoprolol. These concentrations are used to achieve approximately 50% (Ki) and 100% (100×_Ki) β-adrenoceptor occupation, respectively.
As both carvedilol and bucindolol exert agonist-like binding properties, the coupling of β-adrenoceptors to adenylate cyclase was facilitated by forskolin at a concentration of 0.3 μmol l−1. By this method, intrinsic sympathomimetic activity (ISA) of ligands in human myocardium can be detected (Böhm et al., 1990b). Forskolin increased force of contraction by 54±17% (n_=21). Metoprolol decreased cardiac contractility by 53.4±12.9% and 89.4±2.2% at 50% (Ki) and 100% (100×_Ki) receptor occupation, respectively (Figure 3A P<0.01 vs forskolin). Carvedilol had a negative inotropic effect in six out of seven experiments and a slight positive inotropic effect in only one experiment. The total negative inotropic effect was 6.6±2.5% and 27.1±8.1% at Ki and 100×Ki, respectively (Figure 3C, P<0.05 vs forskolin, P<0.01 vs metoprolol). At concentrations of Ki and 100×Ki, bucindolol decreased force of contraction slightly, but insignificantly (Figure 3E). Bucindolol caused a significant positive inotropic effect in 38% of the muscles (three out of eight), increasing force of contraction by 17.1±8.4% at 100 nmol l−1 (Figure 3F). In the other 62%, bucindolol caused a negative inotropic effect of 36.0±7.0% at the highest concentration. In the presence of bucindolol (100 nmol l−1), the negative inotropic effect of metoprolol could be competitively inhibited (Figure 4).
Figure 3.
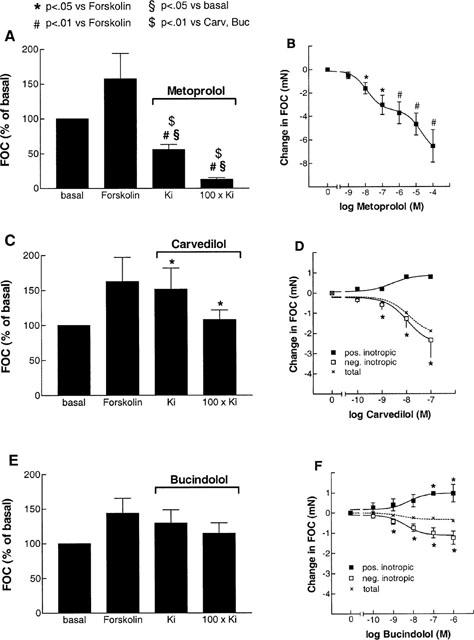
The effects of metoprolol (_n_=6 experiments from six hearts, A, B), carvedilol (Carv, _n_=7/4, C, D) and bucindolol (Buc, n_=8/5, E, F) at Ki and 100×_Ki on forskolin (0.3 μmol l−1) enhanced force of contraction (FOC) in human left ventricular myocardium from patients with heart failure. _Ki_-values are the same as in Figure 2. Bar graphs (A,C,E) give the means of all experiments in per cent of basal FOC at the indicated concentrations. In B,D,F, cumulative concentrations of the respective β-adrenoceptor antagonists are plotted against the change in FOC (mN). In D, F, the total group (dashed line) is divided into one group of muscles with a positive inotropic response to β-blocker (_n_=1 for carvedilol, _n_=3 for bucindolol), and the other group with a negative inotropic response.
Figure 4.
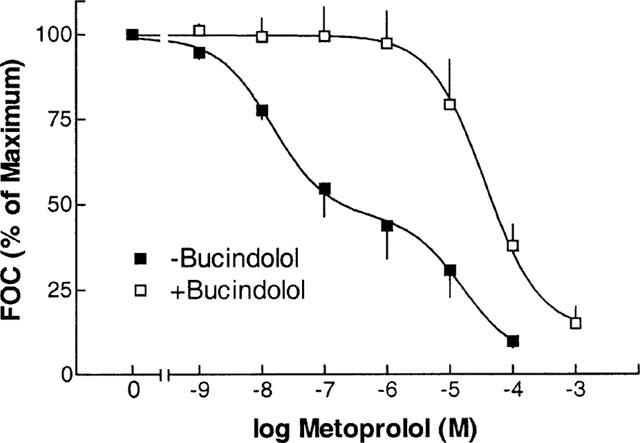
Negative inotropic effect of metoprolol in the presence (+) and absence (−) of bucindolol (0.1 μmol l−1), and in the presence of forskolin (0.3 μmol l−1), respectively. Ordinate: per cent of maximum forskolin-enhanced force of contraction (FOC).
