Altered Extracellular Signal-Regulated Kinase Signaling and Glycogen Metabolism in Skeletal Muscle from p90 Ribosomal S6 Kinase 2 Knockout Mice (original) (raw)
Abstract
The p90 ribosomal S6 kinase (RSK), a cytosolic substrate for the extracellular signal-regulated kinase (ERK), is involved in transcriptional regulation, and one isoform (RSK2) has been implicated in the activation of glycogen synthase by insulin. To determine RSK2 function in vivo, mice lacking a functional rsk2 gene were generated and studied in response to insulin and exercise, two potent stimulators of the ERK cascade in skeletal muscle. RSK2 knockout (KO) mice weigh 10% less and are 14% shorter than wild-type (WT) mice. They also have impaired learning and coordination. Hindlimb skeletal muscles were obtained from mice 10, 15, or 30 min after insulin injection or immediately after strenuous treadmill exercise for 60 min. While insulin and exercise significantly increased ERK phosphorylation in skeletal muscle from both WT and KO mice, the increases were twofold greater in the KO animals. This occurred despite 27% lower ERK2 protein expression in skeletal muscle of KO mice. KO mice had 18% less muscle glycogen in the fasted basal state, and insulin increased glycogen synthase activity more in KO than WT mice. The enhanced insulin-stimulated increases in ERK and glycogen synthase activities in KO mice were not associated with higher insulin receptor or with IRS1 tyrosine phosphorylation or with IRS1 binding to phosphatidylinositol 3-kinase. However, insulin-stimulated serine phosphorylation of Akt was significantly higher in the KO animals. c-fos mRNA was increased similarly in muscle from WT and KO mice in response to insulin (2.5-fold) and exercise (15-fold). In conclusion, RSK2 likely plays a major role in feedback inhibition of the ERK pathway in skeletal muscle. Furthermore, RSK2 is not required for activation of muscle glycogen synthase by insulin but may indirectly modulate muscle glycogen synthase activity and/or glycogen content by other mechanisms, possibly through regulation of Akt. RSK2 knockout mice may be a good animal model for the study of Coffin-Lowry syndrome.
The p90 ribosomal S6 kinase (RSK, or p90RSK) was originally isolated from Xenopus oocytes, where it was found to phosphorylate the S6 ribosomal subunit in vitro (17). Three mammalian homologs of RSK (RSK1-, -2, and -3), each encoded by different genes, have now been cloned (25). These proteins differ from most other protein kinases in that they contain two distinct kinase domains (17). Phosphorylation of RSK substrates is mediated by the N-terminal kinase domain, although the presence of the C-terminal domain is necessary for full activity (6). The C-terminal region contains an autoinhibitory α-helix domain, which prevents autophosphorylation of RSK2 in the basal state (28). RSKs are activated by the extracellular signal-regulated kinases (ERK1 and -2) in vitro and in vivo (39), via phosphorylation on Ser369 and Thr577 (13, 31). The RSK proteins associate with ERK via a short conserved sequence in the extreme C-terminal region of the RSK protein (39), and association with ERK has been shown to be essential for insulin-mediated stimulation of RSK2 in vivo (30). After phosphorylation by ERK, the C-terminal kinase domain autophosphorylates additional sites in the RSK2 molecule, resulting in full activation of the N-terminal kinase domain (13, 31). Although initially thought to be an S6 kinase, more recent studies have shown that the S6 protein is a poor substrate for RSK in vivo (11, 26). Upon activation, RSK2 translocates to the nucleus, where it may phosphorylate various nuclear proteins such as c-Fos, Elk-1, histones, and cAMP-responsive binding-element protein (CREB) (7, 14, 29, 35, 37).
Despite extensive investigation during the last decade, the in vivo functions of RSK remain largely unknown. In the early 1990s, isolated RSK2 from insulin-stimulated rabbit skeletal muscle was found to phosphorylate the G subunit of protein phosphatase 1 (PP1G) in vitro, resulting in increased phosphatase activity toward regulatory phosphorylation sites on glycogen synthase that are dephosphorylated in response to insulin (15). Based on this study, RSK2 was initially believed to mediate the activation of glycogen synthase by activating PP1G, although more recent studies have demonstrated that RSK2 is not involved in regulating glycogen synthase activity in vivo (10, 22). In skeletal muscle, a tissue that expresses high levels of RSK2, insulin and exercise have been shown to be potent regulators of RSK2 activity (20, 40). Since these stimuli are also important activators of gene transcription (19, 24, 27), RSK2 signaling emerges as a likely candidate for transmitting cytosolic signals to the nucleus in skeletal muscle.
To study RSK2 function in vivo, we generated RSK2 knockout (KO) mice by targeted disruption of the rsk2 gene. We found that KO mice are approximately 10 to 15% smaller than wild-type (WT) littermates and have defects in cognition and coordination. Insulin increased glycogen synthase activity in the skeletal muscle, demonstrating that RSK2 is not necessary for stimulating glycogen synthesis with insulin. Treatment with either insulin or exercise resulted in an elevated and prolonged activation of ERK1 and ERK2 phosphorylation in the muscle of KO mice, suggesting that RSK2 plays an important functional role in feedback inhibition of ERK signaling in vivo.
