Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: A relic of early life? (original) (raw)
Abstract
In most Gram-positive bacteria, serine-46-phosphorylated HPr (P-Ser-HPr) controls the expression of numerous catabolic genes (≈10% of their genome) by acting as catabolite corepressor. HPr kinase/phosphorylase (HprK/P), the bifunctional sensor enzyme for catabolite repression, phosphorylates HPr, a phosphocarrier protein of the sugar-transporting phosphoenolpyruvate/glycose phosphotransferase system, in the presence of ATP and fructose-1,6-bisphosphate but dephosphorylates P-Ser-HPr when phosphate prevails over ATP and fructose-1,6-bisphosphate. We demonstrate here that P-Ser-HPr dephosphorylation leads to the formation of HPr and pyrophosphate. HprK/P, which binds phosphate at the same site as the β phosphate of ATP, probably uses the inorganic phosphate to carry out a nucleophilic attack on the phosphoryl bond in P-Ser-HPr. HprK/P is the first enzyme known to catalyze P-protein dephosphorylation via this phospho–phosphorolysis mechanism. This reaction is reversible, and at elevated pyrophosphate concentrations, HprK/P can use pyrophosphate to phosphorylate HPr. Growth of Bacillus subtilis on glucose increased intracellular pyrophosphate to concentrations (≈6 mM), which in in vitro tests allowed efficient pyrophosphate-dependent HPr phosphorylation. To effectively dephosphorylate P-Ser-HPr when glucose is exhausted, the pyrophosphate concentration in the cells is lowered to 1 mM. In B. subtilis, this might be achieved by YvoE. This protein exhibits pyrophosphatase activity, and its gene is organized in an operon with hprK.
Carbon catabolite repression (CCR) is the paradigm of signal transduction systems. The uptake of a rapidly metabolizable carbohydrate such as glucose triggers the signal transduction pathway for CCR and carbon catabolite activation. In Gram-positive bacteria, glucose usage affects the metabolite concentration [decrease of phosphate from 50 to 3 mM; increase of NTP from 1 to 8 mM and of fructose-1,6-bisphosphate (FBP) from 2 to 25–40 mM] (1–3), which leads to the stimulation of the kinase activity of the bifunctional HPr kinase/phosphorylase (HprK/P) (4). HPr, the substrate of HprK/P, is a phosphocarrier protein necessary for the uptake and phosphorylation of sugars by the phospho_enol_pyruvate (PEP)/glycose phosphotransferase system (PTS) (5). To carry out this catalytic function, HPr becomes phosphorylated by PEP and enzyme I at His-15 (6), and histidyl-phosphorylated HPr transfers its phosphoryl group to various sugar-specific enzyme IIAs. The ATP-requiring HprK/P-catalyzed phosphorylation of HPr occurs at Ser-46 (7), and the resulting seryl-phosphorylated HPr (P-Ser-HPr) acts as catabolite corepressor. To carry out this regulatory function, P-Ser-HPr binds to the catabolite control protein A (CcpA) (8, 9), a member of the LacI/GalR repressor family (10). The interaction with P-Ser-HPr allows CcpA to bind to catabolite response elements (9, 11), operator sites preceding most catabolite-repressed or -activated transcription units in Gram-positive bacteria (4, 12). Whole genome analysis with Bacillus subtilis revealed that the expression of ≈400 genes (10% of the genome) is regulated by the P-Ser-HPr:CcpA complex (13, 14).
In addition to its importance for catabolite regulation, formation of P-Ser-HPr is also used to control PTS-catalyzed carbohydrate uptake. Mutants, in which the bifunctional HprK/P had lost its phosphorylase activity but kept almost normal kinase activity, converted almost all HPr to P-Ser-HPr, even when grown in the absence of glucose. These mutants exhibited permanent CCR and were therefore unable to use most PTS and non-PTS substrates (15). Inactivating CcpA in these mutants prevented CCR and restored growth on non-PTS, but not on PTS, substrates. This result established that increasing the intracellular amount of P-Ser-HPr inhibits PTS-catalyzed carbohydrate transport. This effect of P-Ser-HPr is probably a consequence of its ≈500-times slower phosphorylation by PEP and enzyme I compared to the phosphorylation of HPr (16). P-Ser-HPr also mediates inducer exclusion in Gram-positive organisms. In many bacteria, rapidly metabolizable carbohydrates such as glucose were found to inhibit the uptake of less favorable carbon sources. In Gram-negative bacteria, this regulatory function is carried out by the PTS protein EIIAGlc, which can bind to non-PTS permeases and inhibit their transport activity (5). For several Gram-positive organisms, it has been shown that the inhibitory effect of glucose on non-PTS transporters disappeared in ptsH1 (Ser-46 of HPr replaced with Ala) or hprK mutants (17–19), both unable to form P-Ser-HPr. These results suggested that in these bacteria, P-Ser-HPr interacts with several non-PTS permeases and inhibits their transport activity (20).
According to in vitro experiments, the HprK/P-catalyzed formation of the multifunctional regulator P-Ser-HPr in Gram-positive organisms is controlled in a complex manner by at least three compounds: ATP, FBP, and phosphate (21–23). The recently determined crystal structure of Lactobacillus casei HprK/P had revealed that phosphate can bind to the Walker motif A (24), the nucleotide-binding site in kinases and nucleotide-binding proteins (25). This finding explains why phosphate inhibits the ATP-dependent phosphorylation of HPr in a competitive manner. In contrast, FBP stimulates P-Ser-HPr formation. As a consequence, in the presence of FBP much higher phosphate concentrations are required to inhibit the ATP-dependent phosphorylation of HPr (17, 21). Phosphate not only inhibited the kinase activity, but it also was reported to stimulate the dephosphorylation of P-Ser-HPr by HprK/P up to 20-fold (15, 17, 26). This was surprising, because phosphate was thought to be the product of P-Ser-HPr dephosphorylation and was therefore expected to rather inhibit this reaction. We demonstrate here that phosphate is not an activator of P-Ser-HPr dephosphorylation, but that it serves as substrate for this reaction. The nucleophilic attack on the phosphoryl group of P-Ser-HPr is carried out by a phosphate ion instead of a water molecule and leads to the formation of pyrophosphate (phospho–phosphorylase reaction).
