Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala (original) (raw)
Abstract
Extensive evidence indicates that drugs and stress hormones act in the basolateral amygdala (BLA) to modulate memory consolidation. The BLA projects to the nucleus basalis magnocellularis (NBM), which sends broad cholinergic projections to the neocortex. NBM-cortex projections have been implicated in learning, memory storage, and plasticity. The current study investigated whether the cholinergic NBM-cortex projections are involved in BLA-mediated modulation of memory consolidation. Bilateral cholinergic cell lesions of the NBM were induced in rats with infusions of 192 IgG-saporin (0.1 μg/0.5 μl per side). Additionally, cannulae were implanted bilaterally in the BLA. One week after surgery, the rats were trained in an inhibitory avoidance task and, immediately after training, norepinephrine (0.3 μg, 1.0 μg, or 3.0 μg in 0.2 μl) or vehicle (PBS) was infused bilaterally into the BLA. Norepinephrine infusions produced a dose-dependent enhancement of 48-h retention (0.3 μg and 1.0 μg doses enhanced) in nonlesioned rats but did not affect retention in NBM-lesioned rats. Choline acetyltransferase assays of frontal and occipital cortices confirmed the NBM lesions. These findings indicate that cholinergic NBM-cortex projections are required for BLA-mediated modulation of memory consolidation.
Keywords: acetylcholine‖inhibitory avoidance‖memory modulation‖rat
Systemic administration of drugs, including adrenergic agonists (1–3) and GABAergic antagonists (4–6), given immediately after training, enhances memory for several kinds of training. The basolateral amygdala (BLA) (4, 8–12) and its major efferent pathway, the stria terminalis (13–16), are critically involved in the modulation of memory consolidation induced by posttraining pharmacological treatments. In addition, considerable evidence indicates that memory modulation by systemic drugs requires β-adrenergic activation in the BLA (17–21).
Inhibitory avoidance (IA) tasks have been used most extensively in memory modulatory studies, as the use of a single training trial enables selective modulation of the consolidation phase of memory. Memory for IA training can be modulated by posttraining intra-BLA infusions of adrenergic (21–25), GABAergic (6, 26), opioid peptidergic (19, 27, 28, 87), glucocorticoid (21, 30, 31), or cholinergic drugs (32–35). As with systemic treatments, memory modulation induced by posttraining intra-BLA drug infusions acting on glucocorticoid, GABA, or opioid receptors requires concurrent β-adrenergic activation in the BLA (19, 21, 36, 37). Furthermore, it was shown recently in our laboratory that levels of amygdalar norepinephrine assessed after training predict long-term retention of IA (unpublished findings). The adrenergic-dependent memory modulation further depends upon muscarinic cholinergic activation in the BLA (31–33, 38, 39), which likely originates from the major amygdalopetal projection of the nucleus basalis magnocellularis (NBM) (37).
Extensive evidence indicates that the BLA mediates the modulation of memory traces that are processed and/or stored in other brain regions (40) such as the caudate nucleus (41), the hippocampus (11, 37, 41) and the entorhinal cortex (unpublished findings). A possible mode of influence for the BLA on memory storage processes in other brain regions may be its excitatory projection to the cholinergic cells in the NBM (42, 43). The cholinergic cells in the NBM project widely to neocortex (44). This projection is strongly implicated in cortical plasticity, learning, and memory (45–47). Electrical stimulation of the amygdala induces low-voltage fast activity in the neocortex that is blocked by cholinergic antagonism in the neocortex (48) or lidocaine inactivation of the NBM (49). Furthermore, stimulation of the amygdala potentiates the cortical response to somatosensory stimulation or NBM stimulation (50, 51).
The current experiment investigated whether the NBM-cortex projections are involved in memory modulatory processes mediated by the BLA. Rats were given bilateral NBM-lesions with the p75 NGF receptor-mediated immunotoxin 192 IgG-saporin or sham infusions and implanted with bilateral cannulae aimed at the BLA. Eight days after surgery, rats were trained in the IA task and given intra-BLA infusions of norepinephrine or vehicle immediately after training. If the NBM-cortex pathway is involved in BLA-mediated memory-modulatory processes, then the lesions should interfere with norepinephrine-induced memory enhancement as assessed by performance on a 48 h retention test.
Methods
Subjects.
Male Sprague–Dawley rats (Charles River Breeding Laboratories) weighing ≈300 g at the time of surgery were used for this experiment. The rats were individually housed in a temperature-controlled (22°C) vivarium on a 12-hr light/12-hr dark cycle, with food and water freely available, and were allowed 1 week to habituate to the vivarium before surgery.
