Two modes of FEN1 binding to PCNA regulated by DNA (original) (raw)
Abstract
The FEN1 nuclease functions during Okazaki fragment maturation in the eukaryotic cell. Like many other proliferating cell nuclear antigen (PCNA)-binding proteins, FEN1 interacts with the interdomain connector loop (IDCL) of PCNA, and PCNA greatly stimulates FEN1 activity. A yeast IDCL mutant pcna-79 (IL126,128AA) failed to interact with FEN-1, but, surprisingly, pcna-79 was still very active in stimulating FEN1 activity. In contrast, a C-terminal mutant pcna-90 (PK252,253AA) showed wild-type binding to FEN1 in solution, but poorly stimulated FEN1 activity. When PCNA was loaded onto a DNA substrate coupled to magnetic beads, it stabilized retention of FEN1 on the DNA. In this DNA-dependent binding assay, pcna-79 also stabilized retention of FEN1, but pcna-90 was inactive. Therefore, in the absence of DNA, FEN1 interacts with PCNA mainly through the IDCL. However, when PCNA encircles the DNA, the C-terminal domain of PCNA rather than its IDCL is important for binding FEN1. An FF→GA mutation in the PCNA-interaction domain of FEN1 severely decreased both modes of interaction with PCNA and resulted in replication and repair defects in vivo.
Keywords: DNA replication/FEN1/PCNA/RAD27
Introduction
FEN1 is a 5′–3′ flap exo-/endonuclease that plays an important role in multiple DNA metabolic processes. In DNA replication, it is required for Okazaki fragment maturation where it participates in the removal of RNA–DNA primers (for a review see Bambara et al., 1997). In DNA repair processes, FEN1 is required for non-homologous end joining of double-stranded DNA breaks and for long-patch base excision repair (Klungland and Lindahl, 1997; Kim et al., 1998; Wu et al., 1999). In the yeast Saccharomyces cerevisiae, FEN1 is encoded by the RAD27 (RTH1) gene. Genetic studies have shown that the gene is dispensable for growth; however, RAD27 deletion strains (_rad27_Δ) are temperature sensitive for growth and show a terminal phenotype consistent with a defect in DNA replication (Reagan et al., 1995; Sommers et al., 1995). Repetitive sequences are destabilized in the deletion strains and they are strong mutators (Johnson et al., 1995; Tishkoff et al., 1997b; Freudenreich et al., 1998; Kokoska et al., 1998; Schweitzer and Livingston, 1998). _rad27_Δ strains are also defective in telomere maintenance and sensitive to DNA damage (Reagan et al., 1995; Sommers et al., 1995; Parenteau and Wellinger, 1999). The lesions that accumulate in the absence of FEN1 require homologous recombination for repair as RAD27 deletion results in poor growth or lethality in recombin ation-defective backgrounds (Tishkoff et al., 1997b; Johnson et al., 1998; Symington, 1998). Some, but not all of the _rad27_Δ phenotypic defects are suppressed by overexpression of the EXO1 gene, which encodes a related nuclease (Tishkoff et al., 1997a; Parenteau and Wellinger, 1999).
The replication clamp proliferating cell nuclear antigen (PCNA) was identified initially as a factor required for in vitro SV40 DNA replication, and as a processivity factor for DNA polymerase δ (Pol δ) (Tan et al., 1986; Prelich et al., 1987). PCNA also interacts with FEN1 and stimulates its nuclease activity (Li et al., 1995; Chen et al., 1996). PCNA–FEN1 interaction domains have been localized to the interdomain connector loop (IDCL) of PCNA and the C-terminus of FEN1 (Warbrick et al., 1997; Jonsson et al., 1998). This interaction is analogous to that between the human replication inhibitor p21 and PCNA. FEN1 competes with p21 for binding to PCNA (Waga et al., 1994; Chen et al., 1996; Gulbis et al., 1996; Warbrick et al., 1997). The interaction between PCNA and p21 has been studied in detail by mutational analysis. The amino acids in p21 important for binding to PCNA localize to a short octapeptide motif: Q1XXM4XXF7Y8 (Nakanishi et al., 1995; Warbrick et al., 1995). The crystal structure of the complex between human PCNA and a p21 peptide shows extensive interactions through the formation of a β-sheet between the peptide and the IDCL of PCNA (Gulbis et al., 1996). Ile126 and Leu128 in the IDCL are part of a hydrophobic cavity into which Met4 and Tyr8 of the p21 motif are inserted. The cavity is closed off by Phe7. Mutation of Met4 or Tyr8 in the PCNA-binding motif to alanine eliminates binding of p21 to PCNA, whereas mutation of Gln1 or Phe7 severely reduces binding. Conversely, mutations in the hydro phobic core of the IDCL of PCNA eliminate binding to p21 (Jonsson et al., 1998; Oku et al., 1998).
The PCNA-binding motif (Q1XX[ILM]4XXF7[FY]8) has been identified in a large number of proteins involved in DNA metabolic processes ranging from DNA methylation, base excision repair, nucleotide excision repair, mismatch repair, DNA replication, chromatin assembly and cell cycle control (reviewed in Warbrick, 1998; Tsurimoto, 1999). In general, the motif is found either at the N- or the C-terminus, frequently at the extreme terminus of the polypeptide. For yeast FEN1, the sequence QGRLDGFF is located at amino acids 340–347 of the 382 amino acid polypeptide. In the archaebacterium Pyrococcus furiosus, a FEN1 analog has been identified with the motif (QSTLESWF) at amino acids 330–337 of the 340 amino acid polypeptide (Figure 1B). The crystal structure of the protein shows that this motif is at the end of an α-helix that extends away from the folded protein, allowing easy docking to the IDCL of PCNA, and insertion of the hydrophobic amino acids into the hydrophobic cavity at the IDCL of PCNA (Hosfield et al., 1998). As FEN1 from yeast and P.furiosus show 40% identity over the entire amino acid sequence, and 70% identity in the C-terminal helical domain containing the PCNA-binding motif, it is likely that the structure of yeast FEN1 is closely similar to that from the archaebacterium.
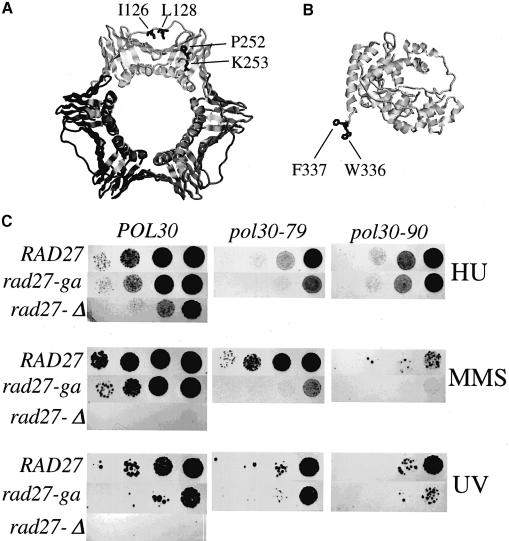
Fig. 1. Structure of PCNA and FEN1 and genetic analysis of mutants. (A) Structure of S.cerevisiae PCNA and the location of the mutations used in this study (shown in only one subunit of the homotrimer); pcna-79, IL126,128AA; and pcna-90, PK252,253AA (Krishna et al., 1994). (B) Structure of P.furiosus FEN1 and the location of the amino acids (W336 and F337) analogous to those mutated in yeast fen1-ga (FF346,347AA) (Hosfield et al., 1998). (C) Sensitivity of yeast strains to hydroxyurea and DNA-damaging agents. Serial 10-fold dilutions were made of the single or double mutants or of the isogenic wild-type yeast strain. Approximately 102, 103, 104 and 105 cells were spotted on YPDA plates containing 100 mM hydroxyurea (HU) or 0.005% MMS, or on YPDA plates and irradiated with 100 J/m2 of UV light and grown at 30°C for 3–4 days.
