Safety and Shedding of an Attenuated Strain of Listeria monocytogenes with a Deletion of actA/plcB in Adult Volunteers: a Dose Escalation Study of Oral Inoculation (original) (raw)
Abstract
Listeria monocytogenes is an intracellular bacterial pathogen which causes bacteremia and has a tropism for the central nervous system and a propensity to cause maternofetal infection. L. monocytogenes has been shown to be an effective prophylactic and a therapeutic vaccine vector for viral and tumor antigens in animal models. L. monocytogenes mutants lacking the ActA protein, which is essential for intracellular movement, are attenuated but retain immunogenicity in mice. Given the pathogenic potential of L. monocytogenes, we created an attenuated mutant strain bearing double deletions in the actA and plcB virulence genes for an initial clinical safety study of a prototype L. monocytogenes vector in adults. Twenty healthy volunteers received single escalating oral doses (106 to 109 CFU, 4 volunteers per dose cohort) of this attenuated L. monocytogenes, designated LH1169. Volunteers were monitored in the hospital for 14 days with frequent clinical checks and daily blood and stool cultures, and they were monitored for six additional weeks as outpatients. There were no positive blood cultures and no fevers attributable to the investigational inoculation. Most volunteers shed vaccine bacteria for 4 days or less, without diarrhea. One volunteer had a late positive stool culture during outpatient follow-up. Three volunteers had abnormal liver function test results temporally associated with inoculation; one could be reasonably attributed to another cause. In the highest-dose cohort, humoral, mucosal, and cellular immune responses to the investigational organism were detected in individual volunteers. Attenuated L. monocytogenes can be studied in adult volunteers without serious long-term health sequelae.
Listeria monocytogenes is a gram-positive bacterium which has long been studied in mice to elucidate mechanisms of cellular immune responses to intracellular pathogens (29, 35). Schafer and colleagues initially proposed that escape of L. monocytogenes from the phagocytic vacuole into the eukaryotic cytoplasm might make these organisms efficient vectors for delivery of antigens to the major histocompatibility complex class I-restricted antigen processing pathways (48). L. monocytogenes vectors have been used to deliver lymphocytic choriomeningitis virus (LCMV) nucleoprotein antigens to mice, with subsequent protection against fatal challenge with LCMV (21, 52, 53). Recombinant L. monocytogenes expressing cottontail rabbit papillomavirus E1 antigen has been successfully used as a therapeutic immunogen in animals bearing papillomavirus-induced cutaneous tumors (28). L. monocytogenes is being pursued as a vector for antigens derived from human papillomaviruses (23) and human immunodeficiency virus type 1 (HIV-1) (40, 41). A murine study showed that oral inoculation of L. monocytogenes expressing HIV-1-gag induced mucosal and systemic immunity to this viral antigen (45). After careful review of available data on oral inoculation of wild-type L. monocytogenes in primates (17) and farm animals, (34, 42, 43) clinical data available from case series (reviewed in reference 44), and reports of large outbreaks of listeriosis (2, 10, 47), we designed an initial safety study of rationally attenuated L. monocytogenes in adult volunteers.
L. monocytogenes organisms are immunostimulatory and lack lipopolysaccharide, and molecularly defined attenuated mutants have been studied in animals. Murine studies show that L. monocytogenes mutants lacking listeriolysin (LLO), a central virulence factor, are avirulent and cannot protect animals against subsequent challenge with wild-type organisms (4, 62). However, mutants lacking ActA (responsible for actin polymerization and resultant movement within eukaryotic cells and intercellular spread) are attenuated but retain immunogenicity (5, 7, 12, 15, 30). ActA mutants have been repeatedly shown in studies to be highly attenuated, including studies with germ-free (38) and interferon (IFN)-deficient mice (25), and they were a logical choice for an initial safety and feasibility study with humans. In the absence of any prior studies on humans and in order to begin safety studies with a double deletion mutant with lower potential for reversion, we added a second attenuating mutation to a well-studied ActA mutant strain. The plcB gene, which encodes a phospholipase or lecithinase (59), has been demonstrated to be important for secondary vacuolar escape (39), neurovirulence (49), and NF-κ B-mediated inflammatory responses in mice (51). We generated a mutant of the serotype 1 strain 10403S with defined in-frame deletions in both actA and plcB. This new mutant, designated LH1169, was more attenuated than wild-type organisms or either single deletion alone. The safety of oral inoculation of single, oral escalating doses of L. monocytogenes LH1169 was evaluated for 20 adult volunteers, and we report here safety, shedding, and early immunogenicity data for humans.
(This work was presented in part at Vaccines for Enteric Diseases 2001, Tampere, Finland, 12 to 14 September, 2001.)
MATERIALS AND METHODS
Bacterial strains.
L. monocytogenes 10403S, a serotype 1 strain widely used in laboratory research, was used as a parental wild-type strain. Defined, in-frame deletions were created within the actA and plcB genes by allelic replacement as described previously (52); the resultant genotype is shown in Fig. 1. This streptomycin-resistant strain was chosen in part in order to allow inclusion of streptomycin in blood agar plates to inhibit the many confounding beta-hemolytic organisms present in human fecal samples. Oligonucleotide primers used to amplify the actA locus were AAG CTT GGG AAG CAG TTG and TGC TTT TAT CGT TAC CGG. For the plcB locus the primers used were AGA CCG CAC CAA AGC TAG and TTT TAA GCA TTT TCA TAG ATG. All sequences shown are 5′ to 3′, with forward primers listed first.
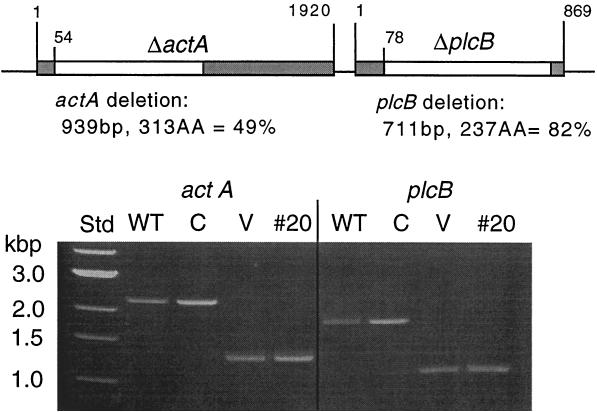
FIG. 1.
Genotype of _actA/plcB_-deleted L. monocytogenes LH1169. In-frame-defined deletions (denoted by white segments) were created as shown for the actA and plcB genes. The photograph shows a 1% agarose gel of PCR-amplified loci from wild-type L. monocytogenes 10403S (WT), a clinical isolate (C), the vaccine strain LH1169 (V), and the last fecal isolate obtained from volunteer 20 (#20). Lanes designated V and #20 show the expected truncated versions of amplicons spanning the actA locus (1.3 kb versus 2.3 kb for WT) and the plcB locus (1.1 kb versus 1.8 kb for WT). Std, 1-kb molecular size standard.
Preclinical evaluation.
