SPARC Inhibits Epithelial Cell Proliferation in Part through Stimulation of the Transforming Growth Factor-β–Signaling System (original) (raw)
Abstract
_S_ecreted _p_rotein, _a_cidic and _r_ich in _c_ysteine (SPARC) is a multifunctional secreted protein that regulates cell–cell and cell–matrix interactions, leading to alterations in cell adhesion, motility, and proliferation. Although SPARC is expressed in epithelial cells, its ability to regulate epithelial cell growth remains largely unknown. We show herein that SPARC strongly inhibited DNA synthesis in transforming growth factor (TGF)-β–sensitive Mv1Lu cells, whereas moderately inhibiting that in TGF-β–insensitive Mv1Lu cells (i.e., R1B cells). Overexpression of dominant-negative Smad3 in Mv1Lu cells, which abrogated growth arrest by TGF-β, also attenuated growth arrest stimulated by SPARC. Moreover, the extracellular calcium-binding domain of SPARC (i.e., SPARC-EC) was sufficient to inhibit Mv1Lu cell proliferation but not that of R1B cells. Similar to TGF-β and thrombospondin-1, treatment of Mv1Lu cells with SPARC or SPARC-EC stimulated Smad2 phosphorylation and Smad2/3 nuclear translocation: the latter response to all agonists was abrogated in R1B cells or by pretreatment of Mv1Lu cells with neutralizing TGF-β antibodies. SPARC also stimulated Smad2 phosphorylation in MB114 endothelial cells but had no effect on bone morphogenetic protein-regulated Smad1 phosphorylation in either Mv1Lu or MB114 cells. Finally, SPARC and SPARC-EC stimulated TGF-β–responsive reporter gene expression through a TGF-β receptor- and Smad2/3-dependent pathway in Mv1Lu cells. Collectively, our findings identify a novel mechanism whereby SPARC inhibits epithelial cell proliferation by selectively commandeering the TGF-β signaling system, doing so through coupling of SPARC-EC to a TGF-β receptor- and Smad2/3-dependent pathway.
INTRODUCTION
_S_ecreted _p_rotein, _a_cidic and _r_ich in _c_ysteine (SPARC) (also known as osteonectin and BM-40) belongs to the matricellular family of secreted proteins, which also includes thrombospondins 1 and 2, osteoponin, and tenascins C and X (Lane and Sage, 1994; Sage, 1997; Yan and Sage, 1999). Members of this nonhomologous protein family mediate cell–cell and cell–matrix interactions without contributing to extracellular matrix structures (Yan and Sage, 1999; Bradshaw and Sage, 2001). Although structurally dissimilar, matricellular proteins are functionally similar in their ability to inhibit cell adhesion by disrupting cell–matrix interactions, particularly during tissue development, remodeling, and repair. In addition to its counteradhesive function, SPARC stimulates angiogenesis, matrix metalloproteinase expression, and extracellular matrix production, and modifies the activity of cytokines and growth factors such as platelet-derived growth factor, vascular endothelial growth factor, and transforming growth factor (TGF)-β (Sage, 1997; Yan and Sage, 1999; Bradshaw and Sage, 2001).
SPARC is also an inhibitor of cell cycle progression, particularly in endothelial, smooth muscle, and mesangial cells (Yan and Sage, 1999; Bradshaw and Sage, 2001; Motamed et al., 2002). Similarly, the proliferation of epithelial, endothelial, and hematopoietic cells is inhibited by the potent tumor suppressor TGF-β (Massague, 1998; Blobe et al., 2000). It is noteworthy that several studies have shown that SPARC and TGF-β engage one another in a reciprocal relationship. For instance, SPARC induces TGF-β expression in mesangial cells (Francki et al., 1999; Bassuk et al., 2000), and, conversely, TGF-β stimulates SPARC expression in a number of cell types, including fibroblasts, keratinocytes, smooth muscle cells, and endothelial cells (Wrana et al., 1991; Ford et al., 1993; Reed et al., 1994; Shiba et al., 1998; Shanker et al., 1999). Although SPARC and TGF-β are both expressed in epithelial cells, the relationship between these signaling molecules in regulating epithelial cell activities remains to be addressed. This question is especially important because many of the activities ascribed to SPARC are also regulated by TGF-β. We therefore hypothesized that many SPARC activities, particularly the inhibition of epithelial cell proliferation, may be mediated in part through its regulation of TGF-β.
We show for the first time that Mv1Lu cells express SPARC and TGF-β in a reciprocal manner and that SPARC inhibits Mv1Lu cell proliferation in large part through stimulation of the TGF-β signaling system. We further show that SPARC couples to stimulation of TGF-β signaling system via its C-terminal extracellular calcium (EC)-domain (i.e., SPARC-EC). Indeed, SPARC and SPARC-EC not only stimulated Smad2 phosphorylation and Smad2/3 nuclear translocation but also TGF-β–responsive reporter gene expression by activating TGF-β receptors and their downstream targets Smads 2 and 3. The stimulation of TGF-β signaling by SPARC is not restricted to epithelial cells and is also shown to occur in endothelial cells; however, in both cell types, SPARC selectively stimulated TGF-β-regulated Smads, not BMP-regulated Smads. Together, our findings have identified a novel mechanism whereby SPARC inhibits epithelial cell proliferation by commandeering the TGF-β signaling system, doing so through coupling of SPARC-EC to a TGF-β receptor/Smad2/3-dependent pathway.
MATERIALS AND METHODS
Materials
Recombinant human TGF-β1 and bone morphogenetic protein (BMP)-7 were obtained from R & D Systems (Minneapolis, MN). The mammalian and retroviral cDNA constructs encoding dominant-negative FLAG-tagged Smad3/3A (pXL-Smad3/3A and pMX-Smad3/3A-IRES-GFP, respectively) were kindly provided by Dr. Xuedong Liu (University of Colorado, Denver, CO). The TGF-β–inducible p3TP-luciferase construct was generously supplied by Dr. Joan Massague (Sloan Kettering, New York, NY). Purified bovine SPARC, human thrombospondin-1, and monoclonal anti-SPARC antibodies were purchased from Hematologic Technologies (Essex Junction, VT). Mouse brain microvascular MB114 endothelial cells were kindly provided by Dr. Michael Hart (University of Wisconsin, Madison, WI). All additional supplies or reagents were routinely available.
SPARC Plasmids
Full-length human and murine SPARC cDNAs were polymerase chain reaction (PCR) amplified from ESTs AI879344 and AI73865, respectively. The PCR products were engineered to contain unique _Hind_III (N terminus) and _Sac_II (C termini) restriction sites for subcloning into the corresponding sites in pcDNA3.1/Myc-His B vector (Invitrogen, Carlsbad, CA) to C-terminally tag SPARC with a Myc- and (His)6-tag.
A retroviral SPARC vector was synthesized by PCR amplifying the full-length Myc-His–tagged murine SPARC cDNA by using oligonucleotides containing _Bgl_II (N terminus) and _Xho_I (C termini) restriction sites. The resulting PCR product was ligated into corresponding sites located immediately upstream of the internal ribosome entry site (IRES) in the bicistronic retroviral vector pMSCV-IRES-GFP (Ghaffari et al., 1999; Schiemann et al., 2002).
Retroviral vectors containing the N-terminal acidic, calcium-binding domain (i.e., SPARC-NT; nucleotides 52–156), the central follistatin (FS)-like domain (i.e., SPARC-FS; nucleotides 157–411), or the C-terminal extracellular calcium-binding (i.e., SPARC-EC; nucleotides 412–912) were constructed by first shuttling the PCR-amplified SPARC-NT, -FS, and -EC cDNA fragments through the pSecTag vector (Invitrogen) at _Pst_I (N terminus) and _Not_I (C terminus) restriction sites. In addition to C-terminally tagging these cDNA fragments with the Myc- and (His)6-tags, the pSecTag vector also appended the Igκ leader sequence to their N termini, thus permitting the secretion of their protein products when introduced into mammalian cells. Afterward, the resulting tagged SPARC-NT, -FS, and -EC cDNA fragments were PCR amplified using oligonucleotides containing _Bgl_II (N terminus) and _Hpa_I (C termini) restriction sites and subsequently ligated into identical sites in pMSCV-IRES-GFP.
All SPARC and SPARC domain cDNA inserts were sequenced in their entirety on a 377A DNA sequencing machine (Applied Biosystems, Foster City, CA).
SPARC Protein Expression Analysis
Quiescent mink lung Mv1Lu epithelial cells cultured on 10-cm plates were metabolically labeled with [35S]methionine for 12 h at 37°C in 5 ml of serum-free media supplemented with or without TGF-β1 (5 ng/ml). Afterward, 500 μl of naïve and TGF-β-conditioned media (CDM) was collected, clarified by centrifugation, and concentrated by trichloroacetic acid/deoxycholate precipitation. Duplicate samples were fractionated through 10% SDS-PAGE before visualization of SPARC by silver staining, and by Western blotting with monoclonal anti-SPARC antibodies (10 ng/ml). Alternatively, Mv1Lu cells were cultured onto six-well plates at a density of 350,000 cells/well and allowed to adhere overnight. The next morning, the cells were washed twice in PBS and incubated for an additional 24 h in 1 ml of serum-free media. Afterward, the cells were stimulated with TGF-β1 (5 ng/ml) for 0–24 h at 37°C, and the resulting CDM was collected and concentrated as above. SPARC expression was subsequently detected by immunoblotting with monoclonal anti-SPARC antibodies.