In preliminary investigations of the interactions between PCNA and FEN1, we observed that a PCNA mutant in which the hydrophobic character of the IDCL had been affected (pcna-79: IL126,128AA; Figure 1A) was still able to stimulate FEN1 activity. Surprisingly, however, a C-terminal PCNA mutant (pcna-90: PK253,254AA) was inactive in this assay (Eissenberg et al., 1997). In order to arrive at a mechanistic understanding of these paradoxical results, we investigated the DNA-independent and DNA-dependent interactions between PCNA and FEN1 and their respective mutants, and the functional consequences of these interactions. Our data indicate that the PCNA-binding motif of FEN1 mediates binding to the IDCL of PCNA in the absence of DNA. However, once PCNA encircles the DNA, proper function of FEN1 requires interaction with the C-terminus rather than the IDCL of PCNA.
Results
Mutation of the PCNA-binding motif of FEN1 shows in vivo defects
Studies with human FEN1 have localized the PCNA-binding motif, QxxLxxFF, to the C-terminus of the protein (Warbrick et al., 1997). The two phenylalanine residues within this motif are essential for FEN1 interaction with PCNA. In order to evaluate the role of this motif, the two phenylalanine residues within the conserved motif were mutated to a glycine and an alanine (FF346,347GA, rad27-ga) by site-directed mutagenesis of the yeast gene for FEN1, RAD27. As will be shown below, this mutant interacts with PCNA with >100-fold reduced affinity.
To examine the effects of the mutation in vivo, we constructed a set of isogenic haploid yeast strains in which the chromosomal copy of the RAD27 gene was disrupted and the strains were complemented with a plasmid containing either the wild-type RAD27 allele or the mutant allele (rad27-ga). Previous studies have shown that deletion of RAD27 results in conditional lethality, the mutant failing to grow at 37°C (Reagan et al., 1995; Sommers et al., 1995). No growth defects were detected in the rad27-ga mutant at any temperature, nor was the mutant sensitive to growth in the presence of the replication inhibitor hydroxyurea (data not shown and Figure 1C). In addition, although the PCNA-interaction mutant did show an increased sensitivity to DNA-damaging agents, it was much less sensitive than the deletion mutant.
Previously, we had shown that PCNA mutants with mutations in the IDCL (pol30-79, IL126,128AA) or in the C-terminus (pol30-90, PK252,253AA) are inviable in a _rad27_Δ background (Figure 1A) (Eissenberg et al., 1997). When combined with the rad27-ga mutation, the double mutant strains were viable. They were slightly more sensitive to hydroxyurea or UV irradiation than either the single POL30 or RAD27 mutants. However, both double mutants showed a greatly increased sensitivity to methylmethane sulfonate (MMS) (Figure 1C). These data indicate a minor repair defect in the rad27-ga mutant which is strongly enhanced in the pol30-79 and pol30-90 mutants.
Bi-molecular interaction between FEN1 and PCNA
Wild-type and mutant FEN1 were expressed in Escherichia coli strain BL21 (DE3) under the control of the inducible bacteriophage T7 promoter system, and purified as described before (Harrington and Lieber, 1994). Both preparations were essentially homogeneous (data not shown).
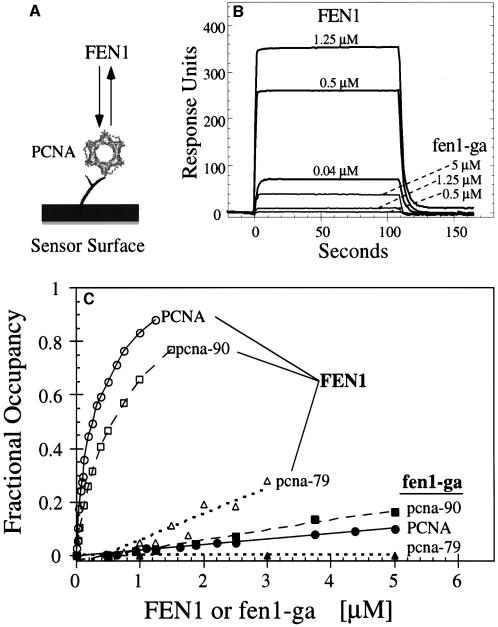
Surface plasmon resonance was used to quantitate the interaction between PCNA and FEN1 and mutants in either protein (Figure 2A). The measurements were carried out under similar solution conditions, i.e. 8 mM MgCl2 and 125 mM NaCl, as are used in the PCNA-dependent FEN1 nuclease activity assays (see below). PCNA was attached to a dextran chip and increasing concentrations of FEN1 were flowed across the chip. Rapid binding, a steady-state equilibrium and rapid dissociation were observed (Figure 2B). The mutant fen1-ga only showed measurable binding to the PCNA–chip at >100-fold higher concentrations, indicating that the two phenylalanines in the PCNA-binding motif are crucial for binding to PCNA (compare 0.04 µM FEN1 with 5 µM fen1-ga in Figure 2B). The binding studies were repeated with chips to which pcna-79 (IL126,128AA) and pcna-90 (PK252,253AA) had been bound to a similar density as wild-type PCNA (Figure 2C). In agreement with all previous studies suggesting the importance of the hydrophobic pocket made by Ile126 and Leu128 in the IDCL region, the pcna-79–chip bound FEN1 very poorly and was completely defective for binding fen1-ga (Figure 2C). On the other hand, the mutations in the C-terminus in pcna-90 did not greatly affect binding of FEN1 or fen1-ga in comparison with wild-type PCNA.
Fig. 2. Binding of FEN1 to PCNA by surface plasmon resonance. (A) Schematic of sensor chip with PCNA immobilized on the chip surface. Either wild-type PCNA, pcna-79 or pcna-90 was immobilized on the surface of a dextran B1 chip, and increasing concentrations of FEN1 or fen1-ga were passed over the chip. (B) Sensorgrams of the response of the indicated concentrations of either FEN1 or fen1-ga to immobilized PCNA. (C) Concentration–response curves for binding of FEN1 or fen1-ga to chips loaded with either PCNA (600 RU), pcna-79 (570 RU) or pcna-90 (440 RU). The data were recalculated to fractional occupancies to correct for differences in loading densities of the three PCNAs to the chip. Open symbols, FEN1 passed over the PCNA chips; filled symbols, fen1-ga passed over the chips.
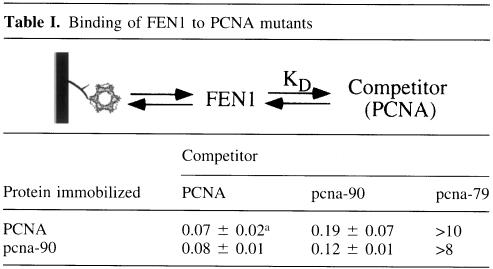
Quantitative determinations of _K_D values are difficult to obtain by direct binding measurements because of chip surface effects. Because the interaction between PCNA and FEN1 is in rapid equilibrium on the time scale of the measurement (see Figure 2B), competition binding experiments can be carried out to obtain true solution _K_D values (Table I). Binding of FEN1 to the PCNA–chip was carried out in the presence of increasing concentrations of competitor PCNA, pcna-79 or pcna-90, and the same set of experiments was carried out with the pcna-90–chip (Table I). Virtually identical results were obtained with either chip. The calculated _K_D value for the FEN1–PCNA interaction is 0.07–0.08 µM whereas for FEN1-pcna-90 it is 0.12–0.17 µM. These results are in agreement with the direct binding studies in Figure 2C. pcna-79 competed very poorly for binding of FEN1 to the PCNA–chip. A lower limit of 10 µM was estimated for the FEN1–pcna-79 interaction (Table I). Binding of fen1-ga to the PCNA chip was too low for quantitative determination of _K_D values.
Table I. Binding of FEN1 to PCNA mutants.