In Figure 5, the effects of 100% receptor occupation by bucindolol on forskolin enhanced FOC is compared to the effects of receptor occupation by carvedilol (Figure 5A) and metoprolol (Figure 5B) in the hearts from the same patients, respectively. There was a close correlation between the effects of bucindolol with the effects of carvedilol (Figure 5A) and metoprolol (Figure 5B).
Figure 5.
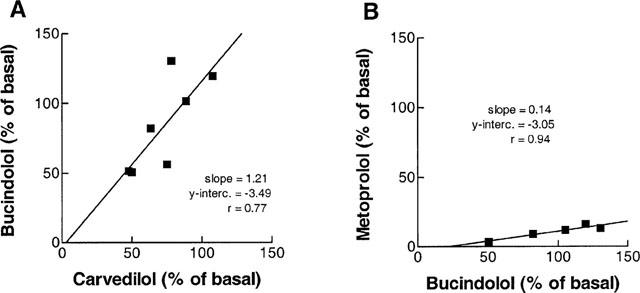
Comparison of intrinsic activity of bucindolol, carvedilol and metoprolol. (A) FOC in per cent of basal values after 100×Ki of carvedilol (abscissa) is plotted against FOC in per cent of basal values after 100×Ki of bucindolol (ordinate) in preparations from the same hearts, after forskolin (0.3 μmol l−1) prestimulation of left ventricular myocardium from patients with heart failure. (B) bucindolol (abscissa) against metoprolol (ordinate), the same conditions as in A.
Discussion
Recent studies in different cell systems have demonstrated that the effects of β-blocking agents on adenylate cyclase stimulation, chronotropy or inotropy are strongly dependent on the examined system. For a number of β-blockers, no definite classification as partial agonist or inverse agonist can be made, since their effects were variable in different systems. In the present study we examined the intrinsic activity of the compounds bucindolol, carvedilol and metoprolol directly in human failing myocardium, since these agents are frequently used in patients with heart failure.
The examination of intrinsic activity in intact human myocardium is complicated by several mechanisms. In myocardium from patients with heart failure, the total amount of β-adrenoceptors is reduced (Bristow et al., 1982), and the remaining receptors are desensitized to agonist stimulation (Hausdorff et al., 1990). In addition, increased membrane concentrations of inhibitory G-protein α-subunits (Böhm et al., 1990a) might also reduce partial agonist or inverse agonist responses. Therefore, in contraction experiments forskolin was added to the organ bath. This diterpene facilitates the coupling of GSα to the catalytic unit of adenylate cyclase, and responses of both partial agonists (Böhm et al., 1990b) and inverse agonists (Mewes et al., 1993) become amplified and detectable.
Using this approach, it could be demonstrated that bucindolol behaved as a partial agonist in 38% of the experiments and as an inverse agonist in 62% of the experiments. Also carvedilol displayed slight partial agonist activity in one experiment. This might be due to different initial activation states of the receptors in the different tissue samples. In β2-adrenoceptor-expressing Sf9 cells, dichloroisoproterenol was shown to act as either a partial agonist or inverse agonist, depending on the degree of isoprenaline-induced desensitization of the system (Chidiac et al., 1996). In Figure 5, the effects of 100% receptor occupation by bucindolol on forskolin enhanced FOC is compared to the effects of receptor occupation by carvedilol (Figure 5A) and metoprolol (Figure 5B) in the hearts from the same patients, respectively. Assuming that the initial state of receptor activation is similar in samples that come from the same tissue, these plots indicate that the activation state of the β-adrenoceptors has an influence on the functional response to the respective ligands. In a study on the pithed rat, bucindolol but not carvedilol behaved as a partial agonist by producing a dose-related increase in heart rate (Willette et al., 1998). This positive chronotropy of bucindolol was also detected in several other in vivo studies (Deitchman et al., 1980; Marwood et al., 1986), whereas in a study on human myocardium, no increase in adenylate cyclase activity or force of contraction in response to bucindolol or carvedilol could be detected (Hershberger et al., 1990). In another study on human atrial myocardium, small amounts of partial agonism of bucindolol, but not carvedilol could be detected after muscle preparations had been depleted of catecholamines (Trochu et al., 1999). In a very recent study on constitutively active mutants of the β1-adrenoceptor, metoprolol displayed inverse agonist activity, whereas carvedilol had partial agonist activity (Lattion et al., 1999). Bucindolol had not been investigated. These variable results, but also the fact that in our study even under identical experimental conditions the intrinsic activity of bucindolol and carvedilol was variable, may be related to different basal activation states of the β-adrenoceptors in the respective tissues.
