Differences in Expression of Toll-Like Receptors and Their Reactivities in Dendritic Cells in BALB/c and C57BL/6 Mice (original) (raw)
Abstract
We have previously reported that differences in early production of interleukin 12 (IL-12) by dendritic cells (DC) underlies the difference between the susceptibilities to Listeria monocytogenes of C57BL/6 and BALB/c mice. To elucidate mechanisms for the different abilities of DC to produce cytokine in C57BL/6 and BALB/c mice, we examined Toll-like receptor (TLR) expression by DC and their responses in vitro to known microbial ligands for TLRs. We found that DC isolated from the spleens of naive C57BL/6 mice preferentially expressed TLR9 mRNA, whereas DC from naive BALB/c mice strongly expressed TLR2, -4, -5, and -6 mRNAs. C57BL/6 DC produced a higher level of IL-12p40 in response to the ligands for TLR4 (lipopolysaccharide), TLR2 (lipoprotein), and TLR9 (CpG), whereas BALB/c DC responded to these ligands by producing a larger amount of monocyte chemoattractant protein 1. C57BL/6 DC expressed higher levels of CD40 and Stat4 than BALB/c DC did, suggesting that naive C57BL/6 mice contained more-mature subsets of DC than naive BALB/c mice. Differences in reactivities of DC to microbial molecules through TLRs may be associated with susceptibility and resistance to Listeria infection in BALB/c and C57BL/6 mice.
Dendritic cells (DC), one of the earliest cell types exposed to pathogens, are the most potent antigen-presenting cells (APC), with a unique ability to induce primary immune responses against microbial infection (3, 21). First, immature DC capture antigen (Ag) via high levels of endocytic and phagocytic activity, and then they migrate to regional lymph nodes, where they develop into mature DC and become APCs able to activate Ag-specific naive lymphocytes. Immature DC express low levels of major histocompatibility complex (MHC) class II, CD83, and costimulatory molecules, such as CD80, CD86, and CD40, and mature DC are characterized by high levels of expression of such a specific array of marker molecules (22, 24, 27, 36). Immature DC showed strong ability to release tumor necrosis factor alpha (TNF-α), whereas mature DC had a great ability to release interleukin 12 (IL-12) in response to bacterial stimuli (48). There are two categories of DC based on their abilities to mount a Th1 or Th2 response (21, 22, 30, 37). DC1 produce IL-12, which stimulates naive CD4+ T cells to develop into CD4+ Th1 cells, whereas DC2 skew CD4+-T-cell differentiation toward the production of Th2 cells (37). Although DC2 have been reported to be able to directly produce IL-4, which is important for the development of polarized Th2 responses (8), it still remains to be elucidated which molecules from DC2 induce Th2 responses. DC can also be separated into two distinct subsets according to their myeloid or lymphoid origin. In mice, the myeloid-related DC are CD11c+ CD11b+ CD8α− DEC205− and the lymphoid-related DC are CD11c+ CD11b− CD8α+ DEC205+ in phenotype (2, 24, 30). There are several lines of CD8α+ DC which showed a greater ability to produce IL-12 than CD8α− DC (25, 32, 35), and CD8α− DC also released it in the maturation stage (4, 38). Thus, it is not clear that myeloid- and lymphoid-derived DC play different roles in the polarization of the immune responses toward the Th1-pathway.
Microbial molecules activate immature DC to become mature DC characterized by cytokine production, up-regulation of costimulatory molecules, and an increased ability to activate T cells through Toll-like receptors (TLRs) (18, 19). Over the years, TLRs have been identified as ancient receptors that confer specificity on the host innate immune system, allowing the recognition of pathogen-associated molecular patterns (26). TLR4 has been reported to function as a receptor for lipopolysaccharide (LPS), an integral component of the outer membranes of gram-negative bacteria (34, 42). TLR2 is reported to be specialized to recognize lipoprotein from diverse species of bacteria, including Mycobacterium tuberculosis, Mycoplasma fermentans, Treponema pallidum, and Borrelia burgdorferi (1, 5, 15, 39, 43, 45, 46). TLR6, in combination with TLR2, recognizes zymosan and peptidoglycan (12, 33, 44). TLR9 and TLR5 have been shown to recognize bacterially derived CpG DNA (14) and flagellin (13), respectively. Visintin et al. recently reported that TLR gene expression was down-regulated in human DC during the course of maturation from monocytes (48). Although TLR expression at the protein level is not relevant to TLR gene expression, TLR gene expression may reflect the stage of DC maturation.
It has been reported that the difference in early production of IL-12 by DC may underlie the difference in resistance and susceptibility to Listeria monocytogenes of C57BL/6 and BALB/c mice (23). In the present study, to elucidate mechanisms for differences in the ability of DC to produce cytokine in C57BL/6 and BALB/c mice, we compared various TLR gene expressions and the in vitro responses to the corresponding ligands by DC freshly isolated from the spleens of C57BL/6 and BALB/c mice. We found that DC from C57BL/6 mice expressed a higher level of TLR9 mRNA but lower levels of TLR2, -4, -5, and -6 mRNAs. IL-12 production was much higher in C57BL/6 DC in response to LPS, lipoprotein, and CpG, whereas monocyte chemoattractant protein 1 (MCP-1) production was significantly higher in BALB/c DC in response to these microbial products. The implications of our findings for the expression of distinct sets of TLR genes and the differences in reactivity to microbial molecules in BALB/c and C57Bl/6 mice are discussed.