Surgery.
The rats were anesthetized with sodium pentobarbitol (50 mg/kg i.p.; Sigma) and were given atropine sulfate (0.1 mg, i.p.) to maintain respiration and sterile saline (2.5 ml, s.c.) to prevent dehydration. Each rat was placed in a small-animal stereotaxic frame (Kopf Instruments, Tujunga, CA) and given bilateral infusions of 192 IgG-saporin solution (0.1 μg in 0.5 μl of 0.1 M PBS; Chemicon) or sham infusions into the NBM region of the substantia innominata, according to the following coordinates based on the atlas of Paxinos and Watson (52): 1.4 mm posterior and 2.7 mm lateral to bregma, 7.8 mm below the dura. The dose and infusion volume of 192 IgG-saporin chosen for this experiment were based on a dose-response study of intraparenchymal NBM-infusions of 192 IgG-saporin (53). Each rat then was placed in a second stereotaxic frame and implanted with bilateral cannulae (15 mm, 23 gauge) aimed at the BLA. The cannulae were positioned ≈2 mm above the BLA with the following coordinates: 2.8 mm posterior and 5 mm lateral to bregma, 6.5 mm below the skull surface (53). Dental cement fixed the cannulae and two anchoring surgical screws to the skull. Stylets (15-mm long 00 insect-dissection pins) then were inserted into the cannulae to maintain patency. The rat was retained in an incubator until it recovered from the anesthesia. Rats were allowed 1 week to recover from the surgery before behavioral training and were handled for 1 min on each of the 4 days preceding training to habituate them to the infusion procedure.
IA Task.
The IA apparatus is a trough-shaped alley divided by a sliding door into two compartments, a lit safe compartment (31 cm long) and a darkened shock compartment (60 cm long). During training, each rat was placed into the lit safe compartment facing away from the dark compartment, with the sliding door open. The rat was allowed to enter the darkened shock compartment. After each rat completely entered the dark compartment with all four paws, the sliding door was closed, and the animal received a mild inescapable foot shock (0.5 mA, 1.0 s) and remained in the dark compartment for 15 s after the shock. The rat then was given the appropriate infusions into the BLA.
Forty-eight hours after IA training, retention for the training was tested. During testing, each rat was placed in the lit safe compartment, as it was in training. The latency for the rat to enter the dark shock compartment was recorded. The maximum latency cutoff was 600 s. Better retention was inferred from longer latencies to enter the shock compartment during the retention test.
Drug Administration.
Immediately after IA training, rats received bilateral infusions (0.2 μl per side) of (+/−)-norepinephrine HCl (Sigma) or vehicle (0.1 M PBS) into the BLA. This conservative volume has previously been shown to provide selective infusion into the BLA (6, 30). The doses of norepinephrine (0.3 μg, 1.0 μg, and 3.0 μg) were chosen on the basis of unpublished dose-response data from this laboratory and, as expected, were of slightly higher concentrations than those of (−)-norepinephrine HCl (discontinued) used in previous studies (17, 54). Solutions were infused over 25 s into the BLA by automated syringe pumps (Sage Instruments, Boston) by means of 30-gauge injection needles (17 mm) attached to 10-μl Hamilton microsyringes (by polyethylene tubing, PE-20). Needles were left in place for an additional 30 s after the infusions to allow diffusion into the BLA. After drug infusions, the stylets were replaced into the cannulae, and the rats were replaced in their home cages.
Biochemistry: Choline Acetyltransferase Activity.
One day after the IA retention test, rats were killed by decapitation. The brains were rapidly removed, chilled in saline solution (0.9%) and dissected on ice. Frontal and occipital cortex samples were collected from each hemisphere of all rats. Parietal cortex regions were taken with the histological blocks for confirmation of infusions sites (described below) and were, therefore, unavailable for biochemical processing. Samples were kept frozen (−70°C) until analyzed. Each sample was sonicated in 400 μl of 50 mM PBS (pH 7.4) and processed for the assay of choline acetyltransferase (ChAT) by the method of Fonnum (55). Protein content of the homogenates was determined by the method of Lowry et al. (56) and expressed in nmol of acetylcholine formed per h per mg of protein. Data from lesioned rats whose ChAT levels were within the range of the controls were excluded.
Histology.
After the cortical dissection for biochemical analysis, the brains were fixed in 4% (wt/vol) formaldehyde solution for 2 days. Brains then were stored in 30% (wt/vol) sucrose in saline solution until slicing. Slices (40 μm) including the NBM and the BLA were collected on a freezing microtome and stained with thionine. Stained sections were analyzed with a light microscope to determine the infusion sites. Rats that did not receive both posttraining infusions within the BLA were excluded from the data analysis.
