PKCε controls the traffic of β1 integrins in motile cells (original) (raw)
Abstract
Protein kinase C (PKC) has been implicated in β1 integrin-mediated cell migration. Expression of the novel PKC isoform, PKCε, in PKCε–/– cells is shown here to stimulate directional migration of cells towards β1 integrin substrates in a manner dependent on PKC catalytic activity. On PKC inhibition, integrin β1 and PKCε become reversibly trapped in a tetraspanin (CD81)-positive intracellular compartment, correlating with reduced haptotaxis. Immunofluorescence and pulse labelling studies indicate that this is a previously uncharacterized recycling compartment trapped by inhibition of PKC. Electron microscopy demonstrated the co-localization of PKCε and integrin β1 on the vesicular membranes. Finally, using a reconstituted in vitro system, the dissociation of PKCε from these vesicles is shown to be dependent on both the presence of cytosolic components and energy, and on PKC catalytic activity. The evidence presented indicates that PKCε controls an internal traffic step that under uninhibited conditions permits the recycling of β1 integrin, contributing to cell motility.
Keywords: integrin/migration/protein kinase C/trafficking
Introduction
Adhesion of cells to the extracellular matrix (ECM) is mediated by the integrin family of heterodimeric transmembrane receptors (Giancotti and Ruoslahti, 1999). They function in linking matrix proteins to the cellular cytoskeleton and signal transduction apparatus, orchestrating signals that regulate different aspects of cell behaviour. Integrins mediate cell migration towards matrix proteins (haptotaxis) or soluble effectors like cytokines or growth factors (chemotaxis) (Horwitz and Parsons, 1999). Different types of adhesive structures are found in motile cells. Small and transient focal complexes are often found in protruding filopodia and lamellipodia, while large and stable focal adhesions usually underlie the body of the cell and are localized at the ends of actin stress fibres (Hall, 1998). The coordinate regulation of the formation and turnover of adhesion sites is critical for establishing directional cell motility.
Recent studies have also shown that protein kinase C (PKC) phosphorylation/activity is regulated by cell adhesion (Parekh et al., 2000; England et al., 2001). PKCε has been shown to translocate to membranes on matrix adhesion (Chun et al., 1996), and there is substantial evidence that members of the PKC family regulate integrin-mediated cell spreading and migration (Woods and Couchman, 1992; Klemke et al., 1994). Studies using pharmacological agents have suggested that PKCs are involved in the activation of integrins, and consequently in cell adhesion, spreading and migration (Rigot et al., 1998; Ng et al., 1999; Sun and Rotenberg, 1999). Both classical and novel PKCs have been implicated as positive regulators of cell adhesion. PKCα has been shown to interact directly with β1 integrins, thus controlling β1 integrin-dependent cell motility (Ng et al., 1999). PKCε and PKCµ (PKD1) are also implicated in fatty acid-enhanced, β1 integrin-mediated adhesion of human breast carcinoma cells to collagen (Palmantier et al., 2001).
It has been hypothesized that membrane internalized from the cell surface is recycled to the leading edge of the migrating cells, providing directionality (Bretscher, 1996). In order to facilitate cell migration, integrin-containing adhesion sites need to be constantly internalized at the rear of the cell and subsequently transported to the leading edge (Palecek et al., 1996). However, little is known about the exact mechanisms regulating this integrin traffic. We have previously shown that stimulation of PKCα induces cell migration and internalization of β1 integrin in a Ca2+/PI 3-kinase/dynamin-I-dependent manner, and that the enhanced migratory response is sensitive to blockade of endocytosis. In addition, integrins have recently been shown to traffic through a Rab11-positive endocytic recycling compartment (Ng et al., 1999; Roberts et al., 2001).
In the present study, we have found PKCε to be a regulator of β1 integrin-dependent migration and the traffic of these receptors through the cell. Inhibition of PKCε reduces cell motility and leads to the accumulation of large β1 integrin/PKCε-containing vesicular structures that appear to represent a normally transient recycling compartment. Removal of PKC inhibition results in the dissipation of these PKCε/β1 integrin-positive vesicles. We have isolated these vesicular structures using fractionation techniques and developed a cell-free system to analyse the requirements for dissipation of these vesicles. The release of PKCε from these vesicles requires ATP, GTP and cytosolic components, and is dependent on PKCε kinase activity. The data identify a critical role for PKCε catalytic function in regulating β1 integrin traffic in cells.
Results
Previous studies have shown that several isoforms in the PKC family of protein kinases promote cell migration. These include PKCα (Platet et al., 1998; Ng et al., 1999), PKCδ (Kiley et al., 1999), PKCθ (Tang et al., 1997) and PKCε (Palmantier et al., 2001). In the present study, we used PKCε knockout (PKCεKO) cells stably transfected with vector alone and PKCε re-expressing (PKCεRE) cells to characterize the effect of PKCε expression on integrins.
Mouse embryo fibroblasts (MEFs) from a PKCε–/– embryo (Castrillo et al., 2001) were isolated and stable isogenic lines established by reintroduction of PKCε (R.D.H.Whelan and P.J.Parker, unpublished data). The levels of expression of PKCε in two isolates (PKCεRE clones 5 and 17) and knockout cells (PKCεKO) are shown in Figure 1A; PKCε expression is 4- to 10-fold higher than in MEFs, which are wild type (wt) for the expression of PKCε (GD25 cells). The cell surface integrin expression pattern of these cells was characterized using a FACS scan. There were no significant differences in integrin cell surface expression between the PKCεKO and PKCεRE cells. The observed integrin pattern included α4, α5, α6, αV, β1 and β3 subunits; α1, α2 and α3 integrin subunits were not expressed on the cell surface of these cells (data not shown).
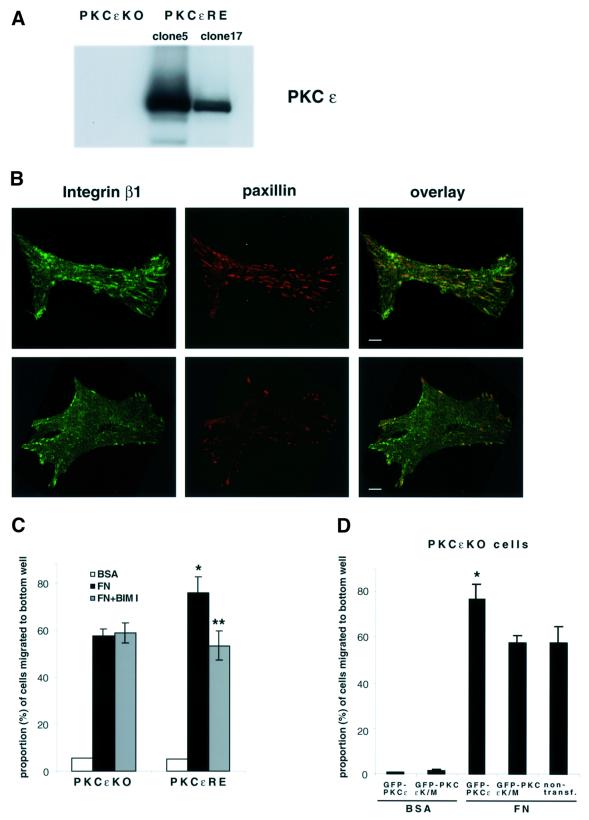
Fig. 1. PKC-driven changes in integrin-controlled functions. (A) MEFs from a PKCε–/– embryo were isolated and isogenic lines established by transfection with PKCε (PKCεRE) or control vector (PKCεKO). The level of PKCε in two different isolates (clone 5 and 17) is shown; no PKCε is detected in the vector control line. (B) The focal adhesions of PKCεKO (top panels) and PKCεRE (lower panels) cells plated on fibronectin (FN). Immunofluorescence staining of β1 integrin (green) and paxillin (red) is shown as representative confocal images of 0.8 µm sections taken from the basal side of the cell. The bar represents 10 µm. (C) PKCε expression enhances haptotactic response towards FN. The bottom of Transwell filters was coated using 10 µg/ml BSA or FN. Serum-starved PKCεKO and PKCεRE cells were allowed to migrate in the absence or presence of the PKC inhibitor BIM I (1 µM) for 24 h. The cells on different sides of the filter were trypsinized and counted. Shown are the precentage of cells that had migrated to the bottom well (mean ± SEM, n = 8). The data were analysed using the _t_-test in order to assess statistical significance (*, difference between PKCεKO and PKCεRE cell migration towards FN, p = 0.04; **, difference between PKCεRE cells migrating towards FN ± BIM I, p = 0.005). (D) Kinase-dead PKCε fails to enhance haptotactic response towards FN. PKCεKO cells were transiently transfected with GFP–PKCε or GFP–PKCεK/M for 24 h before preparation for Transwell assay. As above, the cells were allowed to migrate for 24 h. The cells on different sides of the filter were trypsinized, fixed, transferred to microscope slides by cytospin and 50 fields of each slide were scored for GFP-positive and non-transfected cells. Shown are the percentages of GFP-positive or non-transfected cells that had migrated to the bottom well (mean ± SEM, three separate experiments). The data were analysed using the _t_-test in order to assess statistical significance (*, difference between migration of GFP–PKCε-positive PKCεKO cells and non-transfected and PKCεKO cells towards FN, p = 0.04).
