Nup2p, a Yeast Nucleoporin, Functions in Bidirectional Transport of Importin α (original) (raw)
Abstract
Import of proteins containing a classical nuclear localization signal (NLS) into the nucleus is mediated by importin α and importin β. Srp1p, the Saccharomyces cerevisiae homologue of importin α, returns from the nucleus in a complex with its export factor Cse1p and with Gsp1p (yeast Ran) in its GTP-bound state. We studied the role of the nucleoporin Nup2p in the transport cycle of Srp1p. Cells lacking NUP2 show a specific defect in both NLS import and Srp1p export, indicating that Nup2p is required for efficient bidirectional transport of Srp1p across the nuclear pore complex (NPC). Nup2p is located at the nuclear side of the central gated channel of the NPC and provides a binding site for Srp1p via its amino-terminal domain. We show that Nup2p effectively releases the NLS protein from importin α-importin and β and strongly binds to the importin heterodimer via Srp1p. Kap95p (importin β) is released from this complex by a direct interaction with Gsp1p-GTP. These data suggest that besides Gsp1p, which disassembles the NLS-importin α-importin β complex upon binding to Kap95p in the nucleus, Nup2p can also dissociate the import complex by binding to Srp1p. We also show data indicating that Nup1p, a relative of Nup2p, plays a similar role in termination of NLS import. Cse1p and Gsp1p-GTP release Srp1p from Nup2p, which suggests that the Srp1p export complex can be formed directly at the NPC. The changed distribution of Cse1p at the NPC in nup2 mutants also supports a role for Nup2p in Srp1p export from the nucleus.
Protein transport across the nuclear envelope is mediated by soluble receptors of the importin β family. The receptors interact with their cargo either directly or via an adapter molecule (for reviews, see references 13 and 30). Nuclear import and nuclear export signals within the proteins are recognized by the receptors. The transport complexes disassemble after translocation through the nuclear pore complex (NPC), and then the receptors return to the other side of the nuclear envelope. The receptors interact with proteins of the NPC (nucleoporins) and with the Ran GTPase, the major regulator of nucleocytoplasmic transport. Ran is an abundant protein that also shuttles between the nucleus and the cytoplasm. The Ran-specific GTPase activation protein (Rna1p, RanGAP1) is located in the cytoplasm, whereas the guanine nucleotide exchange factor (Prp20p, RCC1) is found in the nucleus. Accordingly, Ran should be bound to GTP in the nucleus and bound to GDP in the cytoplasm. This asymmetric distribution of Ran's nucleotide-bound state is thought to regulate cargo binding and release and, consequently, the transport direction of transport complexes (reviewed in references 13, 30, and 32). Import and export receptors both bind specifically to the GTP-bound form of Ran (Ran-GTP) but respond in different ways. Import receptors bind to their substrates in the cytoplasm, where the concentration of Ran-GTP is low, and release them in the nucleus upon binding to Ran-GTP. The import receptor–Ran-GTP complexes then migrate back to the cytoplasmic compartment. Export receptors, on the other hand, require the simultaneous binding of Ran-GTP in the nucleus for efficient association with their substrates. The dissociation of receptor–Ran-GTP complexes after export requires RanBP1/Yrb1p, a cytoplasmic protein that tightly binds to Ran-GTP and stimulates Rna1p-mediated GTP hydrolysis by Ran.
The NPC, a 60-million to 125-million-Da complex composed of 35 to 100 different nucleoporins, is the only site of macromolecular exchange in the nuclear envelope (reviewed in references 10 and 48). As shown by electron microscopic techniques, the NPC is a cylindrical structure characterized by an octagonal symmetry. The core structure with the 9-nm central channel is embedded in the nuclear envelope. Eight flexible filaments protrude ∼50 nm into the cytoplasm. On the nuclear side, eight ∼100-nm fibers connected by the terminal ring form a basket-like structure. Many nucleoporins contain more or less degenerate peptide repeats with the characteristic FG motif. The repeat domains of GLFG and FXFG nucleoporins (two major subclasses of the FG Nups) represent preferential sites of interaction with transport receptors (2, 36, 38, 46). Three of the ∼35 known nucleoporins of the yeast Saccharomyces cerevisiae, Nsp1p (31), Nup1p (7), and Nup2p (27), contain between 15 and 29 copies of FXFG repeats.
Proteins containing a classical nuclear localization signal (NLS) contain a short cluster of basic amino acid residues (monopartite NLS) or two basic patches separated by ∼10 residues (bipartite NLS) (8). A prototype of the monopartite NLS is the sequence PKKKRKV of the simian virus 40 (SV40) large T-antigen (21). The NLS is recognized by the adapter protein importin α (karyopherin α) in the cytoplasm (11, 29, 51). Importin β (karyopherin β) associates with importin α and stimulates NLS binding (6, 11, 36). Importin β mediates the translocation of the NLS protein-importin α-importin β (NLS-α/β) complex through the NPC. The import complex dissociates in the nuclear compartment upon Ran-GTP binding to importin β (14, 36). It is not known how the NLS is released from importin α.
Nuclear import receptors different from importin β bind to their substrates directly without the involvement of an adapter. Nine out of the fourteen importin β-like proteins in S. cerevisiae were identified as import receptors (reviewed in references 34 and 43). Examples are Mtr10p, the importer of the mRNA binding protein Npl3p (35, 45), or Pse1p and Yrb4p/Kap123p, the importers of some ribosomal proteins (39, 42).
Importin α is exported from the nucleus back to the cytoplasm by the importin β-related receptor CAS/Cse1p (19, 25, 26, 47). Human CAS and Ran-GTP mediate export from the nucleus of permeabilized cells (26). Srp1p (yeast importin α) accumulates in the nucleus of cse1 mutants. Due to the limited amounts of Srp1p in the cytoplasm, NLS protein import is also inhibited in cse1 mutants (25, 47). The GTP-bound form of Gsp1p (yeast Ran) and Srp1p cooperatively bind to Cse1p, and the trimeric Srp1p–Cse1p–Gsp1p-GTP export complex is dissociated by cytoplasmic Yrb1p (47). Only the NLS-free form of importin α binds to CAS/Cse1p (26, 47), suggesting that NLS binding induces a conformational change within importin α.
Here we show a role of Nup2p in both nuclear import and export of Srp1p. Like cse1 cells, nup2 mutants specifically accumulate Srp1p within the nucleus and NLS proteins in the cytoplasm. By immunoelectron microscopy, we show that Nup2p resides on the nuclear side of the central channel of the NPC and provides a major binding site for Srp1p. In vitro assays with recombinant proteins reveal interactions which suggest an order of events within the NPC. Nup2p displaces the NLS protein from the NLS-α/β import complex. Importins α and β remain bound to Nup2p. Kap95p (yeast importin β) is released upon binding to the GTP-bound form of Gsp1p. Finally, Srp1p destined for export is released from Nup2p by Cse1p and Gsp1p-GTP. We also demonstrate that Nup1p plays a role similar to that of Nup2p in the import but not in the export of Srp1p.
MATERIALS AND METHODS
Strains and plasmids.
To construct a NUP2 deletion strain containing an integrated allele of SRP1-GFP (encoding a fusion of Srp1p to green fluorescent protein), we crossed cells of strain GSY413 expressing SRP1-GFP (47) to cells of the NUP2::HIS3 strain JLY506 (GSY432) (27). One resulting tetrad was GSY618 (NUP2::HIS3 SRP1-GFP MATα ura3-52 leu2 his3 trp1). Similarly, strain GSY777 (NUP2::HIS3 GFP-CSE1 MATα ura3 leu2 trp1 his3 ade2) was isolated after crossing GSY580 (47) to JLY506. Strains expressing integrated fusions of NUP2 (GSY627, NUP2-GFP MATα ura3 leu2 trp1 his3) and NUP1 (GSY652, NUP1-GFP MATa ura3 leu2 trp1 his3 lys2) to GFP were constructed by homologous recombination. The NUP2 and NUP1 genes with engineered restriction sites at the stop codon were inserted into the URA3 plasmid pRS306. After insertion of GFP, the plasmids were linearized and transformed into a wild-type strain. Integration at the correct NUP locus was confirmed by Southern blotting, PCR, and immunoblotting with anti-GFP antibodies. After passage over plates containing 5-fluoro-orotic acid, the pop-out strains expressing the NUP-GFP fusions were identified microscopically.