Northern Blotting
Mv1Lu cells were incubated in the absence or presence of TGF-β1 for 0–24 h and subsequently were harvested in RNAzol reagent (Tel-Test, Friendswood, TX) to isolate total RNA. Ten μg of total RNA was then electrophoresed through 1.2% agarose/formaldehyde gels and transferred to nylon membrane. The immobilized RNA was probed with a 32P-labeled human SPARC cDNA probe (nucleotides 485–888) for 1 h at 68°C in ExpressHyb (BD Biosciences Clontech, Palo Alto, CA). Afterward, the membrane was washed according to the manufacturer's instructions, and SPARC mRNA was visualized by autoradiography.
TGF-β1 Protein Expression Analysis
TGF-β1 production and secretion was determined by enzyme-linked immunosorbent assay (ELISA) analysis as described previously (Huynh et al., 2002). Briefly, Mv1Lu cells or their TGF-β–resistant derivatives R1B (Boyd and Massague, 1989) were cultured onto 96-well plates at a density of 25,000 cells/well. The next morning, the cells were washed twice in phosphate-buffered saline (PBS) and serum starved for 2 h before overnight stimulation with purified bovine SPARC (30 μg/ml; 1 μM). Afterward, 80 μl of naïve or SPARC-CDM was acidified to pH 2 and incubated for 2 h to activate total TGF-β1. After sample neutralization, TGF-β1 was captured with monoclonal anti-TGF-β antibodies (MAB1835; R & D Systems) and detected by sequential incubations with biotinylated anti-TGF-β1 antibodies (BAF240; R & D Systems), followed by avidin-horseradish peroxidase, and finally 3,3′,5,5′tetramethylbenzidine/H2O2 to initiate color development. TGF-β1 concentration differences were determined by spectrophotometry at 450 nm on a Wallac Victor microplate reader (PerkinElmer Biosciences, Boston, MA). Data are the mean (± SEM) basal and SPARC-stimulated TGF-β1 concentrations obtained in two independent experiments.
Retroviral Infections
Control (i.e., pMSCV-IRES-GFP), SPARC, or dominant-negative Smad3/3A retroviral supernatants were produced by EcoPack2 retroviral packaging cells (BD Biosciences Clontech) and used to infect Mv1Lu cells as described in Schiemann et al. (2002). Infected cells were analyzed 48 h postinfection; the highest 5% of green fluorescent protein (GFP)-expressing cells were collected on a MoFlo cell sorter (Cytomation, Fort Collins, CO) and expanded to yield stable polyclonal populations of control, SPARC-, or Smad3/3A-expressing cells having GFP positivity rates of ≥90%. SPARC-infected Mv1Lu cells were also analyzed 48 h postinfection by fluorescence-activated cell sorting (FACS) analysis, and cells expressing low, medium, or high levels of GFP were subsequently collected to establish stable polyclonal populations of Mv1Lu cells expressing low, medium, or high levels of SPARC.
[3H]Thymidine Incorporation Assays
Control or SPARC-expressing Mv1Lu cells, or R1B cells, were cultured onto 96-well plates at a density of 7500 cells/well. Afterward, the cells were stimulated with TGF-β1 (0–5 ng/ml), purified bovine SPARC (30 μg/ml; 1 μM), recombinant SPARC-NT (30 μg/ml; 2.58 μM), recombinant SPARC-FS (30 μg/ml; 1.93 μM), or recombinant SPARC-EC (30 μg/ml; 1.26 μM) for 48 h at 37°C. Cellular DNA was radiolabeled with [3H]thymidine and subsequently quantified by scintillation counting as described previously (Schiemann et al., 2002). Data are the mean (± SEM) of two or more independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated cells.
Purification of Recombinant SPARC Domains
Human embryonic 293T kidney cells were cultured onto 10-cm plates and allowed to adhere overnight. The cells were then transiently transfected by CaPO4 precipitate containing 20 μg/plate of SPARC-NT, -FS, or -EC retroviral construct. Twenty-four hours posttransfection, the cells were incubated in serum-free DMEM for 48 h at 37°C. Afterward, the conditioned media was collected and the pH adjusted to 8.5 before affinity purification of the SPARC-EC protein over Ni2+-agarose. After imidazole elution, the protein containing fractions were concentrated in PBS in a Vivaspin concentrator (MWCO 5000; Vivascience, Stonehouse, United Kingdom). This purification scheme routinely resulted in protein purities of ∼85–90% as determined by Coomassie staining.
Immunofluorescence of Smad3 Nuclear Accumulation
Mv1Lu or R1B cells were allowed to adhere to glass coverslips overnight in 24-well plates (100,000 cells/well). The cells were stimulated the next day with either TGF-β1 (2.5 ng/ml), purified bovine SPARC (30 μg/ml; 1 μM), recombinant SPARC-EC (30 μg/ml; 1.26 μM), or purified human thrombospondin-1 (30 μg/ml; 200 nM) for 0–60 min at 37°C. When present, neutralizing TGF-β antibodies (5 μg/ml) or cycloheximide (10 μg/ml) was incubated with the cells for 60 or 30 min, respectively, before agonist stimulation. Afterward, the cells were washed once with ice-cold PBS supplemented with 0.5 mM MgCl2 and 0.9 mM CaCl2 (PBS++) and immediately fixed in 4% paraformaldehyde for 30 min at room temperature. Fixed cells were permeabilized for 5 min in PBS++ containing 0.2% Triton X-100, followed by incubation with 200 μg/ml goat γ-globulin (Jackson Immunoresearch Laboratories, West Grove, PA) in PBS++ supplemented with 2% fatty acid-free bovine serum albumin (BSA) (PBS++/BSA) to block nonspecific binding sites. Endogenous Smads 2 and 3 were labeled by sequential 45-min incubations in PBS++/BSA supplemented first with rabbit anti-Smad2/3 antibody at 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA), followed by 5 μg/ml biotinylated goat anti-rabbit antibody (Jackson Immunoresearch Laboratories), and finally with Alexa-streptavidin (Molecular Probes, Eugene, OR) at 1.2 μg/ml. After extensive washing with PBS++, the coverslips were mounted on glass slides with Prolong mounting media (Molecular Probes). Images were captured on a Diaphot microscope (Nikon, Tokyo, Japan). Photomicrograph data are also presented graphically as the mean (± SEM) percentage of Smad2/3-stained nuclei.
Inhibition of protein synthesis by cycloheximide was determined by culturing Mv1Lu cells overnight in 24-well plates at a density of 200,000 cells/well. The next morning, the cells were washed twice in DMEM/2.5% fetal bovine serum and immediately treated with or without 10 μg/ml cycloheximide for 30 min at 37°C. Afterward, the cells were labeled with [35S]methionine (400 μCi/ml) for 60 min at 37°C, and subsequently lysed in 200 μl of buffer H/1% Triton X-100 (Schiemann et al., 1999). The ensuing whole cell extracts were clarified by microcentrifugation and subsequently precipitated by trichloroacetic acid/deoxycholate. The resulting protein pellets were resuspended and quantified by scintillation counting.
Smad2 Phosphorylation Analysis
Mv1Lu or MB114 cells were cultured onto 24-well plates at a density of 200,000 cells/well and allowed to adhere overnight. The next morning, the cells were washed twice in PBS and serum starved for 2 h before stimulation with TGF-β1 (5 ng/ml), recombinant BMP-7 (2 μg/ml), purified bovine SPARC (30 μg/ml; 1 μM), recombinant SPARC-EC (30 μg/ml; 1.26 μM), or purified human thrombospondin-1 (30 μg/ml; 200 nM) as indicated. Afterward, the cells were washed twice in ice-cold PBS and lysed in 200 μl of buffer H/1% Triton X-100. After incubation on ice for 30 min, the resulting whole cell extracts were clarified by microcentrifugation and subsequently concentrated by acetone precipitation before fractionation through 10% SDS-PAGE. The fractionated proteins were immobilized electrophoretically to nitrocellulose and subsequently probed with a 1:200 dilution of either anti-phospho-Smad2 or -Smad1 polyclonal antibodies (Cell Signaling Technology, Beverly, MA). The resulting immunocomplexes were visualized by enhanced chemiluminescence. Differences in protein loading were monitored primarily by reprobing stripped membranes with anti-extracellular signal-regulated kinase (ERK)1 antibodies (1:1000 dilution; Santa Cruz Biotechnology) because anti-Smad2 monoclonal (1:500 dilution; Cell Signaling Technology) or -Smad2/3 polyclonal (1:250 dilution; Santa Cruz Biotechnology) antibodies reacted poorly to TGF-β–regulated Smads expressed in Mv1Lu cells. The poor Smad2/3 immunoreactivity of these antibodies during immunoblotting procedures likely resulted from species epitope differences. In contrast, anti-ERK1/2 antibodies reacted strongly to Mv1Lu cell ERK1/2, whose expression levels were unchanged by the aforementioned agonist treatments. Immunocomplexes were visualized as described above.
Luciferase Reporter Gene Assays
Analysis of agonist-stimulated luciferase activity driven by the synthetic p3TP promoter was performed as described previously (Schiemann et al., 2002). Briefly, Mv1Lu or R1B cells were cultured onto 24-well plates at a density of 45,000 cells/well and allowed to adhere overnight. The cells were then transiently transfected by overnight exposure to FuGENE 6 (Roche Diagnostics, Indianapolis, IN) liposomes containing 300 ng/well p3TP-luciferase and 100 ng/well of CMV-β-gal, with or without 100 ng/well of pXL-Smad3/3A (Liu et al., 1997). The next morning, the cells were washed twice with PBS and stimulated in serum-free media with TGF-β1 (5 ng/ml), purified bovine SPARC (30 μg/ml; 1 μM), or recombinant SPARC-EC (30 μg/ml; 1.26 μM) for 24 h at 37°C. Afterward, luciferase and β-gal activities contained in detergent-solubilized cell extracts were determined. Data are the mean (± SEM) luciferase activities of three independent experiments normalized to untreated cells.