Materials and Methods
Protein Purification and Preparation of [32P]P-Ser-HPr.
His-tagged B. subtilis HPr and HprK/P were purified as described (22). The Lactobacillus casei wild-type and G270E mutant HprK/P (the latter exhibits normal kinase but almost no phosphorylase activity), also were purified with an N-terminal His-tag (15). [32P]P-Ser-HPr was prepared by incubating 40 μM His-tagged HPr from B. subtilis with 150 nM L. casei G270E mutant HprK/P, 70 μM [γ-32P]ATP (10 μCi), and 3 mM MgCl2 in 25 mM Tris⋅HCl buffer (pH 7.4) for 15 min at 37°C. HprK/P was subsequently heat-inactivated (10 min at 70°C) before [32P]P-Ser-HPr was separated from [γ-32P]ATP by chromatography on nickel-nitrilotriacetic acid (Qiagen, Chatsworth, CA) and PD-10 (Pharmacia) columns.
TLC.
Samples (1–5 μl) were spotted on polyethyleneimine cellulose sheets. To separate pyrophosphate from phosphate and P-Ser-HP, ascending TLC was performed with 0.3 M potassium phosphate, pH 7.4 (4 vol of 0.3 M K2HP04/1 vol of 0.3 M KH2P04) as solvent, whereas 0.3 M KH2PO4 (pH 4) allowed separation of 1-[32P]FBP from the other radioactive products (Fig. 2, lanes 1–4). Chromatography was carried out for 90 min before the sheets were dried and radioactive signals detected with a PhosphorImager (Molecular Dynamics).
Figure 2.
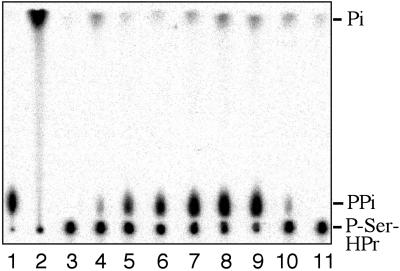
Identification of the [32P]P-Ser-HPr dephosphorylation product. The reaction products of HprK/P-catalyzed [32P]P-Ser-HPr dephosphorylation were separated by TLC using 0.3 M KH2PO4 as solvent. Radioactive standards for pyrophosphate and P-Ser-HPr (lanes 1 and 4, respectively); 1-[32P]FBP formed from fructose-6-P and either [32P]pyrophosphate or [γ-32P]ATP (lanes 2 and 3, respectively). Dephosphorylation of [32P]P-Ser-HPr with B. subtilis HprK/P was carried out in the presence of fructose-6-P (lane 5), pyrophosphate-dependent phosphofructokinase (lane 6), and fructose-6-P and pyrophosphate-dependent phosphofructokinase (lane 7).
[32P]P-Ser-HPr Dephosphorylation.
[32P]P-Ser-HPr (10 or 100 μM) was incubated at 37°C in 20 μl of 50 mM Tris⋅HCl (pH 7.4) with 0.15 μM B. subtilis HprK/P and 3 mM MgCl2 in the absence or presence of 5 mM phosphate. Reactions were stopped by spotting aliquots on polyethyleneimine cellulose sheets. The previously used heat inactivation of HprK/P (15) was omitted to preserve pyrophosphate prone to hydrolysis at higher temperature. To test the dependence of P-Ser-HPr dephosphorylation on the presence of phosphate, a solution containing 100 μM [32P]P-Ser-HPr, 20 μM phosphate and 3 mM MgCl2 in 20 μl of 50 mM Tris⋅HCl (pH 7.4) was incubated for 10 min at 37°C with 10 mM maltose and 0.8 unit of maltose phosphorylase (Molecular Probes), which lowers the amount of inorganic phosphate to ≈10 nM. Then 0.1 μg of HprK/P (0.15 μM final concentration), also treated with maltose/maltose phosphorylase, was added to this assay mixture, which was subsequently incubated for 15 min at 37°C. As a control, an identical reaction was carried out in the absence of maltose and maltose phosphorylase. To determine the kinetic parameters of P-Ser-HPr dephosphorylation, 10-μl assay mixtures were prepared containing 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, 15 nM HprK/P, and either 5 mM phosphate and varying concentrations of [32P]P-Ser-HPr (10, 20, 40, 60, 80, and 100 μM) or 400 μM [32P]P-Ser-HPr and varying concentrations of phosphate (0.01, 0.05, 0.2, 1, 5, and 25 mM). The assay mixtures were incubated at 37°C, and 1-μl aliquots were withdrawn after 1, 2, 3, 4, and 5 min and loaded on polyethyleneimine cellulose sheets. After chromatography with 0.3 M potassium phosphate (pH 7.4), radioactivity was detected with a PhosphorImager, and the program imagequant, Ver. 4.2a (Molecular Dynamics) was used for quantitative calculations. Deviations of the mean values of three independent experiments were <10%.
Phosphofructokinase Reactions.