Yeast plasmids encoding NUP2 (pGS248) and NUP2-N (pGS259) (27), as well as reporter plasmids pGS420 and pGS422, coding for NLS–glutathione _S_-transferase (GST)–GFP (47), and pGS304, containing an L251–49-LacZ fusion (40), were described before. A _Bam_HI/_Xho_I fragment containing the PCR-generated coding sequence of MATα2 was inserted into the URA3 vector YCpGAL-GST-GFP (pGS846), which resulted in pGS854, coding for the 78-kDa GST-GFP-Matα2p reporter. To construct GST fusion proteins for recombinant expression, fragments of NUP1 and NUP2 were inserted into pGEX-4T-1 (Pharmacia). Plasmids pGS272 (pGEX-4T-NUP2-N, Nup2p residues 1 to 174), pGS278 (pGEX-4T-NUP2-M, residues 175 to 563), and pGS273 (pGEX-4T-NUP2-C, residues 566 to 720) contain PCR-derived _Bam_HI fragments. The insert of pGS274 (pGEX-4T-NUP1-N, residues 24 to 286) derived from a 792-bp _Dra_I fragment of plasmid pLD1 (7). Plasmids pGS276 (pGEX-4T-NUP1-M, residues 431 to 815) and pGS277 (pGEX-4T-NUP1-C, residues 958 to 1076) also contain PCR-generated inserts. The coding sequence of PRP20 was PCR amplified from genomic DNA and inserted as a 1,620-bp _Eco_RI fragment into pGEX-4T-1 (pGS269). His6-Prp20p was purified using pGS818, which was constructed by inserting the _Eco_RI fragment into pProEX-HTa (Gibco BRL). The GST-NLS fusion vector (pGS418) derived from plasmids pGS388 and pGS422 (47). A plasmid encoding His6-tagged tobacco etch virus (TEV) protease (TEV NIa proteinase) (33) was a gift from Elena Conti (EMBL, Heidelberg, Germany). The coding sequences of KAP95 (pGS962) and NUP2-N (pGS815) were inserted into pGEX-4TEV (pGS804), a derivative of pGEX-4T-1 that contains a TEV cleavage site (Glu-Asn-Leu-Tyr-Phe-Gln/Gly) in place of the thrombin cleavage site. The construction of pGS467 (pQE9-GSP1) and pGS468 (pQE9-GSP1Q71L) will be described elsewhere. Plasmid pGEX-5G-SRP1 (pGS390) for GST-Srp1p production was described before (47). For purification of N-terminal His6 fusion proteins, the coding regions of SRP1 (pGS459) and CSE1 (pGS675) were inserted as _Bam_HI fragments (47) into pQE9 (Qiagen).
Protein analysis.
Srp1p, Cse1p, Gsp1p, and the GTPase-deficient mutant Gsp1pQ71L fused to N-terminal His6 tags were purified with nickel-nitrolotriacetic acid agarose (Qiagen). Srp1p and Cse1p were further purified with a Mono Q column (Pharmacia). The GDP-bound and GTP-bound forms of Gsp1p and Gsp1pQ71L were separated on a Mono S column (Pharmacia). High-pressure liquid chromatography analysis (14) showed that the Gsp1pQ71L-GTP preparation contained 97% GTP-bound and 3% GDP-bound protein, whereas the Gsp1pQ71L-GDP fraction as well as the Gsp1p-GDP fraction contained 100% GDP-bound protein (not shown). In this study, we used the wild-type Gsp1p-GDP and Gsp1pQ71L-GTP preparations. His6-TEV protease was purified by Ni-nitrolotriacetic acid agarose and Mono S chromatography and then stored at 0.3 mg/ml in 25 mM Tris-HCl (pH 7.5)–75 mM KCl–0.5 mM EDTA–2.5 mM dithiothreitol–25% glycerol–0.33% Triton X-100. GST fusion proteins were purified with glutathione-Sepharose columns (Pharmacia). The GST-Nup fusion eluates were gel filtered using PBSKMT buffer (25 mM sodium phosphate, 150 mM NaCl, 3 mM KCl, 1 mM MgCl2, 0.1% Tween 20 [pH 7.3]). GST-Kap95p was further purified with a Mono Q column. Fusions of GST to Kap95p and to the amino-terminal 174 amino acid residues of Nup2p (Nup2-N) containing a TEV protease cleavage site at the C terminus of GST were used to purify Kap95p and Nup2-N. The fusion proteins were bound to glutathione-Sepharose and cleaved with TEV protease (at a TEV protease/fusion ratio of 1:100) overnight at 4°C in 50 mM Tris-HCl (pH 8.0)–0.5 mM EDTA. Kap95p present in the eluate was further purified on Mono Q. Solution binding assays with immobilized GST fusion proteins were performed as described before (47). Purified proteins or Escherichia coli lysates were incubated with glutathione-Sepharose (Pharmacia) for 30 min at 4°C. After three washes with 1 ml PBSKMT, proteins or NLS peptides were added to a total volume of 300 μl as indicated in the figure legends. After washing, bound proteins were eluted with sodium dodecyl sulfate (SDS) sample buffer. Unbound proteins from the first supernatant were concentrated by acetone precipitation. Western blot analysis was carried out according to the Amersham ECL kit guidelines using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; Sigma) as the secondary antibody.
Microscopy.
Antibodies used for immunofluorescence microscopy, preparation of cells by formaldehyde fixation, and staining of cells with DAPI (4′,6′-diamidino-2-phenylindole) were described before (42, 47). Immunoelectron microscopy was carried out as described previously (22, 53). Briefly, yeast cells were harvested by centrifugation and fixed for 1 h with 4% formaldehyde and 0.5% glutaraldehyde under culture conditions (pH 5.5, 30°C), cryoprotected by a mixture of 25% polyvinylpyrrolidone (PVP K15; molecular weight, 10,000; Fluka) and 1.6 M sucrose (50) for 3 h, and frozen in liquid nitrogen. Ultrathin cryosections were prepared with glass knifes and transferred to formvar-carbon-coated copper grids using the cryoprotectant mixture. Primary antibodies were affinity-purified rabbit anti-Srp1p IgG (42) and polyclonal anti-GFP IgG (Clontech 8363-1). Labeling with primary antibodies and secondary antibody-gold complexes (10 nm; Dianova) was performed as described elsewhere (16). Finally, the sections were stained and stabilized by a freshly prepared mixture of 3% tungstosilicic acid hydrate (Fluka) and 2.5% polyvinyl alcohol (molecular weight, 10,000; Sigma) (50). To quantify the distribution of proteins at the NPC, we measured the distance of individual gold particles from the vertical axis (20-nm increments) and the horizontal axis (10-nm increments) in micrographs (primary magnification, ×22,000) that were further magnified 24-fold. We analyzed only sections perpendicular to the nuclear envelope plane with well-defined double membranes flanking the nuclear pores.
RESULTS
Export of Srp1p and Cse1p and import of NLS proteins are defective in nup2 mutants.
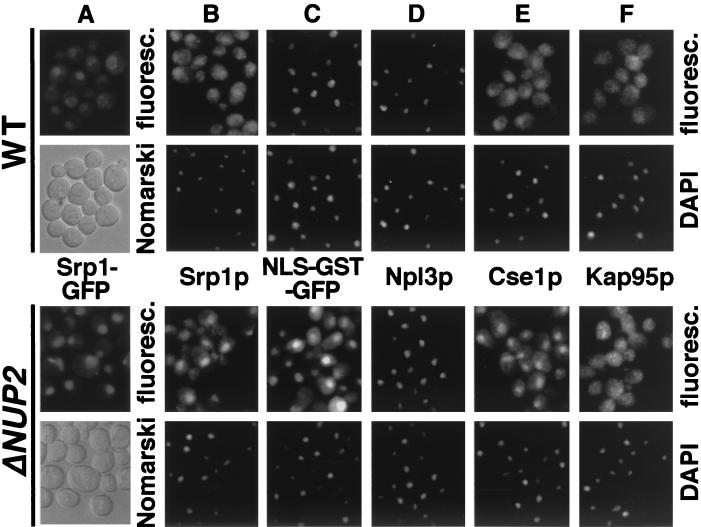
Mutations in CSE1, the yeast homologue of human CAS, lead to a nuclear accumulation of Srp1p (yeast importin α) and to a concomitant defect in NLS protein import (25, 47). Since the nucleoporin Nup2p (molecular mass, 78 kDa) was previously shown to interact with Srp1p (3, 12), we tested whether nup2 mutants also show a mislocalization of Srp1p in living cells using a functional fusion (47) of Srp1p to GFP. Cells lacking Nup2p display no defects in the structure of the nuclear envelope (27). Srp1-GFP is concentrated at the nuclear envelope but also present in the nucleus and cytoplasm of wild-type cells (Fig. 1A). As was recently shown for formaldehyde-fixed cells (5, 18), Srp1p accumulated in the nuclei of cells lacking NUP2. This nuclear accumulation was accompanied by a complete loss of nuclear rim staining (Fig. 1A), indicating that Nup2p is required for targeting of Srp1p to the NPC. A plasmid encoding Nup2-N largely rescued the mislocalization phenotype (data not shown). This amino-terminal domain was previously shown to be essential for Nup2p function (27).
FIG. 1.