MATERIALS AND METHODS
Generation of RSK2 KO mice.
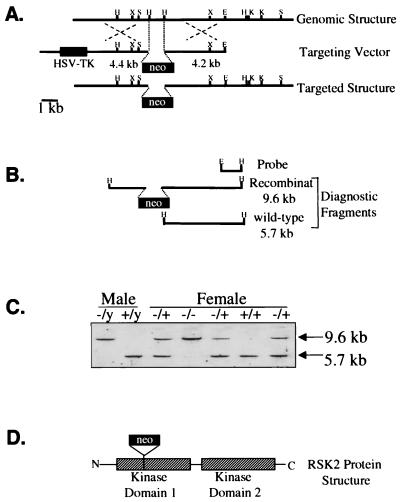
A section of the rsk2 gene (cloned from a 129 mouse library) encoding 31 amino acids of the N-terminal kinase domain was substituted with a _PGK_-neomycin resistance gene neo cassette, resulting in multiple in-frame stop codons in the rsk2 gene. A herpes simplex virus thymidine kinase cassette and parent plasmid sequences were also included in the vector (Fig. 1A). The vector was electroporated into embryonic stem (ES) cells derived from 129 mice followed by selection with G418 and I-131-2′-deoxy-2′-fluoro-b-d-arabinofuranosyl-5-iodouracil (FIAU). Positive clones were identified by Southern blotting, wherein the WT genotype gives a 5.7-kb _Hin_dIII restriction fragment and the targeted gene results in a 9.6-kb fragment (Fig. 1B). ES cells carrying the altered rsk2 gene were microinjected into recipient blastocysts (from C57BL mice). Blastocysts were then implanted into pseudopregnant mice, and chimeric offspring were produced. Mice with a high degree of chimerism (estimated based on coat color; brown for ES cell derived and black for C57BL recipient cell-derived mice) were backcrossed with C57BL mice for three to five generations. The RSK2 gene in humans and mice is localized on the tip of the X chromosome (5). Male mice therefore only have one copy, while females have two copies. Mice with the KO allele were identified by Southern blot of _Hin_dIII-digested genomic DNA obtained from tail biopsy (Fig. 1C). The disrupted rsk2 gene in KO mice results in truncation of the RSK2 protein in the N-terminal kinase domain (Fig. 1D). All mice used in this study were male offspring of a female mouse heterozygous for the KO allele and a WT male mouse. The male progeny of this mating were 50% KO and 50% WT, since the rsk2 gene is located on the X chromosome (observations made during this study). KO mice were identified by PCR amplification of a 300-bp segment of neo (present only in KO mice) from DNA obtained from a tail biopsy. The genotype of mice was later confirmed by immunoblotting muscle lysates for RSK2 protein expression (see below).
FIG. 1.
Gene-targeting strategy. (A) Construction of targeting vector comprised of a plasmid containing a disrupted fragment (neo insertion) of the RSK2 gene. This vector also comprises the herpes simplex virus thymidine kinase (HSV-TK) gene cassette. H, _Hin_dIII; X, _Xho_I; S, _Sph_I; E, _Eco_RI; K, _Kpn_I. (B) Products of _Hin_dIII restriction fragments used for identification WT and KO mice by Southern blotting. The WT genotype gives a 5.7-kb fragment and the targeted gene results in a 9.6-kb fragment. (C) Southern blot using _Hin_dIII-digested genomic DNA from WT and KO mice (both male and female). (D) Schematic drawing of the disrupted RSK2 protein showing the two kinase domains.
Coordination, learning, and cognitive function.
Twenty-week-old mice were subjected to a coordination test in which they balanced on a rotating stick (1.5-cm diameter, 15 rotations per min) for three trials lasting 60 s each. Testing was repeated on 5 consecutive days, and the percentage of mice in each group that completed all three trials successfully was recorded. To assess learning and cognition, 10-week-old mice were placed on one side of a water-filled chamber (55 by 45 cm) with a clear plastic platform submerged 1.5 cm under water on the opposite side. The inner walls of the chamber were marked to provide spatial orientation, and the animals were timed in their ability to locate the submerged platform on three separate occasions.
Insulin and exercise treatments.
For insulin-stimulated effects on ERK signaling, glycogen synthase activity, and c-fos gene transcription, 9- to 18-week-old mice were injected intraperitoneally (i.p.) with porcine insulin (0.05 U/g of body weight [BW]), and control mice were injected with a corresponding volume of saline. Mice were killed by cervical dislocation 10 or 30 min after injection, and gastrocnemius, soleus, and quadriceps muscles were dissected, pooled, and quick frozen in liquid N2. The gastrocnemius, soleus, and quadriceps muscles from one leg were used for immunoblotting, and the same muscles from the other leg were used for isolation of RNA. For studies of glycogen synthase, mice were anesthetized with an i.p. injection of pentobarbital (90 mg/kg of BW) followed by i.p. injection of porcine insulin (0.1 U/g of BW) or a corresponding volume of saline. Mice were killed by cervical dislocation 15 min after insulin injection, and extensor digitorum longus (EDL) muscles from both legs were pooled and used for glycogen and glycogen synthase measurements. For exercise treatment, 25-week-old mice were run on a motorized rodent treadmill for 60 min at 0.9 miles/h up an 8% grade, while untreated littermates served as controls. Hindlimb muscles were rapidly dissected and frozen as described for the insulin treatment.