We first determined the localization of β1 integrin and the focal adhesion protein paxillin in PKCεKO and PKCεRE cells (clone 5). In control cells plated on fibronectin, β1 integrin was found in characteristic paxillin-positive focal adhesions underlying the cell body. In contrast, cells expressing PKCε showed many fewer focal adhesions and these were largely distributed around the perimeter of the cell (Figure 1B).
Prominent focal adhesions are usually indicative of reduced cell motility. We analysed the migratory behaviour of these cells in a Transwell chamber assay. The cells were allowed to migrate towards fibronectin or BSA for 24 h in the absence of serum and the proportion of cells migrating out of the total number of cells was scored (Figure 1C). Associated with the altered distribution of β1 integrin, PKCεRE clone 5 cells were found to migrate more towards fibronectin when compared with vector control cells. Random motility through the filter to BSA was found to be minimal. Notably, the increased migratory behaviour consequent to PKCε expression was inhibited by the PKC inhibitor bis-indolylmaleimide I (BIM I) (Figure 1C), indicating that some catalytic property of PKCε conferred this migratory difference.
To further assess the requirement for PKCε catalytic activity in the induction of haptotaxis, we analysed the migratory behaviour of transfected PKCεKO in a Transwell chamber assay. PKCεKO cells were transiently transfected with GFP–PKCε or GFP–PKCεK/M (kinase inactive) and the proportion of transfected and non-transfected cells migrating out of the total number of cells was scored (Figure 1D). Transient expression of wt PKCε induced migration of PKCεKO cells towards fibronectin. Consistent with the BIM I data, the kinase-dead mutant of PKCε failed to induce motility. Interestingly, the mutant also did not show any dominant effects on the motility of PKCεKO cells. Neither construct induced random motility towards BSA (Figure 1D).
Inhibition of PKC causes the accumulation of β1 integrin and paxillin in distinct vesicular compartments
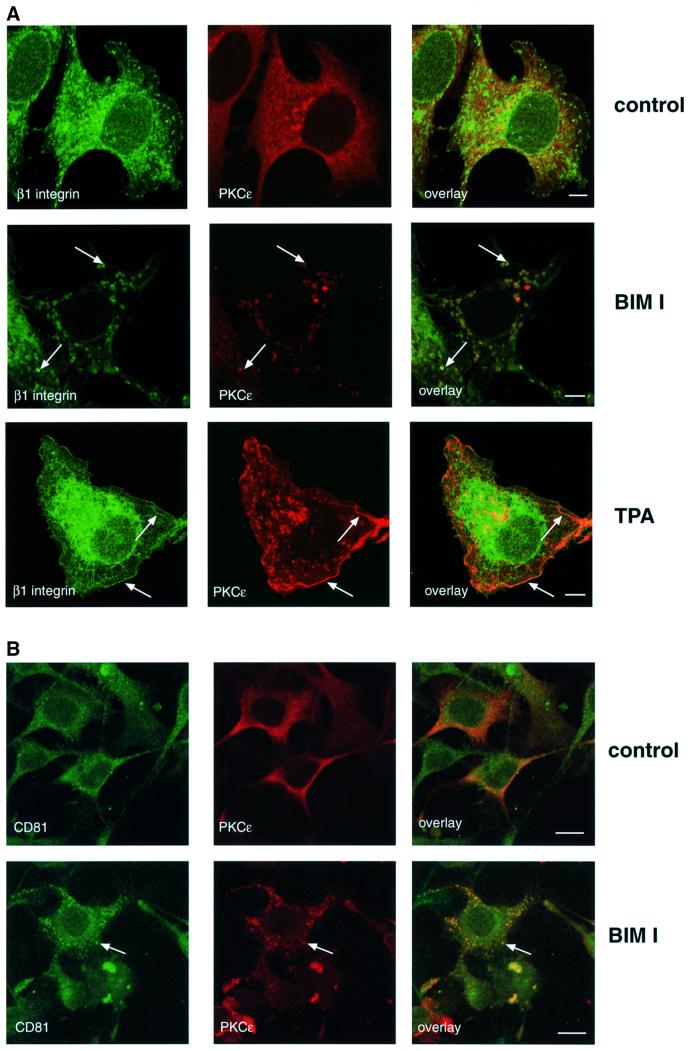
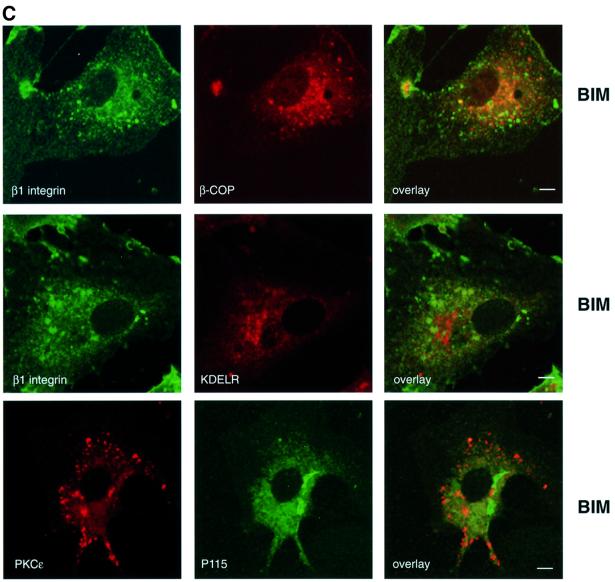
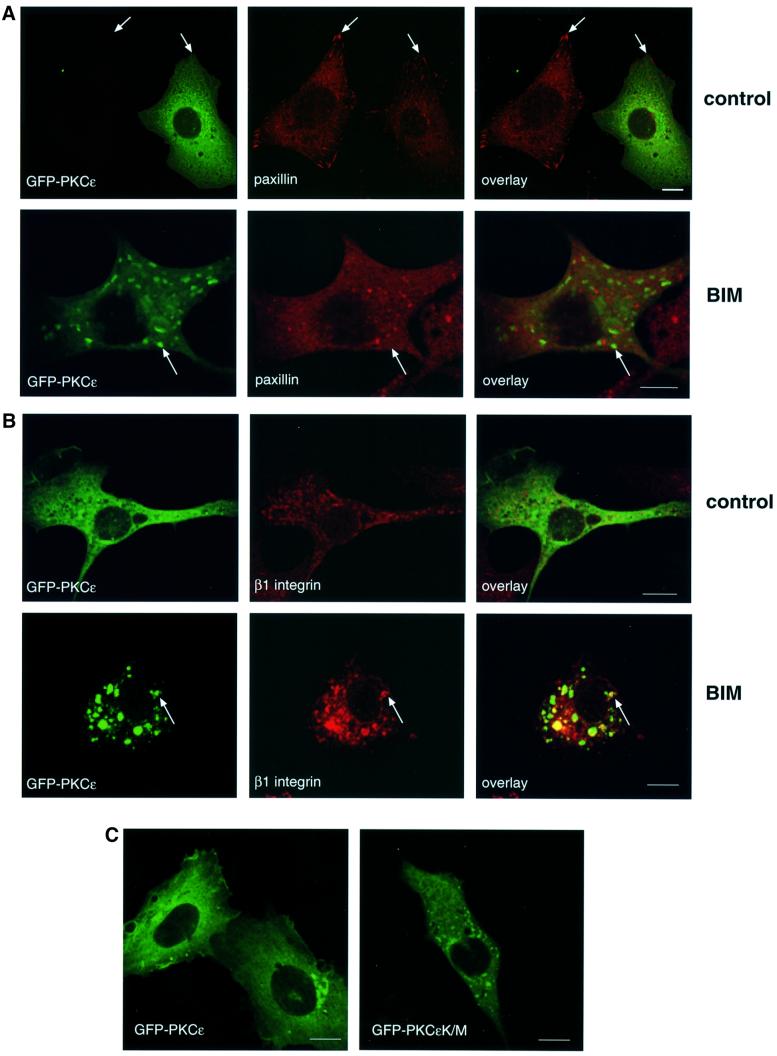
To determine how PKCε inhibition influences β1 integrin, the distribution of β1 integrin and PKCε itself were compared in spreading cells before and after BIM I treatment. The cells were plated on fibronectin-coated coverslips, allowed to spread for 30 min followed by a further 90 min incubation with or without the PKC inhibitor BIM I. In the absence of the inhibitor, β1 integrin was dispersed in punctate structures and in lamellipodia. As previously shown for MCF-7 cells (Ng et al., 1999), β1 integrin was also found in a perinuclear compartment with tubulovesicular structures distributed through the cytoplasm (Figure 2A, top). PKCε is broadly distributed throughout the cytoplasm with some concentration in a perinuclear compartment and no evidence of overlap with β1 integrin (Figure 2A, top). However, upon inhibition of PKC catalytic activity with BIM I, PKCε and β1 integrin accumulate in large cytoplasmic vesicular structures (Figure 2A, middle). In contrast, treatment of cells with the PKC activator TPA caused a partial redistribution of PKCε and β1 integrin to the plasma membrane where they co-localize in ruffles (Figure 2A, bottom). Integrins have been suggested to traffic through