Export of Srp1p and Cse1p as well as NLS protein import are defective in cells lacking NUP2. (A) Yeast cells carrying the SRP1-GFP allele and a deletion of NUP2 (Δ_NUP2_ strain GSY618) were transformed with plasmid pGS248 encoding Nup2p (top) or with the CEN LEU2 vector pRS315 (bottom). Cells were grown in liquid medium at 30°C. Srp1-GFP was visualized by fluorescence microscopy (fluoresc.) and also viewed by Nomarski optics. (B to F) Wild-type (WT; W303), Δ_NUP2_ (JLY506) cells or the same cells transformed with the reporter plasmid pGS422, encoding SV40 NLS-GST-GFP (C), were grown at 30°C. Synthesis of NLS-GST-GFP was induced by 2% galactose for 3 h (C). Cells were prepared for immunofluorescence microscopy and incubated with antibodies against Srp1p (B), GFP (C), Npl3p (D), Cse1p (E), and Kap95p (F). Antibodies were detected by Texas red-conjugated IgG, and DNA was stained with DAPI.
We then analyzed Δ_NUP2_ cells for possible defects in other nucleocytoplasmic transport pathways by indirect immunofluorescence microscopy. The nuclear accumulation of Srp1p was again detected with Srp1p-specific antibodies (Fig. 1B). In contrast to cse1-1 mutants (47), however, some Srp1p is still present in the cytoplasm. Furthermore, Δ_NUP2_ cells show a cytoplasmic accumulation of a reporter protein containing the SV40 large T-antigen NLS, which is completely nuclear in wild-type cells (Fig. 1C). Similarly, a reporter comprising the SV40 NLS fused to invertase was mislocalized to the cytoplasm (not shown). However, the mRNA binding protein Npl3p (Mtr10p-dependent import) (35, 45) and a reporter protein containing the import signal of the ribosomal protein Rpl25p (Yrb4p/Pse1p-dependent import) (39, 42) were still nuclear in Δ_NUP2_ cells (Fig. 1D and data not shown). We conclude that import pathways different from NLS protein import are not affected by the deletion of NUP2. Furthermore, Δ_NUP2_ cells showed a normal cytoplasmic localization of poly(A)+ RNA, indicating that export of mRNA is also not inhibited (data not shown). The distribution of the nuclear transport factors Kap95p (20, 42) and Yrb1p (44) was the same in wild-type and Δ_NUP2_ cells (Fig. 1F and data not shown). However, we observed a significant accumulation of Cse1p in the nuclei of Δ_NUP2_ cells (Fig. 1E). Taken together, the data indicate that Δ_NUP2_ cells show an inhibition of Srp1p and Cse1p export and are specifically impaired in the nuclear import of NLS proteins. The NLS protein import defect of Δ_NUP2_ cells was again rescued by plasmid-encoded Nup2-N (not shown).
Srp1p, Nup2p, and Nup1p colocalize at the nuclear side of the NPC.
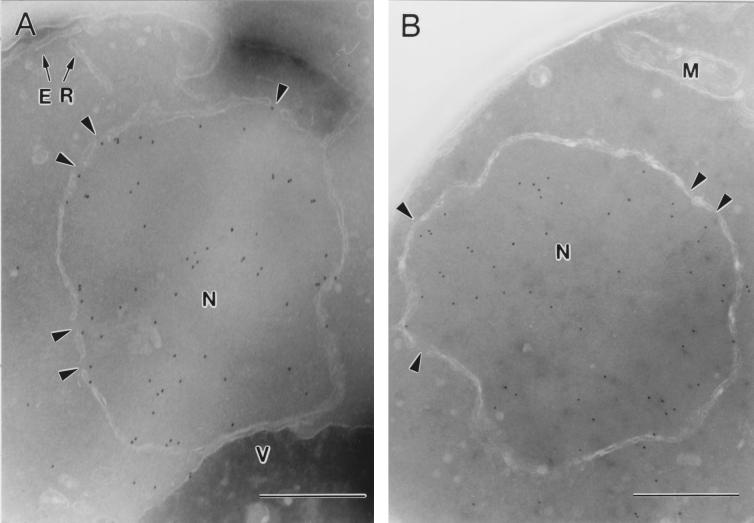
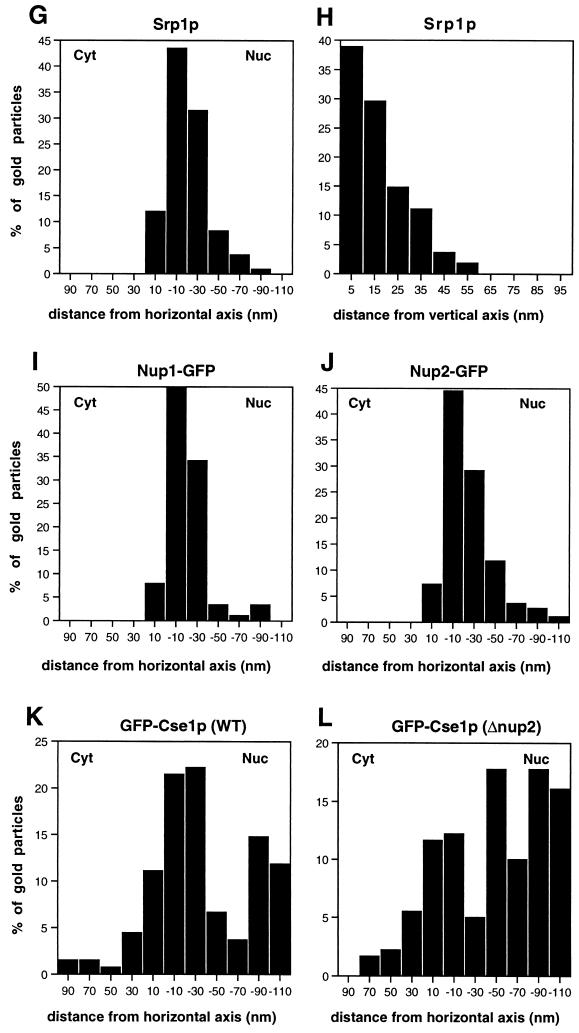
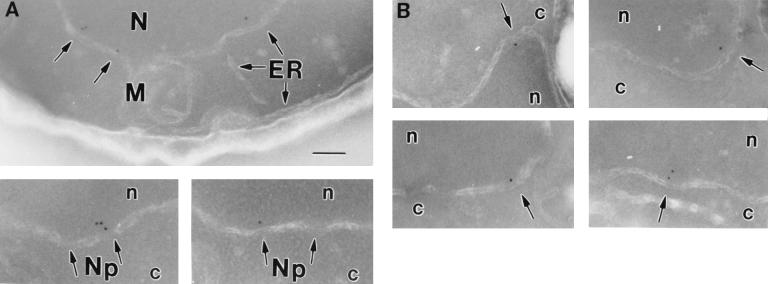
The localization of Srp1p at the NPC was analyzed by immunoelectron microscopy (Fig. 2). Ultrathin cryosections of fixed logarithmically grown wild-type and Δ_NUP2_ cells were incubated with affinity-purified anti-Srp1p antibodies and gold-labeled secondary antibodies. Srp1p was found in the cytoplasm (42% of 633 gold particles quantified), in the nucleoplasm (43%), and at the nuclear envelope (15%) in wild-type cells. The majority (85%) of the gold particles located at the NPC were present at the nuclear side (Fig. 2A, 9B, and 9G). However, the Srp1p population associated with the NPC dropped to 2.5% in Δ_NUP2_ cells (Fig. 2B and data not shown). This is in agreement with the observed loss of Srp1p rim staining in living Δ_NUP2_ cells shown in Fig. 1A. We performed a quantitative analysis of the distribution of NPC-associated gold particles by a method employed by Fahrenkrog and coworkers (9). The distance of gold particles from the horizontal axis (i.e., the central plane) and the vertical axis (i.e., the eightfold symmetry axis) was scored (see Fig. 9G and H). The quantification reveals a major peak of Srp1p in wild-type cells at a distance of ∼20 nm from the horizontal axis facing the nucleoplasm, which corresponds to the nuclear side of the central channel; 70% of the gold particles were found within a radius of 30 nm from the vertical axis. In contrast, no Srp1p was located at the nuclear face of the central channel in Δ_NUP2_ cells, but some NPC-associated Srp1p was found more distal in the basket region (Fig. 2B).
FIG. 2.
Srp1p fails to localize to the nuclear side of the NPC in cells lacking NUP2. Wild-type (W303; A) and Δ_NUP2_ (JLY506; B) cells were grown in liquid medium at 30°C and prepared for immunoelectron microscopy. Ultrathin cryosections of whole cells were incubated with Srp1p-specific antibodies and with secondary antibody-gold complexes (10 nm; Dianova). Intracellular structures: nucleus (N), nuclear pores (arrowheads), mitochondrion (M), endoplasmic reticulum (ER), and vacuole (V). Bars represent 500 nm.