RESULTS
TGF-β Induces SPARC Expression in Mv1Lu Cells
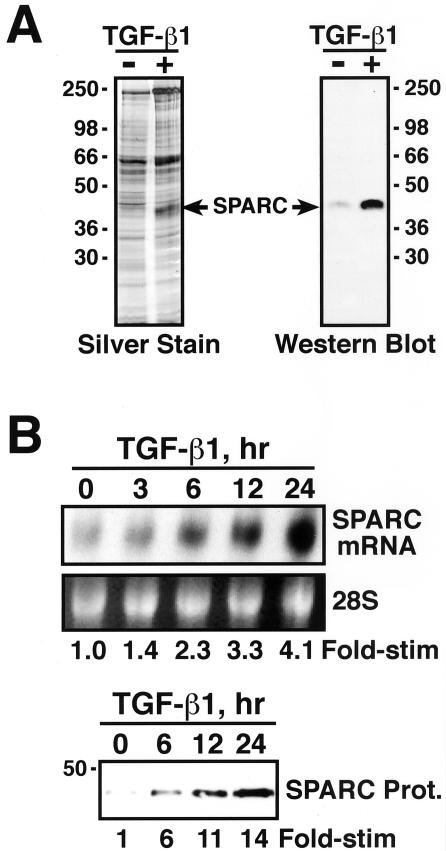
TGF-β induces SPARC expression in fibroblasts, keratinocytes, smooth muscle, and endothelial cells (Wrana et al., 1991; Ford et al., 1993; Reed et al., 1994; Shiba et al., 1998; Shanker et al., 1999); however, its ability to stimulate SPARC expression in epithelial cells remains to be established. Because SPARC is expressed in epithelial cells, and because TGF-β regulates epithelial cell function, we speculated that TGF-β might induce SPARC expression in epithelial cells. To test this hypothesis, naive- and TGF-β–conditioned Mv1Lu cell media were concentrated and fractionated through SDS-PAGE, and SPARC was visualized by silver staining and by Western blotting with monoclonal anti-SPARC antibodies. The results in Figure 1A show that TGF-β treatment of Mv1Lu cells induced significant SPARC protein expression. To confirm that TGF-β stimulated SPARC expression in Mv1Lu cells and to establish the mechanism involved in mediating this effect, we performed Northern blot analysis on total mRNA isolated from TGF-β–treated Mv1Lu cells. As shown in Figure 1B, treatment of Mv1Lu cells with TGF-β stimulated the synthesis of SPARC transcript in a time-dependent manner. The kinetics of TGF-β–stimulated SPARC mRNA expression paralleled those observed for TGF-β to induce SPARC protein expression (Figure 1B). Thus, these findings establish SPARC transcription as a novel target for TGF-β in Mv1Lu cells, ultimately leading to increased production and secretion of SPARC protein.
Figure 1.
TGF-β induces SPARC expression in Mv1Lu cells. (A) Metabolically labeled naive- or TGF-β–conditioned media were collected and concentrated by trichloroacetic acid/deoxycholate precipitation. Duplicate samples were fractionated through 10% SDS-PAGE before visualization of SPARC by silver staining (left) and Western blotting with monoclonal anti-SPARC antibodies (right). (B) Total RNA (10 μg/lane) prepared from TGF-β1–treated Mv1Lu cells was hybridized with a radiolabeled human SPARC cDNA probe. The uniformity of mRNA loading was monitored by ethidium bromide staining to visualize the 28S rRNA (top). Naïve and TGF-β–conditioned media were collected and concentrated by trichloroacetic acid/deoxycholate precipitation before fractionation through 10% SDS-PAGE. SPARC was visualized by Western blotting with monoclonal anti-SPARC antibodies as described above (bottom).
SPARC Induces TGF-β1 Expression in Mv1Lu and R1B Cells
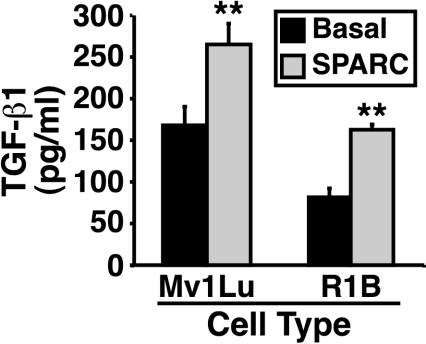
SPARC and TGF-β can engage one another in a reciprocal relationship, with SPARC inducing TGF-β expression and TGF-β inducing that of SPARC (Wrana et al., 1991; Ford et al., 1993; Reed et al., 1994; Shiba et al., 1998; Francki et al., 1999; Shanker et al., 1999; Bassuk et al., 2000). In light of the bidirectional relationship between SPARC and TGF-β and our finding that TGF-β stimulated Mv1Lu cells to express SPARC, we suspected that SPARC treatment of Mv1Lu cells would similarly stimulate their expression of TGF-β. To test this hypothesis, naive- and SPARC-conditioned Mv1Lu cell media were collected and subjected to TGF-β1 ELISA analysis. The graph in Figure 2 shows that SPARC treatment of Mv1Lu cells did indeed stimulate significant TGF-β1 production and secretion. It is important to note that TGF-β can induce its own expression (Kim et al., 1990; Yue and Mulder, 2000), raising the possibility that SPARC may induce Mv1Lu cells to express TGF-β by initiating autocrine TGF-β signaling. We tested this possibility by measuring the ability of SPARC to stimulate TGF-β1 expression in R1B cells, which are chemically induced derivatives of Mv1Lu cells that lack expression of the type I receptor and are thus completely insensitive to TGF-β (Boyd and Massague, 1989). As shown in Figure 2, absolute TGF-β1 production by R1B cells was reduced compared with Mv1Lu cells, indicating that Mv1Lu cells are indeed subjected to autocrine TGF-β signaling. However, the magnitude of SPARC-stimulated production and secretion of TGF-β1 by R1B cells remained unchanged, indicating that SPARC stimulates TGF-β1 expression independent of autocrine TGF-β signaling. Collectively, these findings establish that SPARC and TGF-β engage one another in a reciprocal relationship in Mv1Lu cells, making it plausible that SPARC is essential to some TGF-β functions in these cells and vice versa.
Figure 2.
SPARC induces TGF-β1 expression in Mv1Lu cells. Naïve and SPARC-conditioned Mv1Lu and R1B cell media were collected and acidified to activate total TGF-β. After sample neutralization, TGF-β1 concentrations were determined by ELISA analysis. Data are the mean TGF-β1 concentrations (± SEM) of two independent experiments.
SPARC Inhibits Mv1Lu Cell Proliferation in Part through a TGF-β–dependent Mechanism
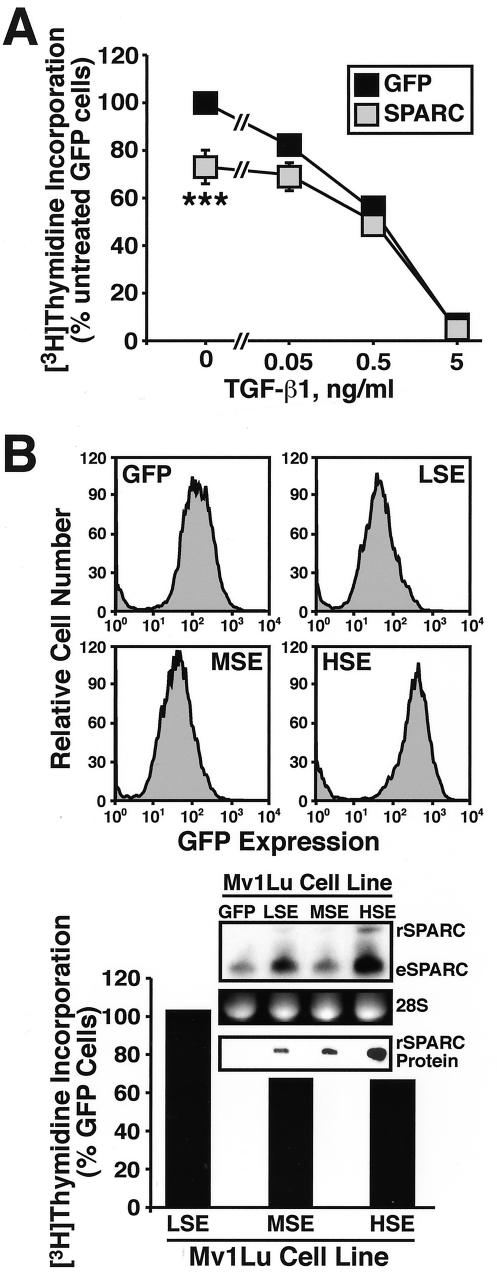
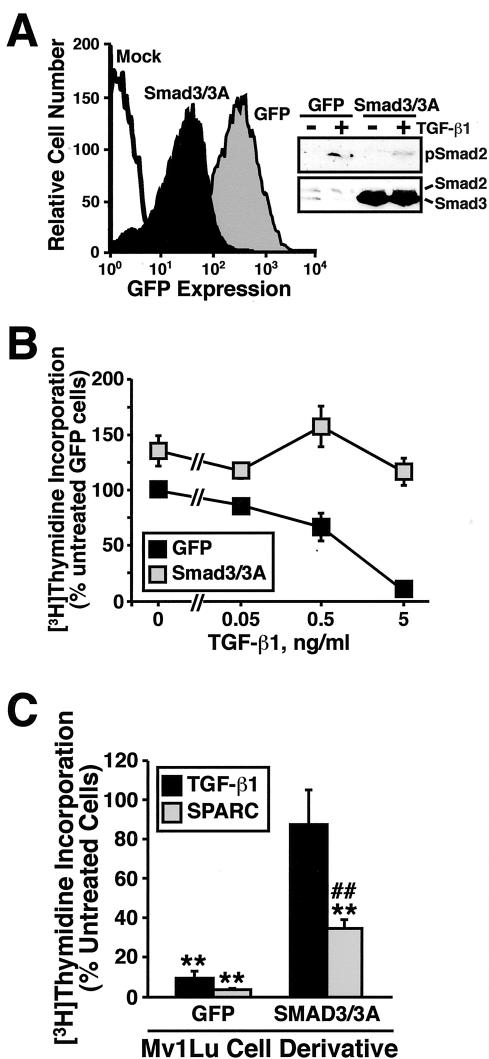
SPARC inhibits the proliferation of several cell types, including fibroblasts, smooth muscle, endothelial, and mesangial cells (Yan and Sage, 1999; Bradshaw and Sage, 2001); however, its role in regulating epithelial cell growth remains unknown. To determine the effect of SPARC on epithelial cell proliferation, stable polyclonal populations of Mv1Lu cells expressing GFP or SPARC at low (LSE; Low SPARC Expresser), medium (MSE; Medium SPARC Expresser), or high (HSE; High SPARC Expresser) levels were generated and compared for their ability to synthesize DNA by a [3H]thymidine incorporation assay. As shown in Figure 3A, TGF-β potently inhibited DNA synthesis in Mv1Lu cells. Although SPARC expression had little effect on the sensitivity of these cells to TGF-β–mediated growth arrest, its expression moderately, but significantly, decreased Mv1Lu cell synthesis of DNA in a dose-dependent manner (Figure 3, A and B). The relatively modest reduction in DNA synthesis in HSE-Mv1Lu cells likely results from our inability to significantly overexpress SPARC by retroviral transduction in Mv1Lu cells. Indeed, recombinant SPARC mRNA is expressed 58 ± 6% (SEM; n = 2) less than steady-state endogenous SPARC mRNA (Figure 3B). However, Mv1Lu cells infected with SPARC exhibited elevated expression of endogenous SPARC transcript (Figure 3B), suggesting that these cells are subjected to autocrine SPARC signaling. Collectively, these findings identify SPARC as an inhibitor of Mv1Lu cell proliferation; they also led us to speculate that greater growth arrest of Mv1Lu cells could be achieved by measures designed to increase their supply of SPARC protein.