Statistical Analysis.
As none of the rats included in the behavioral data analysis after the histological and biochemical analyses had floor (0 s) or ceiling (600 s) retention latencies, parametric tests were used to detect statistically significant treatment effects. The behavioral data were analyzed with a two-way ANOVA with NBM treatment (two levels, sham or lesion) and BLA treatment (four levels, vehicle and three doses of norepinephrine) as the between-subject variables. To confirm that the 192 IgG-saporin lesion did not block all IA learning, the overall mean training latencies were compared with the mean testing latencies of vehicle-infused groups in a two-way ANOVA with session as the variable (two levels, training or testing). The ChAT assay data for each sample area (cortical region, side) were analyzed with a two-way ANOVA with NBM treatment (two levels, sham or lesion) and cortical region (four levels, left and right frontal, left and right occipital) as the between-subject variable. Fisher's post hoc tests were used to detect the sources of the significances detected by the ANOVAs. In all cases, P values of less than 5% (0.05) were the criteria for significant differences. The number of animals per group are reported in the figure legends.
Results
Histology.
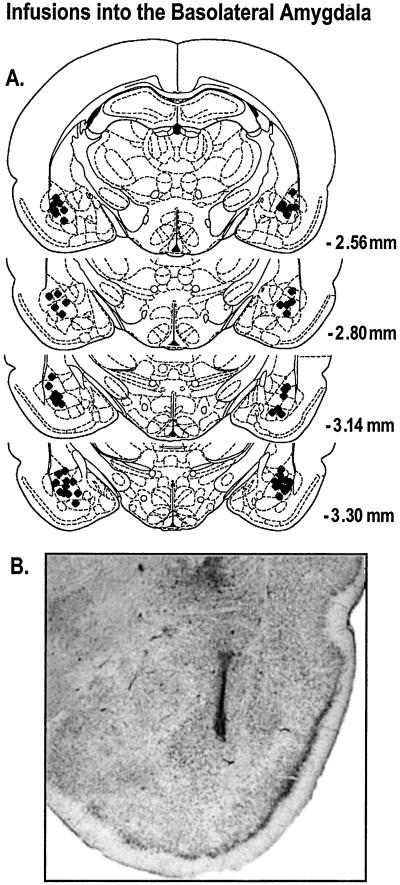
The locations of the posttraining infusion sites are shown in Fig. 1A. A representative example of an infusion needle track terminating within the BLA, per the criteria of this experiment, is shown in Fig. 1B. Data from 13 rats whose infusion needle tracks terminated outside the BLA were excluded from analysis.
Figure 1.
Only data from animals with posttraining infusion sites within the BLA were included in the analysis. (A) ♦ represents the infusion needle tip loci for a random sample of 30 rats included in the behavioral analysis. Coordinates indicate distance posterior to bregma. (B) Photomicrograph of a representative example of an infusion needle terminating within the BLA.
Biochemistry: Choline Acetyltransferase Activity.
As summarized in Table 1, the 192 IgG-saporin lesions of the NBM significantly reduced cortical ChAT activity (F1,300 = 583.05, P < 0.0001). Significantly less ChAT activity was detected in the left and right frontal cortical regions of lesioned animals than in the left and right occipital regions (region effect: F3,300 = 14.41, _P_ < 0.0001; region × NBM treatment effect: F3,300 = 65.45, _P_ < 0.0001). The left and right hemisphere ChAT activity levels did not differ significantly from each other in either cortical area (_P_ > 0.05). The lesions produced a pronounced ChAT reduction in the frontal cortical regions relative to the respective ChAT activity levels of sham animals (P < 0.0001). The mean ChAT levels of the left and right frontal cortices of the lesioned rats were 67.0% and 63.5% less than the mean ChAT level of the controls, respectively. The lesions also produced a reduction of ChAT in the occipital cortical regions relative to the respective ChAT activity levels of sham animals (P < 0.0001). The mean ChAT levels of the left and right occipital cortices of the lesioned rats were 19.8% and 20.4% less than the mean ChAT level observed in the controls, respectively. Data from five rats were excluded from the behavioral analysis because of insufficient ChAT reduction. Only ChAT activity levels of rats included in the behavioral analysis are included in the ChAT activity summary table.
Table 1.
