Natural Killer T Cell Activation Protects Mice Against Experimental Autoimmune Encephalomyelitis (original) (raw)
Abstract
Experimental autoimmune encephalomyelitis (EAE) serves as a prototypic model for T cell–mediated autoimmunity. Vα14 natural killer T (NKT) cells are a subset of T lymphocytes that recognize glycolipid antigens presented by the nonpolymorphic major histocompatibility complex (MHC) class I–like protein CD1d. Here, we show that activation of Vα14 NKT cells by the glycosphingolipid α-galactosylceramide (α-GalCer) protects susceptible mice against EAE. β-GalCer, which binds CD1d but is not recognized by NKT cells, failed to protect mice against EAE. Furthermore, α-GalCer was unable to protect CD1d knockout (KO) mice against EAE, indicating the requirement for an intact CD1d antigen presentation pathway. Protection of disease conferred by α-GalCer correlated with its ability to suppress myelin antigen-specific Th1 responses and/or to promote myelin antigen-specific Th2 cell responses. α-GalCer was unable to protect IL-4 KO and IL-10 KO mice against EAE, indicating a critical role for both of these cytokines. Because recognition of α-GalCer by NKT cells is phylogenetically conserved, our findings have identified NKT cells as novel target cells for treatment of inflammatory diseases of the central nervous system.
Keywords: CD1d, NKT cells, experimental autoimmune encephalomyelitis, autoimmunity, immunotherapy
Introduction
Multiple sclerosis (MS)* is a chronic inflammatory disease of the central nervous system (CNS). Although its precise etiology remains unknown, MS patients typically have circulating T cells directed against the major components of the myelin sheath, suggesting that autoimmunity plays a role in the pathogenesis of MS. Experimental autoimmune encephalomyelitis (EAE) is an autoimmune inflammatory disease that shows many similarities to MS (1). EAE can be induced in susceptible mouse strains by injection of CNS proteins or peptides in adjuvant or by the passive transfer of T cells reactive against such CNS antigens. In both MS and EAE high levels of Th1 cytokines are detected in the CNS at the peak of disease. Most studies have suggested that myelin-specific Th1 cells secreting IFN-γ, TNF-α, and IL-2 mediate EAE (2, 3), whereas myelin-specific Th2 cells producing IL-4 and IL-10 play a regulatory role and are associated with recovery from disease (4–6). Studies with animal models have demonstrated that modulation of immune responses from a Th1-dominant to a Th2-dominant response can effectively protect mice against EAE (for reviews, see references 7 and 8).
Natural killer T (NKT) cells are a unique subset of T lymphocytes that share receptor structures and characteristics with NK cells (for reviews, see references 9–12). A key feature of NKT cells is the expression of a heavily biased TCR, containing Vα14-Jα281 and Vβ8.2, -7, or -2 chains in mice. A similar population of cells, expressing homologous TCR chains (Vα24-JαQ and Vβ11), has been identified in humans (13). NKT cells are abundant in the thymus, liver, and bone marrow, and are also found in peripheral lymphoid organs. One striking property of NKT cells is their capacity to rapidly secrete large amounts of cytokines, including IL-4 and IFN-γ, in response to TCR ligation. Unlike conventional T cells that recognize peptides bound by polymorphic molecules encoded by the MHC, NKT cells are specific for lipid antigens bound by the nonpolymorphic MHC class I–like protein CD1d. Studies with _CD1d_-deficient animals have demonstrated that CD1d expression is required for the development of NKT cells that express the invariant Vα14 TCR (14–16).
Although their precise function remains to be elucidated, NKT cells have been implicated in a variety of immune responses. For example, NKT cells promote protective immunity against the pathogens Borrelia burgdorferi (17), Leishmania major (18), and encephalomyocarditis virus (19) in mice. NKT cells can also play a role in enhancing or suppressing tumor immunity (20–22). A significant amount of evidence indicates that NKT cells play a critical role in the regulation of autoimmune responses. Defects in NKT cell numbers and/or function were noted in a variety of mouse strains that are genetically predisposed for development of autoimmune diseases (23–28). Likewise, abnormalities in the numbers and function of NKT cells have been observed in patients with autoimmune diseases (29–34). NKT cells were also shown to contribute to the induction of tolerance against foreign antigens, including tolerance induced by injection of antigens into the anterior chamber of the eye (35, 36) and tolerance induced against tissue grafts (37, 38). Collectively, these studies have indicated that NKT cells play a critical role in the initiation and regulation of adaptive immunity.
While several glycolipid and phospholipid antigens that can activate NKT cells have been identified (39, 40), the natural ligand recognized by these cells remains to be determined. One NKT cell ligand that has received significant attention is the glycosphingolipid α-galactosylceramide (α-GalCer). α-GalCer was originally isolated from a marine sponge as an agent with profound antimetastatic activities in mice (41). It is now clear that this natural product and its synthetic homologue (KRN7000) are recognized by all CD1d-restricted NKT cells that express the invariant Vα14 TCR (39, 42–45). A number of studies have shown that α-GalCer has profound activities on innate and adaptive immune responses. Administration of α-GalCer to mice leads to the rapid production of a variety of cytokines, including characteristic Th1 and Th2 cytokines (46–49). Stimulation of Vα14 NKT cells in this manner also leads to the rapid activation of NK cells, dendritic cells, B cells, and conventional T cells (46–52). In vivo activation of Vα14 NKT cells with α-GalCer and CD1d polarizes adaptive immune responses for production of Th2 cytokines (48, 49). Hence, α-GalCer may be used to shift the balance from a Th1-dominant toward a Th2-dominant immune response.
Here, we have tested whether activation of NKT cells can modulate immune responses against CNS antigens and protect mice against EAE. Our results demonstrate that α-GalCer protects wild-type but not CD1d knockout (KO) mice against EAE. Prevention of disease correlated with the ability of α-GalCer to promote the generation of CNS antigen-specific Th2 cells. Studies with IL-4 −/− and IL-10 −/− mice further demonstrated that both IL-4 and IL-10 are important for α-GalCer-mediated protection against EAE. Taken together, our findings have identified invariant NKT cells as novel target cells for therapeutic intervention in inflammatory diseases of the CNS.
Materials and Methods
Mice.
Female C57BL/6 (B6), B6.IL-4 −/−, B6.IL-10 −/−, PL/J, and SJL/J (SJL) mice were purchased from The Jackson Laboratory. CD1d −/− mice have been described (14), and were backcrossed 10 times onto the B6 background. At each backcross, heterozygous mice were identified by tail biopsy and Southern blot analysis, as described (14). All mice were bred and maintained in the animal facility at Vanderbilt University School of Medicine (Nashville, TN).
Reagents.
Myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (MOG35–55), MEVGWYRSPFSRVVHLYRNGK, was synthesized by the HHMI biopolymer facility at Duke University (Durham, NC). Myelin basic protein (MBP) was purified from guinea pig spinal cord (Harlan Sprague Dawley Inc.), as described (53). α-GalCer is a glycosphingolipid that was originally isolated as a natural product from the marine sponge Agelas mauritianus (41). In this work a synthetic form of α-GalCer, KRN7000 (54), was used. KRN7000 and synthetic β-GalCer were obtained from Kirin Brewery Co., Ltd.
Detection of NKT Cells by Flow Cytometry.