Immunoblotting.
Muscle was Polytron homogenized as previously described (3). The protein concentrations of the homogenates were measured by the Bradford method (8). Muscle proteins (100 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Nitrocellulose was blocked in Tris-buffered saline with either 5% milk or 5% bovine serum albumin and immunoblotted using antibodies against RSK1 (1 μg/ml), RSK2 (3 μg/ml), RSK3 (1 μg/ml), ERK (1:2,500), dually phosphorylated ERK (1:2,000), or Akt-phospho-Ser473 (1:1,000). The blots were then incubated with a donkey anti-rabbit immunoglobulin G (IgG) antibody conjugated to horseradish peroxidase for 1 h at room temperature followed by enhanced chemiluminescence.
Immunoprecipitation.
Muscle lysates were generated as described above, and 1 mg of protein was incubated with 10 μl of insulin receptor or IRS1 antiserum overnight at 4°C. Immune complexes were precipitated with protein A conjugated to agarose beads and washed twice in ice-cold wash buffer (50 mM HEPES, 100 mM NaF, 2 mM Na3VO4, 1% Triton X-100). IRS1 immunoprecipitates were separated by SDS-PAGE; the portion of membranes containing proteins greater than 111 kDa was immunoblotted using a phosphotyrosine antibody, while the portion containing smaller proteins was immunoblotted for the p85 subunit of phosphatidylinositol 3-kinase. The insulin receptor immunoprecipitates were immunoblotted for phosphotyrosine. Phosphotyrosine blots were incubated with a rabbit anti-mouse secondary antibody. Bound antibody was detected by incubating blots with 125I-labeled protein A for 1 h at room temperature followed by exposure on a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.).
Glycogen synthase activity and muscle glycogen content.
EDL muscle was Polytron homogenized in 19 volumes of ice-cold glycogen synthase buffer (50 mM Tris [pH 7.8], 100 mM NaF, 5 mM EDTA), and glycogen synthase activity was measured as described previously (32). Glycogen synthase activity was expressed as percent I form (enzyme activity without glucose-6-phosphate per activity in presence of 6.7 mM glucose-6-phosphate). To measure glycogen concentrations in the muscle, homogenates from the glycogen synthase assay were incubated in the presence of 2 N HCl for 2 h to hydrolyze glycogen (23). Acid was neutralized with NaOH, and glucose was measured using the hexokinase glucose assay reagent. Glycogen content was expressed as millimolar glycosyl units per kilogram (wet weight) of muscle.
Northern blotting.
RNA was isolated from mouse hindlimb muscle using TriReagent (Molecular Research Center) according to the manufacturer's protocol. Ten micrograms of total RNA was separated by electrophoresis on 1% agarose–6.6% formaldehyde gels and transferred to nylon membranes in 10× SSC (1× SSC is 0.15 M sodium chloride and 0.015 M sodium citrate) via capillary action. Equal loading of RNA was confirmed by ethidium bromide staining of gels. RNA was UV cross-linked to the membrane and prehybridized with nonspecific DNA for 3 h at 42°C. The blots were hybridized with a 32P-labeled 1.8-kb _Eco_RI fragment of the rat c-jun cDNA or a c-fos cDNA probe overnight at 42°C. DNA probes were labeled with [32P]dCTP using a Multiprime DNA labeling kit (Amersham Life Science, Arlington Heights, Ill.). Blots were washed and exposed on a PhosphorImager screen (Molecular Dynamics).
Materials.
Antibodies to phosphotyrosine (pY99), RSK1, and RSK3 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The antibody for ERK1/2 was purchased from Promega (San Luis Obispo, Calif.). The phosphospecific ERK antibody was from Quality Control Biochemicals (Hopkinton, Mass.). The Ser473 phosphospecific Akt antibody was from New England Biolabs (Beverly, Mass.), and the p85 antibody was from Upstate Biotechnology (Lake Placid, N.Y.). Antibodies to the insulin receptor and IRS1 were generously provided by C. Ronald Kahn and Morris White (Joslin Diabetes Center). The RSK2 antibody was generated by injecting rabbits with a synthetic peptide corresponding to amino acid residues 602 to 615 of mouse RSK2 followed by affinity purification of antiserum with the same peptide. Donkey anti-rabbit IgG-horseradish peroxidase secondary antibody, enhanced chemiluminescence reagents, and Multiprime DNA labeling kit were purchased from Amersham. Rabbit anti-mouse IgG and protein A-agarose beads were purchased from Pierce Chemical Company (Rockford, Ill.). 125I-labeled protein A was from ICN Pharmaceuticals (Costa Mesa, Calif.). DNA probes for c-jun and c-fos were provided by Robert Smith (Joslin Diabetes Center). [α-32P]dCTP and [14C]UDP glucose were purchased from New England Nuclear (Boston, Mass.). The hexokinase glucose assay reagent and all other chemicals and reagents were from Sigma Chemical Co. (St. Louis, Mo.).