PCNA-dependent nuclease activity of FEN1
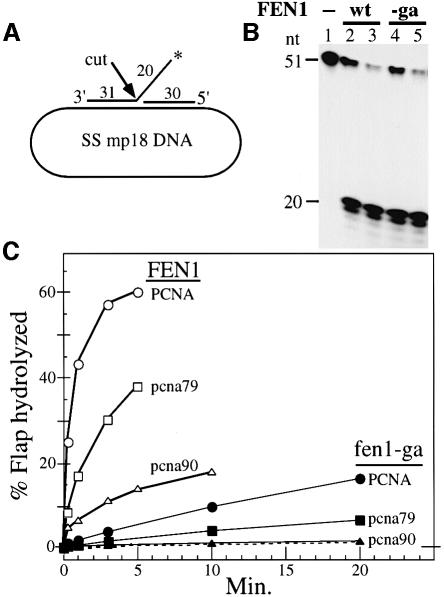
At low salt concentrations, FEN1 displays nuclease activity in the absence of PCNA. However, in the presence of physiological salt concentrations, FEN1 activity requires the presence of PCNA (Li et al., 1995). FEN1 nuclease activity was determined in a FLAP cutting assay in which a 51mer oligonucleotide with 20 nucleotides of 5′-non-complementary DNA and a 5′-adjacent 30mer were hybridized to single-stranded mp18 DNA (Figure 3A). FEN1 cutting at the junction released a labeled 20mer, which was quantitated by denaturing urea–polyacrylamide electrophoresis (Figure 3B) (Harrington and Lieber, 1994). With 10 mM NaCl in the assay, the activity of fen1-ga was identical to that of wild-type FEN1, indicating that mutation of the PCNA-interaction domain did not affect the nuclease activity of FEN1 (Figure 3B).
Fig. 3. The C-termini of both PCNA and FEN1 are essential for FLAP cleavage at 125 mM NaCl. (A) Diagram of the DNA FLAP substrate. The position of the label is indicated by the asterisk on the FLAP strand. (B) Activity of FEN1 and fen1-ga in low salt conditions. FLAP substrate (10 fmol) was coated with 1 µg of SSB and incubated in assay buffer + 10 mM NaCl with no enzyme (lane 1), 40 or 120 fmol of FEN1 (lanes 2 and 3) or 40 or 120 fmol of fen1-ga (lanes 4 and 5). After incubation at 30°C for 3 min, the reaction products were analyzed by 15% urea–PAGE as described in Materials and methods. The mobility of the cleaved products is 19 and 20 nucleotides. (C) Stimulation of FEN1 activity by PCNA. Assays were performed in 100 µl reactions in FEN1 assay buffer + 125 mM NaCl. The FLAP substrate (10 fmol) was incubated with 1 µg of SSB, 1.4 pmol of PCNA or mutant PCNA, 2 pmol of RF-C and 100 µM ATP at 30°C for 30 s and cooled to 13°C. A 130 fmol concentration of either FEN1 or fen1-ga was added to the reaction and incubation continued at 13°C. At different time intervals, 15 µl aliquots were removed and analyzed by 15% urea–PAGE. The percentage of 5′-labeled cleavage products released was quantitated in a phosphoimager. Open symbols, assays with FEN1; filled symbols, assays with fen1-ga. The dashed line represents background activity of FEN1 in the absence of PCNA.
As shown previously, the interaction between PCNA and FEN1 functionally results in a stimulation of FEN1 activity by PCNA (Li et al., 1995). In order for this stimulation to occur at all, it is necessary that PCNA encircles the DNA. In the present study, PCNA-dependent FEN1 activity was determined at 125 mM NaCl, under which conditions FEN1 alone is inactive (dashed line in Figure 3C). The PCNA-dependent FEN1 assay also requires replication factor C (RF-C) and ATP to load PCNA at the FLAP junction, and a single-stranded DNA-binding protein (SSB), which blocks non-productive association of RF-C and FEN1 to single-stranded DNA. In our studies, identical results were obtained with either E.coli SSB or yeast replication protein A (RP-A).
In the assay in Figure 3C, PCNA was loaded onto the FLAP substrate (Figure 3A) by RF-C and ATP prior to addition of FEN1 at t = 0 min. A rapid cleavage of the substrate was observed even at the reaction temperature of 13°C. Surprisingly, pcna-79, which showed a severe defect in binding FEN1 in the absence of DNA substrate (Figure 2C), still showed ∼40% activity in stimulating FEN1 activity. Conversely, pcna-90, which showed almost no defect in FEN1 binding, was much less active, with ∼5% of the wild-type reaction rate. Stimulation of fen1-ga activity by PCNA was greatly reduced (0.7% of wild-type) but still measurable (Figure 3C). Similarly, pcna-79 stimulated fen1-ga activity at a low but detectable level, whereas no stimulation of fen1-ga by pcna-90 was observed (Figure 3C). These experiments were carried out at 13°C to allow for easier measurements of reaction rates, but a similar pattern of activities was observed at 30°C (data not shown and Figure 4).
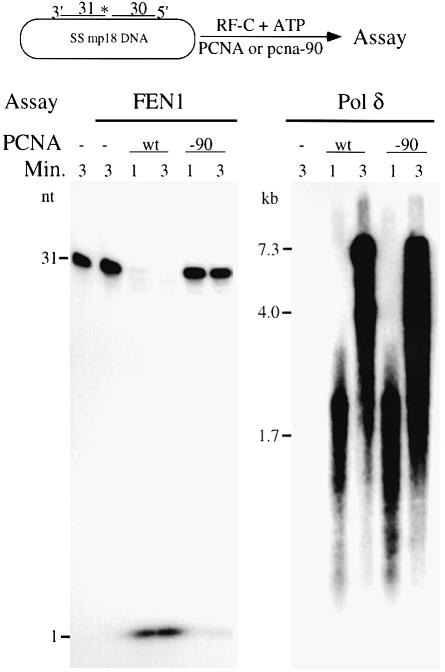
Fig. 4. Pcna-90 is active for stimulation of Pol δ but inactive for FEN1. The nick substrate as described in Materials and methods was used. The 31mer was 5′-32P-labeled. For each 30 µl assay, 10 fmol of DNA substrate were coated with 6 pmol of yeast RPA and incubated with 1 pmol of PCNA or pcna-90 as indicated, 1 pmol of RF-C and 100 µM ATP at 30°C for 30 s. Either 0.5 pmol of FEN1 and 125 mM NaCl (left panel) or 50 fmol of Pol δ together with 100 µM each of dGTP, dCTP and dTTP, 10 µM [α-32P]dATP and 75 mM NaCl (right panel) were added and 14 µl aliquots were removed after 1 and 3 min and analyzed by 15% urea–PAGE (left panel) or by 1% alkaline agarose gel electrophoresis (right panel). Controls contained no enzyme or no PCNA (lanes 1 and 2, left panel), or no PCNA (lane 1, right panel).
The inability of pcna-90 to stimulate FEN1 activity could result from the inability of pcna-90 to interact with FEN1 when loaded onto the DNA. It could be some function of the particular DNA substrate used in these assays or it could be due to improper loading of pcna-90 on these or any DNA substrates. We will address the latter possibilities first. The substrate preference of mammalian FEN1 has been studied extensively. FEN1 shows the greatest activity on substrates containing a FLAP or a nick, and this preference remains unchanged in the presence of PCNA (Harrington and Lieber, 1994; Tom et al., 2000). Mutant pcna-90 failed to stimulate FEN1 activity at a FLAP or at a nick, or on the gapped substrates (data not shown and Figure 4).
Previous studies with the C-terminal mutant pcna-90 have shown that the mutant clamp is loaded appropriately onto singly primed DNA and interacts with Pol δ for processive DNA replication (Eissenberg et al., 1997). The activity of pcna-90 was re-examined on a partial duplex DNA molecule containing a nick, which serves as a dual substrate both for replication by Pol δ and for cutting by FEN1 (Figure 4). Replication assays with Pol δ showed that pcna-90 was as efficient as PCNA in processive DNA synthesis, yet failed to stimulate FEN1 activity.