ATP-requiring phosphofructokinase (0.05 unit) from Bacillus stearothermophilus (Sigma) was used to prepare 1-[32P]FBP from 1 mM fructose-6-P and 20 μM [γ-32P]ATP in the presence of 2 mM MgCl2 and 50 mM Tris⋅HCl (pH 7.4) by incubating the reaction mixture for 30 min at 37°C before heating it for 10 min at 100°C. The pyrophosphate-requiring mung bean phosphofructokinase (0.05 unit) (Sigma) was used to produce 1-[32P]FBP by incubating a solution containing 2.5 mM fructose-6-P, 1 mM phosphate, 50 μM [32P]pyrophosphate, 3 mM MgCl2, and 50 mM Tris⋅HCl (pH 7.4) for 10 min at 37°C. Aliquots from both reactions were used as 1-[32P]FBP standards for TLC (Fig. 2, lanes 2 and 3). To test whether the product of [32P]P-Ser-HPr dephosphorylation was pyrophosphate, 0.05 unit of pyrophosphate-requiring phosphofructokinase was added to a 20-μl solution containing 0.1 mM [32P]P-Ser-HPr, 0.15 μM B. subtilis HprK/P, 2.5 mM fructose-6-P, 2 mM phosphate, 3 mM MgCl2, and 50 mM Tris⋅HCl, pH 7.4. The assay mixture was incubated for 10 min at 37°C before it was separated by TLC on polyethyleneimine cellulose sheets with 0.3 M KH2PO4 (pH 4) as solvent.
Pyrophosphate-Dependent HPr Phosphorylation.
Reaction mixtures (20 μl) containing 3 μM HPr, 0.15 μM B. subtilis HprK/P, 3 mM MgCl2, 50 mM Tris⋅HCl (pH 7.4), and [32P]pyrophosphate ranging from 0.1 to 4 mM were incubated for 10 min at 37°C. The reactions were stopped by adding SDS sample buffer and heating for 5 min at 100°C. The samples were loaded on 0.1% SDS/15% polyacrylamide gels, which after electrophoresis were kept for 10 min in a boiling 15% trichloroacetic acid solution before they were dried and exposed to autoradiography. To determine the kinetic parameters of pyrophosphate-dependent HPr phosphorylation, 10-μl assay mixtures were prepared containing 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, 15 nM HprK/P, and either 5 mM [32P]pyrophosphate and varying concentrations of HPr (2, 5, 10, 20, 50, and 100 μM) or 0.4 mM HPr and varying concentrations of [32P]pyrophosphate (0.01, 0.05, 0.2, 1, 5, and 20 mM). The assay mixtures were incubated at 37°C and 1-μl aliquots were withdrawn after 1, 2, 3, 4, and 5 min and loaded on polyethyleneimine cellulose sheets. Chromatography and quantitative calculations were carried out as described in [32P]P-Ser-HPr dephosphorylation. Deviations of the mean values of three independent experiments were <15%.
To compare the effect of FBP on ATP- and pyrophosphate-dependent HPr phosphorylation, nonradioactive assays were carried out in the absence or presence of 5 mM FBP in reaction mixtures (20 μl) containing 5 μM HPr, 60 nM HprK/P, 50 mM Tris⋅HCl (pH 7.4), 10 mM MgCl2, and various concentrations (0.1, 1, 2, and 5 mM) of either ATP or pyrophosphate. After incubation for 10 min at 37°C, the samples were loaded on nondenaturing 12.5% polyacrylamide gels, which allowed to separate HPr and P-Ser-HPr.
To determine the _K_eq for phosphate-dependent P-Ser-HPr dephosphorylation, 10-μl assay mixtures were prepared containing 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, 150 nM HprK/P, and 50 μM of either [32P]P-Ser-HPr, HPr, phosphate, and pyrophosphate (HPr phosphorylation) or of P-Ser-HPr, HPr, phosphate, and [32P]pyrophosphate (P-Ser-HPr dephosphorylation). To reach equilibrium, the reaction mixtures were incubated for 1 or 2 h and separated by TLC on polyethyleneimine cellulose sheets. The amounts of P-Ser-HPr, HPr, phosphate, and pyrophosphate were determined based on the distribution of the radioactivity, which was measured with a PhosphorImager. To determine in vitro the amount of P-Ser-HPr formed at phosphate and pyrophosphate concentrations measured in cells grown in the absence or presence of glucose, 20-μl reaction mixtures were prepared containing 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, 1 μM HPr, 0.25 μM HprK/P, and either 3 mM phosphate and 6 mM pyrophosphate (glucose condition) or 50 mM phosphate and 1 mM pyrophosphate (glucose absent). The samples were incubated for 1 h at 37°C before HPr and P-Ser-HPr were separated on nondenaturing 12.5% polyacrylamide gels.
Pyrophosphatase Assays with B. subtilis YvoE.
The PCR-amplified B. subtilis yvoE gene with newly created _Bam_HI and _Hin_dIII restriction sites at the 5′- and 3′-ends, respectively, was inserted into pQE30 (Qiagen). Overexpression and purification of His-tagged YvoE were carried out as described (22) for other proteins. Pyrophosphatase assays were performed in 20 μl of 50 mM Tris⋅HCl (pH 7.4), containing 10 μM [32P]pyrophosphate, 1 mM MgCl2, and various concentrations of YvoE (2.5–50 nM). The ability of YvoE to hydrolyze [32P]pyrophosphate produced during [32P]P-Ser-HPr dephosphorylation was tested by incubating 250 nM YvoE for 10 min at 37°C with 0.1 mM [32P]P-Ser-HPr, 0.15 μM B. subtilis HprK/P, 1 mM phosphate, 1 mM MgCl2, and 50 mM Tris⋅HCl, pH 7.4. To determine the kinetic parameters of YvoE-catalyzed pyrophosphate hydrolysis, varying amounts of [32P]pyrophosphate (0.2, 0.5, 1, 2, 5, 10, 20, and 50 μM) were incubated at 37°C in a 10-μl assay mixture containing 50 mM Tris⋅HCl (pH 7.4), 5 mM MgCl2, and 5 nM YvoE. Aliquots of 1 μl were loaded on polyethyleneimine cellulose sheets after 1, 2, 3, 4, 5, 6, and 7 min. Chromatography and quantitative calculations were performed as described in [32P]P-Ser-HPr dephosphorylation. Deviations of the mean values of three independent experiments were <10%.