Figure 3.
Constitutive SPARC expression inhibits Mv1Lu cell proliferation. (A) GFP- or SPARC-expressing Mv1Lu cells were incubated with increasing concentrations of TGF-β1 (0–5 ng/ml) for 48 h. Cellular DNA was radiolabeled with [3H]thymidine and quantitated by scintillation counting. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated GFP-expressing cells. SPARC expression significantly decreased DNA synthesis in Mv1Lu cells (***p < 0.05; Student's t test). (B) SPARC-infected Mv1Lu cells were FACS-sorted by GFP expression to obtain stable polyclonal populations of low (LSE), medium (MSE), or high (HSE) SPARC-expressing cells (top), which then were compared for rates of DNA synthesis by [3H]thymidine assay. Inset shows expression of recombinant (rSPARC) and endogenous (eSPARC) SPARC mRNA by sorted individual Mv1Lu cell populations as detected by Northern blotting. Also shown is the expression of rSPARC captured from conditioned media of individual Mv1Lu cell populations and visualized by anti-Myc immunoblotting. Shown is a representative experiment that was repeated twice with similar results.
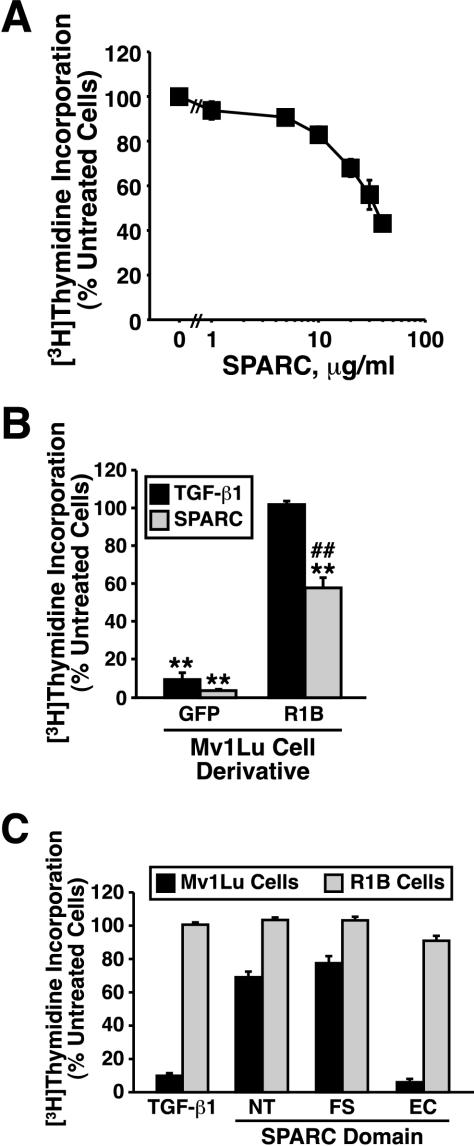
We tested this hypothesis by treating Mv1Lu cells with increasing concentrations of purified bovine SPARC, which dose dependently inhibited their DNA synthesis by ∼60% (EC50 = 15 μg/ml; Figure 4A). Because SPARC induced TGF-β1 production and secretion from Mv1Lu cells (Figure 2), and because TGF-β1 is a potent inhibitor of Mv1Lu cell proliferation, we also measured the ability of SPARC to mediate growth arrest in TGF-β–resistant R1B cells. Interestingly, although SPARC significantly inhibited R1B cell DNA synthesis, its ability to do so in these TGF-β–resistant cells was attenuated significantly compared with Mv1Lu cells (Figure 4B). This finding indicates that SPARC inhibits Mv1Lu cell proliferation in part through activation of the TGF-β signaling system.
Figure 4.
Purified SPARC and recombinant SPARC-EC inhibit Mv1Lu cell proliferation in part through stimulation of the TGF-β–signaling system. (A) Mv1Lu cells were incubated with increasing concentrations of purified bovine SPARC (0–40 μg/ml) as indicated. Changes in DNA synthesis rates were quantified by a [3H]thymidine assay. Data are the mean (± SEM) of two to five independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated cells. Unless shown, all error bars are within individual symbol. (B) GFP-expressing Mv1Lu or R1B cells were treated with TGF-β1 (5 ng/ml) or purified bovine SPARC (30 μg/ml) for 48 h before addition of [3H]thymidine to label cellular DNA. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells. SPARC significantly decreased DNA synthesis in treated cells (**p < 0.05; Student's t test). The decrease in DNA synthesis stimulated by SPARC was significantly attenuated as compared with Mv1Lu cells (##p < 0.05; Student's t test). (C) Mv1Lu or R1B cells were stimulated with TGF-β (5 ng/ml), recombinant SPARC-NT (30 μg/ml), recombinant SPARC-FS (30 μg/ml), or recombinant SPARC-EC (30 μg/ml) as indicated. Cellular DNA was radiolabeled with [3H]thymidine and quantified by scintillation counting. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells.
We next performed structure–function analyses to identify the region in SPARC responsible for its coupling to the TGF-β signaling system. Structurally, SPARC is comprised of three individual domains believed to mediate specific biological activities: 1) an N-terminal acidic, calcium-binding domain that inhibits cell spreading (NT; 1–50 aa); 2) a central FS-like domain that promotes angiogenesis and proliferation (50–130 aa); and 3) a C-terminal EC-binding domain that inhibits proliferation and focal adhesion (131–280 aa) (Sage, 1997; Yan and Sage, 1999; Bradshaw and Sage, 2001). We therefore constructed mammalian expression vectors encoding individual SPARC domains, which then were transiently transfected into human 293T cells. The resulting conditioned media was subjected to Ni2+-agarose chromatography to isolate individual SPARC domains having purities of ∼80–90% (our unpublished data). We then compared the antiproliferative effects of a standard concentration (30 μg/ml) SPARC and recombinant SPARC domains having the following molarities: purified bovine SPARC, 1 μM; recombinant SPARC-NT, 2.58 μM; recombinant SPARC-FS, 1.93 μM; and recombinant SPARC-EC, 1.26 μM. When examined in [3H]thymidine assays, the recombinant NT and FS domains of SPARC were largely ineffective in inhibiting Mv1Lu cell DNA synthesis, whereas addition of its EC domain (i.e., SPARC-EC) strongly inhibited Mv1Lu cell DNA synthesis (Figure 4C). In stark contrast to its effects in Mv1Lu cells, SPARC-EC treatment of R1B cells failed to affect their synthesis of DNA (Figure 4C). Thus, these findings demonstrate that SPARC-EC is the region in SPARC responsible for its coupling to the TGF-β signaling system; they further demonstrate that SPARC can also mediate antiproliferative activity in the absence of TGF-β signaling, pointing toward the existence of a bifurcated SPARC signaling system coupled to regulation of cell proliferation.