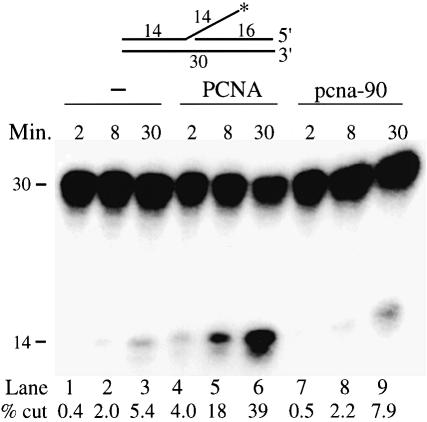
The ATP-dependent loading of PCNA by RF-C involves a highly orchestrated series of steps including ATP binding, clamp opening, template–primer localization, clamp closure, ATP hydrolysis and RF-C dissociation. It is possible that, in the pcna-90 mutant, this process is disturbed in such a manner that the interaction with FEN1 but not with Pol δ is affected, e.g. failure of RF-C to dissociate might affect FEN1 binding but not Pol δ binding. Therefore, we also determined stimulation of FEN1 by PCNA in a reaction which bypassed the requirement for RF-C. In the absence of the clamp loader, PCNA can load, albeit inefficiently, onto a linear double-stranded DNA molecule by sliding onto the end (Burgers and Yoder, 1993; Li et al., 1995; Wu et al., 1996). In this end-loading assay, PCNA stimulated FEN1 activity ∼8-fold (Figure 5). In contrast, stimulation by pcna-90 was only 1.5-fold (compare lanes 1–3 with 7–9). These series of experiments indicate that when pcna-90 encircles the DNA, its functional interaction with FEN1 is affected regardless of the presence of RF-C.
Fig. 5. pcna-90 does not stimulate FEN1 activity on an oligonucleotide FLAP. The substrate used in the assay has been described (Li et al., 1995). The assay was performed in a 50 µl reaction containing 50 mM Tris–HCl pH 8.1, 10 mM MgCl2, 1 mM DTT, 0.5 mg/ml BSA, 25 mM NaCl, 50 fmol of FLAP substrate, 100 fmol of FEN1 and either no PCNA, 5.7 pmol of PCNA or 5.7 pmol of pcna-90 as indicated. After various times at 30°C, 15 µl aliquots were analyzed by 15% urea–PAGE. The percentage of FLAP cut was quantitated and is given below each lane.
pcna-90 complexed with DNA does not bind FEN1
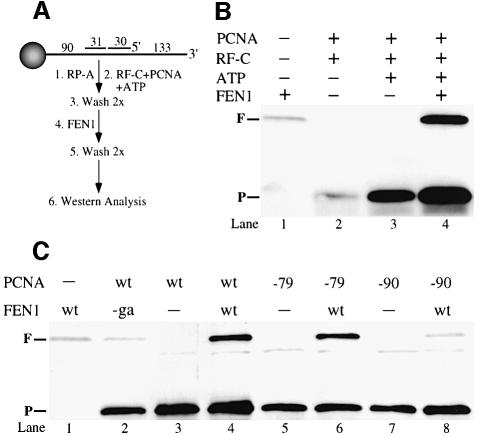
The studies so far suggest that in the absence of DNA, FEN1 binds primarily to the IDCL of PCNA, but that the binding shifts to the C-terminal region of PCNA when both factors are in a complex with DNA. One way to demonstrate such an interaction of FEN1 and PCNA on the DNA would be by showing that PCNA stabilizes FEN1 binding to the DNA. Recently, a bead assay has been applied to measuring PCNA loading, and we adapted that assay for measuring PCNA–FEN1 interactions on the DNA (Waga and Stillman, 1998). Magnetic beads with a 286 nucleotide DNA substrate attached were used for our studies (Figure 6A).
Fig. 6. pcna-79, but not pcna-90 stabilizes binding of FEN1 on DNA. (A) Outline of the assay. The DNA was attached to magnetic beads via a biotin–streptavidin linkage. The nick substrate was incubated in a stepwise fashion with the indicated proteins and processed for immunoblot analysis. (B) Binding of PCNA requires ATP (lanes 2 and 3) and binding of FEN1 requires PCNA (lanes 1 and 4). The positions of FEN1 (F) and PCNA (P) are indicated. (C) Stable complexes are not formed with fen1-ga (lane 2), nor with pcna-90 (lane 8). For details see Materials and methods and Results.
The specificity of the binding assay is presented in Figure 6B. Incubation of the DNA–beads with FEN1 gave low level binding (0.5–1 fmol of FEN1/30 fmol of DNA substrate, lane 1). Similarly, little or no PCNA was retained on the beads when ATP was omitted (lane 2), indicating that PCNA is loaded properly on the substrate attached to the beads. Incubation of the beads with RF-C, PCNA and ATP resulted in the binding of ∼8–10 fmol of PCNA/30 fmol of substrate (lane 3). Upon further addition of FEN1, both proteins were retained efficiently on the beads in approximately equimolar quantities (10–13 fmol of PCNA and 8–12 fmol of FEN1 in three independent experiments). In the presence of FEN1, we consistently observed that 20–30% more PCNA bound to the beads, perhaps indicating a mutual stabilization of PCNA and FEN1 on the DNA (Figure 6B and C, compare lanes 3 and 4). This complex was stable to multiple washes with wash buffer containing 125 mM NaCl. As expected, PCNA did not stabilize binding of mutant fen1-ga to DNA (Figure 6C, lane 2).
Loading of the mutant PCNAs by RF-C and ATP, or their retention on the DNA during the wash steps, was slightly less efficient than for wild-type PCNA, resulting in a signal of 6–8 fmol of pcna-79 or pcna-90 bound per 30 fmol of DNA substrate (Figure 6C, compare lanes 5 and 7 with lane 3). Nevertheless, the ability of the mutant PCNAs to stabilize FEN1 was in accord with the activity data (Figure 3C): pcna-79 efficiently retained FEN1 whereas pcna-90 retained only background levels of FEN1 (compare lanes 6 and 8 with lane 1).
Discussion
Distinct domains exist on PCNA for interaction with FEN1
A summary of the interations and activities determined in this study is given in Table II. Several previous studies have provided a consistent body of evidence that proteins with a consensus PCNA-binding motif (Q1XX[ILM]4XXF7[FY]8) interact with the IDCL of PCNA. In the particular case of FEN1, the bi-molecular interaction between the two proteins is disrupted by mutations in the IDCL in pcna-79 (Figure 2) (Jonsson et al., 1998). Conversely, mutations in the PCNA-binding motif of FEN1 drastically reduce binding to PCNA (Figure 2) (Gary et al., 1999). The functional analysis of FEN1 mutants with mutations in the PCNA-binding domain underscores the importance of this interaction, as these mutants are defective in PCNA-dependent assays (Figure 3) (Gary et al., 1999).
Table II. Summary of interactions between PCNA and FEN1.
| | No DNA | On the DNA | | | | | | | | ----------- | ---------- | ------------------ | ------- | ---- | ------- | ---- | | | | Bindinga | Bindingb | Nuclease activityc | | | | | | | | FEN1 | fen1-ga | FEN1 | fen1-ga | FEN1 | fen1-ga | | | | PCNA | 100 | 0.4 | 100 | <10 | 100 | 0.7 | | | pcna-79 | 1 | <0.1 | 80 | n.d. | 40 | 0.3 | | | pcna-90 | 60 | 0.6 | <10 | n.d. | 5 | <0.1 | |
Surprisingly, however, we found that the binding to activity correlation did not extend to the PCNA mutants. Although binding of pcna-79 to FEN1 was severely affected, the mutant protein still showed a robust activity in a PCNA-dependent FLAP-cutting assay (Figure 2C). Even more surprising, however, was the observation that the C-terminal mutant pcna-90, which bound FEN1 with close to wild-type affinity (Table I), was functionally inactive (Figures 3C, 4 and 5). Those experiments showed that the defect in pcna-90 was not due to loading or its stability on the DNA once loaded, but specifically in FEN1 stimulation. These paradoxical results suggested to us that the protein–protein contacts between FEN1 and PCNA are distinctly different in solution from when the proteins are in a complex with DNA. Therefore, we developed a binding assay in which binding of PCNA and FEN1 to the DNA substrate immobilized on beads could be measured directly, rather than inferred from its subsequent catalytic competence (Figure 6). Very recently, a band-shift assay was employed to show that PCNA stabilizes FEN1 on a DNA substrate (Tom et al., 2000).