Virulence was assessed in vivo in female BALB/c mice inoculated intravenously in accordance with policies and procedures of the Institutional Animal Care and Use Committee at the University of California, Los Angeles (46). Intercellular spread was assessed in vitro in a fibroblast-like cell line (L929 cells; American Type Culture Collection, Rockville, Md.) plaquing assay designed to measure cell-to-cell spread of L. monocytogenes over 5 days (55). Short-term intracellular growth within the macrophage-like cell line J774 (American Type Culture Collection) was measured by using a 30-min infection with a multiplicity of infection of 10:1 (55).
Volunteer study. (i) Human subjects and volunteer screening.
All clinical procedures were reviewed and approved by the Institutional Review Board at Massachusetts General Hospital, Boston, prior to implementation. Healthy adults 18 to 45 years old were recruited by advertising and were medically screened with a complete physical exam and standard laboratory procedures as described previously (27), with the following minor additions. Women of childbearing potential were excluded, given the known risk of listeriosis in pregnant women and the unknown potential duration of shedding of attenuated L. monocytogenes. Subjects with any risk factors for complications of bacteremia, chronic pain syndromes (e.g., headaches), or close contact with immunosuppressed individuals or pregnant women were excluded. Potential volunteers must have previously tolerated a course of therapy with penicillin or ampicillin. Volunteers were required to have normal iron studies (Fe, total iron binding capacity, ferritin) and a prestudy stool sample which was negative for routine enteric pathogens, ova and parasites, and L. monocytogenes (see below). Control samples were obtained for immunological assessments from two additional groups. Excess sera from 5 patients with culture-proven clinical listeriosis were obtained from clinical laboratories at Massachusetts General Hospital. Healthy uninoculated volunteers were enrolled to provide lymphocytes or paired normal human serum samples for enzyme-linked immunosorbent assay (ELISA) studies, as previously described (1).
(ii) Inoculum preparation.
L. monocytogenes strain LH1169 was grown in Trypticase soy broth (Difco) at 37°C with rotary shaking for 16 h, pelleted by centrifugation, washed twice with normal saline, and resuspended at a specific turbidity for administration to volunteers in 25 ml of normal saline. Volunteers ingested 2 g of sodium bicarbonate USP in 150 ml of distilled water just prior to inoculation. Actual doses given were confirmed by counting colonies from triplicate spread plate cultures.
(iii) Clinical assessments and samples.
Subjects were admitted to the General Clinical Research Center at Massachusetts General Hospital for 14 days and had frequent clinical exams, with vital signs taken at least four times a day. Volunteers had routine safety laboratory tests (complete blood count with differential, platelets, serum transaminases, alkaline phosphatase, total bilirubin, and BUN/Cr) done on study days 0, 4, 7, and 10 and additionally as deemed appropriate for subjects with values outside normal ranges for the hospital clinical laboratory. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood via Ficoll gradient separation on days 0, 4, 7, 10, and 28. After being discharged, volunteers returned once a week for 6 weeks for symptom history, clinical check, stool sample, and immunology samples. Serum samples were obtained on days 0, 7, 10, and 14 and at each weekly outpatient visit.
(iv) Microbiological assessments.
Volunteers had daily blood cultures (10 ml of blood per Bactec 9240 bottle; each set has an aerobic and anaerobic bottle). All stools passed were graded (33); up to three stools per day were sent for L. monocytogenes culture. Fecal samples were plated around the clock directly onto Oxford Listeria agar (Difco, Sparks, Md.) and Columbia nalidixic acid agar (Difco) containing 5% defibrinated horse blood (Remel, Lenexa, Kans.) and 100 μg of streptomycin/ml (Amresco, Solon, Ohio). Stool samples were also heavily inoculated (∼5 g) into 10 ml of UVM Listeria enrichment broth (Difco) (14) and were incubated overnight. Aliquots of suspension were then inoculated onto Oxford and Columbia nalidixic acid/horse blood agar plates. If no stools were passed by 8 p.m. on a given day, a rectal swab was obtained and incubated overnight in UVM enrichment broth. All microbiology samples were incubated at 37°C. Quantitative colony counts were not performed. Both blood cultures and selective agar plates were held for a minimum of 7 days, and plates were examined daily for suspicious colonies. Bacterial isolates from fecal samples were confirmed to be L. monocytogenes by standard phenotypic tests (beta hemolysis, Gram stain, catalase test, and motility test) and automated biochemical (VITEK; BioMerieux, Hazelwood, Mo.) assays. Isolates were confirmed to be the vaccine strain by isolation of genomic DNA (Easy DNA; Invitrogen, Carlsbad, Calif.) and by PCR demonstration that the anticipated genetic deletions shown in Fig. 1 were present.
Laboratory assessments. (i) Antigen preparation.
Antigens for use in immunoassays were generated in the laboratory. Killed bacterial cells were overnight shaker cultures of wild-type L. monocytogenes 10403S grown in brain heart infusion broth, pelleted, washed twice with normal saline, suspended to an optical density at 600 nm (OD600) of 1.0, and killed by incubation for 6 h at 65°C. The suspension was stored at −20°C in phosphate-buffered saline (PBS) with 50% (vol/vol) glycerol. A recombinant fusion protein of maltose binding protein (MBP) and the 411 aminoterminal amino acids of LLO was purified from Escherichia coli from a clone, kindly provided by J.-P. Gaillard (Institute Necker, Paris, France), as described previously (20). Recombinant 6-histidine-tagged LLO was purified from E. coli via nickel affinity chromatography from a clone kindly provided by Daniel Portnoy (University of California, Berkeley) (19). A soluble sonicate suspension was prepared from whole, wild-type L. monocytogenes 10403S as described previously (38).
(ii) ELISPOT studies.
ELISPOT studies were performed as described previously (26) by using freshly isolated PBMC. Cells were incubated atop Millipore HA cellulose ester membrane-bottom plates coated with 10 μg of recombinant proteins/ml and sonicate or 0.1 ml of heat-killed L. monocytogenes suspension. Spots were counted at 20× magnification by using a dissecting microscope and were reported as mean values/106 PBMC; ≥6 spots/106 cells was considered a positive result in this assay (26). Tissue culture supernatants from PBMC were also harvested for quantification of soluble vaccine-specific immunoglobulins by ELISA, as previously described in detail (11).
(iii) ELISA and Western blotting.
Serum samples were studied to quantify immunoglobulin G (IgG) and IgA directed against heat-killed bacteria and recombinant his-tagged LLO. ELISA protocols were developed within the laboratory to compare subjects' samples before and after inoculation. Because volunteers were not selected in any way based upon possible prior Listeria exposure or serum antibody detection, each individual served as his own preimmune control. Antigens were suspended in PBS and used to coat Nunc-Immuno Polysorp 96-well plates (Nalge Nunc International, Roskilde, Denmark) overnight at 4°C. Wells were then washed three times after this and all subsequent steps with PBS. Nonfat dry milk (5% [wt/vol]) in PBS with Tween 20 (0.05% [vol/vol]) was used as a blocking solution (1 h, room temperature). Sera were diluted in blocking solution and serially diluted twofold across microtiter plates. Alkaline phosphatase-labeled secondary antibody (goat anti-human IgG at 1:20,000 dilution or goat anti-human IgA at 1:5,000) was added. Plates were developed with 2 mg of _para_-nitrophenylphosphate/ml in 1 M Tris buffer, pH 8, and read at 405 nm with a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, Calif.). Endpoint dilutions are reported as the highest dilution at which a serum sample was ≥0.15 OD units, an arbitrarily chosen cutoff value. Fourfold or greater increases in endpoint titer were considered a positive result. The differences between geometric means between groups were compared statistically with the Mann-Whitney test.