Determination of FBP and Pyrophosphate Concentrations in B. subtilis.
Cells of the B. subtilis wild-type strain 168 were grown in 100 ml of a 0.5% succinate-containing chemically defined medium (27) in the presence or absence of either 0.5% glucose or 0.5% glycerol. For each growth condition, three independent experiments were carried out. When the cultures reached an OD600 of 0.5, they were rapidly chilled to 4°C by using an ethanol/dry ice bath, and the cells were subsequently harvested by centrifugation. The wet cell pellets were rinsed and resuspended in the 5-fold volume (≈3.5 ml) of 0.6 M cold perchloric acid and subsequently kept on ice for 20 min. Tests with radioactive compounds showed that FBP and pyrophosphate resisted hydrolysis by 0.6 M perchloric acid. The precipitated proteins and cell debris were removed by centrifugation. The pH in the supernatant was subsequently adjusted to 7.4 with a solution of 0.6 M KOH in 100 mM Tris⋅HCl (pH 7.4), and the precipitated KClO4 was removed by centrifugation. Aliquots of the final supernatants were used to measure the amounts of triosephosphates, FBP, and pyrophosphate in a coupled photometric assay. Reaction mixtures of 1 ml were prepared, which contained 0.25 or 0.5 ml of the cell extracts, 50 mM Tris⋅HCl (pH 7.4), 50 units of triosephosphate isomerase, 5 units of glycerol-3-P dehydrogenase, 0.5 mM NADH, 8 mM fructose-6-P, 5 mM MgCl2, 65 μM MnCl2, 6.5 μM cobalt acetate, 3.5 mM EDTA, and 1.5 mM citrate. After 10 min of incubation, which allowed determination of the amount of triosephosphates, 5 units of aldolase were added to measure the concentration of FBP. When no further decrease of the OD340 occurred, the detection of pyrophosphate was started with 0.5 unit of pyrophosphate-dependent phosphofructokinase from Propionibacterium freudenreichii (Sigma). To calculate the intracellular concentration of the various metabolites, we used the previously determined correlation between the cell density of a B. subtilis culture measured at 600 nm and the aqueous cell volume. The cells present in 1 ml of culture exhibiting an OD600 of 1 were reported to have a total aqueous cell volume of 0.85 μl (28).
Results and Discussion
P-Ser-HPr Dephosphorylation Does Not Produce Phosphate.
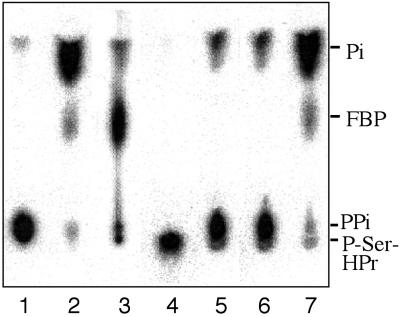
Depending on which effector molecule is bound to the bifunctional HprK/P, it can exist in two different conformations corresponding to its two antagonistic activities. Binding of ATP and FBP favors the kinase conformation, whereas inorganic phosphate gives rise to the phosphorylase form (15, 17, 26). The crystal structure of the phosphorylase form of L. casei HprK/P had revealed that inorganic phosphate binds to the same position in the P-loop as the β phosphate of ATP (24). In addition, in the complex of HprK/P with P-Ser-HPr, the phosphoryl group of P-Ser-HPr is situated close to the position occupied by the γ-phosphate of ATP (29). In HprK/P from Staphylococcus xylosus, a second phosphate was bound to this position (30). We therefore considered the possibility that during P-Ser-HPr dephosphorylation HprK/P might catalyze the formation of pyrophosphate from phosphate bound to the P-loop and the phosphoryl group of P-Ser-HPr, which would explain the unusual stimulatory effect of phosphate on P-Ser-HPr dephosphorylation (15, 17, 26). To identify the product of P-Ser-HPr dephosphorylation, experiments were carried out with radiolabeled B. subtilis [32P]P-Ser-HPr and HprK/P in the presence and absence of phosphate. Phosphate, pyrophosphate, and [32P]P-Ser-HPr were separated by TLC (Fig. 1, lanes 1–3). As reported previously (15, 17, 26), in the absence of phosphate, [32P]P-Ser-HPr was barely dephosphorylated (Fig. 1, lane 4). In the presence of 5 mM phosphate, however, a radioactive dephosphorylation product appeared already after 2 min of incubation, and after 30 min, virtually all [32P]P-Ser-HPr was converted to HPr (Fig. 1, lanes 5–9). Interestingly, the radioactive dephosphorylation product comigrated with the [32P]pyrophosphate and not the [32P]phosphate standard.
Figure 1.
[32P]P-Ser-HPr dephosphorylation by B. subtilis HprK/P. [32P]P-Ser-HPr dephosphorylation was carried out as described in Materials and Methods. The reaction products were separated by TLC with 0.3 M potassium phosphate, pH 7.4. Radioactive standards for pyrophosphate, phosphate, and P-Ser-HPr (lanes 1–3, respectively); 10 μM [32P]P-Ser-HPr incubated with HprK/P for 30, 2, 5, 10, 20, and 30 min (lanes 4–9, respectively) in the absence (lane 4) or in the presence (lanes 5–9) of 5 mM phosphate; lane 10, 0.1 mM [32P]P-Ser-HPr was incubated for 15 min with HprK/P in the presence of 20 μM phosphate, and lane 11, same experiment as in lane 10 except that before adding HprK/P the sample was preincubated for 10 min with maltose and maltose phosphorylase. PPi, pyrophosphate.
Pyrophosphate Is the Product of P-Ser-HPr Dephosphorylation.