The liver was perfused with PBS via the portal vein until blanched and distended, and pressed through a 70-μm cell strainer (Becton Dickinson). Hepatocytes were pelleted by centrifugation at 300 g for 3 min. The remaining liver cells in the supernatant were pelleted at 1,200 g for 5 min and then resuspended in a 40% isotonic Percoll solution (Amersham Pharmacia Biotech). This suspension was underlaid with a 60% isotonic Percoll solution. After centrifugation for 20 min at 1,500 g, mononuclear cells were isolated at the 40/60% interface. The cells were subsequently washed once with RPMI 1640 (Life Technologies) supplemented with 5% FCS (HyClone Laboratories Inc.). Liver cells were stained using anti–TCR-β (H57–597; BD PharMingen) and CD1d/α-GalCer tetramers. CD1d/α-GalCer tetramers were generated essentially as reported (55), with minor modifications (unpublished data). Cell suspensions (106 cells) were stained using combinations of FITC-labeled anti–TCR-β antibodies and APC-labeled CD1d/α-GalCer tetramers and analyzed by flow cytometry using a FACSCalibur™ instrument and CELLQuest™ software (Becton Dickinson).
In Vitro Stimulation of Splenocytes with α-GalCer.
Splenocytes (2 × 105/ well) were incubated with titrated doses of α-GalCer in RPMI 1640 supplemented with 10% FCS, 50 μM 2-mercaptoethanol, 2 mM glutamine, antibiotics, and 10 mM HEPES, for 72 h. For proliferation assays, 1 μCi of [3H]thymidine (PerkinElmer) was then added to the wells, and after an additional 16 h of culture, cells were collected with a cell harvester (Tomtec) and uptake of radioactivity was measured with a betaplate reader (Wallac). For measurement of cytokine levels, culture supernatants were collected and cytokine levels were determined by ELISA.
Induction of EAE in Mice.
Active EAE in mice of the B6 background was induced by immunization of 8- to 10-wk-old female animals subcutaneously with 200 μg of MOG35–55 emulsified in complete Freund's adjuvant (Becton Dickinson) on days 0 and 7. Mice also received 500 ng of pertussis toxin (Life Technologies) intraperitoneally on days 0 and 2. For induction of EAE in PL/J and SJL mice, 8- to 12-wk-old female animals were immunized subcutaneously with 300 μg of MBP emulsified in CFA on day 0. Mice receive 500 ng of pertussis toxin intraperitoneally on days 0 and 2.
Clinical Evaluation of EAE.
Clinical symptoms were monitored daily after the first immunization. The clinical score was graded as follows: 0, no disease; 1, tail limpness; 2, hind limb weakness; 3, hind limb paralysis; 4, fore limb weakness; 5, quadriplegia; 6, death. Cumulative disease scores were calculated by adding daily disease scores from the day after immunization until the end of the experiment.
Treatment of Mice with α-GalCer or β-GalCer.
Unless otherwise noted, three injections of α-GalCer (6 μg/mouse/injection), β-GalCer (6 μg/mouse/injection), or vehicle (0.025% polysorbate-20 in PBS) were performed on days 0, 4, and 7 after immunization with CNS antigens. When performed at the same time as antigen immunization, α-GalCer, β-GalCer, or vehicle were mixed together with antigen and CFA and administered by subcutaneous injection. For other time points, α-GalCer, β-GalCer, or vehicle were injected by the intraperitoneal route.
Measurement of Autoantigen-specific T Cell and Antibody Responses.
11 d after EAE induction, cells (5 × 105) from the inguinal and axillary lymph nodes of immunized mice were cultured in RPMI 1640 supplemented with 10% FCS, and stimulated with titrated doses of MOG35–55 or MBP for 60 h. Supernatants were collected and cytokine levels were measured by ELISA. Total and antigen-specific antibody isotype levels in the serum of immunized mice were measured by ELISA.
ELISA.
A standard sandwich ELISA was used to measure mouse IFN-γ, IL-4, IL-10, and antibody isotype levels. IFN-γ, IL-4, IL-10, IL-13, and total IgE were measured using purified and biotinylated antibody pairs and standards from BD PharMingen. For detection, streptavidin-horse radish peroxidase (HRP) conjugate (Zymed Laboratories) was used in conjunction with the substrate 3,3′,5,5′-tetramethylbenzidine (Dako). Antigen-specific IgE levels were measured similarly, but plates were coated with 10 μg/ml of MOG35–55 peptide instead of capture anti-IgE antibody. For measurement of antigen-specific IgM, IgG1 and IgG2a antibodies, immunoplates (Maxisorp; Nunc) were coated with 10 μg/ml of MOG35–55 peptide in 0.1 M Na2HPO4. After blocking with 1% BSA in PBS, serial dilutions of antiserum were added. Detection was performed with anti–IgM-HRP, anti–IgG1-HRP, and anti–IgG2a-HRP antibodies (all from Southern Biotechnology Associates, Inc.), in conjunction with the substrate _o_-phenylene-diamine (Sigma-Aldrich). Concentrations were calculated on the basis of standard curves of antibody isotypes (all from Southern Biotechnology Associates, Inc.) run in parallel ELISA assays.
Statistical Analysis.
Statistical analyses were performed using Student's t test. P values smaller than 0.05 were considered statistically significant.
Results
NKT Cell Numbers and Function in EAE-susceptible Mice.
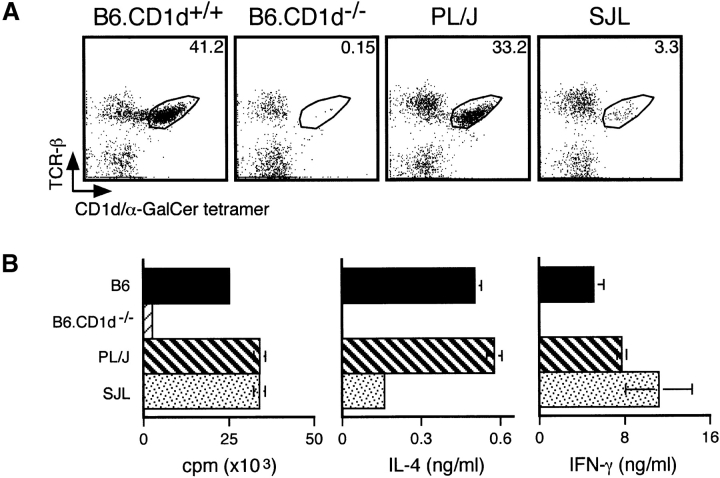
Prior studies have shown that EAE-susceptible SJL mice have numerical and functional defects in NK1.1+ T cells (26). Because recent studies have demonstrated that NK1.1 is not a reliable marker for identification of Vα14 NKT cells (28), we first reevaluated the relative frequency of this cell population in different EAE-susceptible strains. For this purpose we used tetrameric CD1d molecules loaded with α-GalCer, which specifically react with Vα14 NKT cells (55, 56). We found that B6 and PL/J mice have similar numbers of tetramer-positive cells in their liver (Fig. 1 A) and spleen (data not shown), whereas SJL mice have substantially lower numbers of tetramer-positive cells in these organs (Fig. 1 A, and data not shown). Nevertheless, numbers of tetramer-positive cells in SJL mice were significantly higher than in _CD1d_-deficient mice (Fig. 1 A, and data not shown), which have profound defects in Vα14 NKT cell development (14–16, 55, 56).