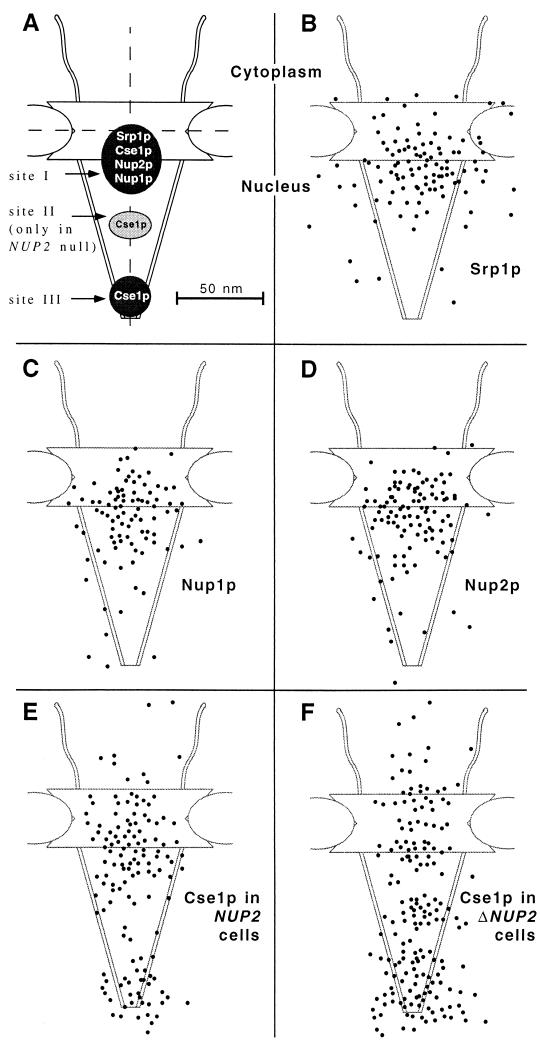
FIG. 9.
(A) Schematic representation of the NPC with the major localization peaks of Srp1p, Nup1p, Nup2p, and Cse1p. All of these proteins colocalize at the nuclear side of the central gated channel in wild-type cells (site I). Cse1p is also present at the distal ring of the nuclear basket (site III). A third Cse1p peak in the central region of the basket (site II) appears only in Δ_NUP2_ cells. The dimensions of the NPC are taken from references 48 and 52. The vertical and horizontal axes are indicated by dashed lines. (B to F) Gold particles corresponding to Srp1p, Nup1p, Nup2p, and Cse1p labeling were projected onto the NPC model. (G to L) Quantification of the distribution at the NPC of gold particles corresponding to Srp1p (128 gold particles), Nup1-GFP (89 gold particles), Nup2-GFP (110 gold particles), GFP-Cse1p in wild-type cells (135 gold particles), and GFP-Cse1p in Δ_NUP2_ cells (180 gold particles).
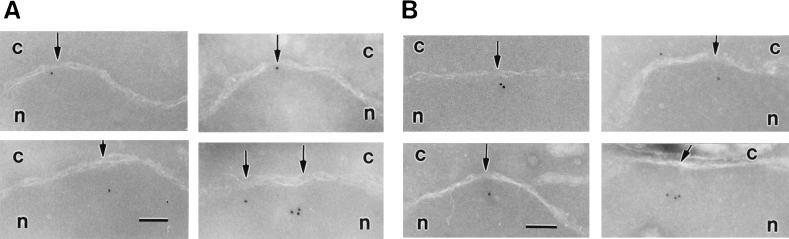
We examined next the distribution of Nup2p at the NPC. We also included Nup1p in this analysis since Nup1p is structurally and functionally related to Nup2p (27) and also interacts with Srp1p (3, 12, 36). NUP1-GFP and NUP2-GFP fusions were integrated into the genome, replacing the wild-type copies of NUP1 and NUP2, respectively. The resulting strains had growth rates identical to those of wild-type cells and exhibited rim staining around the nuclear envelope typical for nucleoporins when analyzed by fluorescence microscopy (not shown). Furthermore, immunoblotting analysis showed that these strains expressed GFP fusions of the expected size (not shown). By immunogold electron microscopy of whole cells with antibodies against GFP, we found 90% of the Nup2-GFP label and 95% of the Nup1-GFP label associated with NPCs (not shown). The two nucleoporins showed very similar distributions at the NPC. Like Srp1p, they are located at the nuclear side of the central channel (Fig. 3). The major peak was observed at a distance of ∼20 nm from the horizontal axis facing the nucleoplasm and within a radius of ∼20 nm from the vertical axis (see Fig. 9C, D, I, and J).
FIG. 3.
Nup2p and Nup1p localize to the nuclear side of the NPC. The genomic copies of NUP2 and NUP1 were replaced by NUP2-GFP (GSY627; A) and NUP1-GFP (GSY652; B), respectively. Cells were grown in liquid medium at 30°C and prepared for immunoelectron microscopy. Ultrathin cryosections were incubated with polyclonal anti-GFP antibodies (Clontech) and with gold-labeled secondary antibodies. Intracellular structures: nucleus (N, n), cytoplasm (c), nuclear pores (Np), mitochondrion (M), and endoplasmic reticulum (ER). The bar represents 100 nm.
NLS protein import is defective in Δ_NUP1_ cells.
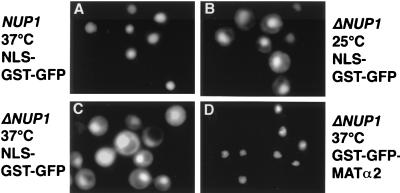
We investigated next whether nup1 mutants, like nup2 mutants, exhibit defects in Srp1p export or NLS protein import. The NUP1 gene is essential for vegetative growth in some but not all strain backgrounds (3, 41). We analyzed a NUP1 deletion strain which is temperature sensitive for growth (23). Indirect immunofluorescence microscopy with Srp1p-specific antibodies demonstrated that Srp1p has the same localization pattern in wild-type and Δ_NUP1_ cells shifted to the nonpermissive temperature of 37°C for 3 h (not shown). However, Δ_NUP1_ cells accumulate the normally nuclear SV40 NLS reporter protein in the cytoplasm at both the permissive and the restrictive temperature (Fig. 4A to C). Likewise, the SV40 NLS-invertase reporter was mislocalized to the cytoplasm (not shown). As a control, we analyzed the nuclear import of the transcription factor Matα2p and of histone H2B, which are both imported into the nucleus independently of Srp1p (M. Greiner and G. Schlenstedt, unpublished data). It was shown before that Δ_NUP1_ cells, which have a normal nuclear envelope morphology, are not defective in import of Matα2p (41). A GST-GFP-Matα2p fusion was found exclusively in the nucleus of wild-type cells (not shown) or Δ_NUP1_ cells at 25 or 37°C (Fig. 4D). Similarly, import of H2B-GFP was not inhibited (not shown). In contrast, nuclear import of H2B was defective in nup1-106 mutants, which display various defects in nuclear functions including structural changes of the nuclear envelope (4). This suggests that the histone H2B import defect in nup1-106 mutants is allele specific. Furthermore, we observed no mislocalization of Cse1p, Yrb1p, and Npl3p in Δ_NUP1_ cells (not shown). We confirmed that nup1 mutants accumulate poly(A)+ RNA in the nucleus (4, 41) (not shown). At present, it is unclear whether this reflects a direct involvement in mRNA export. Taken together, the results indicate that nup1 and nup2 mutants are both specifically defective in NLS protein import. In contrast to nup2 mutants, however, nuclear export of Srp1p is not affected in nup1 mutants.
FIG. 4.
NLS protein import is defective in cells deleted for NUP1. Wild-type (W303; A) and Δ_NUP1_ (LDY461 [23]; B to D) cells were transformed with the reporter plasmid pGS420, encoding NLS-GST-GFP (A to C), or pGS846, encoding GST-GFP-MATα2p (D). Cells were grown in liquid medium containing 2% raffinose and incubated with 2% galactose for 3 h at 25°C (B) or incubated with 2% galactose for 90 min at 25°C and shifted to 37°C for a further 90 min (A, C, and D). GFP fluorescence was visualized microscopically.
Srp1p directly interacts with Nup2p and Nup1p.
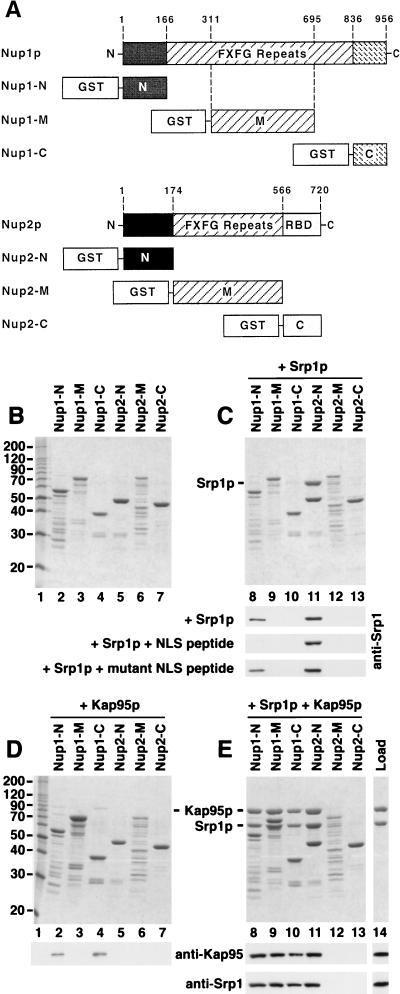
To test for direct interactions of Srp1p with Nup1p and Nup2p, recombinant proteins purified from E. coli were analyzed in liquid binding assays. To map the binding domains, we constructed fusions of GST to the FXFG repeat domains of Nup1p and Nup2p and to the corresponding N- and C-terminal domains that are predicted not to contain FXFG repeats (Fig. 5A). The partially purified fusion proteins, resolved by SDS-polyacrylamide gel electrophoresis (PAGE) are shown in Fig. 5B. The immobilized GST fusion proteins were then incubated with His6-tagged Srp1p. Figure 5C (lane 11) shows a strong binding of Srp1p to Nup2-N. No binding to Nup2-M (middle repeats) or Nup2-C (C terminus) was observed. We detected a weak binding of Srp1p to Nup1-N (Fig. 5C, lane 8), which was confirmed by immunoblotting with anti-Srp1p antibodies. We attempted to further map the Srp1p binding region of Nup2-N (residues 1 to 174) by incubating Srp1p with fusions of GST to smaller overlapping Nup2-N fragments (residues 2 to 84, 43 to 127, and 85 to 175). However, none of these proteins significantly bound to Srp1p, indicating that the whole N-terminal region is required for efficient interaction with Srp1p (not shown).