To determine whether the radioactive product of [32P]P-Ser-HPr dephosphorylation was indeed pyrophosphate, we tested whether it could serve as substrate in a pyrophosphate-requiring kinase reaction. Several plants, protists, and bacteria possess a pyrophosphate-dependent phosphofructokinase (31), and we used the mung bean enzyme for our assays. Theoretically, the pyrophosphate-requiring phosphofructokinase should use one-half of the radioactivity of [32P]pyrophosphate to form 1-[32P]FBP. However, this enzyme is bifunctional and exhibits also fructose-1,6-bisphosphatase activity (32), leading to the formation of inorganic phosphate. Under the experimental conditions used, the main radioactive product was therefore phosphate, and only approximately one-fifth of the radioactivity remained in 1-[32P]FBP (Fig. 2, lane 2). Similarly, approximately one-fifth of the radioactivity was found in 1-[32P]FBP when fructose-6-P and the pyrophosphate-requiring phosphofructokinase were present during [32P]P-Ser-HPr dephosphorylation (Fig. 2, lane 7). No 1-[32P]FBP was formed when one of these two compounds was absent (Fig. 2, lanes 5–6). These results clearly identified the dephosphorylation product of [32P]P-Ser-HPr as pyrophosphate. Pyrophosphate also was obtained when the dephosphorylation of P-Ser-HPr was carried out with His-tagged wild-type HprK/P from L. casei (17) or untagged HprK/P from S. xylosus (30), confirming that this is a general mechanism for P-Ser-HPr dephosphorylation. In agreement with the proposed dephosphorylation mechanism, radioactive pyrophosphate also was formed when P-Ser-HPr was dephosphorylated by HprK/P in the presence of 200 μM [33P]phosphate (data not shown). The _k_cat of P-Ser-HPr dephosphorylation with B. subtilis HprK/P was determined to be 73 s−1, and the _K_m values for P-Ser-HPr and phosphate were 85 and 265 μM, respectively.
P-Ser-HPr Dephosphorylation Strictly Depends on Phosphate.
To test whether P-Ser-HPr dephosphorylation strictly depends on the presence of phosphate, we made use of the phosphate-consuming maltose phosphorylase reaction. When [32P]P-Ser-HPr was dephosphorylated at low phosphate concentrations (20 μM), a radioactive spot comigrating with pyrophosphate could be detected after 15 min of incubation (Fig. 1, lane 10). When HprK/P was added after the reaction mixture had been incubated for 10 min with maltose and maltose phosphorylase, which under the used conditions lowered the amount of free phosphate to ≈10 nM, virtually no radioactivity comigrating with pyrophosphate or phosphate could be observed (Fig. 1, lane 11), establishing that phosphate is essential for P-Ser-HPr dephosphorylation. Under the experimental conditions used, the detection limit for the formation of pyrophosphate was 50 nM, which means we would have observed a radioactive pyrophosphate spot if 0.5% of the P-Ser-HPr had been dephosphorylated. In analogy to phospho-hydrolysis, in which a water molecule attacks a phosphoryl bond, we called the phosphate-requiring dephosphorylation of P-Ser-HPr phospho-phosphorolysis. Based on the recently determined structures of the HPr:HprK/P and P-Ser-HPr:HprK/P complexes, a detailed mechanism for this novel pyrophosphate-producing P-protein dephosphorylation reaction catalyzed by HprK/P has been proposed (29).
HprK/P was found to exhibit sequence and structure similarities to several kinases phosphorylating low molecular weight compounds, including adenylate kinase, cytidylate kinase (24), and PEP carboxykinase (30, 33). Interestingly, PEP carboxykinase has been shown to form pyrophosphate from inorganic phosphate and PEP (34). We observed that in the presence of a few millimolar phosphate, adenylate kinase and cytidylate kinase also form pyrophosphate from their phosphorylation products ADP and CDP, respectively (I.M., J.D., M. Konrad, P. Briozzo, and T. Bernhard, unpublished results). For cytidylate kinase, the molecular activity was 900. Phospho-phosphorolysis therefore seems to be a characteristic of most members of this kinase family.
Pyrophosphate Functions as Phosphoryl Donor for HprK/P-Catalyzed HPr Phosphorylation.
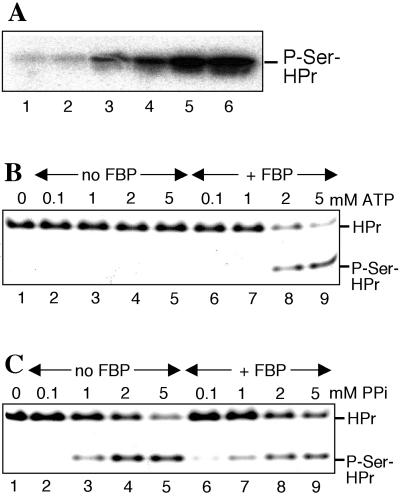
The pyrophosphate-forming dephosphorylation of P-Ser-HPr was found to be reversible, and HprK/P could phosphorylate HPr when pyrophosphate was present. Weak phosphorylation of HPr occurred at 0.1 mM pyrophosphate and increased when its concentration was elevated up to 5 mM (Fig. 3 A and C). The _k_cat of this reaction was 8.3 s−1 and the _K_m values for HPr and pyrophosphate were 78 and 785 μM, respectively. The equilibrium of this reversible reaction was slightly favorable for P-Ser-HPr dephosphorylation and pyrophosphate formation (_K_eq was determined to be 1.9 for the dephosphorylation reaction). By using this constant and the intracellular concentrations of pyrophosphate (see below) and phosphate (2, 3) during growth in the presence and absence of glucose, we calculated that in the presence of glucose 50% of the HPr would be present as P-Ser-HPr, whereas only 1% of P-Ser-HPr would be formed in the absence of glucose. In in vitro phosphorylation experiments, ≈60% of the HPr was converted to P-Ser-HPr when 6 mM pyrophosphate and 3 mM phosphate were present, whereas no P-Ser-HPr could be detected with 1 mM pyrophosphate and 50 mM phosphate. These values are in good agreement with the experimentally determined amounts of P-Ser-HPr in B. subtilis cells (≈60% when grown on glucose, not detectable when grown in the absence of glucose) (15). Interestingly, whereas the ATP-dependent phosphorylation of HPr required FBP (22) (compare Fig. 3B, lanes 2–5 to lanes 6–9), the pyrophosphate-dependent HPr phosphorylation was efficient already in the absence of FBP and was not increased by the presence of this glycolytic intermediate (compare Fig. 3C, lanes 2–5 to 6–9). In addition to pyrophosphate, HprK/P also could use tripolyphosphate to phosphorylate HPr (data not shown).