RESULTS
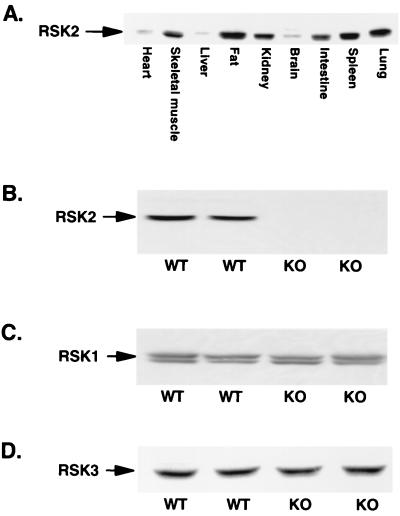
The tissue distribution of the RSK2 protein has not been previously reported; therefore, we first determined the abundance of the protein in numerous mouse tissues. Figure 2A shows that RSK2 is expressed in multiple tissues in normal mice, with highest levels of expression in fat, skeletal muscle, spleen, lung, and kidney. To study the function of RSK2 in skeletal muscle in vivo, we generated mice with targeted disruption of the rsk2 gene as described in Materials and Methods. The absence of the RSK2 protein in KO mice was confirmed by RSK2 immunoblotting of muscle lysates (Fig. 2B). Since there are at least two other RSK isoforms in mammalian tissues, we investigated whether the expression of either of these proteins was altered in muscle from KO animals. As shown in Fig. 2C and D, the expression levels of both RSK1 (320 ± 26 versus 302 ± 13 arbitrary units, n = 5) and RSK3 (116 ± 10 versus 132 ± 13 arbitrary units, n = 12) were similar in skeletal muscle from WT and KO mice, respectively, suggesting that overexpression of these other RSK isoforms does not compensate for the lack of RSK2 in this tissue. KO mice are 10% smaller and 14% shorter than WT mice (Table 1).
FIG. 2.
(A) RSK2 tissue distribution in WT mice. Tissue lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted for RSK2. (B to D) RSK2, RSK1, and RSK3 content in WT and KO mice, respectively. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted with antibody specific for either RSK1 (B), RSK2 (C), or RSK3 (D).
TABLE 1.
Characteristics of mice studied
| Group | Mean ± SD | ||||
|---|---|---|---|---|---|
| Wt (g) | Length (cm) | Basal glycogen content (mg/kg of muscle) | Glycogen synthase activity (% I form) | ||
| Basal | 15-min insulin treatment | ||||
| WT | 26.7 ± 0.4 | 8.76 ± 0.12 | 23.2 ± 0.9 | 18.4 ± 0.5 | 24.3 ± 1.0b |
| KO | 23.9 ± 0.4a | 7.55 ± 0.15a | 19.0 ± 1.2a | 17.5 ± 1.2 | 31.5 ± 2.6a |
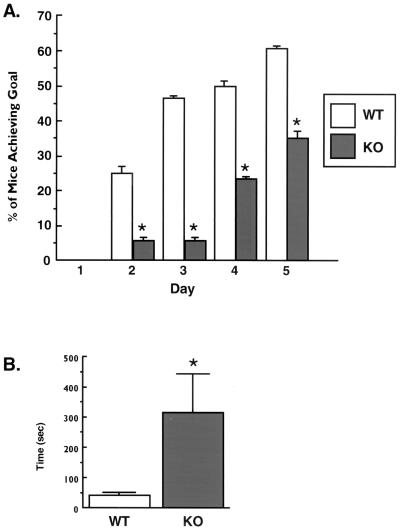
Mutations in the rsk2 gene that disrupt RSK2 activity cause Coffin-Lowry syndrome in humans (33), and this is associated with a severe form of mental retardation (38). Therefore, we assessed possible defects in motor coordination, learning, and cognition in KO mice. Coordination of the mice was assessed over 5 days by testing whether the mice could remain on a rotating stick for three 60-s trials. WT mice consistently demonstrated a greater rate of trial completion during the 5-day testing protocol (Fig. 3A). This suggests that KO animals have poor coordination compared to their WT littermates. Learning and cognition were tested by measuring the time it took mice to find a clear plastic platform submerged 1.5 cm under the water surface in a water-filled chamber. KO mice required about seven times as much time as their WT littermates to find the platform, indicating that they have an impaired learning ability (Fig. 3B).
FIG. 3.