FIG. 5.
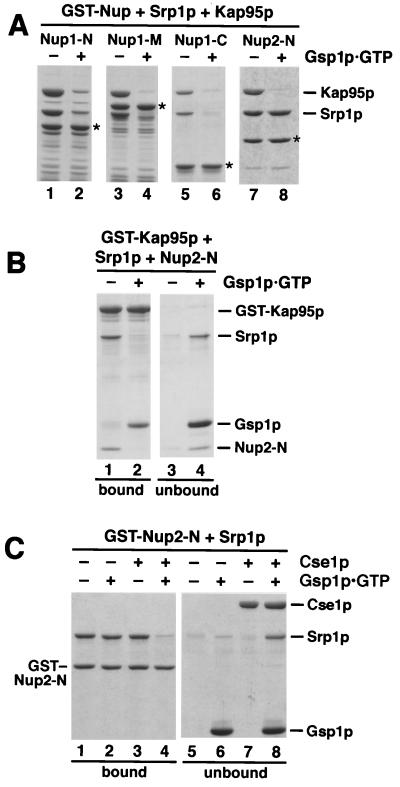
Srp1p and Kap95p directly interact with Nup1p and Nup2p. (A) Domain structures of Nup1p and Nup2p. Nup1p and Nup2p are related in their central domains that contain the FXFG repeats. Nup2p is the only yeast nucleoporin possessing a Ran-binding domain (RBD) of the Yrb1p/RanBP1 type. The residues fused to GST are indicated. The Nup1-M construct contains repeats 6 to 22 of the 29 Nup1p FXFG motifs. Nup2-M contains all 15 Nup2p FXFG repeats. (B to E) Recombinant fusion proteins of GST and the indicated fragments of Nup1p or Nup2p (4 μg) were immobilized to glutathione-Sepharose and incubated with buffer alone (B), with 5 μg of purified Srp1p (C), with 7 μg of purified Kap95p (D), or with Srp1p and Kap95p (E) for 30 min at 4°C. After three washes, bound material was eluted with SDS sample buffer and analyzed by SDS-PAGE and Coomassie blue staining. Positions of molecular weight markers are shown in kilodaltons (lane 1). The eluates as well as Srp1p and Kap95p, representing 25% of the load (lane 14), were also analyzed by immunoblotting with antibodies against Srp1p and Kap95p. GST-Nup fusion proteins were incubated with Srp1p and a 1,000-fold molar excess of SV40 large T-antigen NLS peptides (CTPPKKKRKV) or mutant NLS peptides (CTPPKTKRKV). Bound material was analyzed by immunoblotting with Srp1p-specific antibodies (C).
We then examined the effect of Kap95p on the Srp1p-Nup1/2p interaction. Kap95p alone bound weakly to Nup1-N and Nup1-C but not to Nup2p (Fig. 5D). However, in the presence of Kap95p and Srp1p, both proteins strongly bound to all three Nup1p fragments and to Nup2-N (Fig. 5E, lanes 8 to 11). The formation of 1:1:1 complexes between Srp1p, Kap95p, and Nup1p fragments is therefore the result of a highly cooperative binding of Srp1p and Kap95p. In contrast, Srp1p binds to Nup2-N independently of Kap95p, and Kap95p requires Srp1p for its association with Nup2-N (compare Fig. 5C and E, lanes 11).
Importin α binds simultaneously to importin β and to an NLS protein in the cytoplasm. We next asked whether the NLS-Srp1p-Kap95p complex is capable of binding to Nup1/2p. By using excess amounts of NLS peptides, which drive Srp1p to the NLS-bound form, we showed before that the NLS-bound form of Srp1p is unable to associate with its export receptor Cse1p (47). Similarly, we investigated the effect of SV40 large T-antigen NLS peptides on the binding of Srp1p to Nup1/2p. Figure 5C shows that NLS peptides completely inhibited the binding of Srp1p to Nup1-N, whereas mutant NLS peptides had no effect. On the other hand, the peptides did not affect the binding of Srp1p to Nup2-N. We conclude that the NLS-bound form of Srp1p is unable to bind to Nup1-N. Two possibilities would explain that the NLS peptide did not inhibit the binding of Srp1p to Nup2-N; first, both NLS-bound and NLS-free Srp1p could bind to Nup2p; second, a strong binding of Nup2p to Srp1p could override the inhibitory effect of the peptides. Further experiments showed that the latter explanation is correct (see below).
Nup2p displaces the NLS from Srp1p-Kap95p.
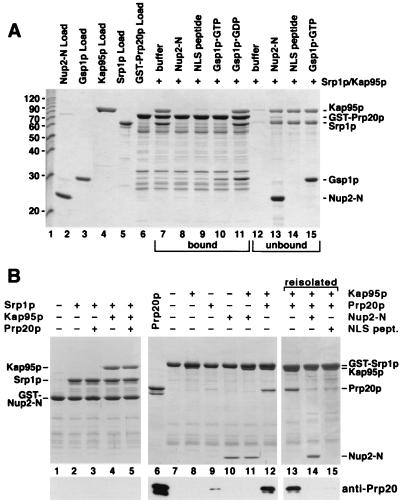
To examine the effect of Nup2p on the NLS-Srp1p-Kap95p complex, we immobilized an NLS protein via the GST tag to glutathione-Sepharose. We used the authentic S. cerevisiae protein Prp20p (Fig. 6A), the yeast guanine nucleotide exchange factor for Gsp1p, which contains a functional NLS (P. Maurer, S. Hahn, and G. Schlenstedt, unpublished data). Trimeric Prp20p-Srp1p-Kap95p complexes formed after incubation with Srp1p and Kap95p that were stable after removal of unbound Srp1p and Kap95p and further incubation for 10 min (Fig. 6A, lanes 7 and 12). As expected (14, 36), Gsp1p-GTP but not Gsp1p-GDP dissociated the trimeric complex (Fig. 6A, lanes 10 and 11). Furthermore, excess amounts of SV40 NLS peptides competed for binding of Srp1p-Kap95p to the immobilized NLS protein (Fig. 6A, lanes 9 and 14). Remarkably, the addition of Nup2-N also resulted in the removal of Srp1p and Kap95p (Fig. 6A, lanes 8 and 13). Only a fourfold molar excess of Nup2-N over immobilized Prp20p completely dissociated the import complex. Purified full-length Nup2p showed the same effect as Nup2-N (not shown). Both Gsp1p-GTP and Gsp1p-GDP also bound directly to Prp20p, the specific nucleotide exchange factor for Gsp1p (Fig. 6A, lanes 10 and 11). We observed essentially the same results when the SV40 large T-antigen NLS was used instead of Prp20p, except that Gsp1p did not bind to GST-SV40 NLS (not shown). Thus, Nup2p is able to displace Srp1p and Kap95p from two different NLSs.
FIG. 6.
Nup2p displaces the NLS protein from the NLS-Srp1p-Kap95p complex. (A) A fusion of GST to Prp20p (8 μg) was immobilized to glutathione-Sepharose and incubated with 8 μg of Srp1p and 12 μg of Kap95p for 30 min at 4°C. After three washes, bound material was further incubated with buffer alone (lanes 7 and 12), 8 μg of Nup2-N (lanes 8 and 13), SV40 NLS peptide (1,000-fold molar excess) (lanes 9 and 13), 10 μg of Gsp1p-GTP (lanes 10 and 15), or Gsp1p-GDP (lane 11). After incubation at 4°C for 10 min, bound (lanes 7 to 11) and unbound (lanes 12 to 15) material was separated and analyzed by SDS-PAGE and Coomassie blue staining. Molecular weight markers (positions indicated in kilodaltons) and loads of recombinant proteins are shown in lanes 1 to 6. (B) GST–Nup2-N (10 μg) (lanes 1 to 5) or GST-Srp1p (8 μg) (lanes 7 to 15) was immobilized to glutathione-Sepharose and incubated for 30 min at 4°C with 8 μg of Srp1p, 12 μg of Kap95p, 10 μg of Prp20p, or 8 μg of Nup2-N as indicated. After three washes, bound material was analyzed by SDS-PAGE and Coomassie blue staining. Samples containing the Prp20p-Srp1p-Kap95p complex were further incubated with buffer (lane 13), with Nup2-N (lane 14), or with SV40 NLS peptide (1,000-fold molar excess) (lane 15). After incubation at 4°C for 10 min, bound material was examined by SDS-PAGE and Coomassie blue staining. Prp20p purified via an N-terminal His6 tag (50% input) was loaded in lane 6. All samples were also analyzed by immunoblotting with anti-Prp20p antibodies.