Rab4-positive early endosomes to a Rab11-positive perinuclear recycling compartment in NIH 3T3 cells (Roberts et al., 2001). On the other hand, the steady-state distribution of β1 integrin in MCF-7 breast cancer cells has been demonstrated to co-localize extensively with that of both endogenous transferrin receptor and fibronectin (Ng et al., 1999). In addition, a substantial fraction of transmembrane proteins of the tetraspanin superfamily (tetraspanins) co-localize with integrins in various intracellular compartments, including multivesicular endosomes and exosomes (reviewed in Berditchevski, 2001). To define the nature of the PKCε/β1 integrin-positive vesicles, PKCεRE cells were treated with BIM I to monitor co-localization of the observed vesicles with different markers. There was no detectable co-localization between vesicular PKCε and labelled transferrin, early endosomal marker EEA1, caveolin or Rab11 (data not shown). However, it was observed that a tetraspanin protein, CD81, accumulated in the PKCε-positive cytoplasmic vesicles in response to BIM I treatment (Figure 2B). Since none of the endocytic markers tested was found to co-localize with the vesicular structures, markers of the early biosynthetic pathway were used (Figure 2C). The lack of any apparent co-localization with β-COP, P115 and KDELR indicates that the vesicular structures observed upon BIM I treatment are neither Golgi nor endoplasmic reticulum.
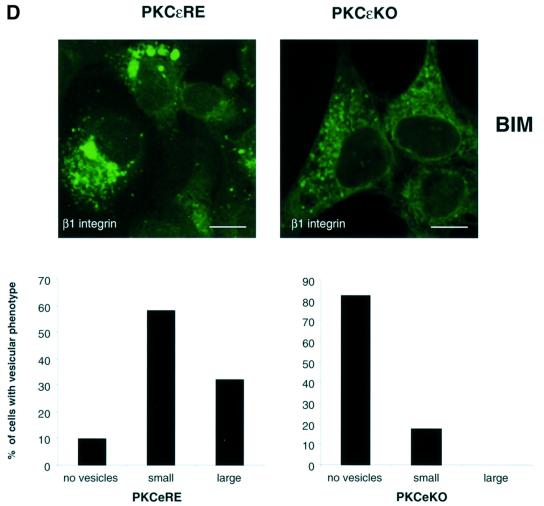
Fig. 2. Modulation of PKCε activity alters the cellular localization of β1 integrin. (A) Immunofluorescence staining of β1 integrin (green) and PKCε (red) in PKCεRE clone 5 cells are shown as representative confocal images of 0.8 µm sections. The cells were plated and allowed to spread on fibronectin for 30 min, following a further 90 min incubation either untreated (top), treated with 1 µM BIM I (middle) or stimulated with 200 nM TPA (bottom). The bar represents 10 µm. The immunofluorescence images suggest that in PKCεRE cells there is co-localization between β1 integrin and PKCε in membrane ruffles upon stimulation, or at specific vesicles following inhibition of PKC. Examples of these sites of co-localization are indicated with arrows. (B) Accumulating PKCε positive vesicles co-localize with the tetraspanin CD81. Immunofluorescence staining of CD81 (green) and PKCε (red) in PKCεRE clone 5 cells treated as above is shown as representative confocal images of 0.8 µm sections. Significant overlap of CD81- and PKCε-positive vesicles is observed in BIM I-treated cells. Examples of these sites of co-localization are indicated with arrows. (C) The lack of co-localization between β1 integrin (green) and β-COP (red, Golgi complex) or KDELR (red, endoplasmic reticulum) and PKCε (red) and P115 (green, Golgi complex) in BIM I-treated clone 5 cells indicates that the vesicles do not originate from the Golgi or the endoplasmic reticulum. (D) BIM I induces significant accumulation of β1 integrin in vesicles only in PKCεRE cells. PKCεKO and PKCεRE cells were treated with 1 µM BIM I as in (A). Staining for β1 integrin is shown in green. In the lower panel is the quantitation of the vesicular phenotype of BIM I-treated PKCεRE and PKCεKO cells scored from eight randomly selected fields each containing ∼10 cells. The vesicles observed were designated small (diameter < 500 nm) or large (diameter > 500 nm).