Figure 1.
Analysis of Vα14 NKT cells in EAE-susceptible mice. (A) Numbers of CD1d/α-GalCer tetramer-positive cells in EAE-susceptible mice. Liver mononuclear cells from the indicated mice were stained with APC-labeled CD1d/α-GalCer tetramers and FITC-labeled anti-TCR-β antibodies and analyzed by flow cytometry. Numbers indicate the percentage of TCR-β+ tetramer+ cells. Representative data are shown from three separate experiments. (B) In vitro responses of spleen cells from EAE-susceptible mice to α-GalCer. Splenocytes (2 × 105) from the indicated mice were cultured with 50 ng/ml of α-GalCer. 3 d later proliferation was measured by [3H]thymidine incorporation and cytokine production in the culture supernatant was measured by ELISA. Data represent the mean ± SE of three mice per group. Representative data are shown from three separate experiments.
To evaluate NKT cell function in EAE-susceptible strains, we measured the proliferative and cytokine responses of splenocytes to in vitro stimulation with α-GalCer. Our results show that these responses from B6 and PL/J splenocytes were very similar, whereas responses were undetectable in CD1d KO mice (Fig. 1 B). In SJL mice, proliferation and IFN-γ production in response to α-GalCer stimulation were comparable to B6 and PL/J mice, whereas IL-4 production was profoundly reduced (Fig. 1 B).
Collectively, these results are in agreement with prior studies (26), indicating that NKT cells in SJL mice are reduced in number and that these cells have functional defects as well.
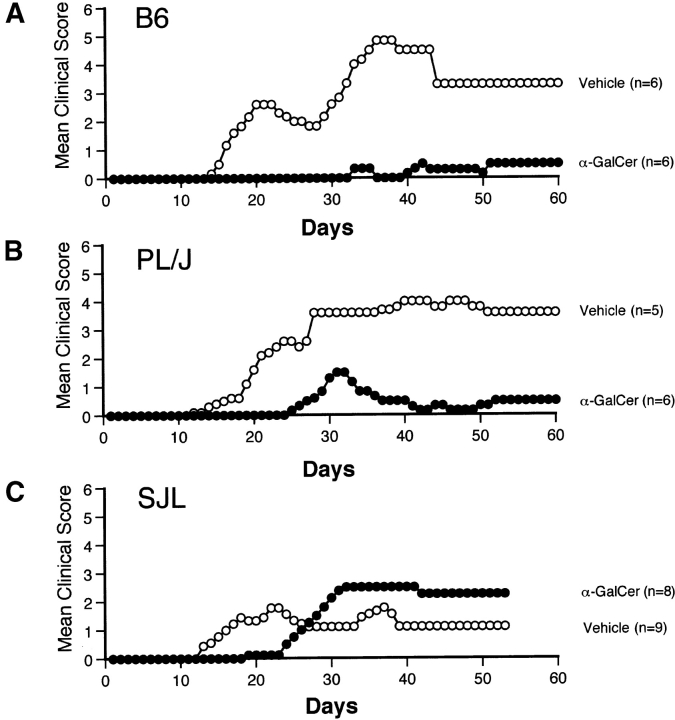
α-GalCer Protects Mice Against EAE.
Next, we tested the ability of α-GalCer to modulate EAE. For these studies, we used three different murine models for EAE: (a) disease induced in B6 (H2b) mice by immunization with the MOG35–55 peptide, (b) PL/J (H2u) mice immunized with MBP, and (c) SJL (H2s) mice immunized with MBP. Representative data from these studies are shown in Fig. 2 and pooled data for a number of separate experiments are summarized in Table I. Results show that α-GalCer effectively prevents EAE in B6 and PL/J mice, but is less effective in SJL mice. In B6 mice, the frequency of disease was 95% in vehicle-treated animals and 28% in α-GalCer–treated animals. The mean onset of disease was 18.0 ± 0.7 d in vehicle-treated B6 mice and 30.5 ± 2.4 d in α-GalCer–treated B6 mice (P < 0.005). Both the mean maximum clinical score (P < 0.005) and the mean cumulative clinical score (P < 0.005) of EAE disease were profoundly reduced in α-GalCer–treated B6 mice (Fig. 2 A, and Table I). Similar results were obtained in PL/J mice, with substantially reduced disease frequency, significant delay in the onset of EAE (P < 0.005), and significant reduction in the mean maximum (P < 0.05) and mean cumulative (P < 0.05) clinical scores in α-GalCer–treated mice, compared with vehicle-treated mice (Fig. 2 B, and Table I). Although α-GalCer treatment significantly delayed disease onset (P < 0.05) in SJL mice, it did not significantly modulate disease frequency and clinical score parameters, and actually enhanced mortality (Table I).
Figure 2.
α-GalCer protects susceptible mice against EAE. EAE was induced in B6 mice (A) by immunization with MOG35–55 peptide, and in PL/J (B) and SJL mice (C) by immunization with MBP, as described in Materials and Methods. Mice were treated with α-GalCer (6 μg/injection) or vehicle (0.025% polysorbate-20 in PBS) on days 0, 4, and 7 after immunization. Representative data from several independent experiments are shown.
Table I.