MATERIALS AND METHODS
Experimental animals.
Male C57BL/6 and BALB/c mice were purchased from Charles River of Japan (Tokyo, Japan), and C3H/HeN and C3H/HeJ mice were purchased from Japan SLC (Shizuoka, Japan). The mice were used in experiments at 6 weeks of age.
Microorganism.
L. monocytogenes strain EGD was used for experiments. Bacterial virulence was maintained by serial passage in BALB/c mice. Fresh isolates were obtained from infected spleens, grown in tryptic soy broth (Difco Laboratories, Detroit, Mich.), washed repeatedly, resuspended in phosphate-buffered saline, and stored at −70°C in small aliquots. The mice were inoculated intravenously with L. monocytogenes in a volume of 0.1 ml of phosphate-buffered saline.
Reagents.
LPS derived from Escherichia coli O55(serotype B6:026) and synthetic lipoprotein [palmitoyl-Cys(RS)-2,3-di(palmitoyloxy)-propyl-Ala-Gly-oh] were obtained from Sigma Chemical Co. (St. Louis, Mo.) and Bachem AG (Bubendorf, Switzerland), respectively. Phosphorothioate-stabilized CpG oligodeoxynucleotide (TCC ATG ACG TTC CTG ATG CT) was purchased from Rikaken Co. (Nagoya, Japan). Zymosan from Saccharomyces cerevisiae was obtained from Wako Pure Chemical Industries (Tokyo, Japan).
Ab.
Fluorescein isothiocyanate (FITC)-conjugated anti-CD40 monoclonal antibody (MAb) or anti-CD8α MAb, phycoerythrin (PE)-conjugated anti-CD86 MAb, biotin-conjugated anti-CD11c MAb, and Cy-chrome-conjugated streptavidin were purchased from PharMingen (San Diego, Calif.). Anti-Stat4 polyclonal antibodies (Ab) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
DC preparation.
DC were prepared from spleens by a modification of the method of Steinmann et al. (40). Briefly, spleen cells were treated with 200 U of collagenase (Wako Pure Chemical Industries)/ml in Hanks' balanced salt solution, and the resultant single-cell suspension was centrifuged in a high density of bovine serum albumin (BSA) (P = 1.08) at 10,000 × g for 20 min. Cells were obtained at the interface and washed with RPMI medium containing 10% fetal bovine serum. After being washed, the cells were incubated in a plastic dish for 90 min at 37°C. Nonadherent cells were removed by pipetting, and the remaining adherent cells were further incubated for 30 min at 37°C. Nonadherent cells were again removed by pipetting, and the remaining adherent cells were further incubated overnight with RPMI 1640 medium at 37°C. After overnight culture, the nonadherent cells were recovered and used as enriched DC. The viability of DC of each strain was >98% as assessed by the trypan blue dye exclusion test.
Reverse transcription (RT)-PCR.
Total RNA was extracted from DC essentially according to the method of Chomczynski and Sacchi (7). First-strand cDNA was synthesized from 2 μg of RNA using reverse transcriptase (SuperScript II RT; Life Technologies, Gaithersburg, Md.) and 20 pmol of a random primer (Life Technologies) in 21-μl reaction mixtures, according to the manufacturer's instructions. The synthesized first-strand cDNA was diluted to a total volume of 20 μl with distilled water. An aliquot of first-strand cDNA was amplified by Ampli-Taq (Perkin-Elmer Cetus, Norwalk, Conn.) in a total volume of 100 μl of reaction buffer consisting of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, and 0.2 mM deoxynucleoside triphosphate. One PCR cycle consisted of denaturation at 94°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 0.5 min. Before the first cycle, an initial denaturation step of 3 min at 94°C was included, and after 23 to 35 cycles, the extension reaction was prolonged to 4 min at 72°C. After amplification, the PCR products were separated by electrophoresis through 1.8% agarose gels and then transferred onto GeneScreen Plus membranes (New England Nuclear, Boston, Mass.). The specific primers were as follows: TLR2 sense, 5′-CAGCTTAAAGGGCGGGTCAGAG-3′; TLR2 antisense, 5′-TGGAGACGCCAGCTCTGGCTCA-3′; TLR4 sense, 5′-AGTGGGTCAAGGAACAGAAGCA-3′; TLR4 antisense, 5′-CTTTACCAGCTCATTTCTCACC-3′; TLR5 sense, 5′-GAATTCCTTAAGCGACGTAA-3′; TLR5 antisense, 5′-GAGAAGATAAAGCCGTGCGA-3′; TLR6 sense, 5′-AGTGCTGCCAAGTTCCGACA-3′; TLR6 antisense, 5′-AGCAAACACCGAGTATAGCG-3′; TLR9 sense, 5′-CCAGACGCTCTTCGAGAACC-3′; and TLR9 antisense, 5′-GTTATAGAAGTGGCGGTTGT-3′.