The distinct BIM I-sensitive migratory behaviour of PKCεRE and PKCεKO cells suggested that in response to BIM I there would be a dissimilar response of β1 integrins. Indeed the BIM I-induced accumulation of β1 integrin (and PKCε) in large cytosolic vesicular structures in the PKCεRE clones contrasted with the response in PKCεKO cells, where β1 integrins were largely not affected in their distribution and are only occasionally observed in small vesicular compartment(s) (Figure 2D). This clearly demonstrates that the absence of PKCε is not equivalent to the presence of catalytically inhibited PKCε (see Discussion).
To ensure that the BIM I-induced accumulation of PKCε in large vesicles was not a clonal artefact, we used a GFP-tagged PKCε and tested the ability of BIM I to induce vesicles in PKCεKO cells transiently transfected with GFP–PKCε. In accordance with the observations made in the isolated, stable clones (Figure 1B), transient expression of PKCε was found to correlate with less prominent paxillin-positive focal adhesions (Figure 3A, top). Upon PKC inhibition, the transiently expressed GFP–PKCε was found to accumulate in vesicles, in a manner indistinguishable from the cloned PKCε-expressing isolate (Figure 3B). To assess the reversibility of the GFP–PKCε vesicles, transiently transfected cells treated with BIM I were followed in a live cell washout experiment. Subsequent to the removal of BIM I, with extensive washing for 2 h (medium changed every 15 min), the vesicles were found to dissipate into the cytoplasm (data not shown). The correlation between the inhibition of PKCε catalytic activity and the formation of vesicular structures was demonstrated further with the introduction of the kinase-dead PKCε into PKCεKO cells (Figure 3C). Cells were transiently transfected with either wt GFP–PKCε or the inactive GFP–PKCεK/M. As shown in the right hand panel, GFP–PKCεK/M showed a vesicular expression in untreated cells. In addition, both β1 integrin and CD81 were found to co-localize with GFP–PKCεK/M in these vesicles in untreated cells (data not shown).
Fig. 3. Cellular localization of GFP–PKCε and paxillin in PKCεKO cells. (A) PKCεKO cells were transiently transfected with GFP–PKCε for 24 h before preparation for microscopy. The cells were plated and allowed to spread on fibronectin for 30 min, followed by a further 90 min incubation either untreated (top) or treated with 1 µM BIM I (bottom). Representative confocal images showing 0.8 µm sections of cells positive or negative for GFP–PKCε also stained with anti-paxillin mAb (red) are shown. The bar represents 10 µm. PKCε decreases the number of prominent focal adhesions detected with paxillin staining (arrows, top) and confirms the accumulation of PKCε in vesicles upon BIM I treatment (arrows, bottom). Note that paxillin is not localized to the GFP–PKCε-positive vesicles but is present in distinct vesicular structures. (B) Integrin β1 co-localizes with GFP–PKCε in vesicles observed in PKCεKO cells upon BIM I treatment. Cells were transfected, plated on fibronectin and either left untreated (top) or treated with BIM I (bottom) as above. Shown are confocal images of cells positive for GFP–PKCε and stained with anti-integrin mAb (red). Examples of sites of co-localization are indicated with arrows. (C) Kinase-dead mutant of GFP–PKCε is expressed in vesicles in untreated cells. PKCεKO cells were transfected with GFP–PKCε or GFP–PKCεK/M for 36 h before preparation for microscopy.
Recent studies have indicated a connection between paxillin and the regulation of membrane recycling (reviewed in de Curtis, 2001). Interestingly, paxillin redistributed from a cytoplasmic/focal adhesion localization to a vesicular/cytoplasmic localization following BIM I treatment (Figure 3A, bottom). However, these vesicles do not overlap with those containing PKCε. These effects of BIM I indicate that PKCε may control the traffic through/exit of β1 integrin from a vesicular compartment, such that they co-accumulate in this compartment on exposure to BIM I. How paxillin accumulates in a distinct vesicular compartment apparently devoid of PKCε is at present unclear. However, its absence from the β1 integrin-containing vesicles suggests that it is not involved in this step of integrin traffic.
Recycling of β1 integrins
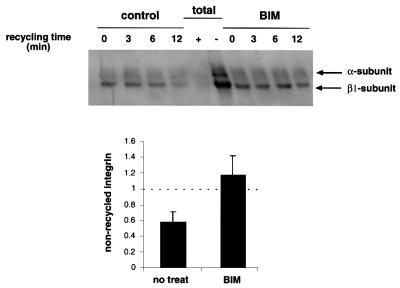
We used a pulse–chase approach (see Materials and methods) to monitor the recycling of internalized integrins back to the plasma membrane to determine whether the inhibition of PKC alters this process. Quantitative analysis of four separate experiments shows that BIM I treatment efficiently inhibited the recycling of β1 integrins back to the plasma membrane, while in the untreated PKCεRE clone 5 cells, 42 ± 10.5% of the internalized integrin had returned to the plasma membrane within 12 min (Figure 4).
Fig. 4. The effect of BIM I treatment on the recycling of β1 integrins. Cells were surface labelled, and internalization was allowed to proceed for 15 min at 37°C, followed by 15 min at 37°C in the presence or absence of 1 µM BIM I. Biotin was removed from receptors remaining on the cell surface by treatment with MesNa at 4°C. Cells were then rewarmed to 37°C for the times indicated in the presence or absence of 1 µM BIM I, to allow recycling to the plasma membrane, followed by a second reduction with MesNa. Cells were lysed, and β1 integrins were immunoprecipitated and analysed by 6% SDS–PAGE under non-reducing conditions, followed by western blotting with peroxidase-conjugated avidin. The positions of the β1 integrin and the associated α-subunits are indicated. As controls, cells were either lysed after the labelling without MesNa reduction, to determine the amount of total biotinylated integrin (total, –), or lysed after the first reduction before internalization, to control the efficiency of the MesNa reduction (total, +).
Characterization of BIM I-induced vesicles
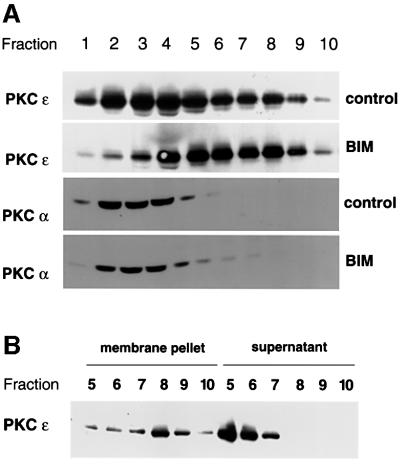
In order to determine the basis of the accumulation in/lack of exit of proteins from this BIM I-induced vesicular compartment, we set out to isolate these structures. Extracts of control or BIM I-treated cells (PKCεRE, clone 5) were fractionated on a sucrose gradient (Dittie et al., 1996) and the vesicular compartment was characterized (Figure 5). As illustrated in Figure 5A, BIM I treatment caused PKCε to shift from a broad distribution that included the cytosol (fractions 1–4) to a more dense compartment. By contrast, PKCα, which can also be inhibited by BIM I, remained unchanged in its cytosolic location. Similar results were obtained with fractionations of BIM I-treated PKCεRE clone 17 cells (data not shown). The PKCε present in the denser eluting fractions was found to be stably associated with membranes, as following dilution and centrifugation at 100 000 g the protein was recovered in the particulate fraction. PKCε eluting in the lighter fractions was recovered predominantly in the supernatant and does not appear to be stably connected with a membrane compartment (Figure 5B). These observations indicate that BIM I induces the membrane association of PKCε, a part of which is in a stable membrane-bound form that correlates with a lipid-independent activity (see Supplementary figure 1 available at The EMBO Journal Online). This is indicative of an effector-bound form of PKCε.