Modulation of EAE by α-GalCer in Wild-Type and Knockout Mice
| Mouse strain | Treatment | Numberof mice | Onset ofdisease(d) | Diseasefrequency(%) | Mortality(%) | Meanmaximumscore | Meancumulativescore |
|---|---|---|---|---|---|---|---|
| B6 | Vehicle | 21 | 18.0 ± 0.7 | 95 | 24 | 4.0 ± 0.3 | 117.0 ± 17.3 |
| α-GalCer | 25 | 30.5 ± 2.4a | 28 | 8 | 1.3 ± 0.3a | 35.8 ± 14.3a | |
| PL/J | Vehicle | 11 | 15.6 ± 1.5 | 80 | 36 | 3.3 ± 0.8 | 144.0 ± 50.8 |
| α-GalCer | 10 | 26.9 ± 1.3a | 37 | 0 | 0.97 ± 0.7b | 18.3 ± 8.3b | |
| SJL | Vehicle | 9 | 15.8 ± 1.5 | 67 | 11 | 3.3 ± 0.7 | 78.9 ± 33.0 |
| α-GalCer | 8 | 23.8 ± 1.5b | 63 | 38 | 3.4 ± 1.1c | 56.7 ± 27.6c | |
| B6 | Vehicle | 5 | 20.4 ± 2.0 | 100 | 20 | 2.5 ± 0.7 | 108.0 ± 24.1 |
| α-GalCer | 7 | 28.0 ± 3.1b | 40 | 0 | 1.2 ± 1.0b | 10.0 ± 7.1a | |
| β-GalCer | 5 | 12.4 ± 0.7a | 100 | 60 | 5.0 ± 0.8c | 187.6 ± 53.5c | |
| B6.CD1d + / + | None | 8 | 12.9 ± 1.7 | 88 | 13 | 3.4 ± 0.6 | 71.4 ± 28.8 |
| B6.CD1d − / − | None | 9 | 11.7 ± 0.9c | 100 | 22 | 3.6 ± 0.5c | 87.3 ± 25.9c |
| B6.CD1d + / + | α-GalCer | 16 | 18.3 ± 2.1 | 25 | 0 | 0.4 ± 0.2 | 5.5 ± 4.2 |
| B6.CD1d − / − | α-GalCer | 19 | 13.1 ± 1.2a | 90 | 5 | 2.2 ± 0.3a | 31.3 ± 7.6a |
| B6.IL-4 − / − | Vehicle | 11 | 15.9 ± 1.2 | 100 | 64 | 5.0 ± 0.4 | 150.2 ± 28.2 |
| α-GalCer | 11 | 13.5 ± 1.1c | 100 | 100 | 6.0 ± 0.0b | 282.5 ± 9.0a | |
| B6.IL-10 −/− | Vehicle | 11 | 14.6 ± 1.0 | 100 | 64 | 5.1 ± 0.4 | 188.8 ± 37.3 |
| α-GalCer | 11 | 15.5 ± 0.8c | 100 | 55 | 4.2 ± 0.6c | 155.7 ± 32.1c |
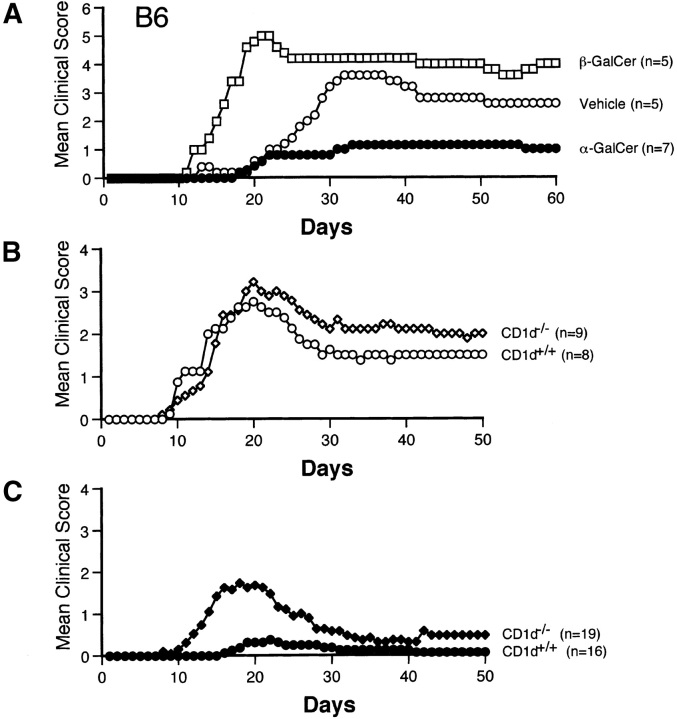
Disease Protection Mediated by α-GalCer Requires a Functional CD1d Antigen Presentation Pathway.
To provide evidence that the activities of α-GalCer on EAE are mediated by stimulation of CD1d-dependent Vα14 NKT cells we tested the ability of β-GalCer, which binds with CD1d but does not activate NKT cells (39), to modulate EAE induced in B6 mice by the MOG35–55 peptide. Our results show that β-GalCer not only fails to protect mice against EAE, but actually enhances disease (Fig. 3 A, and Table I). As compared with vehicle-injected mice, β-GalCer–treated animals had quicker onset of disease (P < 0.005) and increased mean maximum and cumulative clinical scores, although differences for the latter disease parameters did not reach statistical significance. Mortality in β-GalCer–treated animals, compared with vehicle-treated mice, was enhanced (Table I).
Figure 3.
The capacity of α-GalCer to protect mice against EAE is mediated by activation of CD1d-restricted NKT cells. (A) β-GalCer, which binds with CD1d but does not activate NKT cells, fails to protect mice against EAE. B6 mice were immunized with MOG35–55 peptide and treated with α-GalCer (6 μg/injection), β-GalCer (6 μg/injection), or vehicle. (B) CD1d deficiency does not exacerbate EAE in B6 mice. B6.CD1d +/+ and B6.CD1d −/− mice from the 10th backcross to B6 were immunized with MOG35–55 peptide. (C) Protection against EAE requires CD1d expression in mice. EAE was induced in B6.CD1d +/+ and B6.CD1d −/− mice by immunization with MOG35–55. Mice were treated with α-GalCer (4 μg/injection) on days 0 and 7.
To further investigate the role of the CD1d antigen presentation pathway for the activities of α-GalCer on EAE, we examined its ability to modulate disease in _CD1d_-deficient mice that were backcrossed 10 times to the B6 strain. While none of the disease parameters were significantly different among untreated B6.CD1d +/+ and B6.CD1d −/− mice (Fig. 3 B, and Table I), α-GalCer effectively protected B6.CD1d +/+ but not B6.CD1d −/− mice against EAE (Fig. 3 C, and Table I).
These results provide strong evidence that α-GalCer protects mice against EAE by the CD1d-dependent activation of Vα14 NKT cells.
α-GalCer Promotes CNS Antigen-specific Th2 Responses in EAE-induced Mice.
To investigate the immunological mechanisms for disease protection mediated by α-GalCer we measured Th cell responses directed against the immunizing CNS antigens. B6 and PL/J mice were immunized with myelin antigens and treated with vehicle or α-GalCer. 11 d after induction of EAE, we measured T cell–mediated cytokine responses against MOG35–55 peptides and MBP in B6 and PL/J mice, respectively. In B6 mice immunized with MOG35–55, α-GalCer induced a sharp reduction in IFN-γ production and a significant increase in IL-10 production by lymph node cells in response to in vitro restimulation with MOG35–55 peptide, but did not significantly influence IL-4 production by these cells (Fig. 4 A). In MBP-immunized PL/J mice, α-GalCer treatment reduced IFN-γ, enhanced IL-4 and IL-10 (Fig. 4 B), and did not significantly influence IL-13 production (data not shown) by lymph node cells in response to in vitro restimulation with MBP. Hence, we conclude that α-GalCer induces a shift from a Th1-dominant toward a Th2-dominant myelin antigen-specific response.
Figure 4.
α-GalCer promotes antigen-specific Th2 responses in mice immunized with CNS antigens. B6 mice (A) were immunized with MOG35–55 and PL/J mice (B) were immunized with MBP to induce EAE. Mice were treated with α-GalCer or vehicle as described in the legend to Fig. 2. At day 11 after immunization cells from inguinal and axillary lymph nodes were cultured with the indicated concentrations (in μg/ml) of MOG35–55 peptide (A) or MBP (B). After 60 h of culture, supernatants were collected and cytokine levels were measured by ELISA. Data shown represent the mean values ± SE of 6 mice in each experimental group. Representative data are shown from three separate experiments.
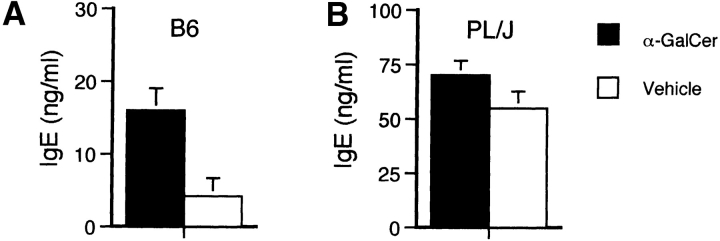
To evaluate effects of α-GalCer on humoral immune responses in mice immunized with CNS antigens, we measured total serum and MOG35–55–specific Ig isotype levels 11 d after immunization and α-GalCer treatment. We observed a significant increase in total IgE levels in α-GalCer–treated B6 mice immunized with MOG35–55 (Fig. 5 A), but no significant α-GalCer–induced alterations in serum IgE levels in PL/J mice immunized with MBP (Fig. 5 B). α-GalCer did not significantly alter total serum IgM, IgG1 and IgG2a levels in B6 and PL/J mice (data not shown). Likewise, no significant differences were observed for MOG35–55–specific IgG1 and IgG2a levels among α-GalCer– and vehicle-treated mice, and MOG35–55–specific IgE levels were undetectable in all mice (data not shown).