Northern blot analysis.
Aliquots (10 μg) of the total RNAs were fractionated on a 1% agarose gel containing 20 mM MOPS (morpholinepropanesulfonic acid), 5 mM sodium acetate, 1 mM EDTA (pH 7.0), and 6% (vol/vol) formaldehyde and transferred to a nylon membrane. After UV cross-linking, the membranes were soaked in prehybridization solution (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's reagent, 0.5% sodium dodecyl sulfate [SDS], 100 mg of denatured salmon sperm DNA/ml, and 50% formamide) for 3 h at 42oC, followed by incubation with 32P-labeled probe in hybridization solution (6× SSC, 0.5% SDS, 100 mg of denatured salmon sperm DNA/ml, and 50% formamide) for 14 h at 42°C. The membranes were washed twice in 2× SSC-0.1% SDS for 10 min each time at room temperature and twice in 0.1× SSC-0.1% SDS for 10 min each time at 50°C and were exposed to Fuji (Tokyo, Japan) RX-U film. cDNA fragments of the coding regions of mouse TLR2 and TLR4 were used as specific probes (42).
Extract preparation and immunoblotting.
Cells were lysed in phospholipase C lysis buffer (50 mM HEPES [pH 7.0], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM NaPPi, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml) at 108 cells/ml. The lysates were separated on SDS-polyacrylamide gels and then electrotransferred to Immobilon polyvinylidene difluoride membranes (Millipore Corp., Bedford, Mass.). The membranes were blocked for 2 h in 2% BSA-TBST (20 mM Tris-HCl [pH 7.6], 0.15 M sodium chloride, 0.1% Tween 20), incubated with primary Ab in TBST for 1 h, washed three times with TBST, and incubated for 1 h with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin (Amersham Pharmacia Biotech) diluted 1:10,000 in TBST. After three washes in TBST, the blot was developed with the enhanced chemiluminescence system (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Cytokine assays.
The cell-free culture supernatants were collected at the appropriate times. The cytokine activity in the culture supernatant was assayed by an enzyme-linked immunosorbent assay (ELISA) using mouse IL-12p40, gamma interferon (IFN-γ), MCP-1, IL-4, and TNF-α DuoSet ELISA Development Systems (Genzyme Diagnostics, Cambridge, Mass.).
Fluorescence-activated cell sorter analysis.
Cells were stained with PE-conjugated MAb, FITC-conjugated MAb, and biotinylated MAb followed by Cy-chrome-conjugated streptavidin (Becton Dickinson, San Jose, Calif.) at 4°C for 40 min. The stained cells were analyzed by FACScan flow cytometry (Becton Dickinson).
Statistics.
The data were analyzed using Student's t test. A P value of <0.05 was taken as the level of significance. Analyses were performed using Stat-View version 4.5 software (Abacus Concepts, Berkeley, Calif.) and Power Macintosh 7200 computers (Apple Computer, Cupertino, Calif.).
RESULTS
Expression of coaccessory molecules and Stat4 by DC isolated from BALB/c and C57BL/6 mice.
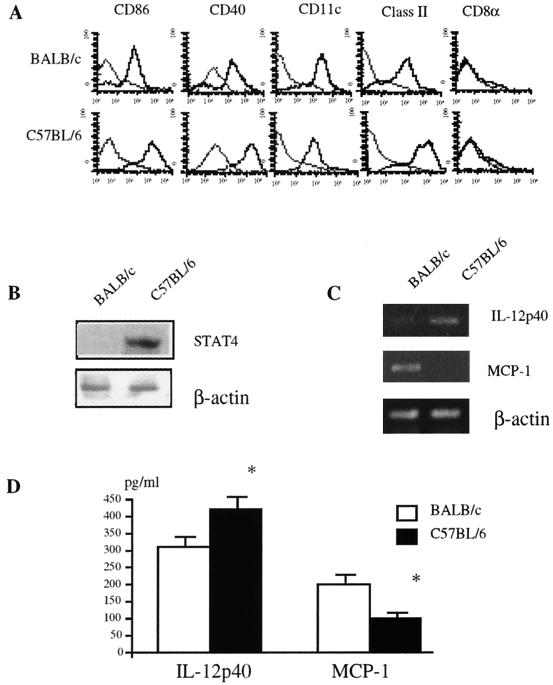
DC are characterized by the expression of accessory and costimulatory molecules, including CD40, CD80, and CD86, as well as MHC class II molecules and CD83, and their intensities increase in a maturation-dependent manner (3, 24). DC were purified from spleen-adherent cells of naive C57BL/6 and BALB/c mice by the BSA method. More than 75% were of the CD11chigh CD11blow CD8α− phenotype. Although a small number of CD11c− CD11b− cells existed in the cell population, CD8α+ cells were <2% in both strains. Typical staining profiles for CD11c, CD40, CD86, and CD8α are shown in Fig. 1A. DC isolated from naive C57BL/6 mice expressed higher levels of CD86 and CD40 than those from BALB/c mice. Expression of Stat4, a key intermediate in IL-12 signaling, has also been reported to be regulated in DC in a maturation-dependent manner (9). We next compared the expression levels of Stat4 in DC from C57BL/6 and BALB/c mice. As shown in Fig. 1B, DC from naive C57BL/6 mice expressed a higher level of Stat4 than those from naive BALB/c mice.