The findings described above predict that effector molecules activated by TGF-β should also be activated by SPARC. A corollary states that inactivation of TGF-β-stimulated signaling molecules should negatively impact SPARC signaling. To test this hypothesis, stable polyclonal populations of Mv1Lu cells expressing either GFP or dominant-negative Smad3/3A (Liu et al., 1997) were generated and compared for their ability to synthesize DNA in the absence or presence of either TGF-β or SPARC. As was shown previously (Liu et al., 1997), overexpression of dominant-negative Smad3/3A abrogated TGF-β–stimulated Smad2 phosphorylation and growth arrest in Mv1Lu cells (Figure 5, A and B). In accordance with our hypothesis, Smad3/3A overexpression also significantly attenuated, but not blocked, the growth inhibitory effects of SPARC on Mv1Lu cells (Figure 5C). Indeed, the response of Smad3/3A-expressing Mv1Lu cells to SPARC mirrored that measured in R1B cells (Figure 4B), indicating the necessity of both TGF-β receptors and TGF-β-regulated Smads 2 and 3 for maximal inhibition of Mv1Lu cell proliferation by SPARC. Collectively, these findings show for the first time that SPARC, like TGF-β, is a negative regulator of Mv1Lu cell growth. Importantly, these findings also demonstrate that SPARC inhibits cell cycle progression in part through its stimulation of the TGF-β signaling system.
Figure 5.
Dominant-negative Smad3/3A expression reduces the antiproliferative activities of SPARC on Mv1Lu cells. (A) GFP- or dominant-negative Smad3/3A-expressing Mv1Lu cells were isolated by FACS-sorting for GFP expression. Expression of Smad3/3A insignificantly reduced Smad2 phosphorylation stimulated by TGF-β1 (inset). (B) GFP- or Smad3/3A-expressing Mv1Lu cells were incubated in the absence or presence of increasing concentrations of TGF-β1 as indicated. Cellular DNA was radiolabeled with [3H]thymidine and quantitated by scintillation counting. Data are the mean (± SEM) of two independent experiments presented as the percentage of [3H]thymidine incorporation normalized to untreated GFP-expressing cells. (C) GFP- or Smad3/3A-expressing Mv1Lu cells were stimulated with TGF-β1 (5 ng/ml) or purified bovine SPARC (30 μg/ml) as indicated. Data are the mean (± SEM) of three independent experiments presented as the percentage of [3H]thymidine incorporation normalized to appropriate untreated cells. SPARC significantly decreased DNA synthesis in treated cells (**p < 0.05; Student's t test). The decrease in DNA synthesis stimulated by SPARC was significantly attenuated compared with Mv1Lu cells (##p < 0.05; Student's t test).
SPARC and SPARC-EC Stimulate Smad2/3 Nuclear Translocation in Mv1Lu Cells
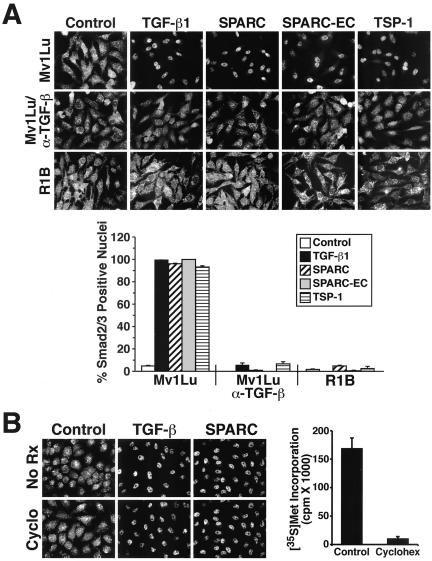
Our findings thus far demonstrate that SPARC and SPARC-EC are able to stimulate long-term (i.e., 24–48 h) TGF-β–dependent biological activities. Mechanistically, these effects may be due to their ability to induce TGF-β1 production and secretion by Mv1Lu cells (Figure 2), thus initiating autocrine TGF-β signaling. However, given that the matricellular family member thrombospondin-1 activates latent TGF-β and consequently TGF-β signaling independent of TGF-β production (Crawford et al., 1998; Ribeiro et al., 1999; Poczatek et al., 2000), it is conceivable that SPARC may also couple to TGF-β signaling independent of TGF-β production. To test this possibility, we performed indirect immunofluorescence on Mv1Lu cells to monitor changes in Smad2/3 subcellular localization in response to 30-min stimulations with TGF-β1, SPARC, SPARC-EC, or thrombospondin-1. As expected, TGF-β1 induced Smad2/3 nuclear translocation in Mv1Lu cells, with nearly 100% of TGF-β–stimulated cells exhibiting Smad2/3-positive nuclei at 30 min (Figure 6A). In contrast, a complete absence of Smad2/3-positive nuclei was observed in R1B cells stimulated by TGF-β (Figure 6A). Thrombospondin-1 also failed to induce Smad2/3 nuclear translocation in R1B cells; however, in Mv1Lu cells, it strongly stimulated Smad2/3 nuclear accumulation. This response to thrombospondin-1 was abrogated by addition of neutralizing TGF-β antibodies (Figure 6A), a finding consistent with its ability to activate latent TGF-β. The slides in Figure 6A further show that SPARC and SPARC-EC also induced Smad2/3 nuclear translocation in essentially 100% of treated Mv1Lu cells, but not in R1B cells. Similar to their effects on thrombospondin-1 signaling, neutralizing TGF-β antibodies also prevented Smad2/3 nuclear accumulation stimulated by TGF-β, as well as that stimulated by SPARC and SPARC-EC (Figure 6A). We also pretreated Mv1Lu cells with cycloheximide (10 μg/ml for 30 min) to test whether protein synthesis was required for Smad2/3 nuclear translocation stimulated by SPARC. This treatment protocol reduced Mv1Lu cell protein synthesis by 95% but had no effect on their ability of to translocate Smad2/3 into the nucleus in response to TGF-β or SPARC (Figure 6B). Thus in addition to stimulating long-term TGF-β–dependent activities, SPARC and SPARC-EC also activate short-term TGF-β signaling and Smad2/3 nuclear translocation through a pathway dependent upon TGF-β and its receptors, but independent of protein synthesis.
Figure 6.
SPARC and SPARC-EC stimulate Smad2/3 nuclear translocation in Mv1Lu Cells. (A) Mv1Lu or R1B cells were incubated in the absence or presence of neutralizing TGF-β antibodies (5 μg/ml) for 60 min before stimulation with TGF-β1 (2.5 ng/ml), purified bovine SPARC (30 μg/ml), recombinant SPARC-EC (30 μg/ml), or thrombospondin-1 (30 μg/ml) for an additional 30 min at 37°C. Afterward, the cells were fixed in 4% paraformaldehyde and processed for indirect immunofluorescence with anti-Smad2/3 antibodies as described under MATERIALS AND METHODS. Images were captured on a Diaphot microscope (40× magnification). Data are from a representative experiment that was repeated two or more times with identical results. Accompanying graph shows the mean (± SEM) percentage of Smad2/3-positive nuclei observed in all photomicrographs. (B) Mv1Lu cells were incubated in the absence or presence of cycloheximide (10 μg/ml) for 30 min before stimulation with TGF-β1 (2.5 ng/ml) or purified bovine SPARC (30 μg/ml) for an additional 30 min at 37°C. Afterward, the cells were fixed and processed for indirect immunofluorescence with anti-Smad2/3 antibodies. Right, cycloheximide-mediated inhibition of Mv1Lu cell protein synthesis as measured by [35S]methionine in vivo labeling as described under MATERIALS AND METHODS.
SPARC and SPARC-EC Stimulate Smad2 Phosphorylation in Mv1Lu and MB114 Cells
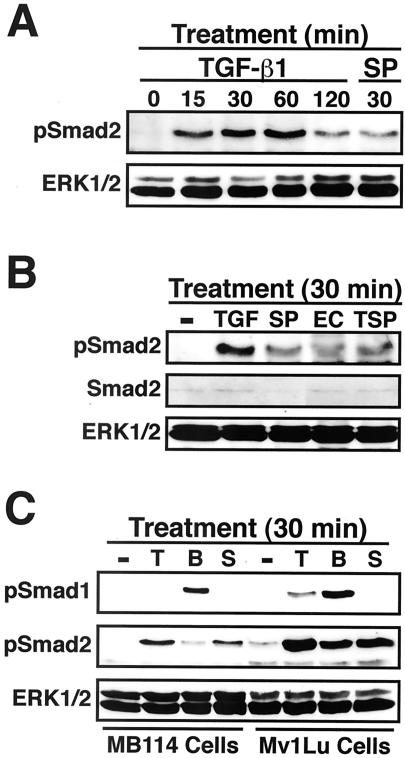
Activated TGF-β type I receptors stimulate Smads 2 and 3 by phosphorylating these transcription factors within their C-terminal SSXS motifs. Once stimulated, Smads 2 and 3 undergo a conformational change that facilitates their association and subsequent nuclear translocation with Smad4 (Massague, 1998; Blobe et al., 2000). The ability of SPARC and SPARC-EC to rapidly stimulate Smad2/3 nuclear translocation (i.e., within 30 min) through a TGF-β– and TGF-β receptor-dependent pathway suggests that these latent transcription factors are activated by phosphorylation within their SSXS motifs. To test this hypothesis, Mv1Lu cells were serum starved for 2 h before stimulation with TGF-β1, SPARC, SPARC-EC, or thrombospondin-1 for various lengths of time. Afterward, the activation status of Smad2 was determined by immunoblot analysis by using phospho-specific Smad2 antibodies. As shown in Figure 7A, TGF-β treatment of Mv1Lu cells stimulated the rapid and sustained phosphorylation of Smad2. In accordance with our hypothesis, SPARC and SPARC-EC also stimulated Smad2 phosphorylation in Mv1Lu cells (Figure 7, A and B).
Figure 7.