Western blotting was performed with commercially available reagents and 6-his-tagged LLO or the fusion protein of MBP and the 411 amino acid fragment of LLO as target antigens. A goat anti-human secondary antibody conjugated to horseradish peroxidase was used with a chemiluminescent detection system to develop blots per the manufacturer's instructions (LumiGLO substrate and secondary antibodies were from KPL Laboratories, Gaithersburg, Md.).
(iv) Cellular immunology.
IFN-γ-secreting cells were quantified by ELISPOT after exposure to _Listeria_-specific antigens using a modification of techniques described by others (31, 58). For these studies, previously frozen aliquots of bulk PBMC isolated on days 0 and 28 after inoculation were thawed, revived overnight in RPMI tissue culture medium with 10% human AB serum (Sigma, St. Louis, Mo.), and exposed for 48 h to recombinant LLO, the MBP-LLO fusion antigen, or a control mitogen phytohemagglutinin or tetanus toxoid at various concentrations (1 to 10 μg/ml). Antigen-exposed cells were then evaluated for IFN-γ secretion by a capture sandwich ELISPOT technique using a monoclonal antibody to IFN-γ (Endogen, Woburn, Mass.) applied to Millipore Immobilon-P membrane-bottom wells. Nonadherent cells were removed by being washed, and IFN-γ secreting cells were enumerated by being developed with a second anti-IFN monoclonal antibody labeled with biotin and visualized with a streptavidin-alkaline phosphatase conjugate, 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) and nitroblue tetrazolium (both from Bio-Rad, Hercules, Calif.).
RESULTS
Preclinical evaluation.
Passage of the strain in vitro demonstrated that the actA and plcB deletions were stably maintained over 30 serial passages on agar medium. PCR analysis of chromosomal DNA from passaged strains demonstrated the expected, smaller amplification products (data not shown). Although formal clinical break points are not available for all agents tested, strain LH1169 was demonstrated to be resistant to streptomycin but susceptible to the clinically useful antibiotics gentamicin, tobramycin, penicillin, ampicillin, and trimethoprim-sulfamethoxazole and by disk diffusion testing and microtiter broth dilution for beta-lactam agents (data not shown). The wild-type parental strain, single _actA_- or _plcB_-deleted strains, and actA/plcB double mutants were compared in a macrophage survival assay and also in a 5-day assay of cell-to-cell spread in L929 fibroblasts. Over a 6-h time frame, survival of the mutant bacteria in J774 cells was nearly indistinguishable from that of the wild-type parental control strain (Fig. 2), indicating efficient phagosomal escape and unimpaired intracytoplasmic growth of the mutant strains (55). As expected, the single and double mutants demonstrated graded decreases in the ability to spread from cell to cell, reflected by plaquing ability within L929 fibroblast cells (Fig. 2). The _actA/plcB_-deleted strain LH1169 was attenuated in the BALB/c mouse, a standard model for evaluation of L. monocytogenes virulence (50% lethal dose [LD50] for strain 10403S, 3 × 104 CFU per BALB/c mouse; LD50 for strain LH1169, 4 × 107 CFU per BABL/c mouse).
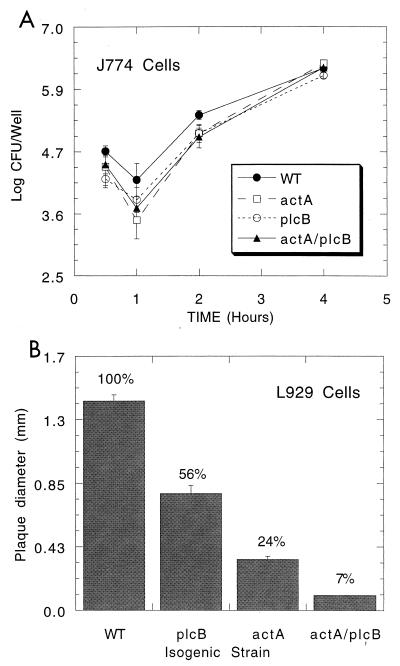
FIG. 2.
Survival of isogenic L. monocytogenes mutants within macrophage-like J774 cells and plaquing within fibroblast-like L929 cells. (A) J774 cells were infected for 30 min with wild type (WT), single mutants, or double mutants at a multiplicity of infection of 10:1 and were subsequently treated with gentamicin (20 μg/ml) to eliminate extracellular bacteria. Cells were lysed and bacteria were serially diluted and spread plated for CFU determinations on brain heart infusion agar. Data are presented as means of 3 wells ± standard errors. (B) L929 cells were similarly infected and overlaid with soft agar containing gentamicin (20 μg/ml). After 4 days of culture cells were overlaid with agar containing vital red dye, which stains live cells, for another day. Unstained, devitalized cell plaques are measured in millimeters. Mean plaque sizes ± standard errors of 10 plaques/strain are reported. Percentages reflect the percent of wild-type plaque size for mutants and show that the double mutant is highly limited in cell-to-cell spread, more so than either single mutant.
Clinical outcomes.
Volunteers were inoculated with escalating single oral doses (shown in Table 1). No serious adverse events occurred. There were no fevers during the 2-week inpatient observation period (maximum temperature for all was 99.3°F). No bacteremia was detected. No volunteers had diarrhea, though 3 out of 20 had slightly looser than usual stools (taking the shape of the container but not being a liquid) within the first 24 h after inoculation only. No volunteer received antibiotics in order to treat clinical listeriosis or symptoms suggestive of listeriosis. Subjects 1 and 8 (106 and 107 CFU, respectively), who were studied during the winter season, had febrile illnesses (temperatures, 101 to 102°F) during the 6-week outpatient follow-up period and had additional outpatient clinical and laboratory examinations, including additional blood cultures. Volunteer number 1 had coryza, cough, headache, and prominent myalgias 4 weeks after inoculation, without photophobia or other evidence of neurological involvement by history or exam. The clinical diagnosis was viral respiratory infection, possibly influenza. Two blood cultures were performed and subsequently were negative, and a complete blood count with differential was unremarkable; influenza antigen testing was not done. These complaints resolved within days with symptomatic therapy only. Volunteer 8 (age 19) had a febrile illness with sore throat, malaise, and prominent cervical adenopathy 7 weeks after inoculation. Pharyngeal erythema was prominent, but there was no exudate. A throat culture, heterophile antibody, 3 blood cultures obtained over 24 h, and HIV viral load were all negative. The subject reported prior episodes of streptococcal pharyngitis and was presumptively treated with 10 days of amoxicillin. One volunteer, number 19, in the highest-dose cohort was well at day 28 and declined to return for any further follow-up evaluation; all others completed all study visits.
TABLE 1.