Fig. 5. BIM I treatment induces accumulation of active, membrane-associated PKCε in a dense compartment. (A) PKCεRE cells were treated for 90 min with BIM I (1 µM) or left untreated, followed by a sucrose gradient fractionation (see Materials and methods). The proteins in the fractions were recovered by TCA precipitation and subjected to western blot analysis. Upon BIM I treatment, PKCε was found to accumulate in a dense compartment (fractions 7–9). Note that PKCα does not co-sediment with PKCε even upon BIM I treatment. (B) The membrane association of PKCε in fractions, prepared as above from PKCεRE cells treated with BIM I, was determined by sedimentation of proteins at 100 000 g. The proteins remaining in the supernatant were recovered with TCA precipitation and equivalent samples of each fraction were subjected to western blot analysis.
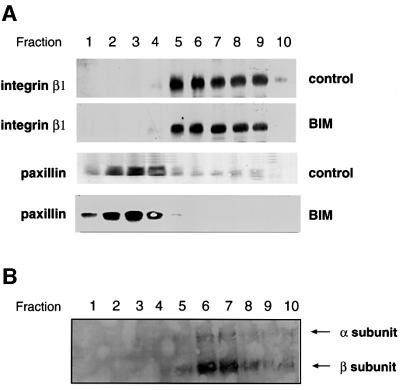
Since, in intact cells, the β1 integrin became predominantly localized in the PKCε vesicular structures, we determined the distribution of β1 integrin in these fractionated extracts. The distribution of the integral membrane protein, β1 integrin, was largely unchanged by BIM I treatment, indicating that its steady-state redistribution does not substantially alter the buoyant density of the compartment in which it resides. However, it is evident that following BIM I treatment, β1 integrin substantially overlapped the shifted PKCε localization, consistent with the immunofluorescence (Figure 6A). Furthermore, paxillin was found to be predominantly cytosolic (fractions 1–4) and was not shifted down the gradient upon BIM I treatment.
Fig. 6. Plasma membrane-derived β1 integrin is present in the BIM I-induced dense compartment. (A) PKCεRE cells were treated for 90 min with BIM I (1 µM), or left untreated and then fractionated (see legend to Figure 5). The proteins in the fractions were recovered with TCA precipitation and subjected to western blot analysis following resolution by SDS–PAGE (8% polyacrylamide) and transfer to membrane. Upon BIM I treatment, β1 integrin was found to accumulate in a dense compartment (fractions 7–9), in a manner similar to PKCε (Figure 5). An equally dense β1 integrin-positive compartment apparently exists also under control conditions. Note that paxillin does not co-sediment with β1 integrin (or PKCε) even upon BIM I treatment. (B) PKCεRE cells were surface labelled with NHS-SS-biotin, allowed to recover at 37°C for 30 min, followed by a 90 min treatment with BIM I. Biotin was released from proteins remaining at the cell surface by MesNa treatment at 4°C, the cells were subjected to fractionation, and the vesicles were isolated as described above. The recovered vesicles were resuspended to a small volume of buffer followed, by refractionation on an equilibrium gradient. The proteins in the fractions were analysed by 6% SDS–PAGE under non-reducing conditions, followed by western blotting with peroxidase-conjugated avidin or anti-PKCε IgG (data not shown). The positions of the β1 integrin and the associated α-subunits are indicated.
To determine whether any of the β1 integrin found in this dense compartment originated from the plasma membrane, a pulse–chase experiment was performed (see Materials and methods). The dense compartment was isolated from these surface-labelled cells and membrane-associated proteins recovered. Strikingly, only two major biotinylated proteins were present in the membrane-bound material under non-reducing conditions. The fact that only one (the 125 kDa band) was observed when analysing the same sample under reducing conditions is characteristic of the behaviour of the integrin α/β heterodimer and enabled us to identify the two bands as integrin α and β subunits, respectively. PKCε was also detected in these same fractions by western blotting with anti-PKCε IgG (Figure 6B; data not shown). This indicates that the BIM I-induced vesicles contain internalized integrins that accumulate within an internal compartment upon inhibition of PKC.
A detailed morphological analysis of these vesicles was carried out by electron microscopy and immunogold labelling of PKCε and β1 integrin (see Supplementary figure 2). This analysis confirms that PKCε and β1 integrin co-localize in a vesicular compartment, consistent with the immunofluorescence data.
Release of PKCε from dense vesicles requires PKCε activity
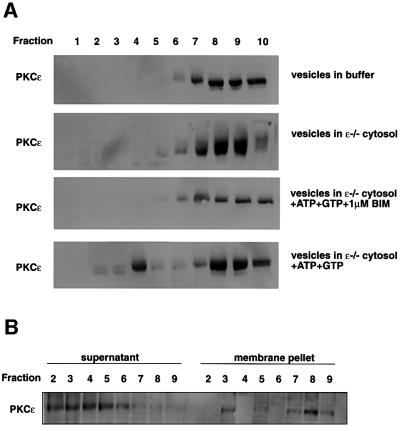
In intact cells, the retention of the PKCε and β1 integrin in the enlarged vesicular compartment was dependent upon inhibition of PKC activity, since the accumulation was reversed by removal of inhibitor. This behaviour is consistent with the notion that PKCε activity is required for the dissipation of these vesicles. This might reflect a catalytic requirement for the release of PKCε itself with consequent β1 integrin traffic or a requirement for PKCε activity in the traffic itself, e.g. a budding step. In either scenario, the prediction is that PKCε release from this dense vesicle fraction will require PKCε activity (in the absence of a change in β1 integrin compartment density it is not informative to monitor β1 integrin per se as a marker). To determine the properties of the system, a reconstitution assay was set up to define under what conditions PKCε could be displaced to lighter fractions.
Vesicles derived from BIM I-treated cells were isolated and concentrated by centrifugation. These were then incubated in buffer at 37°C with various additions, followed by re-fractionation on a sucrose gradient (see Figure 7A). It was evident that in the combined presence of cytosol and ATP + GTP, there was a significant release of PKCε from the vesicle fraction (Figure 7A); this released PKCε was not membrane associated (Figure 7B). As summarized in Table I, the combination of cytosol/ATP/GTP produced a consistent 20% release of PKCε during a 1 h incubation. Cytosol/ATP (no added GTP) was less effective. Interestingly, the release was energy dependent, since cytoplasm alone was not able to promote dissipation of PKCε, suggesting a possible requirement for a kinase reaction (Figure 7). To demonstrate whether PKCε activity was indeed necessary for PKCε release, similar experiments were performed in the presence of BIM I. Inhibition of PKC activity completely blocked PKCε release, paralleling behaviour in intact cells (Figure 7A; Table I).
Fig. 7. Cytosol-, energy- and PKC kinase activity-dependent reconstitution of PKCε release from vesicles. (A) Vesicles were isolated from BIM I-treated PKCεRE cells using fractionation. Fractions 7–9 from an equilibrium gradient were pooled, diluted in buffer and membrane-bound proteins were sedimented at 100 000 g. The recovered vesicles were resuspended to a small volume of buffer and incubated for 1 h at 37°C in reaction mixtures as indicated, followed by refractionation on the equilibrium gradient. The proteins in the fractions were recovered with TCA precipitation and subjected to western blot analysis following resolution by SDS–PAGE (10% polyacrylamide). (B) The membrane association of PKCε following the release reaction, in fractions prepared as above, was determined by sedimentation of membrane-associated proteins at 100 000 g. The proteins remaining in the supernatant were recovered with TCA precipitation and equivalent samples of each fraction were suspended in sample buffer and subjected to western blot analysis following resolution by SDS–PAGE (10% polyacrylamide).