SPARC and SPARC-EC stimulate Smad2 phosphorylation in Mv1Lu and MB114 cells. (A–C) Mv1Lu or MB114 cells were stimulated for 0–120 min with 5 ng/ml TGF-β1 (TGF or T), or for 30 min with 30 μg/ml purified bovine SPARC (SP or S), recombinant SPARC-EC (EC), thrombospondin-1 (TSP), or 2 μg/ml BMP-7 (B) as indicated. The activation status of Smad2 and Smad1 was determined by immunoblot analysis by using phospho-specific Smad2 and Smad1 antibodies. Differences in protein loading were monitored by reprobing stripped membranes with either anti-Smad2 or -ERK1 antibodies. Data are from a representative experiment that was repeated twice with similar results.
SPARC regulates a variety of endothelial cell activities, including their adhesion (Sage et al., 1989), motility (Hasselaar and Sage, 1992), and proliferation (Hasselaar and Sage, 1992; Sage et al., 1995), as well as their expression of fibronectin (Lane et al., 1992), thrombospondin-1 (Lane et al., 1992), and plasminogen activator inhibitor-1 (PAI-1) (Hasselaar et al., 1991; Lane et al., 1992). SPARC also interacts physically with endothelial cells via its EC domain (Yost and Sage, 1993). Given these facts, we asked whether SPARC could induce Smad2 phosphorylation in mouse brain microvascular MB114 endothelial cells. We also asked whether Smad phosphorylation stimulated by SPARC was unique to TGF-β–regulated Smads or was instead a more generalized regulatory mechanism of Smad superfamily members. As shown in Figure 7C, treatment of MB114 cells with either SPARC or TGF-β1 stimulated Smad2 phosphorylation, but failed to stimulate that of Smad1. In contrast, BMP-7 was a strong stimulator of Smad1 phosphorylation in MB114 cells, but only a weak stimulator of Smad2 phosphorylation (Figure 7C). Applying this same treatment protocol to Mv1Lu cells showed that BMP-7 and TGF-β1 were both capable of stimulating Smad1 and Smad2 phosphorylation (Figure 7C). However, as in MB114 cells, treatment of Mv1Lu cells with SPARC only stimulated Smad2 phosphorylation, not Smad1 (Figure 7C). Collectively, these findings indicate that SPARC selectively stimulates the phosphorylation of TGF-β–regulated Smads; they also demonstrate that this SPARC activity is not restricted to Mv1Lu cells but may instead represent a more generalized cellular response to SPARC.
SPARC and SPARC-EC Stimulate TGF-β–responsive Reporter Gene Expression through a TGF-β Receptor/Smad3-dependent Pathway
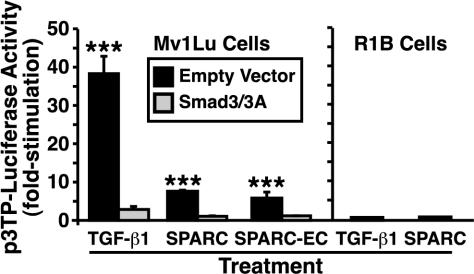
We thus far have shown that SPARC and SPARC-EC inhibit epithelial cell proliferation through their stimulation of the TGF-β signaling system (i.e., this SPARC response requires TGF-β receptors and TGF-β–regulated Smads). Moreover, activation of this pathway by SPARC also resulted in the stimulation of Smad2 phosphorylation and Smad2/3 nuclear translocation. Given these results, we hypothesized that SPARC and SPARC-EC would induce the expression of TGF-β–responsive genes in Mv1Lu cells. To test this hypothesis, we measured changes in luciferase expression driven by the synthetic p3TP-luciferase reporter gene (Wrana et al., 1992), which is induced strongly by TGF-β. As expected, TGF-β treatment of Mv1Lu cells stimulated their expression of luciferase driven by the synthetic p3TP promoter: this stimulation was abrogated by overexpression of dominant-negative Smad3/3A (Figure 8). In accordance with our hypothesis, treatment of Mv1Lu cells with either SPARC or SPARC-EC also significantly stimulated p3TP-driven luciferase activity in a Smad2/3-dependent manner (Figure 8). Moreover, SPARC required TGF-β receptors to stimulate expression of the synthetic p3TP promoter, because like TGF-β, it was unable to induce reporter gene expression in TGF-β-resistant R1B cells (Figure 8). Collectively, these findings demonstrate that SPARC and SPARC-EC induce TGF-β-responsive gene transcription by coupling to the TGF-β receptor and Smad2/3 signaling system.
Figure 8.
SPARC via its EC domain stimulates TGF-β–responsive reporter gene activity through a Smad3- and TGF-β receptor-dependent pathway. Mv1Lu or R1B cells were transiently transfected with p3TP-luciferase and pCMV-β-gal, together with or without dominant-negative Smad3/3A. The transfectants were stimulated with TGF-β1 (5 ng/ml), purified bovine SPARC (30 μg/ml), or recombinant SPARC-EC (30 μg/ml) for 18 h as indicated, and subsequently processed to measure luciferase and β-gal activities. Data are the mean (± SEM) luciferase activities of three independent experiments presented as the fold-stimulations of untreated cells.
DISCUSSION
The matricellular family member, SPARC, is a multifunctional secretory protein that controls a variety of biological activities, including cell adhesion, motility, and angiogenesis, as well as extracellular matrix remodeling (Lane and Sage, 1994; Sage, 1997; Yan and Sage, 1999; Bradshaw and Sage, 2001). SPARC has also been shown to both positively and negatively regulate cell proliferation (Funk and Sage, 1991; Sage, 1992; Funk and Sage, 1993; Sage et al., 1995; Kupprion et al., 1998; Motamed and Sage, 1998; Motamed et al., 2002). Although the activities attributed to SPARC are many, the molecular mechanisms whereby SPARC mediates these effects are largely unknown. The biology of SPARC is further complicated by the fact that SPARC and TGF-β can mediate overlapping activities, as well as induce one another's expression (Wrana et al., 1991; Ford et al., 1993; Reed et al., 1994; Shiba et al., 1998; Francki et al., 1999; Shanker et al., 1999; Bassuk et al., 2000). Indeed, while both molecules are capable of inhibiting cell proliferation, the relationship underlying their ability to do so in epithelial cells has never been studied. We therefore tested the hypothesis that SPARC inhibits epithelial cell proliferation by activating the TGF-β signaling system.
In addressing this question, we found that SPARC is indeed a novel gene target for TGF-β in Mv1Lu epithelial cells (Figure 1). Equally important, we show that SPARC stimulated Mv1Lu cells to produce and secrete TGF-β1 (Figure 2), thereby establishing that SPARC and TGF-β engage one another in a reciprocal relationship in Mv1Lu cells. The finding of a SPARC:TGF-β axis in Mv1Lu cells suggests that SPARC is essential to some TGF-β functions, and conversely, that TGF-β is essential to some SPARC functions. Identifying which SPARC functions depend upon TGF-β and which do not will significantly enhance our understanding of the molecular mechanisms underlying the biology and pathology of SPARC.
In terms of regulating growth control, we show for the first time that SPARC inhibits Mv1Lu cell proliferation in part through its stimulation of the TGF-β signaling system (Figures 3, 4, 5). Although additional TGF-β components are certainly involved, we demonstrated herein the necessity of TGF-β receptors and TGF-β–regulated Smads for maximal growth arrest stimulated by SPARC. In addition, structure–function analyses identified the EC domain of SPARC as the region responsible for its coupling to the TGF-β–signaling system (Figure 4). The duration of SPARC stimulation necessary for inhibiting Mv1Lu cell proliferation is consistent with its ability to induce the production and secretion of TGF-β1 by Mv1Lu cells (Figure 2). Indeed, it is plausible that SPARC inhibits Mv1Lu cell proliferation by stimulating TGF-β1 expression, thus initiating autocrine TGF-β signaling. However, the ability of SPARC to retain partial (and significant) antiproliferative activity in TGF-β–resistant R1B (Figure 4) or Smad3/3A-expressing Mv1Lu cells (Figure 5) indicates the existence in these cells of an alternative SPARC-stimulated signaling pathway that may or may not be subjected to regulation by TGF-β. Future studies comparing the growth inhibitory responses of Mv1Lu and R1B cells to SPARC may facilitate the identification and characterization of this unknown SPARC pathway.
Previous studies have implicated SPARC solely in regulating TGF-β expression (Francki et al., 1999; Bassuk et al., 2000). Although we too find that SPARC stimulated TGF-β1 expression in Mv1Lu cells, we also found that SPARC and SPARC-EC couple to TGF-β signaling independent of protein synthesis and TGF-β1 production (Figure 6). Indeed, short-term (i.e., 30-min) stimulation of Mv1Lu cells with SPARC or SPARC-EC induced Smad2 phosphorylation and Smad2/3 nuclear translocation, ultimately leading to expression of TGF-β-responsive genes (Figure 8). Thus, SPARC governs TGF-β signaling via a two-tiered mechanism involving 1) long-term regulation leading to elevated TGF-β expression, and 2) short-term regulation leading to rapid stimulation of TGF-β signaling.
Our demonstration that SPARC inhibits Mv1Lu cell growth by commandeering the TGF-β signaling system has provided new mechanistic insights into the physiological importance of the relationship between SPARC and TGF-β, and into the nature of the overlapping biological activities mediated by these secretory factors. Besides their ability to regulate angiogenesis, cell migration, and invasion, and extracellular matrix remodeling, SPARC and TGF-β both are potent inhibitors of cell proliferation, arresting cell cycle progression in mid- to-late G1 phase (Massague, 1998; Yan and Sage, 1999; Blobe et al., 2000; Bradshaw and Sage, 2001). Structure–function studies using synthetic SPARC peptides previously identified four individual SPARC sequences that regulate cell proliferation: FS domain peptides 2.1 (55–74 aa) and 2.3 (114–130 aa) that inhibit and stimulate DNA synthesis, respectively; and EC domain peptides 4.0 (248–285 aa) 4.2 (255–274 aa) that both inhibit DNA synthesis (Funk and Sage, 1991, 1993; Sage et al., 1995; Kupprion et al., 1998; Motamed et al., 2002). Collectively, these studies have shown SPARC to be a structurally complex protein, one that contains multiple independent protein sequences possessing redundant and/or antagonist activities.