Cohorts and doses
| Cohort | n | Dose (CFU) |
|---|---|---|
| 1 | 4 | 1 × 106 |
| 2 | 4 | 1.2 × 107 |
| 3 | 4 | 3.7 × 107 |
| 4 | 4 | 1.2 × 108 |
| 5 | 4 | 0.5 × 109 to 1.0 × 109a |
Clinical bacteriology.
Naturally occurring L. monocytogenes was not detected in fecal samples of any of the 40 potential subjects who completed medical screening to the point of submitting a sample for prestudy culture. No other enteric pathogens or parasites were detected in those who participated in the full study. After inoculation, L. monocytogenes was detected in fecal samples from the majority of subjects (15 out of 20); in 5 individuals the investigational strain was never detected. Stool culture results are shown by volunteer in Fig. 3. Most subjects shed bacteria for 4 days or less, and in many stool samples the strain was only detectable after overnight incubation in UVM enrichment broth. By studying fecal samples spiked with known inocula of LH1169 (target inocula of 2, 20, and 200 CFU) we found that the enrichment culture technique used was able to detect 20 to 80 CFU of the vaccine organism in ∼5 g of feces approximately 70% of the time (data not shown). Up to 3 stools per day were cultured to maximize likelihood of finding the investigational strain, if present. No volunteer passed more than 3 stools per day on any day, so in fact every stool passed was cultured. Volunteer 4 in the lowest-dose cohort had a late positive stool culture obtained at the last outpatient follow-up visit, 8 weeks after inoculation. He received 3 days of oral amoxicillin and had 6 subsequent fecal cultures which were negative for L. monocytogenes. This late isolate, and all other last-positive samples from other volunteers, was analyzed genotypically by PCR; in each instance fecal clones were found to contain the anticipated deletions in both actA and plcB (a representative example is shown in Fig. 1).
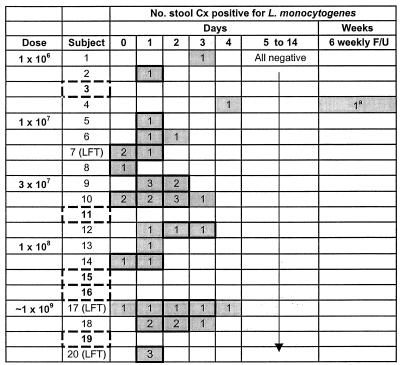
FIG. 3.
Fecal cultures by volunteer and day. The numbers represent the total number of stool samples positive for L. monocytogenes on that day. Shading denotes days on which at least one sample grew L. monocytogenes. A bolded outline represents days on which at least one culture was positive by direct plating of feces, an indication of larger organism burden. On days without bolded outlines, enrichment broth incubation was needed to detect the organism. Five volunteers (dashed) never had L. monocytogenes detected in any fecal sample. Volunteers with abnormal transaminases are noted (LFT). a, Only one volunteer (number 4) had a late positive culture at the sixth outpatient follow-up visit, after multiple previous samples had been negative (see the text for details).
Laboratory safety data.
No volunteer had leukocytosis, lymphopenia, or left shift of the leukocyte differential after inoculation. One volunteer (number 7) had a small, transient monocytosis only on day 4 after inoculation in the absence of an absolute leukocytosis (total leukocytes, 4,600/mm3; 51% neutrophils, 26% lymphocytes, 8% reactive lymphocytes, 3% eosinophils, and 12% monocytes, with 11% being upper normal percentage; the volunteer had an absolute monocyte count of 600/mm3, with upper normal being 400/mm3). There were no abnormalities in renal function in any volunteer over the inpatient stay as measured by serum blood urea nitrogen and creatine (all determinations within normal ranges). Three volunteers had abnormal serum transaminases temporally associated with receipt of the vaccine inoculum, as shown in Fig. 4. For volunteer number 17, we believe that these increases could have been caused exclusively by initiation of vigorous exercise (calisthenics and a stationary bicycle used for diversion during the tedious inpatient stay), as reported for other healthy subjects in vaccine studies (36). This individual had been sedentary, began to exercise to the point of muscle soreness, and was found to have a concurrent increase in serum creatinine phosphokinase (CPK) which was fourfold greater than his baseline, though still within the normal range. Both transaminases and CPK decreased rapidly after cessation of exercise. Additional longer-term follow-up of these values were not obtained for this individual, as he felt completely well.
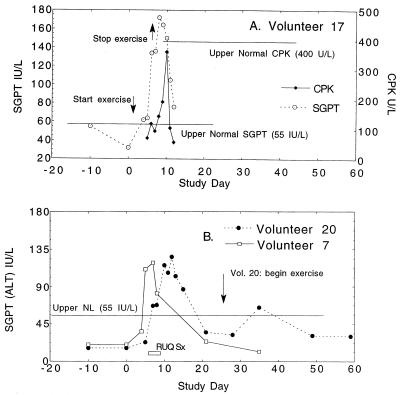
FIG. 4.
Time course of serum alanine aminotransferase (SGPT) and CPK in three volunteers with abnormal findings. Volunteers were inoculated on day 0 and had serum studies performed on the days indicated. (A) Volunteer 17, who received approximately 109 CFU, had a reasonable alternative explanation for aminotransferase elevations (vigorous exercise) and a concurrent elevation in CPK, a muscle enzyme. This individual was asymptomatic, and abnormalities resolved promptly with cessation of exercise. (B) Volunteer 7 (107 CFU) and volunteer 20 (109 CFU) were not exercising during the inpatient stay and had CPKs below 100. Volunteer 20 was minimally symptomatic, with intermittent right-upper-quadrant discomfort (horizontal bar); volunteer 7 was asymptomatic. Alkaline phosphatase and bilirubin determinations were within normal ranges for all subjects. Aspartate aminotransferases (ALT/SGOT) paralleled changes in SGPT in magnitude and time course and are not shown.
Volunteer numbers 7 (107 CFU) and 20 (109 CFU) had transaminase elevations which could not be otherwise explained and were attributed to the inoculation. Volunteer 7 was asymptomatic. Volunteer 20 had mild intermittent right-upper-quadrant abdominal symptoms: transient pain and mild punch tenderness on exam without hepatomegaly on days 7 to 9 after inoculation, as well as mild malaise and lack of energy. This individual had no fevers or bacteremia and no diarrhea or neurological symptoms over the entire course of the study. Transaminases resolved without any therapy for both of these subjects. Volunteer 20 resumed weight lifting upon discharge and had a small second increase in transaminases. Both subjects were immune to hepatitis B at onset of the study. Both subjects were hepatitis C seronegative at the onset of study and after transaminase elevations were found, and there were no symptoms or exposures suggestive of other viral or toxic causes of transaminase elevations. There were no temperature trends or other clinical findings in these subjects suggesting bacteremia or active infection. Additional diagnostic workup was not pursued, given the spontaneous resolution.
Immune responses. (i) ELISPOT studies.