Figure 3.
Pyrophosphate-dependent HprK/P-catalyzed phosphorylation of HPr. (A) HPr was phosphorylated with B. subtilis HprK/P and [32P]pyrophosphate at various concentrations (0.1, 0.2, 0.4, 1, 2 ,and 4 mM for lanes 1–6, respectively). The samples were separated on a 12.5% polyacrylamide gel containing 0.1% SDS. (B and C) HPr was phosphorylated with B. subtilis HprK/P in the absence (lanes 1–5) or presence (lanes 6–9) of 5 mM FBP with varying concentrations of ATP (B) or pyrophosphate (C), and samples were separated on nondenaturing polyacrylamide gels.
YvoE Exhibits Pyrophosphatase Activity.
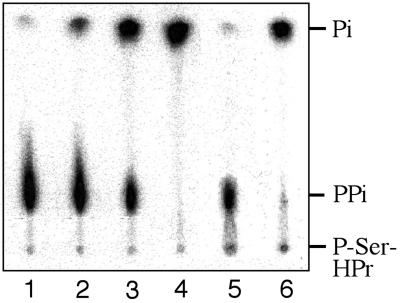
Pyrophosphate is often a by-product of energetically unfavorable biosynthetic reactions, such as aminoacyl-tRNA, argininosuccinate, or acyl CoA synthesis. It is split by pyrophosphatases to make the overall reaction energetically favorable. Similarly, to efficiently dephosphorylate P-Ser-HPr, which can reach intracellular concentrations of several hundred micromolar (15), the resulting pyrophosphate will need to be hydrolyzed. Interestingly, the yvoE gene located behind the lgt gene in the hprK operon of B. subtilis and three other bacilli (Bacillus anthracis, Bacillus halodurans, and B. stearothermophilus) encodes a protein exhibiting strong similarity to members of the P-glycolate phosphatase family (22). Because, in addition, B. subtilis YvoE has been reported to stimulate P-Ser-HPr dephosphorylation (22), we tested whether YvoE could function as pyrophosphatase hydrolyzing pyrophosphate formed during P-Ser-HPr dephosphorylation. B. subtilis YvoE could indeed hydrolyze [32P]pyrophosphate in a dose-dependent manner (Fig. 4, lanes 2–4). When YvoE was present during HprK/P-catalyzed dephosphorylation of [32P]P-Ser-HPr, the pyrophosphate resulting from P-Ser-HPr dephosphorylation (Fig. 4, lane 5) was completely hydrolyzed to phosphate (Fig. 4, lane 6). The _K_m for pyrophosphate was determined to be 5.1 μM, and the _k_cat was 0.06 s−1.
Figure 4.
YvoE-catalyzed hydrolysis of pyrophosphate. [32P]Pyrophosphate (10 μM) was incubated with 0, 2.5, 25, and 50 nM YvoE (lanes 1–4, respectively). Phosphorolysis of 0.1 mM [32P]P-Ser-HP by HprK/P in the absence of YvoE (lane 5) and in the presence of 250 nM YvoE (lane 6). Samples were separated by TLC.
Elevated Amounts of FBP and Pyrophosphate in Glucose-Grown B. subtilis Cells.
The in vitro results of P-Ser-HPr dephosphorylation and pyrophosphate-dependent HPr phosphorylation suggested that in addition to phosphate, ATP, and FBP, pyrophosphate also might regulate the phosphorylation state of HPr in Gram-positive bacteria. By using various techniques, the FBP concentration has been determined in Lactococcus lactis cells. Concentrations between 25 and 45 mM have been reported for glucose-grown cells, whereas ≈2 mM FBP was detected in cells grown in the absence of glucose (1–3). For B. subtilis, FBP concentrations below 2 mM have been reported for glucose-grown cells approaching early stationary phase (OD600 = 1.5) (28), which would not lead to a strong stimulation of HprK/P activity, as in vitro HPr phosphorylation with B. subtilis HprK/P was barely detectable at 2 mM FBP (35). Fluorescence studies had revealed that binding of FBP to B. subtilis HprK/P is maximal at ≈12 mM FBP (35). Pyrophosphate concentrations between 0.5 and 1.5 mM were measured for Escherichia coli cells growing in the presence of an energy source such as glucose. The pyrophosphate concentration dropped below 0.1 mM when glucose was exhausted (36, 37). To find out whether growth on glucose enhances the pyrophosphate concentration also in B. subtilis and to obtain more reliable values for the FBP concentration in this organism, we decided to measure the concentration of both metabolites in exponentially growing cells metabolizing either succinate, succinate and glucose, or succinate and glycerol. The first step of the procedure described in Materials and Methods allowed also to determine the concentration of triosephosphates, which was nearly identical in succinate and succinate plus glucose grown cells (1.34 and 1.26 mM, respectively), but was 5.1 mM in glycerol-grown cells. By contrast, the FBP concentration was 10-fold higher in glucose-grown cells (14.2 mM) compared to cells grown on succinate (1.4 mM). These values are much higher than those previously reported for B. subtilis (0.12 and 1.7 mM) (28). The reason for these differences is probably that we harvested the cells at mid-log phase (OD600 = 0.5), whereas in the previous report, cells were allowed to approach stationary phase (OD600 = 1.5). The presence of glycerol also increased the FBP concentration but only to 6.1 mM. This probably explains why growth of B. subtilis cells on glycerol leads to 2- to 3-fold weaker CCR than growth on glucose (38). In vitro experiments had shown that ATP-dependent HPr phosphorylation by B. subtilis HprK/P is negligible below 1 mM and maximal above 12 mM FBP (35).