Activity of choline acetyltransferase
| Cortical region | Activity (nmol Ach/h/mg protein) | Difference, % | |
|---|---|---|---|
| Control | 192 IgG-saporin | ||
| Frontal, left | 33.20 ± 0.84 | 10.98 ± 0.86 | 67.0 decrease* |
| Frontal, right | 32.37 ± 0.77 | 11.51 ± 1.08 | 64.5 decrease* |
| Occipital, left | 28.36 ± 0.53 | 22.75 ± 0.72 | 19.8 decrease* |
| Occipital, right | 28.83 ± 0.68 | 22.95 ± 0.85 | 20.4 decrease* |
Inhibitory Avoidance.
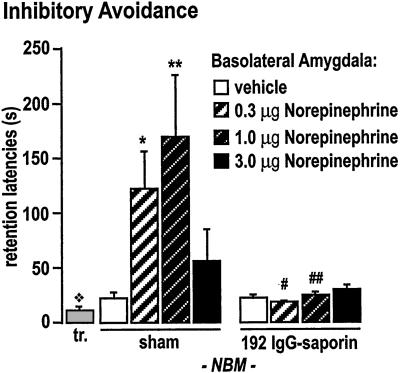
An ANOVA (n = 77) revealed a significant effect of the 192 IgG-saporin NBM-lesion (F1,69 = 12.82, P < 0.001), a significant effect of the intra-BLA norepinephrine infusions (F3,69 = 2.83, _P_ < 0.05) and a significant interaction of the lesion and the BLA-norepinephrine treatment on IA retention (F3,69 = 3.02, _P_ < 0.05). The 192 IgG-saporin NBM-lesions did not affect training latencies in any group or retention latencies in vehicle-infused groups. That is, vehicle-treated lesioned rats (20.7 ± 3.8 s) performed like vehicle-treated sham rats (21.9 ± 6.3 s; _P_ > 0.05) on the retention test, and there was no effect of the NBM lesion on training latency (F1,69 = 0.40, P > 0.05). The vehicle-infused sham and lesion groups had significantly longer latencies to enter the shock compartment on the retention test than the mean training latency (14.17 ± 1.03; F1,108 = 6.49, P < 0.05), indicating that the lesion did not block all IA learning. Fisher's _post hoc_ tests indicated that the 0.3 μg (_P_ < 0.01) and 1.0 μg (_P_ < 0.001) doses of norepinephrine significantly enhanced retention in the sham groups (121.2 ± 35.0 s and 167.9 ± 57.8 s, respectively) compared with the vehicle-infused sham group (Fig. 2). However, the mean retention latencies (17.4 ± 3.0 s and 22.9 ± 4.1 s, respectively) for the lesioned groups that received these doses of norepinephrine (which were memory enhancing in sham rats) did not differ from those of the vehicle infused lesioned group (_P_ > 0.05). The mean retention latencies of the 3.0 μg dose norepinephrine groups (sham, 54.0 ± 30.4; lesion, 28.0 ± 4.6 s) did not differ from those of their respective controls (P > 0.05).
Figure 2.
Intra-BLA infusions of norepinephrine immediately after IA training produce a dose-dependent enhancement of 48-h retention in sham-operated rats. The gray bar (tr.) indicates the mean training latency across all groups, which did not differ between any of the groups (P > 0.05). The mean training latency was significantly less than the retention latencies of the sham and lesion vehicle-infused groups ( , P < 0.05), which did not differ from each other (_P_ > 0.05). An ANOVA revealed significant main effects of the norepinephrine infusions, the 192 IgG-saporin lesion, and a significant norepinephrine infusion × lesion interaction effect (P < 0.05). The 0.3 μg (*, _P_ < 0.01) and the 1.0 μg (**, _P_ < 0.001) doses significantly enhanced retention relative to the vehicle-infused sham group. Rats with the 192 IgG-saporin NBM-lesions did not show memory enhancement with norepinephrine infusions. The mean retention latencies of the 0.3 μg (#, _P_ < 0.05) and the 1.0 μg (##, _P_ < 0.001) dose lesion groups were significantly shorter than those of their respective dose-matched sham control groups, but did not differ from that of the vehicle-infused sham or lesion groups (_P_ > 0.05). (The number of animals per group are as follows: sham-vehicle, 11; sham-norepinephrine 0.3 μg, 10; sham-norepinephrine 1.0 μg, 10; sham-norepinephrine 3.0 μg, 9; lesion-vehicle, 9; lesion-norepinephrine 0.3 μg, 7; lesion-norepinephrine 1.0 μg, 8; lesion-norepinephrine 3.0 μg, 14.)