FIG. 1.
Comparison of maturation levels in DC from C57BL/6 and BALB/c mice. (A) Fluorescence-activated cell sorter analysis of expression of CD11c, CD40, CD86, CD8α, or MHC class II on DC. DC from naive C57BL/6 and BALB/c mice were stained with FITC-conjugated CD86 MAb, PE-conjugated CD40 MAb, and biotin-conjugated anti-CD11c MAb followed by incubation with streptavidin-Red670-Cy-chrome and were analyzed by FACScaliber. Negative controls (thin lines) were obtained by staining with control Ab. The representative profile from each strain is shown as a single histogram. (B) Expression of Stat4 in DC from naive C57BL/6 and BALB/c mice. Cell extracts from pooled DC from five mice of each strain were subjected to Western blot analysis with anti-Stat4 Abs as described in Materials and Methods. Three independent experiments showed similar results, and the results of a representative experiment are shown. (C) Detection of cytokine mRNA by RT-PCR in DC from C57BL/6 and BALB/c mice. cDNAs were amplified using primers specific for IL-12p40, MCP-1, or β-actin and were separated on a 3% agarose gel containing ethidium bromide. Three independent experiments showed similar results, and the results of a representative experiment are shown. (D) Detection of IL-12p40 and MCP-1 production by DC from C57BL/6 and BALB/c mice. DC were cultured in medium overnight, and the concentrations of IL-12p40 and MCP-1 in the supernatants were measured by ELISA. Four independent experiments showed similar results, and the data are expressed as the mean plus standard deviation of triplicate cultures of each strain in a representative experiment. *, significantly different from the values for BALB/c mice (P < 0.01).
The ability of DC to produce IL-12 is also reported to increase in a maturation-dependent manner (4, 38). Therefore, we also examined IL-12 levels in DC from C57BL/6 and BALB/c mice. As shown in Fig. 1C, the level of IL-12p40 mRNA was much higher in DC from C57BL/6 mice than in those from BALB/c mice. In contrast, mRNA for MCP-1, a CC chemokine involved in mounting Th2 polarization (10), was expressed at a much higher level by BALB/c DC than C57BL/6 DC. To compare these cytokines produced by DC at the protein level, we determined the levels of IL-12p40 and MCP-1 in the supernatants of overnight cultures of DC by ELISA. As shown in Fig. 1D, the IL-12p40 level was significantly higher in C57BL/6 DC, but the MCP-1 level was much lower than in BALB/c mice. Thus, these results suggest that naive C57BL/6 mice have a larger number of DC1 but a smaller number of DC2 in their spleens than do naive BALB/c mice.
Expression of TLR genes in DC from C57BL/6 and BALB/c mice.
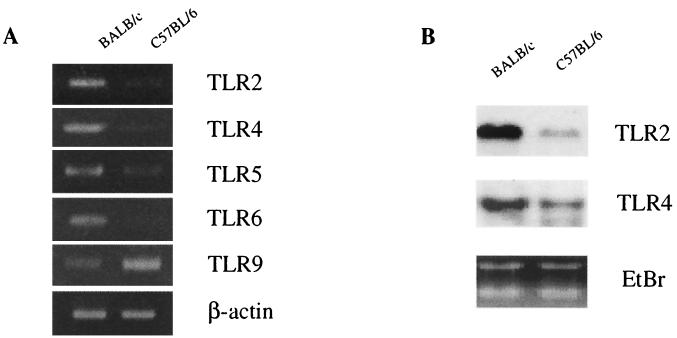
TLR expression levels are reported to decrease with the maturation of human DC (48). We examined the expression of TLR mRNA by DC from BALB/c and C57BL/6 mice by RT-PCR (Fig. 2A). The expression levels of TLR2, TLR4, TLR5, and TLR6 mRNAs were higher in BALB/c mice than in C57BL/6 mice. On the other hand, the TLR9 gene was expressed at a higher level in DC from C57BL/6 mice. Similar results were obtained by Northern blot analysis for TLR2 and TLR4 mRNAs (Fig. 2B). The expression levels of TLR2 and TLR4 mRNAs were higher in BALB/c mice than in C57BL/6 mice. Thus, DC from BALB/c mice and those from C57BL/6 mice express different levels of TLR mRNAs.
FIG. 2.
Detection of TLR mRNA in DC from naive C57BL/6 and BALB/c mice. (A) RT-PCR analysis for TLR mRNA. Total RNAs were extracted from DC of each strain and reverse transcribed. cDNAs were amplified using primers specific for each TLR and separated on a 3% agarose gel containing ethidium bromide. (B) Northern blot analysis for TLR mRNA. Total RNAs from DC were resolved by formaldehyde gel electrophoresis, transferred to a nitrocellulose membrane, and hybridized with a mouse TLR2 or a mouse TLR4 cDNA probe. A picture of the ethidium bromide (EtBr)-stained gel is also shown. Three independent experiments showed similar results, and the results of a representative experiment are shown.