Coordination and cognitive functioning of WT and KO mice. (A) Twenty-week-old WT (n = 22) and KO (n = 22) mice were subjected to a coordination test as described in the text. (B) Ten-week-old WT (n = 5) and KO (n = 5) mice were placed in a water-filled chamber and timed in their ability locate a submerged platform on the opposite side. Data are presented as mean ± standard error swim times from three separate experiments. ∗, P ≤ 0.05 versus WT.
Muscle glycogen concentrations, measured in the fasted state, were significantly lower in the KO mice (Table 1). We next investigated whether the lack of RSK2 affected the activity of glycogen synthase in response to insulin. Glycogen synthase activity was measured in muscle taken from mice 15 min after insulin injection, which represents the time of maximal activation of the enzyme in mouse skeletal muscle. Insulin increased glycogen synthase activity more robustly in the KO mice than in WT mice (Table 1). There was no difference in basal glycogen synthase activity between groups, despite basal glycogen levels being 18% lower in the KO mice. The greater insulin-stimulated glycogen synthase activity was probably responsible for increasing muscle glycogen content to similar levels in KO and WT mice after 30 min of insulin treatment (data not shown).
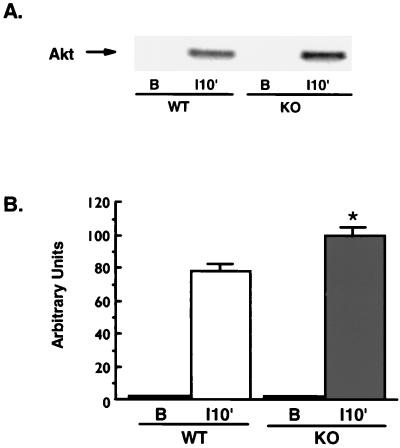
Akt has been suggested to play a role in the activation of glycogen synthesis through its phosphorylation and inactivation of glycogen synthase kinase 3 (GSK3) (12, 34). Akt itself is activated by phosphorylation on both Thr308 and Ser473 (1), and we have confirmed that phosphorylation of Akt on Ser473 closely follows activation of its kinase activity in mouse skeletal muscle (_r_2 = 0.9; P < 0.0001). To test whether the increased insulin-stimulated glycogen synthase activity in the KO mice was associated with greater activation of Akt, we immunoblotted muscle lysates from WT and KO animals with an antibody specific for Akt Ser473 phosphorylation. There was minimal Akt Ser473 phosphorylation in the basal state of both groups of mice, while the phosphorylation was 28% greater in the KO mice with 10 min of insulin treatment (Fig. 4).
FIG. 4.
Akt Ser473 phosphorylation. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted using an antibody specific for Akt only when it is phosphorylated on Ser473, and bands were quantitated by densitometry. (A) Representative immunoblot of muscle lysates from KO (n = 4) and WT (n = 5) mice under basal conditions (lane B) and 10 min after insulin injection (lane 10′). No phosphorylated Akt was detected in basal samples from either group. (B) Quantitation of bands from mice following insulin treatment. A 28% greater insulin-stimulated Akt phosphorylation was observed in lysates from KO mice (∗, P < 0.02 versus WT). Unpaired t tests were used for comparisons between groups.
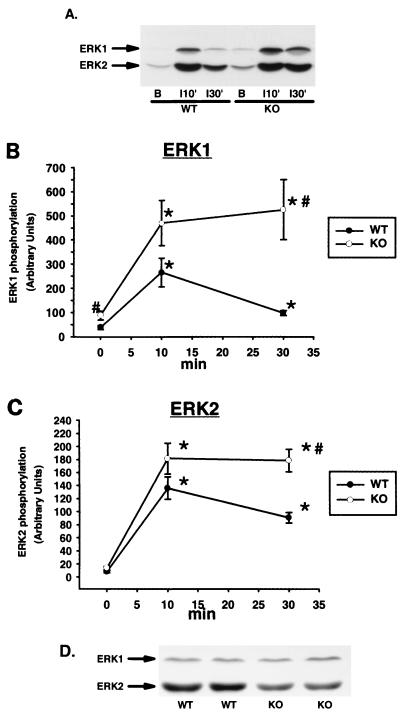
We next assessed ERK activity by immunoblotting muscle lysates with an antibody specific for the dually phosphorylated forms of ERK1 and ERK2. Insulin caused a transient phosphorylation of ERK in the WT mice, with peak phosphorylation occurring 10 min after insulin injection (Fig. 5A to C). Surprisingly, ERK phosphorylation was significantly greater in the KO than in the WT mice in the basal and insulin-stimulated states. Furthermore, insulin resulted in sustained phosphorylation of both ERK isoforms in the KO animals. The greater ERK phosphorylation observed in the KO mice occurred despite lower ERK2 protein expression in these animals (0.69 ± 0.06 versus 0.94 ± 0.06; n = 8 per group; P < 0.01 versus WT), whereas ERK1 levels were not different between groups (Fig. 5D). These data suggest that RSK2 may be involved in feedback inhibition of the ERK pathway in response to insulin treatment.