The bead assay measures the retention of FEN1 on DNA attached to magnetic beads. Efficient retention requires PCNA that is appropriately loaded by RF-C and ATP. In this assay, pcna-90 was loaded onto the DNA but it failed to retain FEN1, providing direct evidence that the functional defect is due to a defective interaction between the two proteins on the DNA. Very importantly, the IDCL mutant pcna-79, which failed to interact with FEN1 in solution, did stabilize FEN1 on the DNA, indicating that positive interactions exist when the proteins are bound to DNA (Figure 6C).
In vivo studies support the in vitro interaction studies
Our genetic studies with the PCNA–FEN1 interaction mutants indicate that this interaction constitutes an important component of genomic stability. However, our interpretation of the results is tempered by the realization that none of the mutants completely destroys the interaction between PCNA and FEN1 (Table II), and that residual interactions may still provide an acceptable degree of complex formation inside the cell. Only the pcna-90 fen1-ga double mutant pair was completely inactive in our in vitro studies (Figure 3C). In this regard, the epistasis data with the replication inhibitor hydroxyurea are illuminating (Figure 1C). The double mutant pol30-90 rad27-ga is not more sensitive to hydroxyurea than the single mutant pol30-90, whereas on the other hand the double mutant pol30-79 rad27-ga is more sensitive to hydroxyurea than pol30-79 alone. These epistasis data indicate that during DNA replication pcna-79 can still maintain a functional interaction with FEN1, which is disrupted in the fen1-ga mutant leading to increased sensitivity, but that pcna-90 does not interact effectively with FEN1 at the fork.
The damage sensitivity studies with UV and MMS, however, are more complex as they indicate repair defects in the double mutants that are greater than in either of the single mutants. Interestingly, the rad27-ga mutant alone shows a phenotype intermediate between that of the wild type and the deletion mutant. Possibly, alternative pathways for loading FEN1 exist, or, more probably, the residual interaction that we demonstrated in the mutant shows partial rescue in vivo.
Recently, Gary et al. provided elegant genetic evidence that PCNA anchors FEN1 at the replication fork (Gary et al., 1999). A RAD27 mutant with a defect in the enzyme’s catalytic site showed more severe growth defects than the _rad27_Δ mutant, implying that the catalytically inactive enzyme was assembled into an inhibitory complex. However, when the catalytic site mutant was also mutated in the PCNA-binding domain, the growth defect was much less severe, presumably because the defective FEN1 was not loaded at the fork in preference to a bypass mechanism, perhaps through an analogous nuclease such as EXO1, which could partially compensate for the absence of FEN1.
A two-domain model for the interaction between PCNA and FEN1
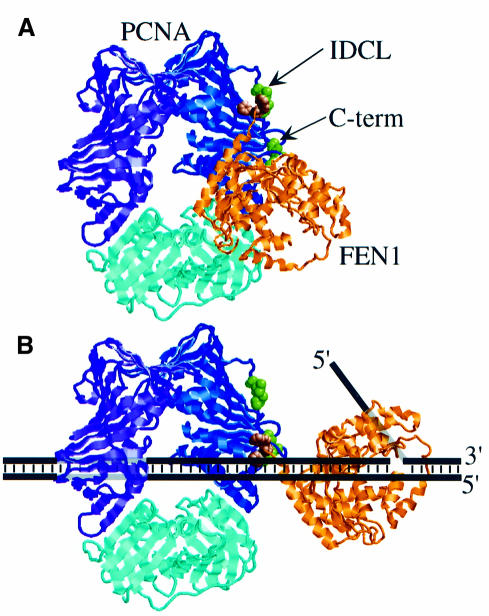
The observation that FEN1, and by extension other DNA-binding proteins containing a PCNA-binding motif, can interact with PCNA in two distinct modes invites two different lines of interpretation. First, both modes of binding could be on the pathway of FEN1 loading. FEN1 could bind initially to the PCNA–DNA complex via the IDCL, followed by a conformational change involving binding of FEN1 to DNA and relocation of its PCNA-binding motif to the C-terminus of PCNA (Figure 7). The minor defect in FEN1 activity with the IDCL mutant pcna-79 may reflect a defect in this initial binding step. Alternatively, each mode of binding may serve a different function in the cell. The PCNA-binding motif of human DNA ligase I and of the RF-C large subunit has been implicated in targeting these proteins to replication foci. Both targeting to replication foci and PCNA binding of DNA ligase I were abolished by mutation of the two phenylalanines in the PCNA-binding motif (Montecucco et al., 1998). Thus, PCNA may serve as a focus-localizing factor for a large number of proteins through interaction with the IDCL. Whether these proteins also interact functionally with PCNA on the DNA, such as detailed here for FEN1, remains to be established.
Fig. 7. A two-domain model for interaction between PCNA and FEN1. (A) A Rasmol ribbon diagram of yeast PCNA is shown with the three identical subunits indicated in blue, purple and cyan. Ile126 and Leu128 in the IDCL (mutated to Ala in pcna-79), and Pro252 and Lys253 in the C-terminus (mutated to Ala in pcna-90) are shown as space-filling residues in green. The donut structure is rotated 45° out of the plane of the paper. The P.furiosus FEN1 in gold with the Phe–Trp dipeptide of the PCNA-interacting domain as a space-filling model in brown was modeled into the PCNA structure analogously to the human PCNA–p21 co-crystal structure (Gulbis et al., 1996). (B) With PCNA encircling the DNA, binding of FEN1 shifts to the C-terminus of PCNA. A model of the interaction on a FLAP substrate is shown.
The PCNA-binding motif of FEN1 is required for binding to the IDCL and to the C-terminus of PCNA, as the fen1-ga mutant (FF336,337GA) is deficient for interaction with PCNA on and off the DNA (Figures 3C and 6C). Therefore, hydrophobic interactions are probably important components of both modes of binding. The lack of an observed interaction between FEN1 and the C-terminus of PCNA in the absence of DNA suggests that this binding domain only becomes exposed when PCNA encircles the DNA (Figure 7). A possible binding site could be formed by three amino acids, Val203, Leu205 and Phe254, which form a surface-exposed hydrophobic patch adjacent to the amino acids mutated in pcna-90 (Krishna et al., 1994).
Our results agree with several studies showing that human p21 inhibits binding of FEN1 to PCNA (Chen et al., 1996; Warbrick et al., 1997; Jonsson et al., 1998). Whether p21 also inhibits PCNA-dependent FEN1 activity has not been reported. However, because the p21–PCNA interactions stretch across the entire PCNA surface from the interdomain connector loop to the C-terminal region of PCNA (Gulbis et al., 1996), it is possible that p21 may inhibit both binding modes of FEN1.