We next examined whether Prp20p also binds to Nup2p-associated Srp1p (Fig. 6B, lanes 1 to 5). Immobilized Nup2-N was incubated with Prp20p and Srp1p or a mixture of Prp20p, Srp1p, and Kap95p. Regardless of the absence or presence of Kap95p, binding of Prp20p was not detected by Coomassie blue staining or immunoblotting (Fig. 6B, lanes 3 and 5). This indicates that Nup2p-associated Srp1p is unable to bind to Prp20p. We then examined the effect of Nup2p on the binding of Prp20p to immobilized Srp1p. As expected, GST-Srp1p bound to Kap95p, which migrates only slightly faster than GST-Srp1p in SDS-gels (Fig. 6B, lane 8). The binding of Nup2-N to Srp1p was the same in the presence or absence of Kap95p (Fig. 6B, lanes 10 and 11). Kap95p greatly stimulated the association of Srp1p with Prp20p (Fig. 6B, lanes 9 and 12). This confirms that efficient NLS recognition by Srp1p requires the simultaneous binding of Kap95p to Srp1p (36). We then incubated the preformed Prp20p-Srp1p-Kap95p complex with buffer, with Nup2-N, or with excess amounts of SV40 NLS peptides (Fig. 6B, lanes 13 to 15). Remarkably, the binding of Nup2-N to Srp1p-Kap95p caused the complete displacement of Prp20p (Fig. 6B, lane 14). Addition of competing NLS peptides also released Prp20p (Fig. 6B, lane 15). These data show that Srp1p associates either with an NLS or with Nup2p and that Nup2p displaces the NLS upon binding to Srp1p.
Gsp1p-GTP displaces Kap95p from Nup2p and Nup1p.
Our data suggest that the NLS-Srp1p-Kap95p import complex can be dissociated at the nuclear side of the NPC when it approaches Nup2p. We investigated next the fate of Srp1p and Kap95p after release of the NLS protein by Nup2p. To this end, we incubated the Nup2p-Srp1p-Kap95p and Nup1p-Srp1p-Kap95p complexes (Fig. 5E) with Gsp1p-GTP. As shown in Fig. 7A (lanes 1 to 6), Gsp1p-GTP released Srp1p and Kap95p from all three Nup1p fragments. This was expected since the binding of Srp1p and Kap95p to Nup1p is highly cooperative and Kap95p cannot bind to Srp1p and to Gsp1p-GTP simultaneously (36). In contrast, Gsp1p-GTP released Kap95p but not Srp1p from the Nup2p-Srp1p-Kap95p complex (Fig. 7A, lanes 7 and 8). Gsp1p-GDP did not affect the stability of Nup1/2p-Srp1p-Kap95p complexes (not shown). To confirm that the release is caused by a direct binding of Gsp1p-GTP to Kap95p, we immobilized a GST-Kap95p fusion, allowed the Nup2-N–Srp1p–Kap95p complex to form (Fig. 7B), and then incubated the sample with buffer alone or with Gsp1p-GTP. The complex was stable in the absence of Gsp1p-GTP but was completely dissociated by Gsp1p-GTP, which itself forms a stoichiometric complex with Kap95p (Fig. 7B).
FIG. 7.
Kap95p complexed to Gsp1p-GTP does not bind to Nup1/2p-associated Srp1p. (A) Fusions of GST to Nup1-N, Nup1-M, Nup1-C, and Nup2-N (asterisks) were incubated with Srp1p and Kap95p as described for Fig. 5E. After three washes, buffer (lanes 1, 3, 5, and 7) or 10 μg of Gsp1p-GTP (lanes 2, 4, 6, and 8) was added, and the reaction mixtures were further incubated for 15 min at 25°C. After washing, bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE and Coomassie blue staining. (B) Immobilized GST-Kap95p (6 μg) was incubated with 6 μg of Srp1p and 4 μg of Nup2-N for 30 min at 4°C. After three washes, the Kap95p–Srp1p–Nup2-N complex was further incubated with buffer alone (lanes 1 and 3) or with 10 μg of Gsp1p-GTP (lanes 2 and 4) for 15 min. After washing, bound (lanes 1 and 2) and unbound (lanes 3 and 4) material was analyzed by SDS-PAGE and Coomassie blue staining. (C) Cse1p and Gsp1p-GTP release Srp1p from Nup2-N. Immobilized GST–Nup2-N (4 μg per reaction) preincubated with 8 μg of Srp1p was incubated with buffer alone (lanes 1 and 5), with 10 μg of Gsp1p-GTP (lanes 2 and 6), with 12 μg of Cse1p (lanes 3 and 7), or with Gsp1p-GTP and Cse1p (lanes 4 and 8) for 15 min at 25°C. Proteins bound to glutathione-Sepharose (lanes 1 to 4) and the unbound material (lanes 5 to 8) was analyzed by SDS-PAGE and Coomassie blue staining.
Nup2p is involved in Cse1p-dependent export of Srp1p.
Export of Srp1p requires the cooperative binding of the predominantly nuclear proteins Cse1p and Gsp1p-GTP (47). We therefore tested whether Cse1p and Gsp1p-GTP are able to displace Nup2p-bound Srp1p. A GST–Nup2-N–Srp1p complex was incubated with Gsp1p-GTP and Cse1p, individually and together (Fig. 7C). Gsp1p-GTP alone or Cse1p alone did not bind to GST–Nup2-N (not shown) or GST–Nup2-N–Srp1p (Fig. 7C, lanes 1 to 4). However, Gsp1p-GTP and Cse1p together effectively released Srp1p from Nup2-N. Accordingly, we recovered Srp1p, Cse1p, and Gsp1p-GTP in the unbound fraction (Fig. 7C, lanes 4 and 8). It is important to note that we did not observe binding of Cse1p and Gsp1p together to Nup2p-associated Srp1p, which indicates that the binding of Srp1p to Nup2p and its binding to Cse1p are mutually exclusive.
We finally investigated the localization of NPC-associated Cse1p in wild-type and Δ_NUP2_ cells by immunogold electron microscopy. We used strains carrying the integrated GFP-CSE1 allele, which is fully functional (47). Cse1p was located in two distinct clusters at the NPC of wild-type cells (Fig. 8A, 9E, and 9K). The first cluster (∼50% of the NPC-associated label) was present at a distance of ∼20 nm from the central plane toward the nucleoplasm, which corresponds to the nuclear side of the central channel and thus resides in a region similar to that where we also found Srp1p, Nup2p, and Nup1p. The second cluster (∼28% of the label) corresponds to the terminal ring of the nuclear basket of the NPC. This pattern is dramatically changed in Δ_NUP2_ cells (Fig. 8B, 9F, and 9L). A third peak (∼20% of the label) appears in the central region of the basket (∼50 nm from the central plane) of Δ_NUP2_ cells. Less than half as many gold particles as in wild-type cells were now found at the position corresponding to the nuclear side of the central channel. The cluster at the terminal ring of the basket becomes the most prominent (∼40% of the label). The reduced presence of Cse1p at the Srp1p-Nup2p site in Δ_NUP2_ cells suggests an important role for Nup2p in Cse1p export through the NPC.
FIG. 8.
Localization of Cse1p at the NPC in wild-type and Δ_NUP2_ cells. Wild-type cells (GSY580; A) and Δ_NUP2_ cells (GSY777; B) carrying genomic copies of GFP-CSE1 were grown in liquid medium at 30°C and prepared for immunoelectron microscopy. Ultrathin cryosections of whole cells were incubated with anti-GFP antibodies and with secondary antibodies conjugated to 10-nm gold particles. Intracellular structures: nucleus (n), cytoplasm (c), and nuclear pores (arrows). Bars represent 100 nm.
DISCUSSION
Importin α mediates nuclear import of NLS proteins (15, 28, 51) but requires transport receptors of the importin β family for its translocation across the NPC. A fraction of Srp1p is found directly associated with the NPC. Three lines of evidence show that Nup2p provides a major binding site for Srp1p at the NPC. First, Srp1p and Nup2p reside in the very same region of the NPC at the nuclear side of the central channel; second, Nup2p directly binds to Srp1p; and third, Srp1p loses its NPC association in the absence of Nup2p. The accumulation of Srp1p in the nucleus of nup2 mutants indicates that Nup2p is essential for efficient export of Srp1p to the cytoplasm. Mutations in the essential CSE1 gene result in the same phenotype (19, 25, 47). nup2 mutants are also defective in NLS protein import, which can be explained by a diminished concentration of Srp1p in the cytoplasm and/or by a direct involvement in NLS import. Surprisingly, the deletion of NUP2 does not result in a significant growth defect (27). This is elucidated by the less severe Srp1p export and NLS import defects of nup2 mutants compared to cse1 mutants.