FIG. 5.
Insulin-simulated ERK phosphorylation and ERK expression. Muscle lysates (100 μg of protein) were separated by SDS-PAGE and immunoblotted with an antibody specific for ERK1 and ERK2 phosphorylated on both tyrosine and threonine residues. (A) Representative immunoblot of muscle lysates from basal (lane B) and insulin-injected (10 and 30 min) KO and WT mice using phosphospecific ERK antibody. (B) Quantitation of multiple ERK1 bands by densitometry. (C) Quantitation of multiple ERK2 samples by densitometry. n = 5 to 16 per group. (D) Representative immunoblot demonstrating ERK expression. Muscle lysates were immunoblotted using an antibody that recognizes ERK regardless of its phosphorylation state. ∗, P < 0.001 versus basal; #, P < 0.05 versus WT. Unpaired t tests were used for comparisons between groups.
We next explored whether the increased stimulation of glycogen synthase, ERK, and Akt by insulin in KO mice was due to increased signaling events proximal to the insulin receptor. To test this hypothesis, we immunoprecipitated the insulin receptor from muscle lysates and immunoblotted with a phosphotyrosine antibody. We found 10-fold stimulation of insulin receptor tyrosine phosphorylation in both WT and KO mice and no differences in basal levels of insulin receptor phosphorylation (data not shown). We also determined whether IRS1 signaling was altered in response to insulin in the KO animals. Muscle lysates were immunoprecipitated with IRS1 antibodies, and the immunocomplexes were immunoblotted using antibodies against phosphotyrosine and the p85 subunit of phosphatidylinositol 3-kinase (data not shown). In both animal groups, insulin treatment resulted in similar increases in IRS1 tyrosine phosphorylation (5-fold above basal) and IRS1-p85 association (4.5-fold above basal) and no changes in basal levels between KO and WT mice (data not shown). These data demonstrate that increased activation of these proximal steps in insulin signaling are not responsible for the increased insulin-stimulated ERK, Akt, and glycogen synthase activity in KO mice.
Since RSK2 has been implicated in the regulation of immediate-early genes, we tested insulin-stimulated induction of c-fos mRNA expression in skeletal muscle from WT and KO mice. RNA was isolated from muscle of WT and KO animals 30 min after injection of insulin or saline, and c-fos mRNA was measured by Northern blot analysis. We found similar two- to threefold increases in c-fos mRNA in both WT and KO animals (data not shown). Thus, RSK2 is not necessary for insulin-stimulated increases in c-fos mRNA concentrations in skeletal muscle in vivo. RSK2 has also been implicated in growth factor-induced phosphorylation of CREB (37). We measured CREB phosphorylation in muscle lysates using an antibody specific for CREB Ser133 phosphorylation and found that CREB phosphorylation was not regulated by insulin treatment in either group at either the 10- or 30-min time point (data not shown). Absolute CREB phosphorylation levels were not different between the two groups.
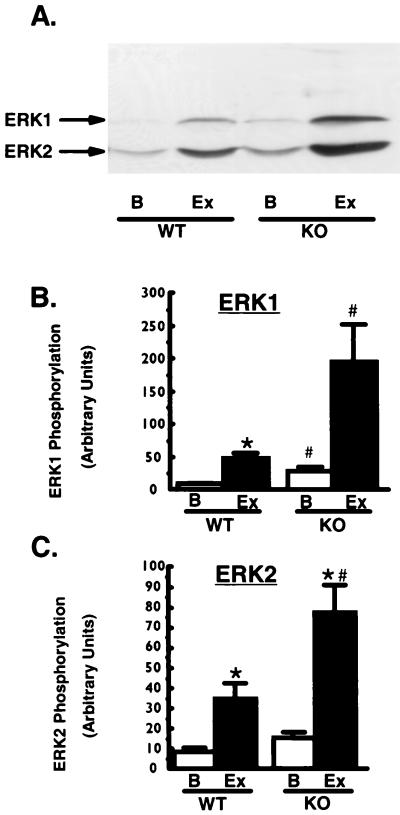
Exercise, another potent stimulator of ERK and RSK2 activity (4, 20), also regulates glycogen metabolism and immediate-early gene transcription in skeletal muscle (36). To study the effects of RSK2 ablation in exercising muscle, we had mice complete 60 min of strenuous treadmill running exercise. Exercise caused a 27% decrease in glycogen levels in both WT and KO mice (data not shown), suggesting similar workload levels for the two groups of animals. Exercise increased ERK phosphorylation in both WT and KO mice (Fig. 6); however, as with insulin treatment, exercise-stimulated ERK phosphorylation was markedly higher in the KO animals. To determine if RSK2 is necessary for exercise-induced increases in c-fos and c-jun gene transcription, we performed Northern blot analysis of RNA isolated from exercised muscle. Exercise induced a 14-fold increase in c-fos mRNA and a 1.5-fold increase in c-jun mRNA in both WT and KO mice. These data demonstrate that RSK2 is not necessary for exercise-induced increases in c-fos and c-jun mRNA in vivo. CREB phosphorylation was not altered by exercise in either group (data not shown).