Clinical studies of attenuated gram-negative organisms suggest that detection of vaccine-specific lymphocytes in peripheral blood shortly after oral inoculation is a sensitive surrogate marker of mucosal immune responses. Surprisingly, no volunteer had IgA-bearing cells specific for vaccine antigens detected after inoculation. One volunteer had a robust IgG response (volunteer 18; see below). Volunteer 11 had 27 lymphocytes directed against recombinant LLO detected on day 0 before receipt of the vaccine inoculum but not at any point afterwards. This was a reproducible finding in multiple wells and dilutions, and negative control wells had no spots. This individual was one of five who never had a fecal culture positive for the vaccine organism. He had no preadmission illnesses by history, and screenings of fecal cultures were not positive for L. monocytogenes. Patients with high anti-streptolysin O (ASLO) titers may have antibodies which cross-react with LLO (20). We speculated that this subject could have had asymptomatic listerial or streptococcal infection before admission, having documented such antecedent, asymptomatic competing or cross-reacting infection in one prior study of an attenuated Salmonella enterica serovar Typhi vaccine (26).
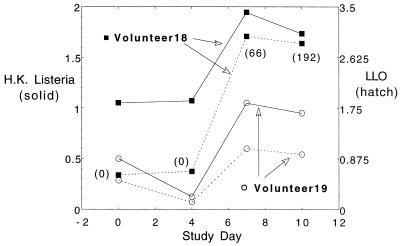
We and others (1, 8, 11) have also measured vaccine-specific Igs in culture media supernatants from lymphocytes cultured at high density (107 cells/ml) for 48 h after isolation. We have hypothesized that these latter studies may be more quantitative, less subjective, and perhaps more sensitive than those involving counting of spots. In prior studies IgG was more easily detected in these assays than IgA (1). Two of four volunteers (numbers 18 and 19) in the highest-dose cohort only had increases in supernatant IgG antibody specific for L. monocytogenes antigens detected on study days 7 and 10 compared to the baseline (Fig. 5). One of these, volunteer 18, also had a modest positive response in the traditional ELISPOT assay, with a maximum of 192 IgG-bearing cells directed against recombinant LLO on study day 10 (last day performed).
FIG. 5.
Measurement of soluble IgG directed against recombinant LLO or heat-killed L. monocytogenes (H.K. Listeria) by ELISA in tissue culture supernatants. PBMC were isolated from volunteers on the days designated and were cultured at 107 cells/ml for 48 h. Tissue culture media were aspirated, centrifuged free of cells, and applied to ELISA plates coated with vaccine antigens; peroxidase-labeled goat anti-human antibody was used to develop wells. Data shown are OD450 values (mean value of 2 wells). Control wells without antigens had OD values of ≤0.05. None of the 18 other volunteers had increases over day 0 values in this assay. Numbers in parentheses are mean IgG-bearing cells by traditional ELISPOT assay in the one volunteer who had positive results in those assays. We have arbitrarily designated a threefold or greater increase over baseline as a positive result in prior studies of this type; although these results are encouraging, they do not consistently meet that criterion, with the exception of volunteer 18's response to recombinant LLO.
(ii) Serum antibodies.
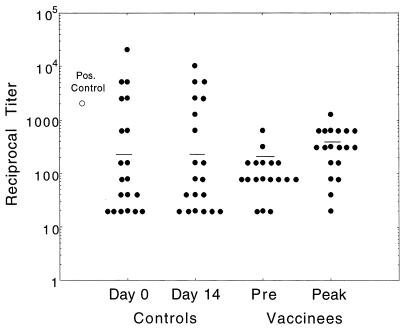
Although antibodies are not the primary protective mechanism against listeriosis, seroconversion can be demonstrated after acute infection with L. monocytogenes, and antibodies may contribute to protection (16, 17, 42, 43). Serum IgG and IgA were assessed before and after inoculation. Baseline levels of IgG directed against whole L. monocytogenes and recombinant LLO varied widely among healthy volunteers (endpoint titer range, 1:20 to 1:20,480). Baseline values did not correlate obviously with shedding, immune responses, or transaminase elevations. None of our inoculated volunteers nor any of a panel of 20 paired sera obtained 14 to 18 days apart demonstrated a fourfold or greater increase in titer directed against whole L. monocytogenes 10403S (Fig. 6). There was a suggestion of a trend toward and increase in geometric mean titer in inoculated volunteers (preimmune versus peak) compared with that of paired sera from uninoculated healthy blood donors, but this was not statistically significant (Fig. 6). Titers of IgG directed against recombinant histidine-tagged LLO increased more than fourfold in 1 of 20 volunteers (volunteer 18, 1:80 to 1:640) and in 1 of 20 sets of paired serum samples from healthy uninoculated individuals (1:20 to 1:80). The time course of volunteer 18's response (i.e., low on screening visit and day 0, increasing gradually until day 21 and remaining persistently elevated) was suggestive of a bona fide serological response. Only 2 of 5 of the positive control sera from patients with microbiologically confirmed listeriosis had marked positive immune responses in these serological assays; all others were comparable or less reactive than our healthy volunteers at baseline. All of these individuals were elderly and/or immunologically abnormal hosts; there were no young healthy subjects without risk factors for listeriosis in this group.
FIG. 6.
Serum IgG by ELISA directed against L. monocytogenes 10403S. Sera on study days 0, 4, 7, 10, and 14 and weekly thereafter for 6 weeks were serially diluted twofold across ELISA plates coated with heat-killed wild-type L. monocytogenes. Total IgG was detected by addition of phosphatase-labeled goat anti-human IgG and _para_-nitrophenylphosphate substrate. Endpoint titers were defined as the final dilution at which OD405 was ≥0.15. Preimmune (Pre) and peak values of reciprocal titers are plotted for inoculated subjects. Control subjects were healthy, uninoculated individuals who agreed to provide serum samples on two separate occasions 14 to 18 days apart. Geometric mean titers are represented by horizontal bars. No subject in either group had a fourfold or greater difference in titer, and the differences between groups (day 0 versus day 14, preimmune to peak values, and day 0 versus preimmune) were not statistically significant by the Mann-Whitney test. The datum for one patient with bacteremic clinical listeriosis who had a prominent response in this assay is also plotted (Pos. Control).
There were no detectable changes in serum IgA directed against whole organisms or recombinant LLO after inoculation (data not shown). Western blotting using either histidine-tagged full-length LLO or MBP-LLO fusion protein did not provide any apparent advantages over ELISA studies (data not shown).
(iii) Cellular immunology.
A secondary goal of this study was to begin to develop measurements of cellular immune responses to L. monocytogenes. Increasingly, both CD4+ and CD8+ T-cell-mediated immune responses are being quantified by intracellular cytokine staining and ELISPOT studies (31, 54, 61). These assays typically evaluate responses to specific peptides or peptide pools, but they have also been performed with larger secreted bacterial protein antigens, such as ESAT-6 and the purified protein derivative of Mycobacterium tuberculosis (58). Despite a plethora of murine data, there are no specific immunological correlates of protection or specific T-cell epitopes of L. monocytogenes known in humans, so we were unable to focus on specific peptide targets. We elected to examine production of IFN-γ in response to soluble listerial antigens in our top two dose cohorts, those receiving ≥108 CFU of attenuated L. monocytogenes, by using bulk PBMC collected before and 28 days after inoculation. We had difficulties in reproducibly reviving frozen PBMC for these assays, and results from the fourth cohort were not interpretable. For subjects receiving 109 CFU we found that, compared with results for no-antigen control wells, all four individuals had levels of detectable IFN-γ-producing cells in response to listerial antigens which were unchanged between pre- and postvaccination samples (days 0 and 28; Table 2).