Role of the C-terminus of PCNA in protein–protein interactions
The bimodal interaction between PCNA and FEN1 may represent a model for several other proteins involved in DNA metabolism. The activity of DNA polymerase ε, which contains a consensus PCNA-binding motif (QTSLTKFF, amino acids 1193–1200), is not stimulated by pcna-90, whereas activity is observed with pcna-79 (Eissenberg et al., 1997). Consensus PCNA-binding motifs are also found in mismatch repair proteins Msh3p and Msh6p. Several POL30 mutants with mutations in the C-terminus, pol30-90 (PK252,253AA), pol30-88 (KF253,254AA) and pol30-104 (A251V), but not IDCL mutant pol30-79, are defective for mismatch repair (Johnson et al., 1996; Eissenberg et al., 1997). These genetic data suggest that the important interactions for mismatch repair, perhaps through Msh3p and Msh6p, are mediated through the C-terminus rather than the IDCL. In addition, many POL30 alleles with mutations in the C-terminal tail, including pol30-90, are cold-sensitive for growth, underscoring the importance of this domain for proper DNA metabolism (Amin and Holm, 1996; Eissenberg et al., 1997).
Not all proteins follow the pattern suggested from the FEN1 studies, S.cerevisiae Pol δ being a notable exception. Human Pol δ has been purified as a two-subunit enzyme. A non-consensus PCNA-binding motif has been identified in the catalytic subunit of human Pol δ (Zhang et al., 1999). Prominent in this domain are three consecutive aromatic amino acids (YFY, amino acids 147–149), and mutations in these amino acids eliminate binding to PCNA. Where on PCNA the catalytic subunit interacts is not known. However, mutations in the IDCL of PCNA both eliminate binding to human Pol δ and fail to stimulate processive synthesis, whereas mutations in the C-terminus have no effect (Fukuda et al., 1995; Jonsson et al., 1998; Zhang et al., 1998).
The three-subunit Pol δ from S.cerevisiae has two PCNA-binding domains: one putative motif in the catalytic subunit (YLY, amino acids 159–161) and a consensus motif in the third subunit, POL32 (QGTLESFF, amino acids 338–345). Deletion of the latter motif does not severely affect processive replication by Pol δ (E.Johansson and P.Burgers, unpublished results). In an apparent direct contradiction to the S.cerevisiae Pol δ data but more in line with the FEN1 results, in vitro replication by Schizosaccharomyces pombe Pol δ is severely affected in the C-terminal PCNA mutant P252A, which is analogous to the pcna-90 (PK252,253AA) mutant used in this study. In addition, the PCNA-binding domain in Cdc27, the S.pombe third subunit of Pol δ, is essential for viability, underscoring the importance of this interaction (Reynolds et al., 2000). Clearly, binding of PCNA to Pol δ is maintained through interactions with at least two subunits of the polymerase. However, the relative importance of the individial interactions may vary between organisms.
In conclusion, our model studies of the interaction between FEN1 and PCNA provide a rationale for the observed importance of the C-terminus of PCNA in the cell. The model predicts that mutations near the C-terminus of PCNA may show defects in other pathways which critically depend on proteins with a consensus PCNA-binding motif.
Materials and methods
Enzymes
The three-subunit Pol δ was purified from a yeast overproduction strain (Burgers and Gerik, 1998). RF-C, PCNA, pcna-79, pcna-90 and RP-A were purified from E.coli overproduction strains as described (Henricksen et al., 1994; Ayyagari et al., 1995; Eissenberg et al., 1997; Gerik et al., 1997). A truncated form of RF-C, in which residues 2–273 from Rfc1p were deleted, was used in this study (Gomes et al., 2000). The E.coli SSB was a gift from Dr T.Lohman of this department. All other enzymes were obtained commercially.
Wild-type and mutant FEN1 were overproduced in E.coli and purified to homogeneity essentially as described before (Harrington and Lieber, 1994).
Plasmids and yeast strains
pET-RAD27 was a gift from Dr M.Lieber. pRS314-RAD27 was a gift from Dr E.Friedberg. Integrating plasmids pBL248-x (pRS305 pol30-x) were described (Eissenberg et al., 1997). pBL171 (pET-rad27-ga with a FF346,347GA mutation in the PCNA-binding motif of RAD27) was constructed by standard site-directed mutagenesis procedures. The mutant rad27-ga from pBL171 replaced the wild-type gene in pRS314-RAD27 to give plasmid pBL172.
Plasmids pBL248-0, pBL248-79 and pBL248-90, containing either wild-type or mutant alleles of POL30, were linearized with Hpa_I within the LEU2 gene and transformed into strain PY58 [MAT_α, ura3-52, trp1_Δ_901, leu2-3,112, can1, rth1_Δ::hisG, pol30_Δ_1_ + pBL211 (ARS1 CEN4 URA3 POL30)] to obtain targeted integration into the leu2-3,112 allele (Eissenberg et al., 1997). The strains with both the integrated wild-type or mutant allele of POL30 and a second copy of POL30 on plasmid pBL211 were transformed with plasmid pRS314 (Bluescript ARSH4 CEN6 TRP1), pRS314-RAD27 or pRS314-rad27-ga (pBL172). Plasmid pBL211 was removed by growing on 5-fluoro-orotic acid-containing medium. The resulting strains contained either wild-type or mutant alleles of POL30 and wild-type RAD27 or rad27-ga. Because both pol30-79 and pol30-90 are synthetic lethal with a RAD27 deletion (Eissenberg et al., 1997), the RAD27 deletion strain was only obtained in a wild-type POL30 background, resulting in a total of seven strains which were tested for sensitivity to DNA-damaging agents as described in Figure 1C.
FEN1 assays
All oligonucleotides were obtained commercially. The oligonucleotides used are all complementary to single-stranded mp18 DNA: COKA8 (nucleotides 6290–6261), DCOKA5 (nucleotides 6230–6260) and HCOKA9 [5′-TTTTTTTTTTTTCCTTCCGA (nucleotides 6230–6260); DCOKA5 with a 20mer 5′-non-complementary tail]. Oligonucleotides HCOKA9 and DCOKA5 were labeled at the 5′ end using [γ-32P]ATP and T4 polynucleotide kinase. To generate the different substrates, primers were mixed with single-stranded bacteriophage M13 mp18 in a 5:1 molar ratio in 50 mM Tris–HCl pH 7.9, 10 mM MgCl2, 100 mM NaCl. Samples were heated to 75°C and cooled slowly to room temperature. The FLAP substrate was generated by hybridizing M13 mp18 with oligonucleotides HCOKA9 and COKA8. The nick substrate was generated using primers DCOKA5 and COKA8. The DNA was passed over a Bio-Gel A-5m column to separate it from free primers.
The assay was performed in an assay buffer containing 30 mM Tris–HCl pH 7.8, 8 mM magnesium acetate, 100 µg/ml bovine serum albumin (BSA) and the indicated amounts of NaCl. DNA substrate (10 fmol) was coated with 1 µg of SSB or 1 µg of yeast RP-A and the indicated amounts of PCNA and RF-C, and 100 µM ATP. The reaction was incubated at 30°C for 30 s, and the indicated amounts of either FEN1 or fen1-ga were added to the reaction. At various time intervals, 15 µl aliquots were removed and the reaction was quenched using 2× loading buffer containing 95% formamide, 20 mM EDTA, 0.05% bromophenol blue and 0.05% xylene cyanol. Samples were heated to 90°C and analyzed by 7 M urea–15% PAGE.
Bi-molecular interaction analysis
Surface plasmon resonance experiments were performed to monitor the interactions between PCNA and FEN1. About 500 response units (RU) (∼2 fmol) of either wild-type PCNA, pcna-79 or pcna-90 were immobilized on the surface of a BIAcore pioneer B1 chip by the carbodiimide coupling method as per the manufacturer’s instructions. The running buffer used in the analysis contained 30 mM HEPES–NaOH pH 7.5, 1 mM EDTA, 0.5% inositol, 0.01% NP-40, 8 mM magnesium acetate and 100 mM NaCl. The PCNA–FEN1 interactions were measured by injecting 55 µl of increasing concentrations of either wild-type FEN1 or mutant fen1-ga over the immobilized wild-type or mutant PCNA chip at a flow rate of 30 µl/min. Under these conditions, binding was not limited by flow rate. To determine the relative affinities of FEN1 for PCNA, steady-state binding levels (RUeq) were plotted as a function of FEN1 concentration.