Table I. Release of PKCε from vesicles.
| Reaction conditions | PKCε released to fractions 2–5 (%) | PKCε remaining in fractions 7–10 (%) | |
|---|---|---|---|
| Buffer | 0.25 ± 0.25 | 96.8 ± 1.9 | (n = 5) |
| Cytoplasm + ATP | 15.5 ± 8.1 | 72.5 ± 1.8 | (n = 3) |
| Cytoplasm + ATP + GTP | 20.8 ± 2.3 | 65.3 ± 3.6 | (n = 5) |
| Cytoplasm + ATP + GTP + BIM I | 0 | 78 ± 4 | (n = 2) |
Discussion
The results here identify a functional relationship between PKCε kinase activity and the traffic of β1 integrins in motile fibroblasts. Expression of PKCε in PKCε-negative cells increases haptotaxis in cells in a manner that is dependent on PKC kinase activity. Associated with PKCε-induced motility, modulation of PKC activity results in redistribution of β1 integrin. On activation, PKCε translocates to the cell membrane and becomes co-localized with β1 integrin at membrane ruffles. In contrast, as shown here, chronic inhibition of PKC results in accumulation of β1 integrin and PKCε in tetraspanin CD81-positive vesicular structures in the cytoplasm. The dramatic accumulation of β1 integrin in vesicles upon inhibition of PKCε implies that there is a PKCε-dependent step in the traffic of integrins. The presence of plasma membrane-derived β1 integrin and the fact that CD81 has been shown to associate with only the mature receptor (Rubinstein et al., 1997) indicate that this is a post-endocytic step. Pulse labelling and chase experiments demonstrate directly that this compartment is part of the recycling pathway. We further report the isolation of these integrin-containing vesicular structures that accumulate in cells upon inhibition of PKCε. Based upon surface labelling, the plasma membrane-derived β1 integrin is shown to co-fractionate with the PKCε following BIM I treatment, consistent with the view that internalized integrin traffics to this compartment and is not degraded. In a reconstituted in vitro reaction, it is shown that the dissociation of PKCε from these vesicles is dependent on the presence of cytosolic components, energy and PKC catalytic activity. The evidence presented indicates that PKCε controls an internal traffic step that under uninhibited conditions permits the recycling of β1 integrin, contributing to cell motility.
In migrating cells, internalized plasma membrane is returned to the leading edge by exocytosis (Bretscher and Thomson, 1983) and there is evidence that some β1 integrins traffic from the plasma membrane through the cell and back to the membrane (Bretscher, 1992). According to studies on integrin dynamics in migratory cells, these receptors are either ripped off the membrane and left behind, or collected into vesicles that are transported along the cell body as the cell migrates (Regen and Horwitz, 1992; Palecek et al., 1996; Laukaitis et al., 2001). The endocytosis and recycling back to the plasma membrane seem to involve Rab4- and Rab11-positive compartments (Roberts et al., 2001). However, the ultimate fate of the internalized integrins after reaching the perinuclear recycling compartment remains unknown. In particular, the transport vesicles carrying recycled integrins have not yet been identified. Here we report that the accumulating vesicular compartment is positive for the tetraspanin CD81. This is potentially interesting since tetraspanins are abundant on various types of intracellular vesicles (Berditchevski, 2001) and have been shown to regulate cell migration through their association with integrins.
Stimulation of conventional and novel PKC isotypes increases cell migration on a range of matrix proteins indiscriminately (Rigot et al., 1998). Interestingly, overexpression of PKCα in epithelial MCF-7 cells enhanced both random motility as well as haptotaxis towards β1 substrates. In contrast, we show here that overexpression of PKCε enhances only directional movement towards matrix. While PKCα expression seems to confer both enhanced directionality and random motility, it is intriguing that PKCε, at least in fibroblasts, seems to enhance only directional movement. It has been hypothesized that the targeting of internalized membranes to the front of the migrating cell contributes to the directional extension of the cell border (Bretscher, 1996). In view of the apparently critical PKCε-dependent step in the exit of integrins from this compartment, it is tempting to speculate the following: while PKCα controls integrin internalization and possibly their final release to the plasma membrane (Ng et al., 1999), PKCε regulates a recycling step of the receptor and may therefore be involved in targeting the recycling receptor specifically to the front of the cell.
The PKC family of signal transducers are characterized by a dependence upon lipids for activity. The apparent lipid independence of PKCε in the dense vesicle compartment described is indicative of an effector-bound form. In addition, the kinase is most likely present in its primed/phosphorylated form, since the lack of phosphorylation at the key priming sites results in a low activity PKC (reviewed in Parekh et al., 2000). The data suggest that BIM I treatment induces accumulation of an active but inhibitor-bound kinase that under normal conditions continuously facilitates exit of recycling β1 integrin from an intracellular compartment, by influencing its own recycling/release. It is of note that the only surface biotinylated protein that accumulates in this intracellular compartment is the β1 integrin, suggesting that other proteins are excluded or are not blocked in their exit from this compartment.
In conclusion, the results presented here indicate that PKCε is responsible for the control of integrin recycling through an intracellular vesicular compartment. The isolation of these vesicles and the reconstitution of regulated PKCε exit from them provide an avenue to defining the underlying components required for controlling β1 integrin traffic.
Materials and methods
Cell culture and plasmid constructs
MEFs were derived from PKCε knockout embryos (R.Whelan and P.J.Parker, unpublished data). Polyclonal PKCεKO cells were transfected with pcDNA3 CMV/IE hygro+ PKCε or vector alone using calcium phosphate. Clonal, PKCεRE stable cell lines were selected using limiting dilution. MEFs were selected and routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum, 100 µg/ml hygromycin at 37°C, in a 10% CO2 atmosphere. The pcDNA3 CMV/IE hygro+ PKCε vector was constructed as follows. The promoter region in pcDNA3 hygro+ was replaced with a 2.1 kb fragment containing CMV/IE promoter derived from p63d. Finally, mouse cDNA coding for the full-length PKCε (3.3 kb fragment) was ligated to the modified pcDNA3 CMV/IE hygro+ vector. Transient transfections were performed using Fugene (Boehringer Mannheim). PKCεKO cells were transfected with either GFP–PKCε plasmid or vector alone. The GFP-tagged PKCε construct was made as follows. In order to fuse PKCε in-frame with the N-terminal GFP tag, the pCDNA3.1 hygro vector containing mouse PKCε was used as a template in a PCR using a 5′ primer 5′-GCGCAGATCTACCATGGTAGTGTTCAATGGC-3′ and a 3′ primer 5′-GCGCGTCGACGCTTGGGAGTATCTCAACACCG-3′. The resulting PCR product containing the full-length sequence of PKCε was digested with _Bgl_II and _Sal_I, and ligated to the corresponding sites of the pEGFP-C1 vector. The K552M mutation was introduced to the GFP-tagged PKCε construct with splicing by overlap extension PCR. Two PCR products bearing the mutation in overlapping complementary ends were produced from the GFP–PKCε with the following primers: (1) 5′-TGCGTTGTCCACAAGCGATGTCA-3′; (2) 5′-TTCTTCAAGACCA TCACAGCGTAG-3′; (3) 5′-CTACGCTGTGATGGTCTTGAAGAA-3′; and (4) 5′-GCGCGTCGACGCTTGGGAGTATCTCAACACCG-3′. The products were gel purified, and extended in a second PCR using primers 1 and 4 to yield the mutant product. The GFP–PKCε and the mutant PCR product were digested with _Sac_I, gel purified, and the 1600 bp mutant segment ligated to the cleaved GFP–PKCε to produce the GFP–PKCεK/M construct. The integrity of the all PCR amplified sequences of PKCε was verified by sequencing.