TABLE 2.
IFN-γ-secreting cells after exposure to antigens, before and after inoculation
| Subject no. | Spot-forming cells per well on days 0 and 28 for the following antigen or reagenta | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | PHAb | T-tox | LLO-MBP | Sonicate | ||||||
| 0 | 28 | 0 | 28 | 0 | 28 | 0 | 28 | 0 | 28 | |
| 17 | 0 | 0 | TNTC | TNTC | 1 | 1 | 5 | 6 | 1 | 1 |
| 18 | 5 | 3 | TNTC | TNTC | 7 | 7 | 13 | 15 | 8 | 8 |
| 19 | 0 | 0 | TNTC | TNTC | 17 | 16 | 30 | 50 | 3 | 2 |
| 20 | 0 | 0 | TNTC | TNTC | 4 | 6 | 7 | 7 | 8 | 10 |
DISCUSSION
To our knowledge, this is the first reported human safety study of a rationally designed L. monocytogenes vector. The strain used was attenuated in mice and relevant in vitro assays. Despite the pathogenic potential of wild-type L. monocytogenes, there were no fevers or evidence of bacteremia or systemic illness in any volunteer. These data are consistent with clinical investigations of outbreaks which show that even when large doses of presumably virulent wild-type Listeria are ingested, immunocompetent subjects generally do not become systemically ill (2, 10). No subject had gastroenteritis, which has been reported after ingestion of large inocula in foodstuffs. One previous dose escalation study of female cynomolgus monkeys (3 to 5 kg) receiving 105 to 109 CFU of wild-type L. monocytogenes showed that only those receiving the highest dose became febrile, visibly ill, and bacteremic (17). Only one of our subjects had a minimal monocytosis, and none had a leukocytosis or lymphopenia, as noted in the primate study. None of our subjects had any suggestion of neurological difficulties or late sequelae of inoculation in the 8-week follow-up period.
In murine models Listeria organisms are rapidly cleared by the liver after intravenous inoculation (9), and humans with iron overload and liver disease are more susceptible to listeriosis. For these reasons and as a matter of routine safety monitoring, liver function tests were monitored. In the absence of any other reasonable explanation, we attributed transaminase elevations to the investigational inoculum in two subjects (volunteers 7 and 20). For volunteer 17, another credible explanation was apparent: vigorous exercise (36). This attribution is supported by an otherwise unexplainable, concurrent elevation in serum CPK. A concurrent elevation of hepatic transaminases related to the investigational inoculum cannot be ruled out, but this would seem to violate rules of parsimony in diagnosis. We found a single study from 1967 in which multiple serum enzymes indicative of tissue damage were measured after intraperitoneal inoculation of mice with L. monocytogenes. These authors described elevations in transaminases and lactate dehydrogenase without increases in CPK, and therefore they believed the serum abnormalities to be hepatic in origin.
No subject with transaminase abnormalities was acutely ill. One was very mildly symptomatic. Hepatic involvement has been described for cases of severe clinical listeriosis. Listeriosis is an unusual cause of granulomatous hepatitis, but hepatic involvement is common in overwhelming neonatal infection with L. monocytogenes, also known as granulomatosis infantisepticum. There are rare case reports of acute listeriosis presenting with a picture consistent with acute hepatitis (18, 24, 63). Unlike our subjects, these patients were severely ill and bacteremic, and most had transaminases in the thousands, presumably related to diffuse bacteremic seeding of the liver. There are also reports of hepatic abscesses (single or multiple) caused by L. monocytogenes (6), in one case in an asymptomatic individual (37). In these descriptions, liver function tests reflect the more cholestatic laboratory findings typically noted for bacterial hepatic abscesses, e.g., modest elevations in alkaline phosphatase and fairly normal transaminases. None of our subjects had elevations in alkaline phosphatase.
We are uncertain of the mechanism by which our inoculum caused transaminase elevations over approximately 2 weeks in subjects 7 and 20. It is possible that these subjects had microscopic seeding of the liver via the splanchnic circulation, which was not evident clinically nor captured on peripheral venous blood culturing. Transaminase elevations could reflect lysosomal autolysis (56), neutrophil-mediated lysis of infected hepatocytes (9), or an inflammatory or toxic reaction to L. monocytogenes or listerial products without actual infection of the liver (56). LLO is a potent cytolysin capable of lysing erythrocyte membranes at concentrations in the nanograms-per-milliliter range under reducing conditions in vitro (60). We hypothesize that in vivo production of LLO could be responsible for transaminase elevations in the absence of other evidence of acute bloodstream or hepatic infection. Published reports of acute gastroenteritis outbreaks after ingestion of L. monocytogenes do not, unfortunately, comment on measurement of hepatic enzymes (2, 10, 47).
Most of our subjects shed detectable L. monocytogenes in feces for 1 to 4 days. Our fecal culture techniques were reproducible and designed to detect fecal shedding in a time frame acceptable for clinical decision making. Our techniques were not as exhaustive as those of some food safety studies, where samples are passed twice in enrichment broth or incubated for up to 7 days in the cold or in enrichment broth (13). We cultured up to 3 stools daily (by happenstance, we cultured every stool passed) in order to enhance our ability to find organisms. There was no evidence of mutation or alteration of strains at the deletion loci in vivo. We detected the vaccine organism 8 weeks after inoculation in one individual who received the lowest dose tested, suggesting that this may be a phenomenon related to the individual rather than to the dose. Unlike other enteric pathogens, there are no good clinical data on the duration of shedding of Listeria organisms in healthy adults, though it may be found in fecal samples of approximately 1 to 5% of adults (22, 50). Our study suggests that the occasional adult may be colonized asymptomatically by L. monocytogenes at levels evading detection for long periods.
Our immunological data are limited by the absence of good positive and negative control samples, and we can only speculate about their significance. Virtually all the patient samples available from clinical cases of listeriosis are from immunocompromised or debilitated elderly patients. Most of the samples we obtained were found to have assay values below those of healthy adults. Similarly, negative controls simply reflect healthy individuals without known listerial infection, but it is likely that most adults are exposed at some point, perhaps frequently, to L. monocytogenes. One European study based upon culturing typical foodstuffs calculated that average adults may ingest 105 L. monocytogenes organisms nearly four times annually and 106 organisms about once annually, though of course this would be heavily dependent upon diet. Healthy subjects' titers against listerial antigens were highly variable in the assays we developed; this could be due to both prior asymptomatic colonization as well as cross-reaction with other gram-positive pathogens or proteins. For example, others have shown that sera with high ASLO titers (evidence of prior streptococcal infection) cross-react strongly with recombinant LLO (20).