Figure 5.
IgE antibody production in the serum of mice immunized with CNS antigens and treated with α-GalCer. EAE was induced in B6 mice (A) by immunization with MOG35–55 peptide and in PL/J (B) mice by immunization with MBP. Mice were treated with α-GalCer or vehicle as described in the legend to Fig. 2. Mice were bled on day 11 after immunization and total IgE levels in the serum were measured by ELISA. Data shown represent the mean values ± SE of four (A) or six (B) mice in each experimental group. Representative data are shown from two separate experiments.
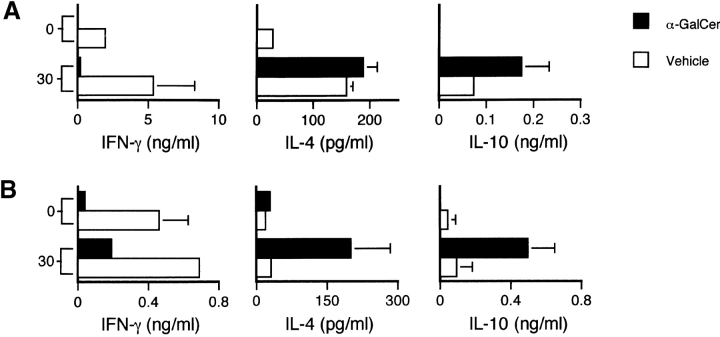
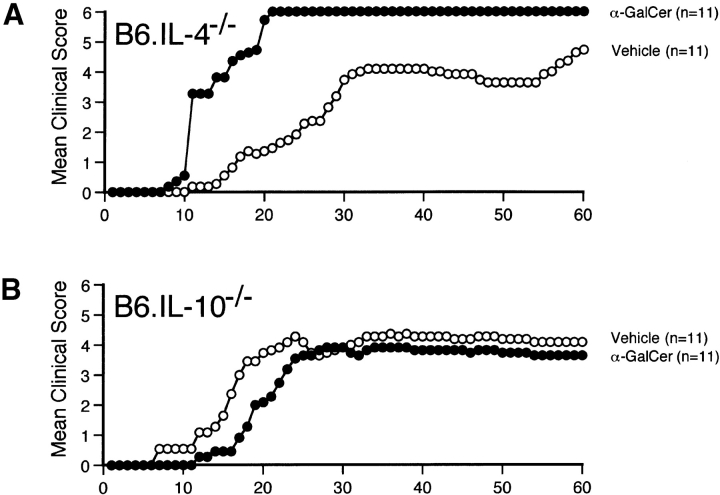
α-GalCer Fails to Protect IL-4 KO and IL-10 KO Mice Against EAE.
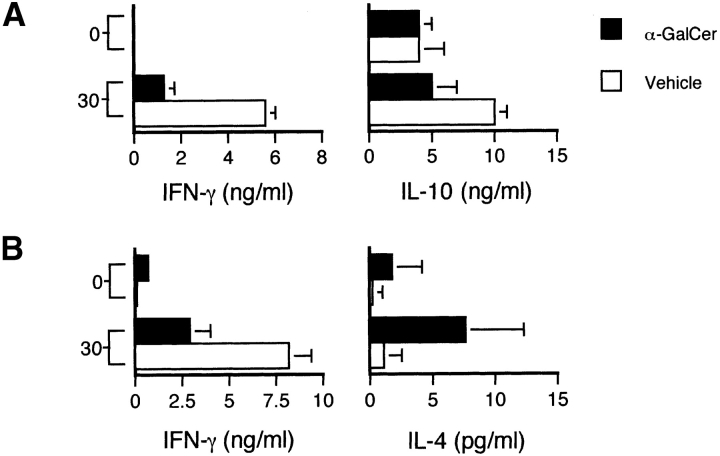
To evaluate the role of the Th2 cytokines IL-4 and IL-10 for the effects of α-GalCer on EAE, we tested the ability of this compound to modulate disease in IL-4 −/− and IL-10 −/− mice crossed onto a B6 background. Results show that α-GalCer is unable to protect either of these cytokine-deficient strains against EAE (Fig. 6, and Table I). In B6.IL-4 −/− mice, α-GalCer actually caused more severe disease, with enhanced mean maximum (P < 0.05) and mean cumulative (P < 0.005) clinical scores and enhanced mortality. While α-GalCer was unable to protect these animals against EAE, it significantly suppressed IFN-γ production by MOG35–55–specific T cells in both IL-4 KO and IL-10 KO mice (Fig. 7), and enhanced IL-4 production by MOG35–55–specific T cells in IL-10 KO mice (Fig. 7 B). These findings indicate a critical role for both IL-4 and IL-10 in the ability of α-GalCer to protect mice against EAE.
Figure 6.
α-GalCer fails to protect IL-4 KO and IL-10 KO mice against EAE. B6.IL-4 −/− (A) and B6.IL-10 −/− (B) mice were immunized with MOG35–55 peptide to induce EAE and treated with α-GalCer or vehicle as described in the legend to Fig. 2. In parallel experiments α-GalCer effectively protected wild-type B6 mice against EAE (data not shown). Representative data are shown from three separate experiments.
Figure 7.
CNS antigen-specific responses in IL-4 KO and IL-10 KO mice immunized with MOG35–55 peptides and treated with α-GalCer. B6.IL-4 −/− (A) and B6.IL-10 −/− (B) mice were immunized with MOG35–55 peptide to induce EAE and treated with α-GalCer or vehicle as described in the legend to Fig. 2. At day 11 after immunization cells from inguinal and axillary lymph nodes were cultured with the indicated concentrations (in μg/ml) of MOG35–55 peptide (A) or MBP (B). After 60 h of culture, supernatants were collected and cytokine levels were measured by ELISA. Data shown represent the mean values ± SE of four mice in each experimental group. Representative data are shown from three separate experiments.
Discussion
EAE serves as a prototypic model for T cell–mediated autoimmunity. At the peak of disease, high levels of Th1 cytokines are detected in the CNS (2, 3). A number of studies have shown that treatment with Th2-promoting cytokines or with monoclonal antibodies directed against Th1-promoting cytokines can effectively protect mice against EAE (for reviews, see references 7 and 8). Here, we have shown that specific activation of Vα14 NKT cells with its ligand α-GalCer provides an alternative way to shift the balance from a pathogenic Th1 toward a protective Th2 response. Disease protection required CD1d expression and was associated with suppressed CNS antigen-specific Th1 responses. We also identified a critical role for the Th2 cytokines IL-4 and IL-10 in the ability of α-GalCer to protect mice against EAE. These findings not only support the proposed role of NKT cells in the regulation of self-tolerance but also highlight the potential use of α-GalCer for therapeutic intervention in a variety of Th1-mediated diseases.