FIG. 6.
Exercise-stimulated ERK phosphorylation. Muscle lysates (100 μg of protein) were immunoblotted using a phosphospecific antibody specific for ERK1 and ERK2 phosphorylated on both tyrosine and threonine residues. (A) Representative immunoblot of muscle lysates. Lanes: B, basal; Ex, after exercise. (B and C) Quantitation of multiple bands. n = 3 to 12 per group. ∗, P < 0.03 versus basal; #, P < 0.02 versus WT. Unpaired t tests were used for comparisons between groups.
DISCUSSION
A striking characteristic of the RSK2 KO mice is the higher and more sustained phosphorylation of ERK1 and ERK2 in response to both exercise and insulin. One hour of treadmill running exercise induced a twofold higher ERK phosphorylation in the KO mice than in WT littermates. Insulin induced a transient phosphorylation of these kinases, with a peak at 10 min in the WT mice, whereas the KO mice displayed a more pronounced ERK phosphorylation at 10 min which did not decrease by 30 min after insulin injection. In addition to sustained activation, the KO animals also have higher specific ERK phosphorylation both in the basal state and after insulin treatment. This strongly suggests that RSK2 plays a role in regulation of ERK signaling upstream of RSK2. We have not yet determined the mechanism by which lack of RSK2 results in increased ERK activation. One possibility is increased expression and/or activity of an ERK phosphatase, since the ERK pathway itself can increase the expression of certain ERK phosphatases (9). If ERK accomplishes this through activation of RSK, the KO mice would be expected to have lower expression of these phosphatases and thus higher ERK phosphorylation. There is also evidence that RSK2 may normally play a role in feedback inhibition of the ERK pathway at an upstream step. For instance, RSK2 has been shown to phosphorylate the guanine nucleotide exchange factor SOS in cells after growth factor stimulation (16). This causes dissociation of SOS from Grb2, leading to decreased activation of Ras and thus ERK (16).
The KO mice had a greater increase in glycogen synthase activity following insulin treatment compared to WT animals. These results are consistent with in vivo studies of RSK2 and glycogen metabolism where RSK2 activity was found to be dissociated from glycogen synthase activity (10, 22). The enhanced insulin-stimulated Akt activity in the KO mice may be responsible for the greater insulin-stimulated glycogen synthase activity in these animals since Akt is known to deactivate GSK3 (12), which would result in less phosphorylation of glycogen synthase (34). RSK2 has recently been reported to bind to PDK1 (18), a kinase that is responsible for phosphorylating and activating Akt (2). Since the KO mice have no RSK2 to bind PDK1, more of this kinase might be available to activate Akt. This mechanism could explain the modest but significant elevation of Akt phosphorylation we observed after insulin stimulation in the KO mice.
RSK2 has also been implicated in the regulation of immediate early genes such as c-fos and phosphorylation of the transcription factor CREB. Our data suggest that RSK2 is not necessary for either of these processes in skeletal muscle in vivo in response to insulin and exercise. However, our data cannot rule out a role for RSK2 in the induction of c-fos gene transcription by other factors in muscle or other tissues. Since ERK itself is known to translocate to the nucleus and activate various immediate-early genes including c-fos, it is possible that the hyperactivation of ERK in the KO mice may compensate for lack of RSK2 in the muscle of these animals.
In humans, mutations in the rsk2 gene that result in disruption of kinase activity have been shown to cause Coffin-Lowry syndrome (33). This disorder is a severe form of mental retardation that is usually accompanied by facial and digital dimorphisms, as well as progressive skeletal deformations (38). In the present study we observed that KO mice have impaired learning and cognitive functions as well as poor coordination compared to WT littermates. Therefore, RSK2 seems to have similar roles in mental functioning both in mice and humans. However, these deficits were not severe enough to be observed without specific testing. Aside from their smaller size, the physical appearance of the RSK2 KO mice is normal up to at least 36 weeks of age.