The effects of metoprolol on cardiac contractility are substantially different to those of bucindolol and carvedilol. In contrast to the latter two agents, metoprolol not only inhibited isoprenaline stimulation, but it further reduced force of contraction to 15% of basal levels. Moreover, when forskolin stimulated cardiac contractility independent from β-adrenoceptor activation, 100% receptor occupation by metoprolol led to nearly complete depression of contractility. Hence, metoprolol is an inverse agonist that primarily stabilizes the inactive conformation of the β-adrenoceptor (R). Consistently, in Sf9 cells transfected with a baculovirus expression system, metoprolol was shown to exert a relatively high amount of inverse agonism at the β2-adrenoceptor compared to carvedilol and bucindolol (Yoshikawa et al., 1996).
The examination of intrinsic activity can be complicated by the presence of contaminating catecholamines in the underlying tissues. The presence of catecholamines could lead to an enhancement of negative inotropic effects of β-adrenoceptor antagonists. However, in experiments with metoprolol in the presence of bucindolol, the combined application of two β-adrenoceptor antagonists would be expected to have additive effects on force of contraction. In contrast, bucindolol is able to inhibit the metoprolol-induced reduction of force of contraction. Thus, the presence of contaminating catecholamines can be ruled out.
The examined β-blockers were shown to affect cardiac β-adrenoceptor regulation in substantially different ways (Gilbert et al., 1993; Yoshikawa et al., 1996). In this study, carvedilol and bucindolol exerted guanine nucleotide modulatable binding at cardiac β-adrenoceptors. Similar results have been obtained by other studies (Yoshikawa et al., 1996; Hershberger et al., 1990; Bristow et al., 1992). In contrast, in our study β-adrenoceptor binding was examined at human ventricular β1- and β2-adrenoceptor subgroups separately by performing experiments in the presence of ICI 118,551 and CGP 20712A, respectively. While for carvedilol, guanine nucleotide modulatable binding had been observed predominantely to β2-adrenoceptors (Bristow et al., 1992), the present study identifies agonist-like binding especially to β1-adrenoceptors. For bucindolol, our study obtains comparable results to those of Hershberger et al. (1990), who also observed complex binding characteristics for bucindolol on both β1- and β2-adrenoceptors.
[125I]-ICYP-metoprolol competition curves were not affected by the presence of Gpp(NH)p. In addition, metoprolol exerted inverse agonism in functional experiments. These results indicate that this compound rather stabilizes the inactive conformation (R) of the β-adrenoceptor. Thus, phosphorylation of the β-adrenoceptor by β-adrenergic receptor kinases and subsequent desensitization or even down-regulation might be effectively prevented. These observations may explain why in vitro models, and also clinical studies observed up-regulation of β-adrenoceptors with consecutive increased haemodynamic response to catecholamine stimulation following metoprolol treatment, but a lack of up-regulation or even further down-regulation of β-adrenoceptors upon treatment with carvedilol and bucindolol (Heilbrunn et al., 1989; Gilbert et al., 1993; Yoshikawa et al., 1996; Böhm et al., 1998).
One possible limitation of the present study could be that it examines effects of β-adrenoceptor antagonists on isolated myocardium. The in vivo effects of these agents could be slightly different. Nevertheless, the study evaluates intrinsic activity of therapeutically relevant β-blockers in intact human myocardium, and thus, might possess some advantage compared to studies in animal or cell models.
The data may be critical to understand differences in regulation of cardiac β-adrenoceptors in patients with heart failure that are treated with bucindolol, carvedilol or metoprolol. Furthermore, different intrinsic activity may provide one explanation for different tolerability or even outcomes due to β-blocker treatment in heart failure. In two recent meta-analyses of the effects of β-blockers on mortality in patients after myocardial infarction, β-blockers with intrinsic sympathomimetic activity (ISA) were associated with a lower risk-reduction compared to agents that lack ISA (Soriano et al., 1997; Freemantle et al., 1999). Xamoterol, a β-blocker with ISA in human myocardium (Böhm et al., 1990b), had detrimental effects on the survival of patients with heart failure (Nicholas et al., 1990). These data indicate that intrinsic activity of β-blockers is of clinical relevance. In general, β-blockers with high intrinsic activity appear to have worse effects on mortality than agents with low intrinsic activity. However, when classifying β-blockers with respect to their intrinsic activity, it should be considered that the effects of the respective agents are very much dependent on the examined system. Thus, it may be difficult to subdivide β-blockers into agents with or without ISA. For human ventricular myocardium, it can be concluded that bucindolol and carvedilol activate the receptor-G-protein complex and display substantial higher intrinsic activity compared to metoprolol. In a part of the experiments, bucindolol even stimulated cardiac contractility, displaying the ability of this agent to behave as a partial agonist in human failing myocardium. Whether this comparable high intrinsic activity may have contributed to the lack of benefit provided by this agent in the BEST-trial (Bristow, 2000) remains to be further elucidated.