Interestingly, similar to the FBP concentration, the concentration of pyrophosphate was also elevated in glucose- (5.8 mM) and glycerol-grown cells (2.8 mM) compared to succinate-grown cells (1.2 mM). In vitro phosphorylation experiments had shown that at concentrations below 1 mM pyrophosphate, HPr is barely phosphorylated by HprK/P (Fig. 3 A, lane 3 and C, lane 2). Optimal HPr phosphorylation was reached at ≈5 mM pyrophosphate (Fig. 3 A, lane 6 and C, lane 5). These results suggest that pyrophosphate plays also a physiological role in regulating the phosphorylation state of HPr. In the absence of glucose, the low intracellular concentration of pyrophosphate and the high concentration of phosphate (60 mM) (1–3) will lead to efficient dephosphorylation of P-Ser-HPr. By contrast, the low concentration of phosphate (≈3 mM) and the large amount of pyrophosphate in cells growing in the presence of glucose will favor HPr phosphorylation. Although the molecular mechanisms controlling the intracellular pyrophosphate pool remain to be determined, the pyrophosphatase activity-exhibiting YvoE might play an important role in this process. Despite its high affinity for pyrophosphate, the _V_max of YvoE-catalyzed pyrophosphate hydrolysis was fairly low. It is therefore tempting to assume that YvoE might be specifically activated when rapidly metabolizable carbohydrates are absent.
The structural and functional similarities among HprK/P, PEP carboxykinase, and several nucleotide kinases (24, 30) suggest that these enzymes evolved from similar ancestors. The predecessors of the latter enzymes probably used oligophosphates, the presumed energy-rich phosphate compounds during prebiotic evolution (39, 40), to phosphorylate early nucleotide-like molecules. This assumption is supported by the finding that, similar to HprK/P, adenylate kinase also can use tripolyphosphate as phosphoryl donor (41). Our results show that, although during evolution HprK/P has gained the ability to use nucleotides for HPr phosphorylation, it retained the capacity to use also oligophosphates, including pyrophosphate, which is also the product of the HprK/P-catalyzed P-Ser-HPr dephosphorylation. It is tempting to assume that primordial protein kinases too catalyzed the dephosphorylation of their phospho-products by reversing the kinase reaction and that specific phosphatases developed later in evolution. Similar to HprK/P, ancient oligophosphate-dependent kinases might have used a phosphate-requiring dephosphorylation mechanism with kinase and phosphorylase functions linked to the same site of the enzyme (15, 24, 30). Depending on whether the concentration of oligophosphates or inorganic phosphate was prevailing, these enzymes might have catalyzed either the kinase or the phosphorylase reaction. HprK/P and its related enzymes are probably descendants of such oligophosphate-using primordial kinases. HprK/P certainly went through extensive changes before becoming the sensor enzyme for catabolite repression in Gram-positive bacteria. However, as the pyrophosphate-producing P-Ser-HPr dephosphorylation mechanism was perfectly suited to respond to the changes of the phosphate concentration during CCR (≈3 mM during growth on glucose, 50 mM when glucose is exhausted) (1–3), the primitive phosphorolysis mechanism of HprK/P was probably preserved. Interestingly, the glycolytic intermediate FBP, which changes its concentration inversely to inorganic phosphate (1, 3), stimulates the ATP-, but not the pyrophosphate-dependent HPr phosphorylation, suggesting that stimulation of HprK/P by FBP developed after this enzyme had gained the capacity to use ATP and GTP, which are both more effective phosphoryl donors when FBP is present (35).
Acknowledgments
This research was supported by the Centre National de la Recherche Scientifique, the Institut National de la Recherche Agronomique, and the Institut National Agronomique–Paris/Grignon. I.M. received fellowships from the French Government and from the Institut National de la Recherche Agronomique.