IL-12 and MCP-1 production by DC of C57BL/6 and BALB/c mice in vitro_._
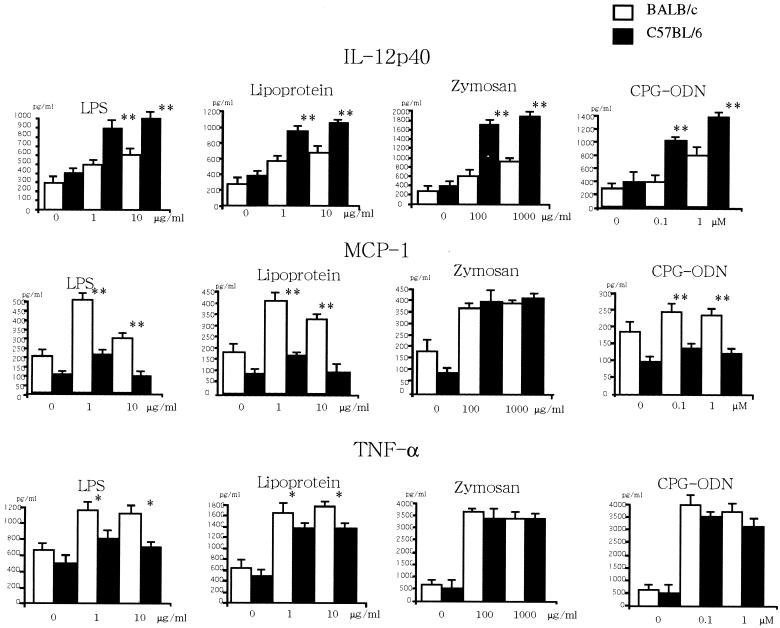
We next studied the in vitro responses of DC from C57BL/6 mice and BALB/c mice to microbial Ags, which trigger TLR2, TLR4, TLR6, or TLR9 signaling. LPS from gram-negative bacteria signals through TLR4 (34, 42), and lipoproteins from gram-positive bacteria signal through TLR2 (1, 5, 15, 39, 43, 45, 46). Bacterial DNAs containing unmethylated CpG motifs signal through TLR9 (14). Zymozan containing β-glucan signals through a heterodimer of TLR2 and TLR6 (12, 33, 44). As shown in Fig. 3, LPS stimulated DC from C57BL/6 mice to produce larger amounts of IL-12p40 but smaller amounts of MCP-1 and TNF-α, whereas LPS stimulated DC from BALB/c mice to produce larger amounts of MCP-1 and TNF-α but smaller amounts of IL-12p40. Similarly, DC from C57BL/6 mice responded to known microbial ligands for TLR2 (lipoproteins) and TLR9 (CpG) by producing larger amounts of IL-12p40, whereas DC from BALB/c mice responded to these ligands by producing larger amounts of MCP-1 and TNF-α. Zymosan stimulated DC from C57BL/6 mice to produce amounts of IL-12p40 larger than those from BALB/c mice, and it stimulated DC from BALB/c mice and C57BL/6 mice to produce an almost equal amount of MCP-1 or TNF-α.
FIG. 3.
. Cytokine production by DC from C57BL/6 and BALB/c mice. DC (106/ml) pooled from five mice of each strain were cultured with various doses of LPS, lipoprotein, zymosan, or CpG-oligodeoxynucleotides (ODN) for 24 h, and the concentration of IL-12p40, MCP-1, or TNF-α in the supernatants was measured by ELISA. Four independent experiments showed similar results, and the data are expressed as the mean plus standard deviation of triplicate cultures of each strain in a representative experiment. * and **, significantly different from the values for BALB/c mice (*, P < 0.05; **, P < 0.01).
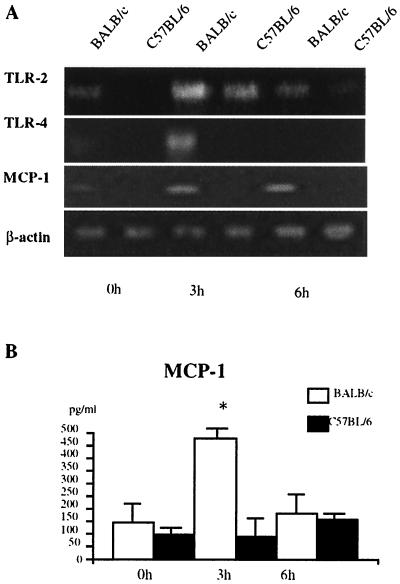
TLR expression and MCP-1 production by DC in vivo at an early stage after L. monocytogenes infection.
It has been reported that CD11blow CD11c+ splenic DC obtained from C57BL/6 mice 3 and 6 h after L. monocytogenes infection expressed higher levels of IL-12p40 mRNA and IL-12p40 protein than did those from BALB/c mice (23). We next examined TLR expression and MCP-1 production by DC in vivo at an early stage after Listeria infection. As shown in Fig. 4, the expression levels of TLR2 and TLR4 mRNAs were higher in DC of BALB/c mice 3 h after Listeria infection. The expression of MCP-1 by DC after Listeria infection was also examined at the transcriptional and protein levels. In contrast to IL-12 production in a previous study (23), levels of MCP-1 mRNA and MCP-1 production were significantly higher in DC of BALB/c mice than in those of C57BL/6 mice 3 h after Listeria infection. Thus, these results suggest that, similar to in vitro responses of naive DC, BALB/c mice showed a predominant DC2-like response in vivo at an early stage after Listeria infection.