Confocal microscopy
Acid-washed glass coverslips were coated with 10 µg/ml fibronectin (Sigma) in PBS overnight at 4°C and blocked using 0.1% BSA (w/v) in PBS for 1 h at 37°C. The cells were trypsinized, washed with medium containing 0.2% (w/v) soybean trypsin inhibitor (Sigma), resuspended in serum-free DMEM and plated on coated coverslips for 30 min. Where indicated, this was followed with a further 90 min incubation in the presence or absence of 1 µM BIM I (Alexis). For antibody staining, cells were washed in PBS, fixed with 4% paraformaldehyde, permeabilized in PBS/0.2% Triton X-100 and washed with PBS/1% (w/v) BSA for blocking. Cells were stained with antibodies to β1 integrin (Transduction Laboratories), paxillin (Santa Cruz), CD81 (Santa Cruz), transferrin receptor (Santa Cruz), EEA1 (Santa Cruz), caveolin (Transduction Laboratories), Rab11 (S.Tooze, Cancer Research UK), KDELR (Tang et al., 1993), β-COP CM1A10 mAb (D.Shima, Cancer Research UK; Shima et al., 1999), p115 (4H1; Waters et al., 1992) and PKCε (polyclonal rabbit antiserum, 130; Schaap and Parker, 1990) with appropriate FITC- and Cy3-conjugated anti-mouse, anti-rabbit and anti-goat antibodies (Jackson ImmunoResearch Laboratories). Cells were mounted, after washing in PBS and H2O, in mowiol [100 mM Tris–HCl pH 8.5, 10% (w/v) mowiol (Calbiochem) and 25% (v/v) glycerol] containing antifade [2.5% (w/v) 1,4-diazadicyclo[2.2.2]octane; Sigma]. For experiments with GFP–PKCε, immunocytochemistry was performed 24 h after transfection of PKCεKO cells using Fugene6. Slides were examined using a confocal laser scanning microscope (Axioplan 2 with LSM 510; Carl Zeiss Inc.) equipped with 63×/1.4 Plan-APOCHROMAT oil immersion objectives. GFP, FITC and Cy3 were excited with the 488 and 543 nm lines of Kr–Ar lasers, respectively, and individual channels were scanned in series to prevent cross-channel bleed through. Each image represents a single ∼1.0 µm ‘Z’ optical section of cells.
Biotinylation of cell surface integrins and recycling
Recycling of the β1 integrins was performed as described by Roberts et al. (2001) with some modifications. The cells were placed on ice, washed once in cold PBS and surface labelled at 4°C with 0.2 mg/ml NHS-SS-biotin (Pierce) in PBS for 30 min. Labelled cells were washed in cold PBS and transferred to DMEM at 37°C for 15 min to allow maximal internalization, followed by a further 15 min incubation in the presence or absence of 1 µM BIM I. The cells were transferred to ice. Biotin was removed from proteins remaining at the cell surface by MesNa reduction and iodoacetamide (IAA) quenching. The internalized integrin fraction was then chased from the cells by returning them to 37°C in DMEM in the presence or absence of 1 µM BIM I. At the indicated times, cells were returned to ice and biotin was removed from recycled proteins by a second reduction with MesNa. The cells were lysed in 200 mM NaCl, 75 mM Tris, 15 mM NaF, 1.5 mM Na3VO4, 7.5 mM EDTA, 7.5 mM EGTA, 1.5% Triton X-100, 10 µg/ml leupeptin and 10 µg/ml aprotinin. Lysates were clarified by centrifugation at 10 000 g for 10 min. Supernatants were corrected to equivalent protein concentrations, integrins were isolated by immunoprecipitation and analysed by SDS–PAGE. As controls, cells were either lysed after the labelling to determine the amount of total biotinylated integrin or lysed after the first reduction before internalization to control for the efficiency of the MesNa reduction.
Cell surface biotinylation for fractionations was performed as described above. Internalization was allowed to proceed at 37°C for 30 min, followed by a further 90 min incubation in the presence of BIM I (1 µM) and a MesNa reduction to remove biotin from non-internalized proteins. The cells were harvested and subjected to fractionation. The proteins in the fractions were resolved under non-reducing or reducing conditions on 6% SDS–PAGE, transferred to a membrane and subjected to western blot analysis with peroxidase-conjugated avidin (Amersham).
Cellular fractionation by sucrose gradient centrifugation
BIM I-treated or untreated PKCεRE cells were harvested, washed and homogenized using a cell cracker in HB buffer [0.25 M sucrose, 10 mM HEPES–KOH pH 7.2 containing 1 mM EDTA and 1 mM magnesium acetate and protease inhibitors (Complete tablet; Boehringer Mannheim)]. The post-nuclear supernatant (PNS) was loaded onto a velocity gradient as described previously (Dittie et al., 1996). PKCε and β1 integrin were enriched in velocity gradient fractions 1–3 (J.Ivaska and P.J.Parker, unpublished data). Equilibrium gradient fractions were subsequently prepared from these fractions as described previously (Dittie et al., 1996) with a slightly modified gradient (1 ml of 1.2 M, 2 ml of 1.0 M, 2 ml of 0.8 M, 2 ml of 0.6 M and 1 ml of 0.4 M sucrose in 10 mM HEPES–KOH pH 7.2). Proteins were precipitated from these fractions in 10% trichloroacetic acid (TCA) for 4 h at 4°C, washed with ethanol/acetone (50/50, v/v), then denatured in Laemmli (Laemmli, 1970) sample buffer (non-reducing where indicated), separated on an 8% polyacrylamide gel and transferred to a PVDF membrane. Incubation with primary antibodies [β1 integrin (Transduction Laboratories); PKCε (Schaap and Parker, 1990); paxillin (Transduction Laboratories); PKCα (Young et al., 1988)] was performed overnight at 4°C. Detection was with enhanced chemiluminescence (ECL; Amersham) according to recommended procedures.
In vitro release reactions
Vesicles were prepared from BIM I-treated PKCεRE cells. Fractions 7–9 from the equilibrium gradient were pooled, diluted in 2 vols of 10 mM HEPES–KOH pH 7.2, and pelleted by centrifugation at 100 000 g for 1 h. The pellets were resuspended in HB buffer at 50× the concentration of the original vesicle pool. The cytoplasm used in the reactions was the PNS extracted from PKCεKO cells adjusted with HB buffer to a protein concentration of 5 mg/ml. The reaction mixtures contained 10 µl of vesicles, 100 µl of HB (buffer control) or cytoplasm supplemented with 10× FB (FB; 20 mM HEPES–KOH pH 7.4, 50 mM KOAc, 3 mM MgCl2, 1 mM DTT) and where indicated an ATP regenerating system (stock solutions 100 mM ATP, 800 mM creatine phosphate, 3200 U/ml creatine phosphokinase that were mixed before use in equal volumes to give a 30× stock) and 300 µM GTP. The reactions were incubated for 1 h at 37°C, diluted in 3 ml of ice-cold 0.35 M sucrose in HEPES–KOH pH 7.2 and loaded on a second equilibrium gradient.