A number of different cell types play a regulatory role in the pathogenesis of EAE. These include Th2 cells (4–6) and other regulatory CD4+ T cells (57, 58), CD8+ T cells (59–61), B cells (62), and NK cells (63). In addition, a regulatory role for NKT cells in the pathogenesis and recovery from EAE was suggested by the finding that SJL mice have reduced numbers of NK1.1+ T cells and defects in the in vivo cytokine response to anti-CD3 stimulation (26). Our CD1d/α-GalCer tetramer staining (Fig. 1 A) and in vitro α-GalCer stimulation (Fig. 1 B) experiments are in agreement with this conclusion. Based on these findings, complete elimination of CD1d-restricted NKT cells in mice would be predicted to exacerbate EAE disease. However, we found that all disease parameters in MOG35–55–immunized B6.CD1d +/+ and B6.CD1d −/− mice were indistinguishable (Fig. 3 B, and Table I). Because the animals used in these experiments had been backcrossed a total of 10 times onto the B6 strain it is unlikely that this result was due to resistance genes that were carried over from the 129 background originally used to generate the KO mice (14). Consistent with this finding, we have recently found that disease in Vα14 transgenic B6 mice is indistinguishable from control animals (unpublished data). Nevertheless, it remains possible that NKT cells play a role in the regulation of EAE induced by other CNS antigens and/or in other mouse strains.
α-GalCer profoundly suppressed all disease parameters in B6 mice immunized with MOG35–55 and PL/J mice immunized with MBP (Fig. 2, A and B, and Table I). In SJL mice immunized with MBP α-GalCer significantly delayed the onset of disease, but did not significantly alter other disease parameters (Fig. 2 C, and Table I). The reduced efficacy of α-GalCer for treatment of EAE disease in SJL mice is consistent with the reduced numbers of Vα14 NKT cells in these animals (Fig. 1 A) and with the functional defects of splenocytes to in vitro stimulation with α-GalCer, in particular the profound reduction in IL-4 responses (Fig. 1 B).
The requirement for CD1d molecules and Vα14 NKT cells in the effects of α-GalCer on EAE was demonstrated by the failure of β-GalCer to protect mice against disease (Fig. 3 A), and the inability of α-GalCer to prevent disease in _CD1d_-deficient mice (Fig. 3 C). Although β-GalCer can bind with CD1d molecules, it does not activate Vα14 NKT cell hybridomas nor does it stimulate in vitro cytokine production by splenocytes (39) (unpublished data). It is therefore surprising that β-GalCer actually enhances disease in B6 mice immunized with MOG35–55 (Fig. 3 A, and Table I). One potential explanation for this unexpected finding is that β-GalCer competes with an endogenous ligand for binding with CD1d and thus prevents or perhaps antagonizes NKT cell recognition of endogenous antigens. The precise mechanism by which β-GalCer exacerbates disease requires further investigation.
Activation of Vα14 NKT cells leads to the rapid production of a variety of cytokines (46, 48, 49, 51), including IL-4 and IL-10, which promote Th2 differentiation, and IFN-γ, which promotes Th1 differentiation. It was also shown that administration of α-GalCer to mice polarizes Vα14 NKT cells for loss of IFN-γ but maintenance of IL-4 production (48, 49). In turn, injection of α-GalCer into mice polarizes Th cell responses for Th2 differentiation (48, 49). In this study, we found that α-GalCer similarly suppresses Th1 and/or enhances Th2 differentiation of CNS antigen-specific T cells (Fig. 4). Because Th2 cytokines can suppress EAE (for reviews, see references 7 and 8), it is tempting to speculate that the activities of α-GalCer on EAE are due to its effects on the differentiation of autoantigen-specific Th cells. The cytokines produced by α-GalCer–activated NKT cells (i.e., IL-4 and IL-10) may directly influence the differentiation of autoantigen-specific Th cells. Alternatively, α-GalCer–activated NKT cells may influence Th cell differentiation through their activities on antigen-presenting cells (47, 48, 51). Our finding that α-GalCer fails to protect IL-4 KO and IL-10 KO mice against EAE (Fig. 6, and Table I) is in agreement with a critical role of Th2 cytokines. Th2 cytokine production by both NKT cells and CNS antigen-specific Th cells may be important for the protective effects of α-GalCer on EAE disease. This issue will be addressed in future studies.
Collectively, our studies suggest the following sequence of events to account for the ability of α-GalCer to protect mice against EAE. α-GalCer activates Vα14 NKT cells and polarizes these cells for loss of IFN-γ production. Alternatively, the NKT cells from α-GalCer–injected mice may lose the capacity to induce IFN-γ production by other cell types, such as NK cells. In turn, these altered early immune responses influence adaptive immune responses against the immunizing CNS antigens, thus effectively shifting the balance from a pathogenic Th1 toward an innocuous or protective Th2 response. Future studies will be aimed at testing this hypothesis.
In addition to its protective effects on EAE, α-GalCer protects non-obese diabetic (NOD) mice against Type 1 diabetes (64–66), and has some efficacy in experimental models for inflammatory bowel disease (67). In the case of Type 1 diabetes, the protective effects of α-GalCer correlated with its ability to suppress IFN-γ production by NKT cells and to promote islet autoantigen-specific Th2 cell responses (64, 65). Thus, α-GalCer may prove useful for treatment of a variety of diseases, including MS, Type 1 diabetes, and rheumatoid arthritis, that are mediated by pathogenic Th1 cells and where a Th2 bias is beneficial.
With regard to the clinical applications of α-GalCer therapy, it should be noted that α-GalCer induces significant liver toxicity in mice (68). However, no adverse effects have been observed in clinical trials for using α-GalCer as a treatment for human cancers (69). In addition, potential toxic effects of α-GalCer therapy may be overcome with analogs of this chemical that are also recognized by NKT cells (39, 54), or by combining α-GalCer therapy with reagents such as IL-7 (70), IL-18 (71), anti-CD86 antibodies (72), and anti-CD154 antibodies (73), that suppress Th1 or enhance Th2 cytokine production by NKT cells. In this context, combined α-GalCer and IL-7 therapy is superior for prevention of autoimmune diabetes in NOD mice (65) and anti-CD86 antibodies synergize with α-GalCer for treatment of EAE disease (72).
It was recently reported that an analogue of α-GalCer, termed OCH, with a truncated sphingosine chain, preferentially stimulates NKT cells to produce IL-4 (74). These investigators further showed that a single injection of the OCH analogue at the time of priming of B6 with MOG35–55 peptides provides partial protection against EAE (74). However, using the same treatment protocol α-GalCer did not alter EAE (72, 74). The latter finding is at variance with the studies presented here and is most likely due to differences in the administration protocol. Yamamura and colleagues gave mice a single α-GalCer injection at the time of priming, whereas our standard treatment protocol involves three injections at days 0, 4, and 7 after EAE induction. In fact, our treatment protocol using α-GalCer provides more robust protection against EAE than the protocol of Yamamura and colleagues using OCH. Thus, administration of OCH according to our treatment protocol should provide more optimal protection against disease than reported by Yamamura and colleagues (74) and described here for α-GalCer.