FIG. 4.
Detection of TLR2 mRNA, TLR4 mRNA, and MCP-1 production by DC from C57BL/6 mice and BALB/c mice infected with L. monocytogenes. (A) Total RNAs extracted from splenic DC from mice at indicated times after an intravenous inoculation with 6 × 104 L. monocytogenes cells. The cDNA was amplified using primers specific for TLR2 or TLR4 and separated on a 3% agarose gel containing ethidium bromide. Three independent experiments showed similar results, and the results of a representative experiment are shown. (B) Concentration of MCP-1 in 24-h culture supernatant of DC were determined by ELISA. Three independent experiments showed similar results, and the results of a representative experiment are shown. The data are expressed as the mean plus standard deviation from triplicate cultures of each strain in a representative experiment. *, significantly different from the values for BALB/c mice (P < 0.01).
DISCUSSION
It has been shown that the differences in very early production of IL-12 by DC after Listeria infection at least partly underlie the resistance and susceptibility of C57BL/6 and BALB/c mice to L. monocytogenes (23). To elucidate mechanisms for differential cytokine production by DC in BALB/c and C57BL/6 mice, we compared their expression of TLR and their reactivities to microbial products. We demonstrated that the expression levels of mRNAs for TLR2, -4, -5, and -6 were higher in DC isolated from the spleens of naive BALB/c mice than in those from naive C57BL/6 mice, whereas the TLR9 mRNA level was much higher in DC from naive C57BL/6 mice than in those from BALB/c mice. DC of naive C57BL/6 mice responded to the known microbial ligands for TLR2, -4, -6, and -9 by producing higher levels of IL-12p40. On the other hand, DC from naive BALB/c mice responded to the microbial ligands by producing larger amounts of MCP-1. Maturation markers, such as CD40, CD86, and Stat4, were expressed at a higher level by DC from C57BL/6 mice than by those from BALB/c mice. Thus, a difference in the composition of DC subsets as assessed by the expression patterns of TLR genes, costimulatory molecules, and transcriptional factors and the cytokine production profile may underlie the resistance and susceptibility of C57BL/6 and BALB/c mice to infection with L. monocytogenes.
The differentiation of monocytes into DC has a critical impact on immune responses (6, 16). During differentiation, DC up-regulate the expression of MHC class I and class II and costimulatory molecules and thus increase their efficiency as APC (3, 21, 22, 24, 27, 36). Fukao et al. have recently reported that Stat4 is up-regulated in DC during maturation (9). Thus, the expression level of Stat4, in addition to the intensities of costimulatory molecules, is a good indicator for determining the DC maturation stage. We found that DC from C57BL/6 expressed higher levels of CD40, CD86, and Stat4 than those from BALB/c mice, suggesting that DC from C57BL/6 mice are more mature than those from BALB/c mice. TLR signaling is important for DC maturation, characterized by cytokine production, up-regulation of costimulatory molecules, and an increased ability to activate T cells (18, 19). Visintin et al. have recently reported that TLR gene expression is down-regulated in human DC during the course of maturation from monocytes (48). Consistent with the expression of TLR genes in human DC in a maturation-dependent manner, DC from C57BL/6 mice expressed decreased levels of most TLRs except TLR9. Taken together, these results suggest that differences in TLR gene expression patterns in DC may reflect differences in the composition of DC subsets at different maturation stages in C57BL/6 and BALB/c mice.
Kadowaki et al. have reported that subsets of human DC precursors express different TLRs and respond to different microbial antigens (17). Lymphoid-related pre-DC strongly expressed TLR7 and -9 genes, whereas immature CD11c+ DC of monocyte origin preferentially expressed TLR1, -2, and -3 genes. Therefore, it is also possible that differences in TLR gene expression patterns may be due to differences in the composition of subpopulations of monocyte-derived CD8− DC and lymphoid CD8+ DC in C57BL/6 and BALB/c mice. Although we found that DC purified from spleens by the BSA method contained only a few CD8+ CD11c+ DC in both C57BL/6 and BALB/c mice, the possibility that differences in TLR expression by DC in BALB/c and C57BL/6 mice may be due to differences in the composition of DC of different lineages in C57BL/6 mice and BALB/c mice cannot be excluded.