Using individual SPARC domains instead of synthetic SPARC peptides, we too find that SPARC to be a structurally complex protein. For instance, treating Mv1Lu cells with either recombinant SPARC-NT or SPARC-FS modestly inhibited DNA synthesis, whereas treating them with SPARC-EC strongly inhibited their synthesis of DNA (Figure 4). This region in SPARC (i.e., peptide 4.2) has been shown previously to inhibit cell proliferation (Sage et al., 1995; Kupprion et al., 1998; Motamed and Sage, 1998; Motamed et al., 2002) and to bind to bovine aortic endothelial cells (Yost and Sage, 1993). Although the molecular mechanisms whereby SPARC inhibits cell cycle progression are not well defined, these studies have shown that SPARC hampers cell growth in part by inhibiting the expression of cyclin A and the Rb-like protein p107, and by diminishing the activities of Rb and cyclin-dependent protein kinase-2 (Cdk2) (Motamed and Sage, 1998; Bradshaw et al., 1999; Motamed et al., 2002). It is noteworthy that TGF-β also inhibits these activities (Reddy et al., 1994; Feng et al., 1995; Reynisdottir and Massague, 1997; Schiemann et al., 2002), and more importantly, that SPARC and SPARC-EC prohibit Mv1Lu cell proliferation in part through a TGF-β-dependent pathway (Figures 3, 4, 5). Thus, SPARC may inhibit the activities of cyclin A, Cdk2, and Rb through its ability to commandeer the TGF-β–signaling system. However, the ability of SPARC to partially inhibit DNA synthesis in TGF-β–resistant cells suggests that SPARC can also regulate the activities of cell cycle machinery independent of TGF-β signaling. Furthermore, because SPARC binds to and inhibits the activities of mitogenic growth factors (e.g., platelet-derived growth factor, vascular endothelial growth factor, and likely basic fibroblast growth factor (Yan and Sage, 1999; Bradshaw and Sage, 2001)], it will be interesting to determine the extent to which SPARC-mediated sequestration of growth factors accounts for its TGF-β–independent inhibitory effects on cell cycle progression.
Finally, future studies will need to address the molecular mechanism whereby SPARC rapidly stimulates the TGF-β–signaling system. Through the use of neutralizing TGF-β antibodies and TGF-β–resistant cells, we demonstrated that the activation of TGF-β signaling by SPARC requires TGF-β, its receptors, and its latent transcription factors Smads 2 and 3. We also showed that this response to SPARC is independent of protein synthesis. Based on these findings, we envision two likely scenarios of SPARC action leading to stimulation of TGF-β signaling. First, SPARC may interact directly with TGF-β, or with other TGF-β-interacting molecules to enhance the binding of TGF-β to its receptors. Such a mechanism has recently been described for TGF-β and connective tissue growth factor, whose direct interaction with TGF-β enhances TGF-β receptor binding (Abreu et al., 2002).
Second, SPARC may be an activator of latent TGF-β. With the exception of platelets, TGF-β is synthesized and secreted from cells as an inactive noncovalent complex comprised of TGF-β and its propeptide, latency-associated peptide (LAP). After secretion, latent TGF-β/LAP complexes are bound by and sequestered to inactive extracellular matrix depots by members of the latent TGF-β binding protein (LTBP) family. Latent TGF–β/LAP complexes can then be activated by several independent mechanisms, including 1) proteolytic cleavage mediated by plasmin, or 2) conformational changes in LAP induced by reactive oxygen species, αvβ6 integrin, and the matricellular family member thrombospondin-1 (Gleizes et al., 1997; Khalil, 1999; Blobe et al., 2000). We have recently attempted ELISA analyses to measure the ability of SPARC to induce the conversion of latent TGF-β from its inactive to active species but have thus far been unable to detect an increase in free TGF-β in conditioned media of SPARC-treated Mv1Lu cells (30–60-min SPARC stimulation; our unpublished data). Although negative, these results are similar to the effects of LTBP1 and thrombospondin-1 on latent TGF-β activation (Taipale et al., 1998). Indeed, both proteins are believed to induce a conformational change in latent TGF–β/LAP complexes that enables TGF-β to access and bind its receptor while remaining complexed to LAP (i.e., no release of active TGF-β). Along these lines, we show herein that thrombospondin-1 and SPARC both stimulate Smad2 phosphorylation and Smad2/3 nuclear translocation in Mv1Lu cells and that neutralizing TGF-β antibodies abrogate these responses in Mv1Lu cells. Recent findings in our laboratory have also shown that pretreatment of Mv1Lu cells with SPARC reduces subsequent binding of iodinated TGF-β1 to cell surface TGF-β receptors by 14.2 ± 0.5% (n = 2; p < 0.05). Although preliminary, it is plausible that SPARC either directly or indirectly activates latent TGF-β, which occupies TGF-β receptors and thus prevents their subsequent binding of iodinated TGF-β1. Future studies clearly need to address and clarify this issue, as well as to determine precisely how SPARC couples to TGF-β signaling. In addition, it will be interesting to determine whether the ability of SPARC to couple to TGF-β signaling is operant in primary cells and whether this mechanism is inappropriately altered during disease development.
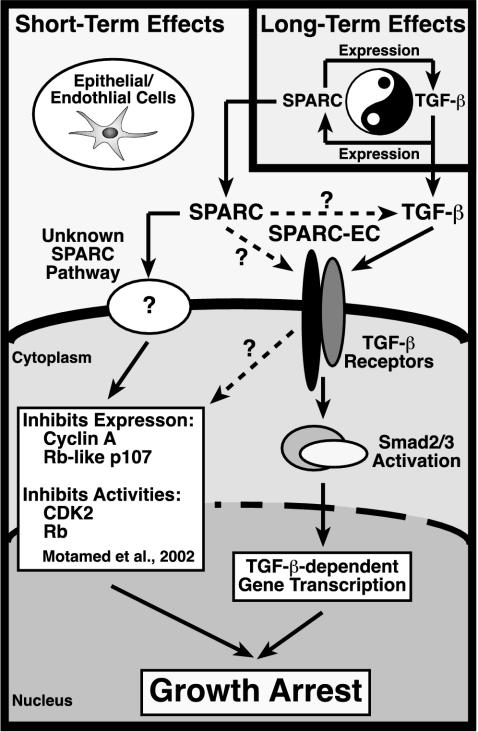
As summarized in Figure 9, we have established SPARC as a novel gene target for TGF-β in Mv1Lu epithelial cells and as a potent inhibitor of Mv1Lu cell proliferation. We further show that SPARC via its EC domain stimulates the phosphorylation and nuclear translocation of TGF-β–regulated Smads, resulting in TGF-β–dependent gene transcription and growth arrest. The effects of SPARC on Smad activation are specific for TGF-β–regulated Smads and occur in epithelial and endothelial cells. In addition, SPARC and TGF-β are shown to engage one another in a reciprocal relationship in Mv1Lu cells, resulting in both long-term and short-term regulation of TGF-β signaling by SPARC. In terms of disease development, we expect elevated SPARC expression to elicit a feed-forward TGF-β signaling loop that exacerbates the processes of tissue remodeling and repair. We therefore are testing the hypothesis that aberrant TGF-β signaling initiated via the inappropriate activation of the SPARC:TGF-β axis may promote the malignant potential of cancer cells that have lost their ability to undergo TGF-β–mediated growth arrest but remain fully competent to undergo TGF-β–stimulated migration and invasion.
Figure 9.
Schematic of SPARC-mediated regulation of and coupling to the TGF-β signaling system. Inset, long-term TGF-β treatment of Mv1Lu cells stimulates their synthesis of SPARC transcript and protein. Conversely, long-term SPARC treatment induces Mv1Lu cells to produce and secrete TGF-β1, presumably leading to enhanced autocrine TGF-β signaling. Dysregulation of this regulatory loop may exacerbate the development and progression of diseases characterized by alterations in tissue remodeling and repair, including cancer. Newly synthesized SPARC is also endowed with the potential to regulate short-term TGF-β signaling. Although it is unknown whether SPARC acts directly on TGF-β (i.e., activation of latent TGF-β complexes; dashed arrow) or its receptors (dashed arrow), these molecules are in fact necessary and required for SPARC to stimulate the TGF-β signaling system. Activation of TGF-β receptors by SPARC stimulates TGF-β–regulated Smads, which translocate into the nucleus to induce or repress TGF-β–responsive genes, ultimately leading to epithelial cell growth arrest. The ability of SPARC to couple to TGF-β signaling is mediated via its EC domain. Alternatively, cells resistant to TGF-β (i.e., loss of TGF-β receptors or Smad inactivation) remain competent to partial growth arrest by SPARC, a response that is not recapitulated by SPARC-EC. Activation of this unknown pathway likely inhibits the expression and activities of cell cycle components (e.g., cyclin A, Rb-like p107, CDK2, and Rb). Although the contribution of TGF-β signaling to regulation of this unknown SPARC pathway remains to be defined (dashed arrow), both systems cooperate to maximally inhibit epithelial cell proliferation by SPARC.