One striking property of the CD1d antigen presentation pathway is its remarkable conservation among different species (42, 44, 45). Thus, α-GalCer binds both mouse and human CD1d and is recognized by invariant NKT cells from either species (39, 42, 44, 45). Therefore, the studies presented here for mice are directly relevant to modulation of human autoimmune diseases.
Acknowledgments
The authors thank Dr. Vipin Kumar for sharing information prior to publication, Kirin Brewery Co., Ltd. for providing synthetic α-GalCer and β-GalCer, and Jie Wei and Michele Nadaf for technical assistance.
This work was supported by the Howard Hughes Medical Institute.
A.K. Singh, M.T. Wilson, and S. Hong contributed equally to this work.
Footnotes
*
Abbreviations used in this paper: α-GalCer, α-galactosylceramide; β-Gal- Cer, β-galactosylceramide; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; HRP, horseradish peroxidase; KO, knockout; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NKT, natural killer T; NOD, non-obese diabetic.
References
- 1.Steinman, L. 1999. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 24:511–514. [DOI] [PubMed] [Google Scholar]
- 2.Powell, M.B., D. Mitchell, J. Lederman, J. Buckmeier, S.S. Zamvil, M. Graham, N.H. Ruddle, and L. Steinman. 1990. Lymphotoxin and tumor necrosis factor-α production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int. Immunol. 2:539–544. [DOI] [PubMed] [Google Scholar]
- 3.Begolka, W.S., C.L. Vanderlugt, S.M. Rahbe, and S.D. Miller. 1998. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J. Immunol. 161:4437–4446. [PubMed] [Google Scholar]
- 4.Issazadeh, S., A. Ljungdahl, B. Hojeberg, M. Mustafa, and T. Olsson. 1995. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J. Neuroimmunol. 61:205–212. [DOI] [PubMed] [Google Scholar]
- 5.Issazadeh, S., V. Navikas, M. Schaub, M. Sayegh, and S. Khoury. 1998. Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J. Immunol. 161:1104–1112. [PubMed] [Google Scholar]
- 6.Khoury, S.J., W.W. Hancock, and H.L. Weiner. 1992. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 176:1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson, C.I., and H.O. McDevitt. 1999. Redirecting Th1 and Th2 responses in autoimmune disease. Curr. Top. Microbiol. Immunol. 238:79–122. [DOI] [PubMed] [Google Scholar]
- 8.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161–166. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 10.Hong, S., D.C. Scherer, N. Singh, S.K. Mendiratta, I. Serizawa, Y. Koezuka, and L. Van Kaer. 1999. Lipid antigen presentation in the immune system: lessons learned from CD1d knockout mice. Immunol. Rev. 169:31–44. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]
- 12.Joyce, S. 2001. CD1d and natural T cells: how their properties jump-start the immune system. Cell. Mol. Life Sci. 58:442–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exley, M., J. Garcia, S.P. Balk, and S. Porcelli. 1997. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J. Exp. Med. 186:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendiratta, S.K., W.D. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. Van Kaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 6:469–477. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y.H., N.M. Chiu, M. Mandal, N. Wang, and C.R. Wang. 1997. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 6:459–467. [DOI] [PubMed] [Google Scholar]
- 16.Smiley, S.T., M.H. Kaplan, and M.J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 275:977–979. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, H., A. Belperron, S.W. Barthold, and L.K. Bockenstedt. 2000. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797–4801. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa, H., H. Hisaeda, M. Taniguchi, T. Nakayama, T. Sakai, Y. Maekawa, Y. Nakano, M. Zhang, T. Zhang, M. Nishitani, et al. 2000. CD4+ Vα14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int. Immunol. 12:1267–1274. [DOI] [PubMed] [Google Scholar]
- 19.Exley, M.A., N.J. Bigley, O. Cheng, S.M. Tahir, S.T. Smiley, Q.L. Carter, H.F. Stills, M.J. Grusby, Y. Koezuka, M. Taniguchi, and S.P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69:713–718. [PubMed] [Google Scholar]
- 20.Smyth, M.J., K.Y. Thia, S.E. Street, E. Cretney, J.A. Trapani, M. Taniguchi, T. Kawano, S.B. Pelikan, N.Y. Crowe, and D.I. Godfrey. 2000. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. [DOI] [PubMed] [Google Scholar]
- 22.Moodycliffe, A.M., D. Nghiem, G. Clydesdale, and S.E. Ullrich. 2000. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1:521–525. [DOI] [PubMed] [Google Scholar]
- 23.Gombert, J.M., A. Herbelin, E. Tancrede-Bohin, M. Dy, C. Carnaud, and J.F. Bach. 1996. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 26:2989–2998. [DOI] [PubMed] [Google Scholar]
- 24.Baxter, A.G., S.J. Kinder, K.J. Hammond, R. Scollay, and D.I. Godfrey. 1997. Association between αβTCR+CD4− CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 46:572–582. [DOI] [PubMed] [Google Scholar]
- 25.Falcone, M., B. Yeung, L. Tucker, E. Rodriguez, and N. Sarvetnick. 1999. A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J. Exp. Med. 190:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimoto, T., A. Bendelac, J. Hu-Li, and W.E. Paul. 1995. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA. 92:11931–11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mieza, M.A., T. Itoh, J.Q. Cui, Y. Makino, T. Kawano, K. Tsuchida, T. Koike, T. Shirai, H. Yagita, A. Matsuzawa, et al. 1996. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 156:4035–4040. [PubMed] [Google Scholar]
- 28.Hammond, K.J., D.G. Pellicci, L.D. Poulton, O.V. Naidenko, A.A. Scalzo, A.G. Baxter, and D.I. Godfrey. 2001. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167:1164–1173. [DOI] [PubMed] [Google Scholar]
- 29.Sumida, T., A. Sakamoto, H. Murata, Y. Makino, H. Takahashi, S. Yoshida, K. Nishioka, I. Iwamoto, and M. Taniguchi. 1995. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J. Exp. Med. 182:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, S.B., S.C. Kent, K.T. Patton, T. Orban, R.A. Jackson, M. Exley, S. Porcelli, D.A. Schatz, M.A. Atkinson, S.P. Balk, et al. 1998. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 391:177–181. [DOI] [PubMed] [Google Scholar]
- 31.Illes, Z., T. Kondo, J. Newcombe, N. Oka, T. Tabira, and T. Yamamura. 2000. Differential expression of NK T cell Vα24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 164:4375–4381. [DOI] [PubMed] [Google Scholar]
- 32.van der Vliet, H.J., B.M. von Blomberg, N. Nishi, M. Reijm, A.E. Voskuyl, A.A. van Bodegraven, C.H. Polman, T. Rustemeyer, P. Lips, A.J. van den Eertwegh, et al. 2001. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 100:144–148. [DOI] [PubMed] [Google Scholar]
- 33.Kojo, S., Y. Adachi, H. Keino, M. Taniguchi, and T. Sumida. 2001. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis. Rheum. 44:1127–1138. [DOI] [PubMed] [Google Scholar]