Monocytes and DC are thought to respond to the known microbial ligands for each TLR in accordance with the TLR expression profile. The expression of distinct sets of TLRs and corresponding differences in reactivity to microbial molecules among subsets of pre-DCs and immature DCs have been reported (17). For example, monocytes preferentially express TLR1, -2, -4, -5, and -8 and respond to LPS and peptidoglycan by producing TNF-α and IL-6, whereas immature DC preferentially express TLR1, -2, and -3 mRNAs and respond to the TLR2 ligand, PGN, by producing a large amount of TNF-α. However, we found that DC from BALB/c mice expressing a lower level of TLR9 mRNA responded to the TLR9 ligand, CpG, by producing larger amounts of MCP-1. Furthermore, IL-12 production in response to microbial molecules was much greater in DC of C57BL/6 mice than in those of BALB/c mice, irrespective of the expression levels of the corresponding TLR mRNA. Although the expression levels of TLRs are not known at the protein level in DC, differences in reactivities of DC to microbial molecules in C57BL/6 and BALB/c mice may not be explained by differences in TLR expression levels alone. Immature DC, which express most TLRs, respond to LPS by producing larger amounts of TNF-α, whereas mature DC, which lack TLR expression, did not produce TNF-α but preferentially produced IL-12 in response to LPS (48). Consistent with this finding, we found that the TLR4 ligand, LPS, induced TNF-α and MCP-1 secretion at a higher level in BALB/c DC expressing a higher level of TLR4 mRNA. This supports the idea that the different reactivities of DC in C57BL/6 and BALB/c mice can be ascribed to differences in the numbers of mature DC in the two strains.
Immature DC can be driven in vitro by treatment of peripheral blood monocytes with granulocyte-macrophage colony-stimulating factor and IL-4, and additional signaling from TNF-α receptor, CD40 ligand, or TLRs is required for complete maturation (6, 16). BALB/c mice are known to contain a larger number of IL-4-producing T cells corresponding to NK1.1+ T (NKT) cells, whereas C57BL/6 mice contain predominantly IFN-γ-producing NK and NKT cells (11, 31). IFN-γ and granulocyte-macrophage colony-stimulating factor enhance the production of IL-12 by DC, whereas cytokines such as IL-10, IL-4, and transforming growth factor β show an inhibitory effect on IL-12 production (28, 41). Therefore, it is possible that the environment in BALB/c mice, in which IL-4 is predominant, affects DC differentiation and modulates signaling from TLRs for the induction of IL-12 gene expression. On the other hand, the environment in C57BL/6 mice, in which IFN-γ is predominant, may affect DC maturation, resulting in up-regulation of IL-12 production following stimulation with ligands for TLRs. Recently, it was reported that receptors for prostaglandin E2 (PGE2) were more abundant in APC from BALB/c mice than in those from C57BL/6 mice (20). Signaling from PGE2 is known to directly inhibit IL-12 production by DC (47). Therefore, it is also possible that signaling from PGE2 receptors may modulate signaling from TLRs for the induction of IL-12 gene expression in BALB/c mice.
Cytokines produced by DC play pivotal roles in the differentiation of naive CD4+ T cells into the Th1 and Th2 subtypes (21, 29, 36). DC1 produce IL-12, which stimulates naive CD4+ T cells to develop into CD4+ Th1 cells, whereas IL-4 is known to play an important role in Th2 cell differentiation (30). Recently, MCP-1, a CC chemokine that attract monocytes, has been associated with the development of polarized Th2 responses (10). In the present study, we demonstrated that DC from BALB/c mice, which tend to produce Th2, produced larger amounts of MCP-1 in response to the known ligands for TLRs. In contrast, DC from resistant C57BL/6 mice, which tend to produce Th1, produced smaller amounts of MCP-1 in response to TLR ligands. These results suggest that a balance of IL-12 and MCP-1 produced by DC is crucial in determining the fate of immune responses.
In conclusion, we have demonstrated that the expression levels of mRNAs for TLR2, -4, -5, and -6 were higher in DC isolated from the spleens of naive BALB/c mice than in those from naive C57BL/6 mice, whereas TLR9 mRNA was preferentially expressed by DC from C57BL/6 mice compared with those from BALB/c mice. DC of C57BL/6 mice responded to the known microbial ligands for TLR2, TLR4, TLR6, and TLR9 by producing higher levels of IL-12p40. On the other hand, DC from BALB/c mice responded to the microbial ligands by producing larger amounts of MCP-1. Maturation markers, such as CD40, CD86, and Stat4, were expressed at higher levels by DC from C57BL/6 mice than by those from BALB/c mice. Thus, differences in the reactivities of DC to microbial molecules through TLRs may underlie the resistance and susceptibility of C57BL/6 and BALB/c mice to L. monocytogenes.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science and Culture (JSPS-RFTF97L00703), the Yamada Science Foundation, and the Yakult Bioscience Foundation.