Transwell chamber haptotactic migration assays
PKCεKO and PKCεRE cells were detached from culture plates with trypsin, washed three times with serum-free medium supplemented with glutamine and 0.5% (w/v) BSA (migration buffer), then replated at 106 cells/ml onto the inserts of 8 µm pore size Transwell chambers (Costar, Cambridge, MA). The underside of the inserts were precoated with either 0.1% (w/v) BSA or 10 µg/ml fibronectin overnight at 4°C and blocked with 0.1% (w/v) BSA for 1 h at 37°C. After 20 h, cells were trypsinized from both the top and underside of the inserts, and washed once with 0.2% (w/v) soybean trypsin inhibitor in migration buffer. The cell suspensions obtained were centrifuged and the recovered cells were counted using a hemocytometer. The number of cells that had migrated through the insert was compared with the number of cells in the non-migrating population that remained in the upper chamber. Data from six independent experiments were pooled and analysed using two-tailed, paired Student’s _t_-test.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We wish to thank Drs David Shima and Giampietro Schiavo for their valuable comments. We also thank Dr Sharon Tooze (Cancer Research UK) for the anti-Rab11 serum. J.I. is an EMBO longterm fellow and is supported by grants from the Academy of Finland, Emil Aaltonen Foundation, Farmos Research Foundation, Finnish Cancer Association, Finnish Cultural Foundation, Irja Karvonen Cancer Foundation, Leiras Research Foundation, Maud Kuistila’s Foundation and Paulo’s Foundation.
References
- Berditchevski F. (2001) Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci., 114, 4143–4151. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. (1992) Circulating integrins: α5β1, α6β4 and Mac-1, α3β1, α4β1 or LFA-1. EMBO J., 11, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M.S. (1996) Moving membrane up to the front of migrating cells. Cell, 85, 465–467. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. and Thomson,J.N. (1983) Distribution of ferritin receptors and coated pits on giant HeLa. EMBO J., 2, 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo A., Pennington,D.J., Otto,F., Parker,P.J., Owen,M.J. and Bosca,L. (2001) Protein kinase Cε is required for macrophage activation and against bacterial infection. J. Exp. Med., 194, 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J.S., Ha,M.J. and Jacobson,B.S. (1996) Differential translocation of protein kinase Cε during HeLa cell adhesion to a gelatin substratum. J. Biol. Chem., 271, 13008–13012. [DOI] [PubMed] [Google Scholar]
- de Curtis I. (2001) Cell migration: GAPs between membrane traffic and the cytoskeleton. EMBO rep., 2, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittie A.S., Hajibagheri,N. and Tooze,S.A. (1996) The AP-1 adaptor complex binds to immature secretory granules from cells and is regulated by ADP-ribosylation factor. J. Cell Biol., 132, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England K., Watson,J., Beale,G., Warner,M., Cross,J. and Rumsby,M. (2001) Signalling pathways regulating the dephosphorylation of Ser729 in the hydrophobic domain of protein kinase Cε upon cell passage. J. Biol. Chem., 276, 10437–10442. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti,E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) G proteins and small GTPases: distant relatives keep in touch. Science, 280, 2074–2075. [DOI] [PubMed] [Google Scholar]
- Horwitz A.R. and Parsons,J.T. (1999) Cell migration—movin’ on. Science, 286, 1102–1103. [DOI] [PubMed] [Google Scholar]
- Kiley S.C., Clark,K.J., Goodnough,M., Welch,D.R. and Jaken,S. (1999) Protein kinase Cδ involvement in mammary tumor cell metastasis. Cancer Res., 59, 3230–3238. [PubMed] [Google Scholar]
- Klemke R.L., Yebra,M., Bayna,E.M. and Cheresh,D.A. (1994) Receptor tyrosine kinase signaling required for integrin αVβ5-directed cell motility but not adhesion on vitronectin. J. Cell Biol., 127, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Laukaitis C.M., Webb,D.J., Donais,K. and Horwitz,A.F. (2001) Differential dynamics of α5 integrin, paxillin and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol., 153, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T., Shima,D., Squire,A., Bastiaens,P.I.H., Gschmeissner,S., Humphries,M.J. and Parker,P.J. (1999) PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J., 18, 3309–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S.P., Schmidt,C.E., Lauffenburger,D.A. and Horwitz,A.F. (1996) Integrin dynamics on the tail region of migrating fibroblasts. J. Cell Sci., 109, 941–952. [DOI] [PubMed] [Google Scholar]
- Palmantier R., George,M.D., Akiyama,S.K., Wolber,F.M., Olden,K. and Roberts,J.D. (2001) _Cis_-polyunsaturated fatty acids stimulate β1 integrin-mediated of human breast carcinoma cells to type IV collagen by activating protein kinases C-ε and -µ. Cancer Res., 61, 2445–2452. [PubMed] [Google Scholar]
- Parekh D.B., Ziegler,W. and Parker,P.J. (2000) Multiple pathways control protein kinase C phosphorylation. EMBO J., 19, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platet N., Prevostel,C., Derocq,D., Joubert,D., Rochefort,H. and Garcia,M. (1998) Breast cancer cell invasiveness: correlation with protein kinase C activity and differential regulation by phorbol ester in estrogen receptor-positive and -negative cells. Int. J. Cancer, 75, 750–756. [DOI] [PubMed] [Google Scholar]
- Regen C.M. and Horwitz,A.F. (1992) Dynamics of β1 integrin-mediated adhesive contacts in motile fibroblasts. J. Cell Biol., 119, 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigot V., Lehmann,M., Andre,F., Daemi,N., Marvaldi,J. and Luis,J. (1998) Integrin ligation and PKC activation are required for migration of colon carcinoma cells. J. Cell Sci., 111, 3119–3127. [DOI] [PubMed] [Google Scholar]
- Roberts M., Barry,S., Woods,A., van der Sluijs,P. and Norman,J. (2001) PDGF-regulated rab4-dependent recycling of αvβ3 integrin endosomes is necessary for cell adhesion and spreading. Curr. Biol., 11, 1392–1402. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Poindessous-Jazat,V., Le Naour,F., Billard,M. and Boucheix,C. (1997) CD9, but not other tetraspans, associates with the β1 integrin precursor. Eur. J. Immunol., 27, 1919–1927. [DOI] [PubMed] [Google Scholar]
- Schaap D. and Parker,P.J. (1990) Expression, purification and characterization of protein kinase C-ε. J. Biol. Chem., 265, 7301–7307. [PubMed] [Google Scholar]
- Shima D.T., Scales,S.J., Kreis,T.E. and Pepperkok,R. (1999) Segregation of COPI-rich and anterograde-cargo-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr. Biol., 9, 821–824. [DOI] [PubMed] [Google Scholar]
- Sun X.G. and Rotenberg,S.A. (1999) Overexpression of protein kinase Cα in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation and motility. Cell Growth Differ., 10, 343–352. [PubMed] [Google Scholar]
- Tang B.L., Wong,S.H., Qi,X.L., Low,S.H. and Hong,W. (1993) Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J. Cell Biol., 120, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Morgan,K.G., Parker,C. and Ware,J.A. (1997) Requirement for protein kinase Cθ for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells. J. Biol. Chem., 272, 28704–28711. [DOI] [PubMed] [Google Scholar]
- Waters M.G., Clary,D.O. and Rothman,J.E. (1992) A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol., 118, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A. and Couchman,J.R. (1992) Protein kinase C involvement in focal adhesion formation. J. Cell Sci., 101, 277–290. [DOI] [PubMed] [Google Scholar]
- Young S., Rothbard,J. and Parker,P. (1988) A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Eur. J. Biochem., 173, 247–252. [DOI] [PubMed] [Google Scholar]