To determine the solution affinity between FEN1 and either wild-type PCNA, pcna-79 or pcna-90, a 250 nM solution of FEN1 was incubated with increasing amounts of PCNA, pcna-79 or pcna-90 ranging in concentration from 10 to 5000 nM, and the solution was injected over the chip containing wild-type PCNA. The equilibrium dissociation constant _K_D was calculated by plotting the decrease of the signal of FEN1 binding to the chip as a function of increasing PCNA concentration, or pcna-79 or pcna-90, using software provided by the manufacturer. Each _K_D value was obtained from at least 10 injections. A similar analysis was carried out with pcna-90 immobilized on the surface of a B1 chip.
The same analysis was carried out with 4500 RU (corresponding to 13 fmol) of PCNA immobilized on a CM5 chip. FEN1 (30 nM) was then incubated with increasing amounts of PCNA ranging in concentration from 1 to 500 nM and flowed across the chip. The _K_D values obtained in this analysis were within 2-fold of those obtained with the B1 chip, and the data in Table I represent the average values.
Isolation of complexes of PCNA and FEN1 on DNA bound to magnetic beads
Single-stranded M13 mp18 DNA was amplified using two primers: biotinylated primer BP-6140 (mp18 nucleotides 6140–6169) and primer P-6423 (mp18 complementary nucleotides 6423–6399). The amplified 286 bp fragment was immobilized onto streptavidin–magnetic beads (Dynabeads) in 10 mM Tris–HCl pH 7.5, 1 mM EDTA and 1 M NaCl by incubation at room temperature for 2–3 h. All washes were carried out using a Dynal magnet with a volume of buffer 100–200 times the bead volume for 1–2 min each at room temperature. The unbound PCR products were washed off the beads twice with wash buffer A (10 mM Tris–HCl pH 7.5, 1 mM EDTA and 1 M NaCl). The duplex DNA was denatured twice with 0.1 M NaOH and the non-biotinylated strand was washed off twice with wash buffer A. The bead-bound single-stranded DNA was hybridized with oligonucleotides DCOKA5 and COKA8 (see above), washed twice with wash buffer A and resuspended in buffer C [30 mM HEPES–NaOH pH 7.5, 8 mM magnesium acetate, 125 mM NaCl, 0.2 mg/ml BSA and 1 mM dithiothreitol (DTT)]. The binding assay was performed in a 20 μl reaction in buffer C. DNA–bead substrate (30 fmol, 1 µl of beads) was coated with 5 pmol of yeast RP-A for 2 min, followed, where indicated, by addition of 5 pmol of PCNA, 1.5 pmol of RF-C and 100 μM ATP. The reaction was incubated at 30°C for 2 min. The beads were washed twice with wash buffer B (30 mM HEPES–NaOH pH 7.5, 5% glycerol, 0.1 mg/ml BSA, 1 mM DTT, 0.01% NP-40, 0.1 mM EDTA and 125 mM NaCl) and resuspended in buffer C. A 1.7 pmol aliquot of either FEN1 or fen1-ga was added to the reaction and incubated at 30°C for 1 min. Then, the beads were washed twice with wash buffer B. The same number of washes was carried out for each experiment regardless of whether specific components were left out of the assay. Bead-bound proteins were boiled in sample loading buffer and separated on a 10% SDS–polyacrylamide gel. The proteins were blotted onto a nitrocellulose membrane using a Mini Trans-Blot electrophoretic transfer cell from BioRad. The blot was probed with a mixture of polyclonal antibodies raised in rabbits against PCNA and FEN1. Detection was carried out using an ECL chemiluminescence kit (Amersham) as recommended by the manufacturer. Different exposures of the blot were photographed with a CCD camera and digitized for quantitation. The blots also contained known and varying amounts of PCNA and FEN1 for calibration purposes.
Acknowledgments
Acknowledgements
The authors thank Rao Ayyagari for the purification of pcna-90, Michael Lieber and Erroll Friedberg for plasmids, and John Majors for critical discussion of the manuscript. This work was supported in part by grant GM32431 from the National Institutes of Health. X.V.G. was supported in part by a fellowship from the W.M.Keck Foundation.
References
- Amin N.S. and Holm,C. (1996) In vivo analysis reveals that the interdomain region of the yeast proliferating cell nuclear antigen is important for DNA replication and DNA repair. Genetics, 144, 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyagari R., Impellizzeri,K.J., Yoder,B.L., Gary,S.L. and Burgers,P.M. (1995) A mutational analysis of the yeast proliferating cell nuclear antigen indicates distinct roles in DNA replication and DNA repair. Mol. Cell. Biol., 15, 4420–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambara R.A., Murante,R.S. and Henricksen,L.A. (1997) Enzymes and reactions at the eukaryotic DNA replication fork. J. Biol. Chem., 272, 4647–4650. [DOI] [PubMed] [Google Scholar]
- Burgers P.M. and Gerik,K.J. (1998) Structure and processivity of two forms of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem., 273, 19756–19762. [DOI] [PubMed] [Google Scholar]
- Burgers P.M.J. and Yoder,B.L. (1993) ATP-independent loading of the proliferating cell nuclear antigen requires DNA ends. J. Biol. Chem., 268, 19923–19936. [PubMed] [Google Scholar]
- Chen U., Chen,S., Saha,P. and Dutta,A. (1996) p21Cip1/Waf1 disrupts the recruitment of human Fen1 by proliferating-cell nuclear antigen into the DNA replication complex. Proc. Natl Acad. Sci. USA, 93, 11597–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J.C., Ayyagari,R., Gomes,X.V. and Burgers,P. (1997) Mutations in yeast proliferating cell nuclear antigen define distinct sites for interaction with DNA polymerase δ and DNA polymerase ε. Mol. Cell. Biol., 17, 6367–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich C.H., Kantrow,S.M. and Zakian,V.A. (1998) Expansion and length-dependent fragility of CTG repeats in yeast. Science, 279, 853–856. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Morioka,H., Imajou,S., Ikeda,S., Ohtsuka,E. and Tsurimoto,T. (1995) Structure–function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. J. Biol. Chem., 270, 22527–22534. [DOI] [PubMed] [Google Scholar]
- Gary R., Park,M.S., Nolan,J.P., Cornelius,H.L., Kozyreva,O.G., Tran,H.T., Lobachev,K.S., Resnick,M.A. and Gordenin,D.A. (1999) A novel role in DNA metabolism for the binding of Fen1/Rad27 to PCNA and implications for genetic risk. Mol. Cell. Biol., 19, 5373–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerik K.J., Gary,S.L. and Burgers,P.M. (1997) Overproduction and affinity purification of Saccharomyces cerevisiae replication factor C. J. Biol. Chem., 272, 1256–1262. [DOI] [PubMed] [Google Scholar]
- Gomes X.V., Gary,S.L. and Burgers,P.M. (2000) Overproduction in E.coli and characterization of yeast replication factor C lacking the ligase-homology domain. J. Biol. Chem., 275, 14541–14549. [DOI] [PubMed] [Google Scholar]
- Gulbis J.M., Kelman,Z., Hurwitz,J., O’Donnell,M. and Kuriyan,J. (1996) Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell, 87, 297–306. [DOI] [PubMed] [Google Scholar]
- Harrington J.J. and Lieber,M.R. (1994) Functional domains within FEN-1 and RAD2 define a family of structure-specific endonucleases: implications for nucleotide excision repair. Genes Dev., 8, 1344–1355. [DOI] [PubMed] [Google Scholar]
- Henricksen L.A., Umbricht,C.B. and Wold,M.S. (1994) Recombinant replication protein A: expression, complex formation and functional characterization [published erratum appears in J. Biol. Chem. 1994, 269, 16519]. J. Biol. Chem., 269, 11121–11132. [PubMed] [Google Scholar]