We were somewhat surprised that we were not able to detect IgA-bearing cells directed against listerial antigens after oral inoculation, as this is a sensitive early test of immune response to Salmonella and other gram-negative bacteria that is administered orally (3, 26, 57). Perhaps these responses are a function of the entry of these other pathogens via M cells and Peyer's patches into the gut-associated lymphoid tissues. This pathway is not preferentially utilized by L. monocytogenes, which invades systemically through the intestinal mucosa via binding of the bacterial surface protein internalin with E-cadherin on eukaryotic enterocytes (32). A recent paper evaluating the mucosal immune response to L. monocytogenes 10403S actA mutants in germ-free mice showed that _Listeria_-specific IgA production by intestinal and mesenteric lymph node fragments peaked relatively late in the course of infection, days 21 to 59 after inoculation (38). Although it is difficult to extrapolate from this animal model to humans, perhaps future studies should include later time points in ELISPOT assays.
This study was not designed to measure cellular immune responses but rather to serve as a pilot study to investigate the feasibility of evaluating such responses in the future. The lack of positive controls, the absence of known immune targets and epitopes, and the lack of a correlate of protection in humans were practical difficulties. We also found recombinant His-tagged LLO highly toxic to cells and difficult to fully inactivate. Volunteers 18 and 19, who had the largest numbers of IFN-γ-secreting cells, also had detectable _Listeria_-specific IgG produced by unstimulated lymphocytes (Fig. 5). Volunteers 17 and 20, who had liver function test abnormalities, did not have prominent responses in this assay. IFN production by bulk PBMC in response to complex, large antigens may represent CD4+ or CD8+ responses, and we did not have adequate frozen samples to further pursue these studies.
Our data demonstrate that _actA/plcB_-deleted attenuated L. monocytogenes may be administered without serious sequelae to carefully monitored adult volunteers. Transaminase elevations occurred in 3 out of 20 volunteers. The pathophysiological mechanism and significance of this finding are uncertain. These clinical data and correlation with in vitro studies may serve as a reference point for other investigators interested in the study of L. monocytogenes vectors. We plan to engineer clinically acceptable derivatives of this _actA/plcB_-deleted strain which will express discrete viral antigens. These strains will be used to more definitively evaluate human immune responses to specific cellular immune epitopes delivered by attenuated L. monocytogenes.
Acknowledgments
This work was supported by Public Health Service grants R01AI45137 to E.L.H., R01CA84008 to J.F.M., and grant M01RR01066-21 to the General Clinical Research Center at Massachusetts General Hospital and by the Claflin Distinguished Scholar Award to E.L.H.
We gratefully acknowledge the essential contributions of the volunteers and staffs of the General Clinical Research Center and the Clinical Microbiology Laboratory of the Massachusetts General Hospital.
REFERENCES
- 1.Angelakopoulos, H., and E. Hohmann. 2000. Pilot study of PhoP/PhoQ-deleted Salmonella enterica serovar Typhimurium in expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 68**:**2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aureli, P., G. C. Fiorucci, D. Caroli, G. Marchiaro, O. Novara, L. Leone, and S. Salmaso. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342**:**1236-1241. [DOI] [PubMed] [Google Scholar]
- 3.Baqar, S., A. A. N. E. Din, D. A. Scott, A. L. Bourgeois, A. S. Mourad, M. T. Keinosky, M. J. Oplinger, and J. R. Murphy. 1997. Standardization of measurement of immmunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4**:**375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, R., H. G. A. Bouwer, D. A. Portnoy, and D. J. Hinrichs. 1992. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 60**:**1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boury, N. M., and C. J. Czuprynski. 1995. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro. Immunol. Lett. 46**:**111-116. [DOI] [PubMed] [Google Scholar]
- 6.Braun, T. I., D. Travis, R. Dee, and R. E. Nieman. 1993. Liver abscess due to Listeria monocytogenes: case report and review. Clin. Infect. Dis. 17**:**267-269. [DOI] [PubMed] [Google Scholar]
- 7.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA 90**:**11890-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, H. S., and D. A. Sack. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8**:**482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan, J. W. 1999. Early host pathogen interactions in the liver and spleen during systemic murine listeriosis: an overview. Immunobiology 201**:**178-187. [DOI] [PubMed] [Google Scholar]
- 10.Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffen. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336**:**100-105. [DOI] [PubMed] [Google Scholar]
- 11.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 2000. Safety and immunogenicity of _phoP/phoQ_-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18**:**449-459. [DOI] [PubMed] [Google Scholar]
- 12.Domann, E. J., M. Wehland, M. Rohde, S. Pistor, M. Hartl, W. Goebel, M. Leineister-Wachter, M. Wuenscher, and T. Chakraborty. 1992. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 11**:**1981-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, C. W. 1999. Conventional methods to detect and isolate Listeria monocytogenes, p. 225-260. In E. T. Ryser and E. H. Marth (ed.), Listeria, Listeriosis and Food Safety. Marcel Dekker, New York, N.Y.
- 14.Donnelly, C. W., and G. J. Baigent. 1986. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl. Environ. Microbiol. 52**:**689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dramsi, S., S. Levi, A. Triller, and P. Cossart. 1998. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread. Infect. Immun. 66**:**4461-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163**:**4087-4090. [PubMed] [Google Scholar]
- 17.Farber, J. M., E. Daley, F. Coates, N. Beausoleil, and J. Fournier. 1991. Feeding trials of Listeria monocytogenes with a nonhuman primate model. J. Clin. Microbiol. 29**:**2606-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebauer, K., J. C. Hall, J. B. Donlon, R. Herrmann, S. Roffe, and C. Platell. 1989. Hepatic involvement in listeriosis. Aust. NZ J. Med. 19**:**486-489. [DOI] [PubMed] [Google Scholar]
- 19.Gedde, M. M., D. E. Higgins, L. G. Tilney, and D. A. Portnoy. 2000. Role of listeriolysin in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68**:**999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gholizadeh, Y., C. Poyart, M. Juvin, J. L. Beretti, J. Croize, P. Berche, and J. L. Gaillard. 1996. Serodiagnosis of listeriosis based upon detection of antibodies against truncated forms of listeriolysin O. J. Clin. Microbiol. 34**:**1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goosens, P. L., G. Milon, P. Cossart, and M. F. Saron. 1995. Attenuated L. monocytogenes as a live vector of induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int. Immunol. 7**:**797-805. [DOI] [PubMed] [Google Scholar]
- 22.Grif, K., I. Hein, M. Wagner, E. Brandl, O. Mpagmugo, J. McLauchlin, M. P. Dierich, and F. Allerberger. 2001. Prevalence and characterization of Listeria monocytogenes in the feces of healthy Austrians. Wien. Klin. Wochenschr. 19**:**737-742. [PubMed] [Google Scholar]
- 23.Gunn, G. R., A. Zubair, C. Peters, Z. K. Pan, T. C. Wu, and Y. Paterson. 2001. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 167**:**6471-6479. [DOI] [PubMed] [Google Scholar]