Abbreviations
CcpA
catabolite control protein A
CCR
carbon catabolite repression
FBP
fructose-1,6-bisphosphate
HprK/P
HPr kinase/phosphorylase
PEP
phospho_enol_pyruvate
PTS
PEP/glycose phosphotransferase system
P-Ser-HPr
seryl-46-phosphorylated HPr
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Thompson J, Torchia D A. J Bacteriol. 1984;158:791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason P W, Carbone D P, Cushman R A, Waggoner A S. J Biol Chem. 1981;256:1861–1866. [PubMed] [Google Scholar]
- 3.Neves A R, Ramos A, Nunes M C, Kleerebezem M, Hugenholtz J, de Vos W M, Almeida J, Santos H. Biotechnol Bioeng. 1998;64:200–212. doi: 10.1002/(sici)1097-0290(19990720)64:2<200::aid-bit9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Deutscher J, Galinier A, Martin-Verstraete I. In: Bacillus subtilis and its Closest Relatives: From Genes to Cells. Sonenshein A L, Hoch J A, Losick R, editors. Washington, DC: Am. Soc. Microbiol.; 2001. pp. 129–150. [Google Scholar]
- 5.Postma P W, Lengeler J W, Jacobson G R. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gassner M, Stehlik D, Schrecker O, Hengstenberg W, Maurer W, Rüterjans H. Eur J Biochem. 1977;75:287–296. doi: 10.1111/j.1432-1033.1977.tb11528.x. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher J, Pevec B, Beyreuther K, Kiltz H-H, Hengstenberg W. Biochemistry. 1986;25:6543–6551. doi: 10.1021/bi00369a031. [DOI] [PubMed] [Google Scholar]
- 8.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones B E, Dossonnet V, Küster E, Hillen W, Deutscher J, Klevit R E. J Biol Chem. 1997;272:26530–26535. doi: 10.1074/jbc.272.42.26530. [DOI] [PubMed] [Google Scholar]
- 10.Henkin T M, Grundy F J, Nicholson W L, Chambliss G H. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Miwa Y, Galinier A, Deutscher J. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson W L, Chambliss G H. J Bacteriol. 1985;161:875–881. doi: 10.1128/jb.161.3.875-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno M S, Schneider B L, Maile R R, Weyler W, Saier M H., Jr Mol Microbiol. 2001;39:1366–1381. doi: 10.1111/j.1365-2958.2001.02328.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K, Kobayashi K, Miwa Y, Kang C M, Matsunaga M, Yamaguchi H, Tojo S, Yamamoto M, Nishi R, Ogasawara N, et al. Nucleic Acids Res. 2001;29:683–692. doi: 10.1093/nar/29.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monedero V, Poncet S, Mijakovic I, Fieulaine S, Dossonnet V, Martin-Verstraete I, Nessler S, Deutscher J. EMBO J. 2001;20:3928–3937. doi: 10.1093/emboj/20.15.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutscher J, Kessler U, Alpert C A, Hengstenberg W. Biochemistry. 1984;23:4455–4460. doi: 10.1021/bi00314a033. [DOI] [PubMed] [Google Scholar]
- 17.Dossonnet V, Monedero V, Zagorec M, Galinier A, Pérez-Martínez G, Deutscher J. J Bacteriol. 2000;182:2582–2590. doi: 10.1128/jb.182.9.2582-2590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viana R, Monedero V, Dossonnet V, Vadeboncoeur C, Pérez-Martínez G, Deutscher J. Mol Microbiol. 2000;36:570–584. doi: 10.1046/j.1365-2958.2000.01862.x. [DOI] [PubMed] [Google Scholar]
- 19.Monedero V, Kuipers O P, Jamet E, Deutscher J. J Bacteriol. 2001;183:3391–3398. doi: 10.1128/JB.183.11.3391-3398.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J-J, Saier M H., Jr Proc Natl Acad Sci USA. 1995;92:417–421. doi: 10.1073/pnas.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kravanja M, Engelmann R, Dossonnet V, Blüggel M, Meyer H E, Frank R, Galinier A, Deutscher J, Schnell N, Hengstenberg W. Mol Microbiol. 1999;31:59–66. doi: 10.1046/j.1365-2958.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 22.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer M-C, Deutscher J, Haiech J. Proc Natl Acad Sci USA. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H, Jr, Hillen W. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 24.Fieulaine S, Morera S, Poncet S, Monedero V, Gueguen V, Galinier A, Janin J, Deutscher J, Nessler S. EMBO J. 2001;20:3917–3927. doi: 10.1093/emboj/20.15.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraste M, Sibbald P R, Wittinghofer A. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 26.Deutscher J, Kessler U, Hengstenberg W. J Bacteriol. 1985;163:1203–1209. doi: 10.1128/jb.163.3.1203-1209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sissler M, Delorme C, Bond J, Ehrlich S D, Renault P, Francklyn C. Proc Natl Acad Sci USA. 1999;96:8985–8990. doi: 10.1073/pnas.96.16.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita Y, Freese E. J Biol Chem. 1979;254:5340–5349. [PubMed] [Google Scholar]
- 29.Fieulaine S, Morera S, Poncet S, Mijakovic I, Galinier A, Janin J, Deutscher J, Nessler S. Proc Natl Acad Sci USA. 2002;99:13437–13441. doi: 10.1073/pnas.192368699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquez J A, Hasenbein S, Koch B, Fieulaine S, Nessler S, Hengstenberg W, Scheffzek K. Proc Natl Acad Sci USA. 2002;99:3458–3463. doi: 10.1073/pnas.052461499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mertens E, Ladror U S, Lee J A, Miretsky A, Morris A, Rozario C, Kemp R G, Muller M. J Mol Evol. 1998;47:739–750. doi: 10.1007/pl00006433. [DOI] [PubMed] [Google Scholar]
- 32.Kemp R G, Tripathi R L. J Bacteriol. 1993;175:5723–5724. doi: 10.1128/jb.175.17.5723-5724.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galinier A, Lavergne J-P, Geourjon C, Fieulaine S, Nessler S, Jault J-M. J Biol Chem. 2002;277:11362–11367. doi: 10.1074/jbc.M109527200. [DOI] [PubMed] [Google Scholar]
- 34.Lochmüller H, Wood H G, Davis J J. J Biol Chem. 1966;241:5678–5691. [PubMed] [Google Scholar]
- 35.Jault J-M, Fieulaine S, Nessler S, Gonzalo P, Di Pietro A, Deutscher J, Galinier A. J Biol Chem. 2000;275:1773–1780. doi: 10.1074/jbc.275.3.1773. [DOI] [PubMed] [Google Scholar]
- 36.Danchin A, Dondon L, Daniel J. Mol Gen Genet. 1984;193:473–478. doi: 10.1007/BF00382086. [DOI] [PubMed] [Google Scholar]
- 37.Kukko-Kalske E, Lintunen M, Inen M K, Lahti R, Heinonen J. J Bacteriol. 1989;171:4498–4500. doi: 10.1128/jb.171.8.4498-4500.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. J Bacteriol. 1994;176:3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kornberg A. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keefe A D, Miller S L. Origins Life Evol Biosphere. 1996;26:15–25. doi: 10.1007/BF01808157. [DOI] [PubMed] [Google Scholar]
- 41.Sanders C R, Tian G, Tsai M-D. Biochemistry. 1989;28:9028–9043. doi: 10.1021/bi00449a011. [DOI] [PubMed] [Google Scholar]