We thank K. Itano and A. Nishikawa for preparing the manuscript.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285**:**736-739. [DOI] [PubMed] [Google Scholar]
- 2.Ardavin, C., L. Wu, C. L. Li, and K. Shortmann. 1993. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 362**:**761-763. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18**:**767-811. [DOI] [PubMed] [Google Scholar]
- 4.Berthier, R., C. Martinon-Ego, A. M. Laharie, and P. N. Marche. 2000. A two-step culture method starting with early growth factors permits enhanced production of functional dendritic cells from murine splenocytes. J. Immunol. Methods 239**:**95-107. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285**:**732-736. [DOI] [PubMed] [Google Scholar]
- 6.Caux, C., C. Dezutter-Dembuyant, D. Schmitt, and J. Banchereau. 1992. GM-CSF and TNF-α cooperate in generation of dendritic Langerhans cells. Nature 360**:**258-261. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162**:**15615-15619. [DOI] [PubMed] [Google Scholar]
- 8.d'Ostiani, C. F., G. Del Seroa, A. Baccia, C. Montagnolia, A. Sprecab, A. Mencaccia, P. Ricciardi-Castagnolic, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans: implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191**:**1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukao, T., D. M. Frucht, G. Yap, M. Gadina, J. J. O'Shea, and S. Koyasu. 2001. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J. Immunol. 166**:**4446-4455. [DOI] [PubMed] [Google Scholar]
- 10.Gu, L., T. Susan, M. R. Horner, G. Tam, M. Loda, and B. J. Rollins. 2000. Control of Th2 polarization by the chemokine monocyte. Nature 404**:**407-411. [DOI] [PubMed] [Google Scholar]
- 11.Guler, M. L., J. D. Gorham, C.-S. Hsieh, A. J. Mackey, R. G. Steen, W. F. Dietrich, and K. M. Murphy. 1996. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science 271**:**984-987. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166**:**15-19. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410**:**1099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408**:**740-745. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163**:**2382-2386. [PubMed] [Google Scholar]
- 16.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of DC from mouse bone marrow cultures supplemented with colony-stimulating factor. J. Exp. Med. 176**:**1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194**:**863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166**:**5688-5694. [DOI] [PubMed] [Google Scholar]
- 19.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends. Immunol. 22**:**78-83. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda, E., T. Sugiura, K. Zeki, Y. Yoshida, and U. Yamashita. 2000. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J. Immunol. 164**:**2386-2395. [DOI] [PubMed] [Google Scholar]
- 21.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and nonpolarized T cells. Nat. Immunol. 1**:**311-316. [DOI] [PubMed] [Google Scholar]
- 22.Lanzavecchia, A., and F. Sallusto. 2001. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 13**:**291-298. [DOI] [PubMed] [Google Scholar]
- 23.Liu, T., H. Nishimura, T. Matsuguchi, and Y. Yoshikai. 2000. Difference in interleukin-12 and -15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57BL/6 mice. Cell. Immunol. 202**:**31-40. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y. J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2**:**585-589. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189**:**587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91**:**295-298. [DOI] [PubMed] [Google Scholar]
- 27.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106**:**255-258. [DOI] [PubMed] [Google Scholar]
- 28.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19**:**683-765. [DOI] [PubMed] [Google Scholar]
- 29.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1**:**199-205. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7**:**145-173. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura, T., K. Santa, T. Yahata, N. Sato, A. Ohta, Y. Ohmi, T. Sato, K. Hozumi, and S. Habu. 1997. Involvement of IL-4-producing Vβ8.2+ CD4+ CD62L− CD45RB− T cells in non-MHC gene-controlled predisposition toward skewing into T helper type-2 immunity in BALB/c mice. J. Immunol. 158**:**5698-5706. [PubMed] [Google Scholar]
- 32.Ohteki, T., T. Fukao, K. Suzue, C. Maki. M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189**:**1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97**:**13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282**:**2085-2088. [DOI] [PubMed] [Google Scholar]
- 35.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C. R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 96**:**1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14**:**495.. [DOI] [PubMed] [Google Scholar]
- 37.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cells and dendritic cell differentiation. Science 283**:**1183-1186. [DOI] [PubMed] [Google Scholar]
- 38.Sato, M., K. Iwakabe, A. Ohta, M. Sekimoto, T. Koda, S. Kimura, and T. Nishimura. 1999. Functional skewing of bone marrow-derived dendritic cells by Th1-or Th2-inducing cytokines. Immunol. Lett. 67**:**63-68. [DOI] [PubMed] [Google Scholar]
- 39.Schroder, N. W., B. Opitz, N. Lamping, K. S. Michelsen, U. Zahringer, U. B. Gobel, and R. R. Schumann. 2000. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids_._ J. Immunol. 165**:**2683-2693. [DOI] [PubMed] [Google Scholar]
- 40.Steinmann, R. M., W. C. van Voorhis, and D. M. Apalding. 1986. Dendritic cells, p. 320. In D. W. Weir and L. A. Herzenberg (ed.), Handbook of experimental immunology, 4th ed. Blackwell Scientific Publications, Oxford, United Kingdom.
- 41.Strobl, H., and W. Knapp. 1999. TGF-β1 regulation of dendritic cells. Microb. Infect. 15**:**1283-1290. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11**:**443-451. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164**:**554-557. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13**:**933-940. [DOI] [PubMed] [Google Scholar]
- 45.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291**:**1544-1547. [DOI] [PubMed] [Google Scholar]
- 46.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401**:**811-815. [DOI] [PubMed] [Google Scholar]
- 47.van der Pouw Kraan, T. C., L. C. Boeije, R. J. Smeenk, J. Wijdenes, and L. A. Aarden. 1995. Prostaglandin E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 181**:**775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166**:**249-255. [DOI] [PubMed] [Google Scholar]