, P < 0.05), which did not differ from each other (_P_ > 0.05). An ANOVA revealed significant main effects of the norepinephrine infusions, the 192 IgG-saporin lesion, and a significant norepinephrine infusion × lesion interaction effect (P < 0.05). The 0.3 μg (*, _P_ < 0.01) and the 1.0 μg (**, _P_ < 0.001) doses significantly enhanced retention relative to the vehicle-infused sham group. Rats with the 192 IgG-saporin NBM-lesions did not show memory enhancement with norepinephrine infusions. The mean retention latencies of the 0.3 μg (#, _P_ < 0.05) and the 1.0 μg (##, _P_ < 0.001) dose lesion groups were significantly shorter than those of their respective dose-matched sham control groups, but did not differ from that of the vehicle-infused sham or lesion groups (_P_ > 0.05). (The number of animals per group are as follows: sham-vehicle, 11; sham-norepinephrine 0.3 μg, 10; sham-norepinephrine 1.0 μg, 10; sham-norepinephrine 3.0 μg, 9; lesion-vehicle, 9; lesion-norepinephrine 0.3 μg, 7; lesion-norepinephrine 1.0 μg, 8; lesion-norepinephrine 3.0 μg, 14.)
Discussion
The present study investigated whether the cholinergic cells in the NBM projecting to cortex are involved in BLA-mediated modulation of memory. ChAT assays confirmed the reduction of cholinergic input to the neocortex induced by infusion of the immunotoxin 192 IgG-saporin into the NBM. Norepinephrine infusions produced a dose-dependent enhancement of retention in sham rats but did not enhance retention in NBM-lesioned rats.
The finding that posttraining intra-BLA norepinephrine infusions enhanced IA retention in nonlesioned controls is consistent with those of numerous reports (14, 17, 18, 22, 24, 25, 54, 57, 58). Furthermore, in vivo microdialysis-HPLC studies have demonstrated that systemic injections of epinephrine or a footshock induce norepinephrine release in the amygdala (59), and that norepinephrine release in the amygdala during IA training correlates highly with performance on a 48-h retention test (unpublished findings). This convergence of evidence from behavioral pharmacological and i_n vivo_ microdialysis-HPLC studies strongly suggests that modulation of memory consolidation is driven by norepinephrine acting on β-adrenergic receptors in the BLA (60).
The current finding that the intraparenchymal 192 IgG-saporin lesions of the NBM did not produce a deficit in IA retention is consistent with previous findings that impairment on this task is produced by intracerebroventricular (icv) administration of saporin that produces > 90% ChAT reduction in the medial septum and diagonal band of Broca in combination with > 80% ChAT reduction in the NBM (61). Generally, memory deficits are only consistently produced with icv cholinergic basal forebrain lesions that disrupt > 75–85% of ChAT activity of the total cholinergic basal forebrain (62–64). With an experiment using a training protocol similar to that of the current study, Wenk et al. (65) found no deficits on several measures of IA, including 48-h retention latency and freezing behavior in rats with saporin lesions of the NBM. However, Torres et al. (66) found a small but significant impairment on IA retention with saporin NBM lesions. The relative ineffectiveness of immunotoxic NBM lesions to impair memory relative to excitotoxic NBM lesions suggests that the robust memory deficits produced with excitotoxic lesions (67–70) may be caused by damaged amygdalopetal cholinergic projections (35, 71, 72) and/or damaged noncholinergic cells (63, 73–76).
The present finding is that 192 IgG-saporin NBM lesions, which did not produce IA impairment, blocked memory enhancement induced by norepinephrine infusions administered into the BLA. This finding strongly suggests that the NBM-cortex projections may mediate BLA-driven modulation of memory storage or processing in the neocortex. Moreover, this finding is consistent with previous reports in indicating that the BLA modulates memory storage in other brain regions (14, 16, 54, 77–80) rather than influencing consolidation of memories within the BLA, and that long-term memory of IA training involves entorhinal and posterior parietal cortices (81–83).
Because the BLA sends and receives projections to and from both the NBM and the cortex (42, 44, 84, 85), there are several plausible mechanisms by which the NBM-cortex projections may be involved in mediating BLA-driven memory modulation. The mechanism of cholinergic NBM-neocortical projection involvement in BLA-mediated memory modulation may simply involve direct excitation of the NBM by the BLA; that is, BLA activation may directly facilitate the NBM-cortex projections and thereby enhance memory storage processes in the cortex. Evidence that stimulation of the BLA produces scopolamine-sensitive, NBM-dependent neocortical desynchronization (48, 49) and potentiates cortical responsivity to somatosensory stimulation or NBM stimulation is consistent with this view (50, 51). Also, it is possible that the BLA-cortex projections may directly influence the cortex in a manner that requires coincident cholinergic activation in the cortex from the NBM to modulate memory storage. Alternatively, it may be that direct cortical input to the BLA is influenced by the level of cholinergic activation the cortex receives from the NBM. Finally, it may be that a combination of these mechanisms is involved. It is worth noting that 192 IgG-saporin selectively lesions corticopetal cholinergic neurons, as the amygdalopetal neurons, which may provide important input to the BLA during memory-modulatory processes (35), do not express the p75 low-affinity NGF receptor to which this immunotoxin binds (64, 65, 76).