According to the current model, the direct interaction of the GTP-bound form of Ran/Gsp1p with importin β is the dissociating step of NLS protein import in the nucleus (13, 30). Kap95p is displaced from the NLS-Srp1p subcomplex upon binding to Gsp1p-GTP. Due to the weaker affinity of Srp1p to the NLS in the absence of Kap95p, the NLS protein is presumably released from Srp1p spontaneously. Our data allow us to hypothesize that an alternative import termination mode could be mediated by Nup2p and Gsp1p-GTP together. Nup2p displaces the NLS protein from the NLS-Srp1p-Kap95p import complex by binding to Srp1p. Subsequently, Kap95p is released by Gsp1p-GTP from the Nup2p-associated Srp1p-Kap95p heterodimer. Nup2p-mediated import termination is expected to be more efficient than termination by Gsp1p alone, as it occurs right after arrival in the nuclear compartment and thus ensures a fast retransport of importins α and β to the cytoplasm. After release of Kap95p from Srp1p-Nup2p, the Kap95p–Gsp1p-GTP complex will be exported first. Srp1p requires the association with Cse1p and Gsp1p-GTP for export. The formation of this complex can also occur at Nup2p, which immobilizes NLS-free Srp1p destined for export at the NPC. Since bindings of Srp1p to Nup2p and to Cse1p are mutually exclusive, the export complex will be released from Nup2p.
Nup1p and Nup2p are related to each other only in their central repeat domains. The cooperative binding of Srp1p and Kap95p to the Nup1p FXFG repeat domain was shown before (36). However, binding of Srp1p-Kap95p to the repeat domain of Nup2p has not been observed. We show here that Nup1-N and Nup1-C, which are predicted not to contain repeat motifs (7), also cooperatively bind to Srp1p and Kap95p. This is best explained by the presence of multiple different binding sites within Nup1p for Srp1p-Kap95p, which evidently represent NLS-like sequences since Srp1p and Kap95p cooperatively associate with Nup1p and binding can be competed with NLS peptides. The Nup1p FXFG repeat domain is able to displace Srp1p-Kap95p from an NLS protein (36). Thus, Nup1p and Nup2p apparently overlap in their function to terminate NLS import. This is illustrated by a specific defect in NLS protein import of both nup1 and nup2 mutants, which could also indicate that Nup1p and Nup2p collaborate in NLS import termination. However, Nup2p acts differently from Nup1p, since efficient binding to Srp1p does not require Kap95p. We found no indication for a role of Nup1p in Srp1p export. Nup1p binds only weakly to Srp1p in the absence of Kap95p, and nup1 mutants are not defective in Srp1p export.
We show that Nup2p binds tightly to the NLS-free form of Srp1p and displaces an NLS protein from Srp1p. An open question is whether Nup2p mediates NLS displacement by competition or by an active release mechanism. Several lines of evidence indicate that Nup2p acts not merely as a strong NLS. First, no clear NLS motif is found within the N-terminal 174 residues. Second, the entire Nup2-N domain is necessary for efficient binding to Srp1p. Third, Nup2p associates with Srp1p independently of Kap95p, whereas Srp1p and Kap95p cooperatively bind to the NLS. Further experiments are necessary to clarify whether Nup2p binds to the NLS recognition site or/and to another region of Srp1p. A recent study also reported on the involvement of Nup2p in nuclear export of Srp1p (5). As in the present study, the authors show the nuclear accumulation of Srp1p in Δ_NUP2_ cells, the binding of Srp1p to Nup1p fragments and to Nup2-N by overlay assays, and the release of Srp1p from Nup2p by Cse1p and Gsp1p. Using SV40 NLS peptide conjugates, a simultaneous binding of Srp1p to NLS peptides and to Nup1p and Nup2p was observed (5). In contrast, we show by different approaches (Fig. 5C, 6A, and 6B) that bindings of Srp1p to an NLS and to Nup1p or Nup2p are mutually exclusive. Moreover, as discussed above, the NLS is released from Srp1p by Nup1p and by Nup2p.
The majority of yeast nucleoporins is located on the cytoplasmic as well as on the nuclear side of the NPC (38, 48). Besides Nup2p, only Nup60p and Nup1p (38) are exclusively found on the nuclear side of the NPC. Most nucleoporins have been localized by pre-embedding labeling methods. To avoid the drawbacks of this technique (antibody accessibility problems and detergent treatment when whole cells are used), we applied postembedding labeling of cryosections. The recently reported localization peak for Nup1p of 53 nm distal of the central plane (38) differs significantly from the value of ∼20 nm distal of the central plane that we obtained. Rout and coworkers applied pre-embedding labeling of nuclear envelope fragments treated with dimethyl sulfoxide and heparin (24, 38). We used postembedding immunolabeling of aldehyde-fixed whole cells, which allows good structure conservation. Therefore, we are convinced that our result more closely reflects the state of the NPC in intact cells. The proposal that Nup1p might be a constituent of the nuclear basket (38) is not supported by our data. In contrast, we found that Nup1p is absent from the basket but present at the nuclear boundary of the central channel. Using whole cells and pre-embedding immunolabeling, Nup2p was recently localized and found at a mean distance of 34 nm distal of the central plane toward the nucleoplasm (18), which is close to the value we observed (∼20 nm). The presence of Nup1p and Nup2p exclusively on the nuclear side of the NPC strongly supports a functional involvement in directional transport processes across the NPC. Besides their role in NLS protein import, Nup1p and Nup2p might also contribute to other import or export pathways by providing specific binding sites for transport factors. Indeed, binding of Nup1p to the Kap95p-related transport receptors Mtr10p, Sxm1p, Nmd5p, and Pdr6p (1, 35, 37, 49) as well as binding of Nup2p to Los1p, Nmd5p, and Pdr6p (1, 17, 49) were detected mostly by overlay assays.
The Srp1p export receptor Cse1p is a mainly nuclear protein but is also found at the NPC and in the cytoplasm. We show that like Srp1p, Cse1p accumulates in the nuclei of Δ_NUP2_ cells. We conclude that Nup2p is necessary for efficient export of Cse1p as well as of Srp1p. Both nup2 export phenotypes combined with the observation that Cse1p and Srp1p tightly associate with Gsp1p suggest that Srp1p is the major if not sole transport substrate of Cse1p and that Cse1p exits the nucleus complexed only to Srp1p. We analyzed the NPC-associated fraction of Cse1p in wild-type cells and nup2 mutants by immunogold electron microscopy. In wild-type cells, the majority of NPC-associated Cse1p colocalizes with Srp1p and Nup2p at the nuclear boundary of the central channel (site I). In nup2 mutants, Nup2p and Srp1p are absent from site I. In the same cells, the presence of Cse1p at site I is reduced to ∼50%. This indicates that half of Cse1p at site I might be associated with Srp1p or Nup2p in wild-type cells. The other half of Cse1p at site I could represent import intermediates. A second localization peak is found at the terminal ring of the basket in wild-type cells (site III). Cse1p accumulates substantially at site III in Δ_NUP2_ cells. A third peak in the central region of the basket (site II) appears only in nup2 mutants. By one model, Cse1p could sequentially move across the NPC toward the cytoplasmic side using certain nucleoporins as preferential binding sites. The population of Cse1p molecules associated with site II might represent a transport intermediate that is detectable only in the absence of Nup2p. The greatly enhanced presence of Cse1p at sites II and III seems to result from a traffic jam within the NPC, which is caused by inefficient export in the mutant.