Acknowledgments
Experimental work was supported by the Deutsche Forschungsgemeinschaft (to M. Böhm). This work contains parts of the Doctoral Thesis of C. Maack (University of Cologne).
Abbreviations
β-AR
β-adrenergic receptor
Bmax
maximum receptor density
°C
degrees centigrade
EC50
concentration achieving 50% of maximum effect
FOC
force of contraction
Gpp(NH)p
guanylylimidodiphosphate
GS
stimulatory G-protein
ICYP
[125I]-Iodocyanopindolol
ISA
intrinsic sympathomimetic activity
Kd, Ki
dissociation constant
NF
nonfailing
nH
slope factor
NYHA
New York Heart Association
RH
receptors in a high-affinity state
s.e.mean
standard error of the mean
References
- BENOVIC J.L., STANISZEWSKI C., MAYOR F., CARON M.G., LEFKOWITZ R.J. β-Adrenergic receptor kinase. J. Biol. Chem. 1988;263:3893–3897. [PubMed] [Google Scholar]
- BEST STEERING COMMITTEE Design of the Beta-blocker Evaluation Survival Trial (BEST) Am J Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- BÖHM M., ETTELBRÜCK S., FLESCH M., VAN GIELST W.H., KNORR A., MAACK C., PINTO Y.M., PAUL M., TEISMAN A.C.H., ZOLK O. β-Adrenergic signal transduction following carvedilol treatment in hypertensive cardiac hypertrophy. Cardiovasc. Res. 1998;40:146–155. doi: 10.1016/s0008-6363(98)00099-6. [DOI] [PubMed] [Google Scholar]
- BÖHM M., GIERSCHIK P., JAKOBS K.-H., PIESKE B., SCHNABEL P., UNGERER M., ERDMANN E. Increase of Giα in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990a;82:1249–1265. doi: 10.1161/01.cir.82.4.1249. [DOI] [PubMed] [Google Scholar]
- BÖHM M., MITTMANN C., SCHWINGER R.H.G., ERDMANN E. Effects of xamoterol on inotropic and lusitropic properties of the human myocardium and on adenylate cyclase activity. Am. Heart J. 1990b;120:1381–1392. doi: 10.1016/0002-8703(90)90252-s. [DOI] [PubMed] [Google Scholar]
- BOND R.A., LEFF P., JOHNSON T.D., MILANO C.A., ROCKMAN H.A., MCMINN T.R., APPARSUNDARAM S., HYEK M.F., KENAKIN T.P., ALLEN L.F., LEFKOWITZ R.J. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., GINSBURG R., MINOBE W., CUBICIOTTI R.S., SAGEMAN W.S., LURIE K., BILLINGHAM M.E., HARRISON D.E., STINSON E.B. Decreased catecholamine sensitivity and β-adrenergic receptor density in failing human hearts. N. Engl. J. Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., LARRABEE P., MINOBE W., RODEN R., SKERL L., KLEIN J., HANDWERGER D., PORT J.D., MÜLLER-BECKMANN B. Receptor pharmacology of carvedilol in the human heart. J. Cardiovasc. Pharmacol. 1992;19 Suppl.1:S68–S80. doi: 10.1097/00005344-199219001-00014. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzyme reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIDIAC P., NOUET S., BOUVIER M. Agonist-induced modulation of inverse agonist efficacy at the β2-adrenergic receptor. Mol. Pharmacol. 1996;50:662–669. [PubMed] [Google Scholar]
- CIBIS II INVESTIGATORS AND COMMITTEES The Cardiac Insufficiency Bisoprolol Study (CIBIS-II): a randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- DE LEAN A., STADEL J.M., LEFKOWITZ R.J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- DEITCHMAN D., PERHACH J.L., SNYDER R.W. Beta-adrenoceptor and cardiovascular effects of MJ 13105 (bucindolol) in anesthetized dogs and rats. Eur. J. Pharmacol. 1980;61:263–277. doi: 10.1016/0014-2999(80)90128-4. [DOI] [PubMed] [Google Scholar]
- FREEMANTLE N., CLELAND J., YOUNG P., MASON J., HARRISON J. β-Blockade after myocardial infarction: systematic review and meta regression analysis. Brit. J. Med. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT E.M., OLSEN S.L., RENLUND D.G., BRISTOW M.R. Beta-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1993;71:23C–29C. doi: 10.1016/0002-9149(93)90083-o. [DOI] [PubMed] [Google Scholar]