- Hosfield D.J., Mol,C.D., Shen,B. and Tainer,J.A. (1998) Structure of the DNA repair and replication endonuclease and exonuclease FEN-1: coupling DNA and PCNA binding to FEN-1 activity. Cell, 95, 135–146. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Kovvali,G.K., Prakash,L. and Prakash,S. (1995) Requirement of the yeast RTH1 5′ to 3′ exonuclease for the stability of simple repetitive DNA. Science, 269, 238–240. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Kovvali,G.K., Guzder,S.N., Amin,N.S., Holm,C., Habraken,Y., Sung,P., Prakash,L. and Prakash,S. (1996) Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J. Biol. Chem., 271, 27987–27990. [DOI] [PubMed] [Google Scholar]
- Johnson R.E., Kovvali,G.K., Prakash,L. and Prakash,S. (1998) Role of yeast Rth1 nuclease and its homologs in mutation avoidance, DNA repair and DNA replication. Curr. Genet., 34, 21–29. [DOI] [PubMed] [Google Scholar]
- Jonsson Z.O., Hindges,R. and Hubscher,U. (1998) Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen. EMBO J., 17, 2412–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Biade,S. and Matsumoto,Y. (1998) Involvement of flap endonuclease 1 in base excision DNA repair. J. Biol. Chem., 273, 8842–8848. [DOI] [PubMed] [Google Scholar]
- Klungland A. and Lindahl,T. (1997) Second pathway for completion of human DNA base excision-repair—reconstitution with purified proteins and requirement for DNase IV (fen1). EMBO J., 16, 3341–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoska R.J., Stefanovic,L., Tran,H.T., Resnick,M.A., Gordenin,D.A. and Petes,T.D. (1998) Destabilization of yeast micro- and minisatellite DNA sequences by mutations affecting a nuclease involved in Okazaki fragment processing (rad27) and DNA polymerase δ (pol3-t). Mol. Cell. Biol., 18, 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna T.S., Kong,X.-P., Gary,S., Burgers,P.M. and Kuriyan,J. (1994) Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell, 79, 1233–1243. [DOI] [PubMed] [Google Scholar]
- Li X., Li,J., Harrington,J., Lieber,M.R. and Burgers,P.M. (1995) Lagging strand DNA synthesis at the eukaryotic replication fork involves binding and stimulation of FEN-1 by PCNA. J. Biol. Chem., 270, 22109–22112. [DOI] [PubMed] [Google Scholar]
- Montecucco A. et al. (1998) DNA ligase I is recruited to sites of DNA replication by an interaction with proliferating cell nuclear antigen: identification of a common targeting mechanism for the assembly of replication factories. EMBO J., 17, 3786–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Robetorye,R.S., Pereira,S.O. and Smith,J.R. (1995) The C-terminal region of p21SDI1/WAF1/CIP1 is involved in proliferating cell nuclear antigen binding but does not appear to be required for growth inhibition. J. Biol. Chem., 270, 17060–17063. [DOI] [PubMed] [Google Scholar]
- Oku T., Ikeda,S., Sasaki,H., Fukuda,K., Morioka,H., Ohtsuka,E., Yoshikawa,H. and Tsurimoto,T. (1998) Functional sites of human PCNA which interact with p21 (Cip1/Waf1), DNA polymerase δ and replication factor C. Genes Cells, 3, 357–369. [DOI] [PubMed] [Google Scholar]
- Parenteau J. and Wellinger,R.J. (1999) Accumulation of single-stranded DNA and destabilization of telomeric repeats in yeast mutant strains carrying a deletion of RAD27. Mol. Cell. Biol., 19, 4143–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura,M., Marshak,D.R., Mathews,M.B. and Stillman,B. (1987) The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature, 326, 471–475. [DOI] [PubMed] [Google Scholar]
- Reagan M.S., Pittenger,C., Siede,W. and Friedberg,E.C. (1995) Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide excision repair gene. J. Bacteriol., 177, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds N., Warbrick,E., Fantes,P.A. and MacNeill,S.A. (2000) Essential interaction between the fission yeast DNA polymerase δ subunit Cdc27 and Pcn1 (PCNA) mediated through a C-terminal p21(Cip1)-like PCNA binding motif. EMBO J., 19, 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J.K. and Livingston,D.M. (1998) Expansions of CAG repeat tracts are frequent in a yeast mutant defective in Okazaki fragment maturation. Hum. Mol. Genet., 7, 69–74. [DOI] [PubMed] [Google Scholar]
- Sommers C.H., Miller,E.J., Dujon,B., Prakash,S. and Prakash,L. (1995) Conditional lethality of null mutations in RTH1 that encodes the yeast counterpart of a mammalian 5′- to 3′-exonuclease required for lagging strand DNA synthesis in reconstituted systems. J. Biol. Chem., 270, 4193–4196. [DOI] [PubMed] [Google Scholar]
- Symington L.S. (1998) Homologous recombination is required for the viability of rad27 mutants. Nucleic Acids Res., 26, 5589–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.K., Castillo,C., So,A.G. and Downey,K.M. (1986) An auxiliary protein for DNA polymerase δ from fetal calf thymus. J. Biol. Chem., 261, 12310–12316. [PubMed] [Google Scholar]
- Tishkoff D.X., Boerger,A.L., Bertrand,P., Filosi,N., Gaida,G.M., Kane,M.F. and Kolodner,R.D. (1997a) Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc. Natl Acad. Sci. USA, 94, 7487–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff D.X., Filosi,N., Gaida,G.M. and Kolodner,R.D. (1997b) A novel mutation avoidance mechanism dependent on S.cerevisiae RAD27 is distinct from DNA mismatch repair. Cell, 88, 253–263. [DOI] [PubMed] [Google Scholar]
- Tom S., Henricksen,L.A. and Bambara,R.A. (2000) Mechanism whereby proliferating cell nuclear antigen stimulates Flap endonuclease 1. J. Biol. Chem., 275, 10498–10505. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T. (1999) PCNA binding proteins. Front. Biosci., 4, 849–858. [DOI] [PubMed] [Google Scholar]
- Waga S., Hannon,G.J., Beach,D. and Stillman,B. (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature, 369, 574–578. [DOI] [PubMed] [Google Scholar]
- Waga S. and Stillman,B. (1998) Cyclin-dependent kinase inhibitor p21 modulates the DNA primer–template recognition complex. Mol. Cell. Biol., 18, 4177–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E. (1998) PCNA binding through a conserved motif. BioEssays, 20, 195–199. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Lane,D.P., Glover,D.M. and Cox,L.S. (1995) A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol., 5, 275–282. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Lane,D.P., Glover,D.M. and Cox,L.S. (1997) Homologous regions of fen1 and p21(cip1) compete for binding to the same site on PCNA—a potential mechanism to co-ordinate DNA replication and repair. Oncogene, 14, 2313–2321. [DOI] [PubMed] [Google Scholar]
- Wu X., Li,J., Li,X., Hsieh,C.L., Burgers,P.M. and Lieber,M.R. (1996) Processing of branched DNA intermediates by a complex of human FEN-1 and PCNA. Nucleic Acids Res., 24, 2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wilson,T.E. and Lieber,M.R. (1999) A role for FEN-1 in nonhomologous DNA end joining: the order of strand annealing and nucleolytic processing events. Proc. Natl Acad. Sci. USA, 96, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Sun,Y., Hsu,H., Zhang,L., Zhang,Y. and Lee,M.Y. (1998) The interdomain connector loop of human PCNA is involved in a direct interaction with human polymerase δ. J. Biol. Chem., 273, 713–719. [DOI] [PubMed] [Google Scholar]
- Zhang P., Mo,J.Y., Perez,A., Leon,A., Liu,L., Mazloum,N., Xu,H. and Lee,M.Y. (1999) Direct interaction of proliferating cell nuclear antigen with the p125 catalytic subunit of mammalian DNA polymerase δ. J. Biol. Chem., 274, 26647–26653. [DOI] [PubMed] [Google Scholar]