ACKNOWLEDGMENTS
J. Solsbacher and P. Maurer contributed equally to this work.
We thank Ralf Bischoff and Richard Zimmermann for helpful discussions and critical comments on the manuscript, Markus Greiner for construction of the MATα2 reporter plasmid, and Sandra Ruprecht, Silke Guthörl, Ellen Roth, and Margit Vogel for expert technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Albertini M, Pemberton L F, Rosenblum J S, Blobel G. A novel nuclear import pathway for the transcription factor TFIIS. J Cell Biol. 1998;143:1447–1455. doi: 10.1083/jcb.143.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-β. Cell. 2000;102:99–108. doi: 10.1016/s0092-8674(00)00014-3. [DOI] [PubMed] [Google Scholar]
- 3.Belanger K D, Kenna M A, Wei S, Davis L I. Genetic and physical interactions between Srp1p and nuclear pore complex proteins Nup1p and Nup2p. J Cell Biol. 1994;126:619–630. doi: 10.1083/jcb.126.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogerd A M, Hoffman J A, Amberg D C, Fink G R, Davis L I. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J Cell Biol. 1994;127:319–332. doi: 10.1083/jcb.127.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth J W, Belanger K D, Sannella M I, Davis L I. The yeast nucleoporin Nup2p is involved in nuclear export of importin α/Srp1p. J Biol Chem. 1999;274:32360–32367. doi: 10.1074/jbc.274.45.32360. [DOI] [PubMed] [Google Scholar]
- 6.Chi N C, Adam J H E, Adam S A. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis L I, Fink G R. The NUP1 gene encodes an essential component of the yeast nuclear pore complex. Cell. 1990;61:965–978. doi: 10.1016/0092-8674(90)90062-j. [DOI] [PubMed] [Google Scholar]
- 8.Dingwall C, Laskey R A. Nuclear targeting sequences—a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 9.Fahrenkrog B, Hurt E C, Aebi U, Panté N. Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes. J Cell Biol. 1998;143:577–588. doi: 10.1083/jcb.143.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gant T M, Goldberg M W, Allen T D. Nuclear envelope and nuclear pore assembly: analysis of assembly intermediates by electron microscopy. Curr Opin Cell Biol. 1998;10:409–415. doi: 10.1016/s0955-0674(98)80018-5. [DOI] [PubMed] [Google Scholar]
- 11.Görlich D, Kostka S, Kraft R, Dingwall C, Laskey R A, Hartmann E, Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- 12.Görlich D, Kraft R, Kostka S, Vogel F, Hartmann E, Laskey R A, Mattaj I W, Izaurralde E. Importin provides a link between nuclear protein import and U snRNA export. Cell. 1996;87:21–32. doi: 10.1016/s0092-8674(00)81319-7. [DOI] [PubMed] [Google Scholar]
- 13.Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–670. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 14.Görlich D, Panté N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 15.Görlich D, Prehn S, Laskey R A, Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths G. Labeling reactions for immunocytochemistry. In: Griffiths G, editor. Fine structure immunocytochemistry. Berlin, Germany: Springer; 1993. pp. 237–275. [Google Scholar]
- 17.Hellmuth K, Lau D M, Bischoff F R, Künzler M, Hurt E, Simos G. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol. 1998;18:6374–6386. doi: 10.1128/mcb.18.11.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood J K, Casolari J M, Silver P A. Nup2p is located on the nuclear side of the nuclear pore complex and coordinates Srp1p/importin-α export. J Cell Sci. 2000;113:1471–1480. doi: 10.1242/jcs.113.8.1471. [DOI] [PubMed] [Google Scholar]
- 19.Hood J K, Silver P A. Cse1p is required for export of Srp1p/importin-α from the nucleus in Saccharomyces cerevisiae. J Biol Chem. 1998;273:35142–35146. doi: 10.1074/jbc.273.52.35142. [DOI] [PubMed] [Google Scholar]
- 20.Iovine M K, Wente S R. A nuclear export signal in Kap95p is required for both recycling the import factor and interaction with the nucleoporin GLFG repeat regions of Nup116p and Nup100p. J Cell Biol. 1997;137:797–811. doi: 10.1083/jcb.137.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalderon D, Roberts B L, Richardson W D, Smith A E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 22.Kärgel E, Menzel R, Honeck H, Vogel F, Böhmer A, Schunck W H. Candida maltosa NADPH-cytochrome P450 reductase: cloning of full length cDNA, heterologous expression in Saccharomyces cerevisiae and function of the N-terminal region for membrane anchoring and proliferation of the endoplasmic reticulum. Yeast. 1996;12:333–348. doi: 10.1002/(SICI)1097-0061(19960330)12:4%3C333::AID-YEA915%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Kenna M A, Petranka J G, Reilly J L, Davis L I. Yeast Nle3p/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol Cell Biol. 1996;16:2025–2036. doi: 10.1128/mcb.16.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraemer D M, Strambio-de-Castillia C, Blobel G, Rout M P. The essential yeast nucleoporin NUP159 is located on the cytoplasmic side of the nuclear pore complex and serves in karyopherin-mediated binding of transport substrate. J Biol Chem. 1995;270:19017–19021. doi: 10.1074/jbc.270.32.19017. [DOI] [PubMed] [Google Scholar]
- 25.Künzler M, Hurt E C. Cse1p functions as the nuclear export receptor for importin alpha in yeast. FEBS Lett. 1998;433:185–190. doi: 10.1016/s0014-5793(98)00892-8. [DOI] [PubMed] [Google Scholar]
- 26.Kutay U, Bischoff F R, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 27.Loeb J D J, Davis L I, Fink G R. NUP2, a novel yeast nucleoporin, has functional overlap with other proteins of the nuclear pore complex. Mol Biol Cell. 1993;4:209–222. doi: 10.1091/mbc.4.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb J D J, Schlenstedt G, Pellman D, Kornitzer D, Silver P A, Fink G R. The yeast nuclear import receptor is required for mitosis. Proc Natl Acad Sci USA. 1995;92:7647–7651. doi: 10.1073/pnas.92.17.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moroianu J, Hijikata M, Blobel G, Radu A. Mammalian karyopherin α1β and α2β heterodimers: α1 or α2 subunit binds nuclear localization signal and β subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci USA. 1995;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 31.Nehrbass U, Kern H, Mutvei A, Horstmann H, Marshallsay B, Hurt E C. NSP1: a yeast nuclear envelope protein localized at the nuclear pores exerts its essential function by its carboxy-terminal domain. Cell. 1990;61:979–989. doi: 10.1016/0092-8674(90)90063-k. [DOI] [PubMed] [Google Scholar]
- 32.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 33.Parks T D, Howard E D, Wolpert T J, Arp D J, Dougherty W G. Expression and purification of a recombinant tobacco etch virus NIa proteinase: biochemical analyses of the full-length and a naturally occurring truncated proteinase form. Virology. 1995;210:194–201. doi: 10.1006/viro.1995.1331. [DOI] [PubMed] [Google Scholar]
- 34.Pemberton L F, Blobel G, Rosenblum J S. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- 35.Pemberton L F, Rosenblum S R, Blobel G. A distinct parallel pathway for the nuclear import of an mRNA-binding protein. J Cell Biol. 1997;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblum S R, Pemberton L F, Blobel G. A nuclear import pathway for a protein involved in tRNA maturation. J Cell Biol. 1997;139:1655–1661. doi: 10.1083/jcb.139.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rout M P, Aitchison J D, Suprapto A, Hjertaas K, Zhao Y, Chait B T. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–652. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rout M P, Blobel G, Aitchison J D. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 40.Schaap P J, van Riet J, Woldringh C L, Raué H A. Identification and functional analysis of the nuclear localization signals of ribosomal protein L25 from Saccharomyces cerevisiae. J Mol Biol. 1991;221:225–237. doi: 10.1016/0022-2836(91)80216-h. [DOI] [PubMed] [Google Scholar]
- 41.Schlaich N L, Hurt E C. Analysis of nucleocytoplasmic transport and nuclear envelope structure in yeast disrupted for the gene encoding the nuclear pore protein Nup1p. Eur J Cell Biol. 1995;67:8–14. [PubMed] [Google Scholar]
- 42.Schlenstedt G, Smirnova E, Deane R, Solsbacher J, Kutay U, Görlich D, Ponstingl H, Bischoff F R. Yrb4p, a yeast Ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J. 1997;16:6237–6249. doi: 10.1093/emboj/16.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlenstedt G, Solsbacher J. Transport between the cytoplasm and the nucleus. Protoplasma. 1999;209:166–172. [Google Scholar]
- 44.Schlenstedt G, Wong D H, Koepp D M, Silver P A. Mutants in a yeast Ran binding protein are defective in nuclear transport. EMBO J. 1995;14:5367–5378. doi: 10.1002/j.1460-2075.1995.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senger B, Simos G, Bischoff F R, Podtelejnikov A, Mann M, Hurt E C. Mtr10p functions as a nuclear import receptor for the mRNA binding protein Npl3p. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah S, Tugendreich S, Forbes D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J Cell Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solsbacher J, Maurer P, Bischoff F R, Schlenstedt G. Cse1p is involved in export of yeast importin α from the nucleus. Mol Cell Biol. 1998;18:6805–6815. doi: 10.1128/mcb.18.11.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoffler D, Fahrenkrog B, Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr Opin Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- 49.Titov A A, Blobel G. The karyopherin Kap122p/Pdr6p imports both subunits of the transcription factor IIA into the nucleus. J Cell Biol. 1999;147:235–246. doi: 10.1083/jcb.147.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokuyasu K T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989;21:163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- 51.Weis K, Mattaj I W, Lamond A I. Identification of hSRP1α as a functional receptor for nuclear localization sequences. Science. 1995;268:1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- 52.Yang Q, Rout M P, Akey C W. Three-dimensional architecture of the isolated yeast nuclear pore complex: Functional and evolutionary implications. Mol Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- 53.Zimmer T, Vogel F, Ohta A, Takagi M, Schunck W H. Protein quality—a determinant of the intracellular fate of membrane-bound cytochromes P450 in yeast. DNA Cell Biol. 1997;16:501–514. doi: 10.1089/dna.1997.16.501. [DOI] [PubMed] [Google Scholar]