- 34.Gausling, R., C. Trollmo, and D.A. Hafler. 2001. Decreases in interleukin-4 secretion by invariant CD4-CD8- Vα24JαQ T cells in peripheral blood of patients with relapsing-remitting multiple sclerosis. Clin. Immunol. 98:11–17. [DOI] [PubMed] [Google Scholar]
- 35.Sonoda, K.H., M. Exley, S. Snapper, S.P. Balk, and J. Stein-Streilein. 1999. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong, S., and L. Van Kaer. 1999. Immune privilege: keeping an eye on natural killer T cells. J. Exp. Med. 190:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikehara, Y., Y. Yasunami, S. Kodama, T. Maki, M. Nakano, T. Nakayama, M. Taniguchi, and S. Ikeda. 2000. CD4+ Vα14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J. Clin. Invest. 105:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seino Ki, K., K. Fukao, K. Muramoto, K. Yanagisawa, Y. Takada, S. Kakuta, Y. Iwakura, L. Van Kaer, K. Takeda, T. Nakayama, et al. 2001. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc. Natl. Acad. Sci. USA. 98:2577–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 40.Gumperz, J.E., C. Roy, A. Makowska, D. Lum, M. Sugita, T. Podrebarac, Y. Koezuka, S.A. Porcelli, S. Cardell, M.B. Brenner, and S.M. Behar. 2000. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 12:211–221. [DOI] [PubMed] [Google Scholar]
- 41.Natori, T., Y. Koezuka, and T. Higa. 1993. Agelasphins, novel α-galactosylceramides from the marine sponge Agelas Mauritianus. Tetrahedron Lett. 34:5591–5592. [Google Scholar]
- 42.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdin, N., L. Brossay, Y. Koezuka, S.T. Smiley, M.J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 161:3271–3281. [PubMed] [Google Scholar]
- 44.Spada, F.M., Y. Koezuka, and S.A. Porcelli. 1998. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieda, M., A. Nicol, Y. Koezuka, A. Kikuchi, T. Takahashi, H. Nakamura, H. Furukawa, T. Yabe, Y. Ishikawa, K. Tadokoro, and T. Juji. 1999. Activation of human Vα24 NKT cells by α-glycosylceramide in a CD1d-restricted and Vα24 TCR-mediated manner. Hum. Immunol. 60:10–19. [DOI] [PubMed] [Google Scholar]
- 46.Carnaud, C., D. Lee, O. Donnars, S.H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647–4650. [PubMed] [Google Scholar]
- 47.Kitamura, H., K. Iwakabe, T. Yahata, S. Nishimura, A. Ohta, Y. Ohmi, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, et al. 1999. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh, N., S. Hong, D.C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373–2377. [PubMed] [Google Scholar]
- 49.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014–2025. [DOI] [PubMed] [Google Scholar]
- 50.Eberl, G., and H.R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985–992. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura, H., A. Ohta, M. Sekimoto, M. Sato, K. Iwakabe, M. Nakui, T. Yahata, H. Meng, T. Koda, S. Nishimura, et al. 2000. α-Galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell. Immunol. 199:37–42. [DOI] [PubMed] [Google Scholar]
- 52.Eberl, G., P. Brawand, and H.R. MacDonald. 2000. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J. Immunol. 165:4305–4311. [DOI] [PubMed] [Google Scholar]
- 53.Bright, J.J., C. Du, M. Coon, S. Sriram, and S.J. Klaus. 1998. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J. Immunol. 161:7015–7022. [PubMed] [Google Scholar]
- 54.Morita, M., K. Motoki, K. Akimoto, T. Natori, T. Sakai, E. Sawa, K. Yamaji, Y. Koezuka, E. Kobayashi, and H. Fukushima. 1995. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 38:2176–2187. [DOI] [PubMed] [Google Scholar]
- 55.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivares-Villagomez, D., Y. Wang, and J.J. Lafaille. 1998. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188:1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van de Keere, F., and S. Tonegawa. 1998. CD4+ T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 188:1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang, H., S.I. Zhang, and B. Pernis. 1992. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 256:1213–1215. [DOI] [PubMed] [Google Scholar]
- 60.Koh, D.R., W.P. Fung-Leung, A. Ho, D. Gray, H. Acha-Orbea, and T.W. Mak. 1992. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 256:1210–1213. [DOI] [PubMed] [Google Scholar]
- 61.Miller, A., O. Lider, A.B. Roberts, M.B. Sporn, and H.L. Weiner. 1992. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl. Acad. Sci. USA. 89:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf, S.D., B.N. Dittel, F. Hardardottir, and C.A. Janeway, Jr. 1996. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, B., T. Yamamura, T. Kondo, M. Fujiwara, and T. Tabira. 1997. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 186:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong, S., M.T. Wilson, I. Serizawa, L. Wu, N. Singh, O.V. Naidenko, T. Miura, T. Haba, D.C. Scherer, J. Wei, et al. 2001. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7:1052–1056. [DOI] [PubMed] [Google Scholar]
- 65.Sharif, S., G.A. Arreaza, P. Zucker, Q.S. Mi, J. Sondhi, O.V. Naidenko, M. Kronenberg, Y. Koezuka, T.L. Delovitch, J.M. Gombert, et al. 2001. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat. Med. 7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 66.Wang, B., Y.B. Geng, and C.R. Wang. 2001. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 194:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saubermann, L.J., P. Beck, Y.P. De Jong, R.S. Pitman, M.S. Ryan, H.S. Kim, M. Exley, S. Snapper, S.P. Balk, S.J. Hagen, et al. 2000. Activation of natural killer T cells by α-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 119:119–128. [DOI] [PubMed] [Google Scholar]
- 68.Osman, Y., T. Kawamura, T. Naito, K. Takeda, L. Van Kaer, K. Okumura, and T. Abo. 2000. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur. J. Immunol. 30:1919–1928. [DOI] [PubMed] [Google Scholar]
- 69.Giaccone, G., C. Punt, Y. Ando, R. Ruijter, N. Nishi, M. Peters, B. von Blomberg, R.J. Scheper, M. Roelvink, J. Beijnen, et al. 2000. KRN7000, an NKT cell enhancer, in patients with solid tumors - a phase I study of the EORTC BTDG. Proc. Am. Soc. Clin. Oncol. 19:1871–1871. [Google Scholar]
- 70.Hameg, A., C. Gouarin, J.M. Gombert, S. Hong, L. Van Kaer, J.F. Bach, and A. Herbelin. 1999. IL-7 up-regulates IL-4 production by splenic NK1.1+ and NK1.1− MHC class I-like/CD1-dependent CD4+ T cells. J. Immunol. 162:7067–7074. [PubMed] [Google Scholar]
- 71.Leite-de-Moraes, M.C., A. Hameg, M. Pacilio, Y. Koezuka, M. Taniguchi, L. Van Kaer, E. Schneider, M. Dy, and A. Herbelin. 2001. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J. Immunol. 166:945–951. [DOI] [PubMed] [Google Scholar]
- 72.Pal, E., T. Tabira, T. Kawano, M. Taniguchi, S. Miyake, and T. Yamamura. 2001. Costimulation-dependent modulation of experimental autoimmune encephalomyelitis by ligand stimulation of Vα14 NK T cells. J. Immunol. 166:662–668. [DOI] [PubMed] [Google Scholar]
- 73.Hayakawa, Y., K. Takeda, H. Yagita, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166:6012–6018. [DOI] [PubMed] [Google Scholar]
- 74.Miyamoto, K., S. Miyake, and T. Yamamura. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 413:531–534. [DOI] [PubMed] [Google Scholar]