The BLA receives projections from many diffusely projecting systems associated with stress and arousal, including noradrenergic (86), dopaminergic (7), and cholinergic projections (44, 85). Thus, the present findings suggest that memory impairment in animals with immunotoxic lesions of the NBM may depend on the magnitude of stress and/or arousal associated with the task, and, therefore, the extent to which the BLA is activated during memory consolidation. This possible role for the cholinergic NBM-cortex projection during memory consolidation is not in conflict with the hypothesis that it may be involved in attentional processes during learning (29, 62, 65, 76).
In summary, the present findings demonstrate a role for the NBM-cortex projections in BLA-driven memory modulatory processes. 192 IgG-saporin lesions of the NBM blocked memory enhancement by posttraining intra-BLA infusion of norepinephrine in an IA task. These data are consistent with previous findings in suggesting that the BLA influences memory storage in other brain areas. These findings further suggest that BLA activation may modulate memory-storage processes in the neocortex by means of a mechanism that requires activation of the NBM-cortex projections.
Acknowledgments
The authors thank Andrew Chen for processing ChAT assays, Anna Litmanovich and ThuyVu Ho for valuable technical assistance, and Norman M. Weinberger for providing the 192 IgG-saporin, and Jan Bures and Paul Gold for their helpful comments on a previous draft. This study was supported by National Institutes of Mental Health Grant MH12526 (to J.L.M.) and National Institutes of Health Grant AG17533 (to L.J.T.).
Abbreviations
BLA
basolateral amygdala
IA
inhibitory avoidance
NBM
nucleus basalis magnocellularis
ChAT
choline acetyltransferase
References
- 1.Gold P E, van Buskirk R. Behav Biol. 1978;23:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg D B, Issacs K, Gold P E, McGaugh J L. Behav Neural Biol. 1985;44:447–453. doi: 10.1016/s0163-1047(85)90856-8. [DOI] [PubMed] [Google Scholar]
- 3.Introini-Collison I B, McGaugh J L. Behav Neural Biol. 1986;45:358–365. doi: 10.1016/s0163-1047(86)80024-3. [DOI] [PubMed] [Google Scholar]
- 4.Brioni J D, Nagahara A H, McGaugh J L. Brain Res. 1989;487:105–112. doi: 10.1016/0006-8993(89)90945-1. [DOI] [PubMed] [Google Scholar]
- 5.Brioni J D, McGaugh J L. Psychopharmacology. 1988;96:505–510. doi: 10.1007/BF02180032. [DOI] [PubMed] [Google Scholar]
- 6.Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh J L. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- 7.Fallon J H, Koziell D A, Moore R Y. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- 8.Cahill L, McGaugh J L. Psychobiology. 1991;19:206–210. [Google Scholar]
- 9.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 10.Roozendaal B, Portillo-Marquez G, McGaugh J L. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 11.Roozendaal B, McGaugh J L. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 12.Roozendaal B, Quirarte G L, McGaugh J L. Ann N Y Acad Sci. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- 13.Introini-Collison I B, Arai Y, McGaugh J L. Psychobiology. 1989;17:397–401. [Google Scholar]
- 14.Introini-Collison I B, Miyasaki B, McGaugh J L. Psychopharmacology. 1991;104:541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- 15.Tomaz C, Dickenson-Anson H, McGaugh J L. Proc Natl Acad Sci USA. 1992;89:3615–3619. doi: 10.1073/pnas.89.8.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roozendaal B, McGaugh J L. Brain Res. 1996;709:243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- 17.Liang K C, Juler R G, McGaugh J L. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 18.Liang K C, Chen L, Huang T E. Chin J Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- 19.Introini-Collison I B, Nagahara A H, McGaugh J L. Brain Res. 1989;476:94–101. doi: 10.1016/0006-8993(89)91540-0. [DOI] [PubMed] [Google Scholar]
- 20.Introini-Collison I B, Sahghafi D, Novack G, McGaugh J L. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-h. [DOI] [PubMed] [Google Scholar]