Acknowledgments
TGF-β1 was generously provided by R & D Systems. We thank Dr. Nai-Wen Chi and members of the Schiemann laboratory for critical reading of the manuscript. We also thank William Townend and Shirley Sobus for expertise and help provided on studies performed in the Cytometry Core Facility (National Jewish Medical and Research Center). This research was supported by a start-up fund from the National Jewish Medical and Research Center and in part by grants from the Cancer League of Colorado, Elsa U. Pardee Foundation, and the Howard Hughes Medical Institute to W.P.S.
References
- Abreu, J.G., Ketpura, N.I., Reversade, B., and De Robertis, E.M. (2002). Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat. Cell Biol. 4, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk, J.A., et al. (2000). Induction of TGF-β by the matricellular protein SPARC in a rat model of glomerulonephritis. Kidney Int. 57, 117–128. [DOI] [PubMed] [Google Scholar]
- Blobe, G.C., Schiemann, W.P., and Lodish, H.F. (2000). Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358. [DOI] [PubMed] [Google Scholar]
- Boyd, F.T., and Massague, J. (1989). Transforming growth factor-β inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J. Biol. Chem. 264, 2272–2278. [PubMed] [Google Scholar]
- Bradshaw, A.D., Francki, A., Motamed, K., Howe, C., and Sage, E.H. (1999). Primary mesenchymal cells isolated from SPARC-null mice exhibit altered morphology and rates of proliferation. Mol. Biol. Cell 10, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, A.D., and Sage, E.H. (2001). SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 107, 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, S.E., Stellmach, V., Murphy-Ullrich, J.E., Ribeiro, S.M., Lawler, J., Hynes, R.O., Boivin, G.P., and Bouck, N. (1998). Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Feng, X.H., Filvaroff, E.H., and Derynck, R. (1995). Transforming growth factor-β (TGF-β)-induced down-regulation of cyclin A expression requires a functional TGF-β receptor complex. Characterization of chimeric and truncated type I and type II receptors. J. Biol. Chem. 270, 24237–24245. [DOI] [PubMed] [Google Scholar]
- Ford, R., Wang, G., Jannati, P., Adler, D., Racanelli, P., Higgins, P.J., and Staiano-Coico, L. (1993). Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes: regulation via a TGF-β-dependent pathway. Exp. Cell Res. 206, 261–275. [DOI] [PubMed] [Google Scholar]
- Francki, A., Bradshaw, A.D., Bassuk, J.A., Howe, C.C., Couser, W.G., and Sage, E.H. (1999). SPARC regulates the expression of collagen type I and transforming growth factor-β1 in mesangial cells. J. Biol. Chem. 274, 32145–32152. [DOI] [PubMed] [Google Scholar]
- Funk, S.E., and Sage, E.H. (1991). The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 88, 2648–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, S.E., and Sage, E.H. (1993). Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J. Cell. Physiol. 154, 53–63. [DOI] [PubMed] [Google Scholar]
- Ghaffari, S., Wu, H., Gerlach, M., Han, Y., Lodish, H.F., and Daley, G.Q. (1999). BCR-ABL and v-SRC tyrosine kinase oncoproteins support normal erythroid development in erythropoietin receptor-deficient progenitor cells. Proc. Natl. Acad. Sci. USA 96, 13186–13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleizes, P.E., Munger, J.S., Nunes, I., Harpel, J.G., Mazzieri, R., Noguera, I., and Rifkin, D.B. (1997). TGF-β latency: biological significance and mechanisms of activation. Stem Cells 15, 190–197. [DOI] [PubMed] [Google Scholar]
- Hasselaar, P., Loskutoff, D.J., Sawdey, M., and Sage, E.H. (1991). SPARC induces the expression of type 1 plasminogen activator inhibitor in cultured bovine aortic endothelial cells. J. Biol. Chem. 266, 13178–13184. [PubMed] [Google Scholar]
- Hasselaar, P., and Sage, E.H. (1992). SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J. Cell. Biochem. 49, 272–283. [DOI] [PubMed] [Google Scholar]
- Huynh, M.L., Fadok, V.A., and Henson, P.M. (2002). Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, N. (1999). TGF-β: from latent to active. Microbes Infect. 1, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Kim, S.J., Angel, P., Lafyatis, R., Hattori, K., Kim, K.Y., Sporn, M.B., Karin, M., and Roberts, A.B. (1990). Autoinduction of transforming growth factor β1 is mediated by the AP-1 complex. Mol. Cell. Biol. 10, 1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupprion, C., Motamed, K., and Sage, E.H. (1998). SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J. Biol. Chem. 273, 29635–29640. [DOI] [PubMed] [Google Scholar]
- Lane, T.F., Iruela-Arispe, M.L., and Sage, E.H. (1992). Regulation of gene expression by SPARC during angiogenesis in vitro. Changes in fibronectin, thrombospondin-1, and plasminogen activator inhibitor-1. J. Biol. Chem. 267, 16736–16745. [PubMed] [Google Scholar]
- Lane, T.F., and Sage, E.H. (1994). The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 8, 163–173. [PubMed] [Google Scholar]
- Liu, X., Sun, Y., Constantinescu, S.N., Karam, E., Weinberg, R.A., and Lodish, H.F. (1997). Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA 94, 10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague, J. (1998). TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Motamed, K., Funk, S.E., Koyama, H., Ross, R., Raines, E.W., and Sage, E.H. (2002). Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J. Cell. Biochem. 84, 759–771. [DOI] [PubMed] [Google Scholar]
- Motamed, K., and Sage, E.H. (1998). SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J. Cell. Biochem. 70, 543–552. [DOI] [PubMed] [Google Scholar]
- Poczatek, M.H., Hugo, C., Darley-Usmar, V., and Murphy-Ullrich, J.E. (2000). Glucose stimulation of transforming growth factor-β bioactivity in mesangial cells is mediated by thrombospondin-1. Am. J. Pathol. 157, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, K.B., Hocevar, B.A., and Howe, P.H. (1994). Inhibition of G1 phase cyclin dependent kinases by transforming growth factor β1. J. Cell. Biochem. 56, 418–425. [DOI] [PubMed] [Google Scholar]
- Reed, M.J., Vernon, R.B., Abrass, I.B., and Sage, E.H. (1994). TGF-β1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J. Cell. Physiol. 158, 169–179. [DOI] [PubMed] [Google Scholar]
- Reynisdottir, I., and Massague, J. (1997). The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11, 492–503. [DOI] [PubMed] [Google Scholar]
- Ribeiro, S.M., Poczatek, M., Schultz-Cherry, S., Villain, M., and Murphy-Ullrich, J.E. (1999). The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J. Biol. Chem. 274, 13586–13593. [DOI] [PubMed] [Google Scholar]
- Sage, E.H. (1992). Secretion of SPARC by endothelial cells transformed by polyoma middle T oncogene inhibits the growth of normal endothelial cells in vitro. Biochem. Cell Biol. 70, 579–592. [DOI] [PubMed] [Google Scholar]
- Sage, E.H. (1997). Terms of attachment: SPARC and tumorigenesis. Nat. Med. 3, 144–146. [DOI] [PubMed] [Google Scholar]
- Sage, E.H., Bassuk, J.A., Yost, J.C., Folkman, M.J., and Lane, T.F. (1995). Inhibition of endothelial cell proliferation by SPARC is mediated through a Ca(2+)-binding EF-hand sequence. J. Cell. Biochem. 57, 127–140. [DOI] [PubMed] [Google Scholar]
- Sage, H., Vernon, R.B., Funk, S.E., Everitt, E.A., and Angello, J. (1989). SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 109, 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann, W.P., Blobe, G.C., Kalume, D.E., Pandey, A., and Lodish, H.F. (2002). Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion: fibulin-5 is induced by TGF-β and affects protein kinase cascades. J. Biol. Chem. 277, 27367–27377. [DOI] [PubMed] [Google Scholar]
- Schiemann, W.P., Pfeifer, W.M., Levi, E., Kadin, M.E., and Lodish, H.F. (1999). A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood 94, 2854–2861. [PubMed] [Google Scholar]
- Shanker, G., Olson, D., Bone, R., and Sawhney, R. (1999). Regulation of extracellular matrix proteins by transforming growth factor β1 in cultured pulmonary endothelial cells. Cell Biol. Int. 23, 61–72. [DOI] [PubMed] [Google Scholar]
- Shiba, H., et al. (1998). Differential effects of various growth factors and cytokines on the syntheses of D.N.A., type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J. Cell. Physiol. 174, 194–205. [DOI] [PubMed] [Google Scholar]
- Taipale, J., Saharinen, J., and Keski-Oja, J. (1998). Extracellular matrix-associated transforming growth factor-β: role in cancer cell growth and invasion. Adv. Cancer Res. 75, 87–134. [DOI] [PubMed] [Google Scholar]
- Wrana, J.L., Attisano, L., Carcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X.F., and Massague, J. (1992). TGF β signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Wrana, J.L., Overall, C.M., and Sodek, J. (1991). Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor β. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur. J. Biochem. 197, 519–528. [DOI] [PubMed] [Google Scholar]
- Yan, Q., and Sage, E.H. (1999). SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 47, 1495–1506. [DOI] [PubMed] [Google Scholar]
- Yost, J.C., and Sage, E.H. (1993). Specific interaction of SPARC with endothelial cells is mediated through a carboxyl-terminal sequence containing a calcium-binding EF hand. J. Biol. Chem. 268, 25790–25796. [PubMed] [Google Scholar]
- Yue, J., and Mulder, K.M. (2000). Requirement of Ras/MAPK pathway activation by transforming growth factor β for transforming growth factor β1 production in a Smad-dependent pathway. J. Biol. Chem. 275, 30765–30773. [DOI] [PubMed] [Google Scholar]