In conclusion, RSK2 is not required for insulin- and exercise-stimulated c-fos and c-jun gene transcription or for increasing glycogen synthesis with insulin in skeletal muscle in vivo. Our findings strongly suggest that RSK2 plays an important role in feedback inhibition of the ERK signaling cascade in skeletal muscle. Lack of RSK2 in mice results in coordination, learning, and cognitive deficits. RSK2 null mice could provide a valuable model for studying Coffin-Lowry syndrome and will be an important tool for further studies of RSK2 function in vivo.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AR-42238 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to L.J.G.). K.E.H. was supported by a fellowship grant from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Aronson D, Boppart M D, Dufresne S D, Fielding R A, Goodyear L J. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res Commun. 1998;251:106–110. doi: 10.1006/bbrc.1998.9435. [DOI] [PubMed] [Google Scholar]
- 4.Aronson D, Violan M A, Dufresne S D, Zangen D, Fielding R A, Goodyear L J. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Investig. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorbaek C, Vik T A, Echwald S M, Yang P Y, Vestergaard H, Wang J P, Webb G C, Richmond K, Hansen T, Erikson R L. Cloning of a human insulin-stimulated protein kinase (ISPK-1) gene and analysis of coding regions and mRNA levels of the ISPK-1 and the protein phosphatase-1 genes in muscle from NIDDM patients. Diabetes. 1995;44:90–97. doi: 10.2337/diab.44.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Bjorbaek C, Zhao Y, Moller D E. Divergent functional roles for p90rsk kinase domains. J Biol Chem. 1995;270:18848–18852. doi: 10.1074/jbc.270.32.18848. [DOI] [PubMed] [Google Scholar]
- 7.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brondello J M, Brunet A, Pouyssegur J, McKenzie F R. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- 10.Chang P Y, Le Marchand-Brustel Y, Cheatham L A, Moller D E. Insulin stimulation of mitogen-activated protein kinase, p90rsk, and p70 S6 kinase in skeletal muscle of normal and insulin-resistant mice. Implications for the regulation of glycogen synthase. J Biol Chem. 1995;270:29928–29935. doi: 10.1074/jbc.270.50.29928. [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Kuo C J, Crabtree G R, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinase. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 12.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 13.Dalby K N, Morrice N, Caudwell F B, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- 14.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 15.Dent P, Lavoinne A, Nakielny S, Caudwell F B, Watt P, Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990;348:302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- 16.Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene. 1997;15:373–383. doi: 10.1038/sj.onc.1201214. [DOI] [PubMed] [Google Scholar]
- 17.Erikson E, Maller J L. A protein kinase from Xenopus eggs specific for ribosomal protein S6. Proc Natl Acad Sci USA. 1985;82:742–746. doi: 10.1073/pnas.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frodin M, Jensen C J, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 20.Goodyear L J, Chang P-Y, Sherwood D J, Dufresne S D, Moller D E. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1996;271:E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence J C, Jr, Roach P J. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- 22.Lin T A, Lawrence J C., Jr Activation of ribosomal protein S6 kinases does not increase glycogen synthesis or glucose transport in rat adipocytes. J Biol Chem. 1994;269:21255–21261. [PubMed] [Google Scholar]
- 23.Lowry O H, Passonneau J V. A flexible system of enzymatic analysis. New York, N.Y: Academic Press, Inc.; 1972. pp. 151–156. [Google Scholar]
- 24.Michel J B, Ordway G A, Richardson J A, Williams R S. Biphasic induction of immediate early gene expression accompanies activity-dependent angiogenesis and myofiber remodeling of rabbit skeletal muscle. J Clin Investig. 1994;90:277–285. doi: 10.1172/JCI117318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moller D E, Xia C H, Tang W, Zhu A X, Jakubowski M. Human RSK isoforms: cloning and characterization of tissue-specific expression. Am J Physiol. 1994;266:C351–C359. doi: 10.1152/ajpcell.1994.266.2.C351. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 27.Pearson A M. Muscle growth and exercise. Crit Rev Food Sci Nutr. 1990;29:167–196. doi: 10.1080/10408399009527522. [DOI] [PubMed] [Google Scholar]
- 28.Poteet-Smith C E, Smith J A, Lannigan D A, Freed T A, Sturgill T W. Generation of constitutively active p90 ribosomal S6 kinase in vivo. Implications for the mitogen-activated protein kinase-activated protein kinase family. J Biol Chem. 1999;274:22135–22138. doi: 10.1074/jbc.274.32.22135. [DOI] [PubMed] [Google Scholar]
- 29.Sassone-Corsi P, Mizzen C A, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis C D. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 30.Smith J A, Poteet-Smith C E, Malarkey K, Sturgill T W. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland C, Campbell D G, Cohen P. Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2: identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur J Biochem. 1993;212:581–588. doi: 10.1111/j.1432-1033.1993.tb17696.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas J A, Schlender K K, Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968;25:486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- 33.Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, Mandel J L, Sassone-Corsi P, Hanauer A. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 34.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 35.Ward G E, Kirschner M W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990;61:561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- 36.Williams R S, Neufer P D. Regulation of gene expression in skeletal muscle by contractile activity. In: Rowell L B, Shepherd J T, editors. Handbook of physiology, section 12. New York, N.Y: Oxford University Press; 1999. pp. 1124–1150. [Google Scholar]
- 37.Xing J, Ginty D D, Greenberg M E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 38.Young I D. The Coffin-Lowry syndrome. J Med Genet. 1988;25:344–348. doi: 10.1136/jmg.25.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Bjorbaek C, Moller D E. Regulation and interaction of pp90(rsk) isoforms with mitogen-activated protein kinases. J Biol Chem. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G X, Meier K E, Buse M G. Sequential activation of two mitogen activated protein (MAP) kinase isoforms in rat skeletal muscle following insulin injection. Biochem Biophys Res Commun. 1993;197:578–584. doi: 10.1006/bbrc.1993.2518. [DOI] [PubMed] [Google Scholar]