- 21.Quirarte G L, Roozendaal B, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatfield T, McGaugh J L. Neurobiol Learn Mem. 1999;71:32–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 23.Ferry B, McGaugh J L. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- 24.Ferry B, Roozendaal B, McGaugh J L. Eur J Pharmacol. 1999;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- 25.Ferry B, Roozendaal B, McGaugh J L. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva M A, Tomaz C. Neuropsychobiology. 1995;32:31–36. doi: 10.1159/000119209. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher M, Kapp B S. Life Sci. 1978;23:1973–1977. doi: 10.1016/0024-3205(78)90565-9. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher M, Kapp B S, Pascoe J P, Rapp P R. In: The Amygdaloid Complex: Proceedings of the International Symposium on the Amygdaloid Complex. Ben-Ari Y, editor. New York: Elsevier/North–Holland; 1981. pp. 343–354. [Google Scholar]
- 29.Waite J J, Wardlow M L, Power A E. Neurobiol Learn Mem. 1999;71:325–352. doi: 10.1006/nlme.1998.3884. [DOI] [PubMed] [Google Scholar]
- 30.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 31.Power A E, Roozendaal B, McGaugh J L. Eur J Neurosci. 2000;12:3481–3487. doi: 10.1046/j.1460-9568.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 32.Dalmaz C, Introini-Collison I B, McGaugh J L. Behav Brain Res. 1993;58:167–174. doi: 10.1016/0166-4328(93)90101-u. [DOI] [PubMed] [Google Scholar]
- 33.Introini-Collison I B, Dalmaz C, McGaugh J L. Neurobiol Learn Mem. 1996;65:57–64. doi: 10.1006/nlme.1996.0006. [DOI] [PubMed] [Google Scholar]
- 34.Vazdarjanova A, McGaugh J L. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power, A. E. & McGaugh, J. L. (2002) Neurobiol. Learn. Mem., in press. [DOI] [PubMed]
- 36.McGaugh J L, Introini-Collison I B, Nagahara A H. Brain Res. 1988;446:37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- 37.Roozendaal B, Nguyen B T, Power A E, McGaugh J L. Proc Natl Acad Sci USA. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salinas J A, Introini-Collison I B, Dalmaz C, McGaugh J L. Neurobiol Learn Mem. 1997;68:51–59. doi: 10.1006/nlme.1997.3776. [DOI] [PubMed] [Google Scholar]
- 39.Introini-Collison I B, McGaugh J L. Psychopharmacology. 1988;94:379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo I, McGaugh J L. Behav Pharmacol. 2000;11:517–534. doi: 10.1097/00008877-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Packard M G, Cahill L, McGaugh J L. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Olmos, Alheid G F, Beltramino C A. In: The Rat Nervous System. Paxinos G, editor. Sydney: Academic; 1985. pp. 223–334. [Google Scholar]
- 43.Russchen F T, Amaral D G, Price J L. J Comp Neurol. 1985;242:1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- 44.Mesulam M-M, Mufson E J, Wainer B H, Levey A I. Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 45.Metherate R, Weinberger N M. Synapse. 1990;6:133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- 46.Bakin J, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutiérrez H, Miranda M I, Bermúdez-Rattoni F. J Neurosci. 1997;17:3796–3803. doi: 10.1523/JNEUROSCI.17-10-03796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dringenberg H C, Vanderwolf C H. Exp Brain Res. 1997;116:160–174. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- 49.Dringenberg H C, Vanderwolf C H. Exp Brain Res. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- 50.Dykes R W. Can J Physiol Pharmacol. 1997;75:535–545. [PubMed] [Google Scholar]
- 51.Dringenberg H C, Saber A J, Cahill L. NeuroReport. 2001;12:2395–2398. doi: 10.1097/00001756-200108080-00022. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 53.Pizzo D P, Waite J J, Thal L J, Winkler J. J Neurosci Methods. 1999;91:9–19. doi: 10.1016/s0165-0270(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 54.Liang K C, McGaugh J L, Yao H. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- 55.Fonnum F. Biochem J. 1969;115:465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowry O H, Rosenberg N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 57.Gallagher M, Kapp B S, Musty R E, Driscoll P A. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- 58.Gallagher M, Kapp B S. Behav Neural Biol. 1981;31:90–95. doi: 10.1016/s0163-1047(81)91130-4. [DOI] [PubMed] [Google Scholar]