- HASH T.W., PRISANT L.M. β-Blocker use in systolic heart failure and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1997;37:7–19. doi: 10.1177/009127009703700103. [DOI] [PubMed] [Google Scholar]
- HAUSDORFF W.P., CARON M.G., LEFKOWITZ R.J. Turning off the signal: Desensitization of β-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- HEILBRUNN S.M., SHAH P., BRISTOW M.R., VALANTINE H.A., GINSBURG R., FOWLER M.B. Increased β-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation. 1989;79:483–490. doi: 10.1161/01.cir.79.3.483. [DOI] [PubMed] [Google Scholar]
- HERSHBERGER R.E., WYNN J.R., SUNDBERG L., BRISTOW M.R. Mechanisms of action of bucindolol in human ventricular myocardium. J. Cardiovasc. Pharmacol. 1990;15:959–967. doi: 10.1097/00005344-199006000-00014. [DOI] [PubMed] [Google Scholar]
- JASPER R.J., MICHEL M.C., INSEL P.A. Molecular mechanism of β-adrenergic receptor blockers with intrinsic sympathomimetic activity. FASEB J. 1988;2:2891–2894. doi: 10.1096/fasebj.2.13.2901994. [DOI] [PubMed] [Google Scholar]
- LATTION A.L., ABUIN L., NENNIGER-TOSATO M., COTECCHIA S. Constitutively active mutants of the β1-adrenoceptor. FEBS Letts. 1999;457:302–306. doi: 10.1016/s0014-5793(99)01064-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARWOOD J.F., STOKES G.S. Studies on the vasodilator actions of bucindolol in the rat. Clin. Exper. Pharmacol. Physiol. 1986;13:59–68. doi: 10.1111/j.1440-1681.1986.tb00316.x. [DOI] [PubMed] [Google Scholar]
- MERIT-HF STUDY GROUP Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- MEWES T., DUTZ S., RAVENS U., JAKOBS K.H. Activation of calcium currents in cardiac myocytes by empty beta-adrenoceptors. Circulation. 1993;88:2916–2922. doi: 10.1161/01.cir.88.6.2916. [DOI] [PubMed] [Google Scholar]
- NICHOLAS G., OAKLEY C., POULEUR H., ROUSSEAU M.F., RYDÉN L.E., WELLENS H., for The Xamoterol In Heart Failure Study Group Xamoterol in severe heart failure. Lancet. 1990;336:1–6. [Google Scholar]
- PACKER M., BRISTOW M.R., COHN J.N., COLUCCI W., FOWLER M.B., GILBERT E.M., SHUSTERMAN N.H., for the US Carvedilol Heart Failure Study Group The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- SAMANA P., COTECCHIA S., COSTA T., LEFKOWITZ R.J. A mutation-induced activated state of the β2-adrenergic receptor. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- SCATCHARD G. The attractions of proteins for small molecules and ions. Ann. N.Y. Acad. Sci. 1949;51:660–672. [Google Scholar]
- SORIANO J.B., HOES A.W., MEEMS L., GROBBEE D.E. Increased survival with β-blockers: importance of ancillary properties. Progress in Cardiovascular Diseases. 1997;39:445–456. doi: 10.1016/s0033-0620(97)80039-4. [DOI] [PubMed] [Google Scholar]
- TROCHU J.N., ERFANIAN M., KHANDOUDI N., BARON O., BRIL A., GAUTHIER C. Carvedilol produces contractile effects different from those of bucindolol in human atria Circulation 1999100I-439(abstract) [Google Scholar]
- WILLETTE R.N., MITCHELL M.P., OHLSTEIN E.H., LUKAS M.A., RUFFOLO R.R.. , JR Evaluation of intrinsic sympathomimetic activity of bucindolol and carvedilol in rat heart. Pharmacology. 1998;56:30–36. doi: 10.1159/000028179. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA T., PORT J.D., ASANO K., CHIDIAC P., BOUVIER M., DUTCHER D., RODEN R.L., MINOBE W., TREMMEL K.D., BRISTOW M.R. Cardiac adrenergic receptor effects of carvedilol. Eur. Heart. J. 1996;17 Suppl. B:8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]