- 24.Hardie, R., and W. Roberts. 1984. Adult listeriosis presenting as acute hepatitis. J. Infect. 8**:**256-258. [DOI] [PubMed] [Google Scholar]
- 25.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN-γ. Immunity 3**:**109-117. [DOI] [PubMed] [Google Scholar]
- 26.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. _phoP/phoQ_-deleted Salmonella typhi (Ty800) is a safe and immunogenic single dose typhoid fever vaccine in volunteers. J. Infect. Dis. 173**:**1408-1414. [DOI] [PubMed] [Google Scholar]
- 27.Hohmann, E. L., C. A. Oletta, and S. I. Miller. 1995. Evaluation of a _phoP/phoQ-_deleted _aroA_-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine 14**:**19-24. [DOI] [PubMed] [Google Scholar]
- 28.Jensen, E. R., R. Selvakumar, H. Shen, R. Ahmed, F. O. Wettstein, and J. F. Miller. 1997. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J. Virol. 71**:**8467-8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11**:**129-163. [DOI] [PubMed] [Google Scholar]
- 30.Kocks, C., J. B. Marchand, E. Gouin, H. Ohayon, and P. Cossart. 1995. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility of Listeria innocua and E. coli, respectively. Mol. Microbiol. 18**:**413-423. [DOI] [PubMed] [Google Scholar]
- 31.Lalavani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186**:**859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292**:**1722-1725. [DOI] [PubMed] [Google Scholar]
- 33.Levine, M. M., J. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103HgR. Lancet **ii:**467-470. [DOI] [PubMed]
- 34.Low, J. C., and W. Donachie. 1991. Clinical and serum antibody responses of lambs to infection by Listeria monocytogenes. Res. Vet. Sci. 51**:**185-192. [DOI] [PubMed] [Google Scholar]
- 35.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116**:**381-406. [PubMed] [Google Scholar]
- 36.Malinoski, F. J. 1992. Strenuous exercise simulating hepatic injury during vaccine trials. Vaccine 10**:**39-42. [DOI] [PubMed] [Google Scholar]
- 37.Manian, F. A. 1994. Liver abscess due to Listeria monocytogenes. Clin. Infect. Dis. 841-842. [DOI] [PubMed]
- 38.Manohar, M., D. O. Baumann, N. A. Bos, and J. J. Cebra. 2001. Gut colonization with _actA_-negative mutant of Listeria monocytogenes can stimulate a humoral mucosal immune response. Infect. Immun. 69**:**3542-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquis, H., V. Doshi, and D. A. Portnoy. 1995. The broad range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63**:**4531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mata, M., and Y. Paterson. 1999. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J. Immunol. 163**:**1449-1456. [PubMed] [Google Scholar]
- 41.Mata, M., Z. J. Yao, A. Zubair, K. Syres, and Y. Paterson. 2001. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine 19**:**1435-1445. [DOI] [PubMed] [Google Scholar]
- 42.Miettinen, A., and J. Husus. 1991. Antibodies to listeriolysin O reflect the acquired resistance to Listeria monocytogenes in experimentally infected goats. FEMS Microbiol. Lett. 61**:**181-186. [DOI] [PubMed] [Google Scholar]
- 43.Miettinen, A., J. Susu, and J. Tuomi. 1990. Serum antibody response to Listeria monocytogenes, excretion, and clinical characteristics in experimentally infected goats. J. Clin. Microbiol. 28**:**340-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mylonakis, E., E. Hohmann, and S. Calderwood. 1998. Central Nervous System infection with Listeria monocytogenes. 33 year's experience at a General Hospital and review of 776 episodes from the literature. Medicine 77**:**313-316. [DOI] [PubMed] [Google Scholar]
- 45.Rayevskaya, M., and F. Frankel. 2000. Systemic and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain to Listeria monocytogenes. J. Virol. 75**:**2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed, L. J., and H. Muench. 1930. A simple method of estimating 50 percent endpoints. Am. J. Hyg. 27**:**493-496. [Google Scholar]
- 47.Salamina, G., E. DalleDonne, A. Niccolini, G. Poda, D. Cesaroni, M. Bucci, R. Fini, M. Maldini, A. Schuchat, B. Swaminathan, W. Bibb, J. Rocourt, N. Binkin, and S. Salmaso. 1996. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol. Infect. 117**:**429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer, R., D. A. Portnoy, S. A. Brassel, and Y. Paterson. 1992. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J. Immunol. 149**:**53-59. [PubMed] [Google Scholar]
- 49.Schluter, D., E. Domann, C. Buck, T. Hain, H. Hof, T. Chakraborty, and M. Deckert-Schluter. 1998. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun. 66**:**5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuchat, A., B. Swaminathan, and C. V. Broome. 1991. Epidemiology of human listeriosis. Clin. Micro. Rev. 4**:**169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarzer, N., R. Nost, J. Seybold, S. K. Parida, O. Fuhrman, M. Krull, R. Schmidy, R. Newton, S. Hippenstiel, E. Domann, T. Chakraborty, and N. Suttorp. 1998. Two distinct phospholipases of Listeria monocytogenes induce ceramide generation, nuclear factor 75 β activation and E-selectin expression in human endothelial. J. Immunol. 161**:**3010-3018. [PubMed] [Google Scholar]
- 52.Shen, H., M. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective antiviral cell mediated immunity. Proc. Natl. Acad. Sci. USA 92**:**3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slifka, M. K., H. Shen, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1996. Antiviral cytotoxic T-cell memory by vaccination with recombinant Listeria monocytogenes. J. Virol. 70**:**2902-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, J. G., X. Liu, R. M. Kaufhold, J. Clair, and M. J. Caulfield. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella virus. Clin. Diagn. Lab. Immunol. 8**:**871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58**:**3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sword, C. P., and M. S. Wilder. 1967. Plasma enzyme changes in Listeria monocytogenes infected mice. J. Infect. Dis. 117**:**387-392. [DOI] [PubMed] [Google Scholar]
- 57.Tacket, C. O., S. M. Kelly, F. Schodel, G. Losonsky, J. P. Nataro, R. Edelman, M. M. Levine, and R. Curtiss III. 1997. Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain exprssing plasmid-encoded hepatitis B antigens stabilized by the _asd_-balanced lethal vector system. Infect. Immun. 65**:**3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulrichs, T., P. Anding, S. Porcelli, S. H. Kaufman, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68**:**6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez-Boland, J., C. Kocks, S. Dramsi, H. Obayan, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60**:**219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walton, C., C. H. We, and G. Y. Wu. 1999. A method for purification of listeriolysin O from a hypersecretor strain of Listeria monocytogenes. Prot. Express. Purif. 15**:**243-245. [DOI] [PubMed] [Google Scholar]
- 61.Wang, R., J. Epstein, F. M. Baraceros, E. J. Gorak, Y. Charoenvit, D. J. Carucci, R. C. Hedstrom, N. Rahardjo, T. Gay, P. Hobart, R. Stout, T. R. Jones, T. L. Richie, S. E. Parker, D. L. Doolan, J. Norman, and S. L. Hoffman. 2001. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 98**:**10817-10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong, H., Y. Tanabe, S. Ohya, and M. Mitsuyama. 1998. Administration of killed bacteria together with listeriolysin O induces protective immunity against Listeria monocytogenes in mice. Immunology 94**:**14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, V. L., W. P. Miller, E. J. Wing, J. M. Romano, C. A. Ruiz, and F. J. Bruns. 1982. Disseminated listeriosis presenting as acute hepatitis. Am. J. Med. 73**:**773-777. [DOI] [PubMed] [Google Scholar]