- 59.Williams C L, Men D, Clayton E C, Gold P E. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- 60.Ferry B, Roozendaal B, McGaugh J L. Biol Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z J, Berbos T G, Wrenn C C, Wiley R G. Neurosci Lett. 1996;203:214–218. doi: 10.1016/0304-3940(95)12282-6. [DOI] [PubMed] [Google Scholar]
- 62.Waite J J, Chen A D, Wardlow M L, Wiley R G, Lappi D A, Thal L J. Neuroscience. 1995;65:463–476. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- 63.Waite J J, Thal L J. Life Sci. 1996;58:1947–1953. doi: 10.1016/0024-3205(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 64.Wrenn C C, Wiley R G. Int J Dev Neurosci. 1998;16:595–602. doi: 10.1016/s0736-5748(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 65.Wenk G L, Stoehr J D, Quintana G, Mobley S, Wiley R G. J Neurosci. 1994;14:5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres E M, Perry T A, Blockland A, Wilkinson L S, Wiley R G, Lappi D A, Dunnet S B. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 67.Salamone J D, Beart P M, Alpert J E, Iverson S D. Behav Brain Res. 1984;13:63–70. doi: 10.1016/0166-4328(84)90030-5. [DOI] [PubMed] [Google Scholar]
- 68.Murray C L, Fibiger H C. Neuroscience. 1985;14:1025–1032. doi: 10.1016/0306-4522(85)90273-8. [DOI] [PubMed] [Google Scholar]
- 69.Robbins T W, Everitt B J, Ryan C N, Marston H M, Jones G H, Page K J. Neuroscience. 1989;28:337–352. doi: 10.1016/0306-4522(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 70.Riekkinen P, Jr, Sirviö J, Hannila T, Miettinen R, Riekkinen P. Brain Res Bull. 1990;24:839–842. doi: 10.1016/0361-9230(90)90148-s. [DOI] [PubMed] [Google Scholar]
- 71.Kesner R P, Crutcher K A, Omana H. Neuroscience. 1990;38:93–102. doi: 10.1016/0306-4522(90)90376-f. [DOI] [PubMed] [Google Scholar]
- 72.Heckers S, Mesulum M-M. Neuroscience. 1994;60:383–397. doi: 10.1016/0306-4522(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 73.Dunnet S B, Whishaw I Q, Jones G H, Bunch S T. Neuroscience. 1987;20:653–669. doi: 10.1016/0306-4522(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 74.Lindefors N, Boatell M L, Mahy N, Persson H. Neurosci Lett. 1992;135:262–264. doi: 10.1016/0304-3940(92)90451-c. [DOI] [PubMed] [Google Scholar]
- 75.Page K J, Sirinathsinghji D J S, Everitt B J. Eur J Neurosci. 1995;7:1012–1021. doi: 10.1111/j.1460-9568.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 76.Wenk G L. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- 77.McGaugh J L, Introini-Collison I B, Juler R G, Izquierdo I. Behav Neurosci. 1986;100:839–844. doi: 10.1037//0735-7044.100.6.839. [DOI] [PubMed] [Google Scholar]
- 78.Parent M B, West M, McGaugh J L. Behav Neurosci. 1994;108:1080–1087. doi: 10.1037//0735-7044.108.6.1080. [DOI] [PubMed] [Google Scholar]
- 79.Packard M G, Teather L A. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 80.Vazdarjanova A, McGaugh J L. Proc Natl Acad Sci USA. 1998;95:15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walz R, Roesler R, Barros D M, de Souza M M, Rodrigues C, Sant'Anna M K, Quevedo J, Choi H K, Neto W P, DeDavid e Silva T L, et al. Behav Pharm. 1999;10:723–730. doi: 10.1097/00008877-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Walz R, Roesler R, Quevedo J, Rockenbach I C, Amaral O B, Vianna M R, Lenz G, Medina J H, Izquierdo I. Behav Brain Res. 1999;105:219–223. doi: 10.1016/s0166-4328(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 83.Walz R, Roesler R, Quevedo J, Sant'Anna M K, Madruga M, Rodrigues C, Gottfried C, Medina J H, Izquierdo I. Neurobiol Learn Mem. 2000;73:11–20. doi: 10.1006/nlme.1999.3913. [DOI] [PubMed] [Google Scholar]
- 84.McDonald A, Jackson T. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- 85.Woolf N J, Butcher L L. Brain Res Bull. 1982;8:751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- 86.Sumal K K, Blessing W W, Joh T H, Reis D J, Pickel V M. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- 87.Introini-Collison I B, Ford L, McGaugh J L. Neurobiol Learn Mem. 1995;63:200–205. doi: 10.1006/nlme.1995.1021. [DOI] [PubMed] [Google Scholar]