Expression of CCL28 by Reed-Sternberg Cells Defines a Major Subtype of Classical Hodgkin’s Disease with Frequent Infiltration of Eosinophils and/or Plasma Cells (original) (raw)
Abstract
Classical Hodgkin’s disease (HD) is characterized by rare neoplastic Hodgkin and Reed-Sternberg (H-RS) cells within abundant reactive cellular backgrounds. In most cases, H-RS cells originate from the B-cell lineage, but their immunophenotypes are unusual. Here we newly found frequent expression of chemokine receptors CXCR6 and CCR10 and their respective ligands CXCL16 and CCL28 in HD-derived cell lines. CCR10 is known to be selectively expressed by plasma cells, whereas CCL28 attracts eosinophils via CCR3 and plasma cells via CCR10 and CCR3. Therefore, we examined their expression in HD tissues by immunohistochemistry. We found that H-RS cells in 15 of 19 cases were positive for CCL28. Among them, seven cases were also positive for CCR10, suggesting a potential autocrine effect. In situ hybridization confirmed the expression of CCL28 mRNA in H-RS cells. The CCL28 positivity in H-RS cells did not significantly correlate with that of LMP-1, CCL17, CCL22, or CCL11. However, it significantly correlated with the background accumulation of eosinophils, plasma cells, and CCR10+ cells. Thus, the production of CCL28 by H-RS cells may play a major role in tissue accumulation of eosinophils and/or plasma cells in classical HD. The frequent expression of CCR10 in H-RS cells themselves also supports their close relationship to plasma cells.
Hodgkin’s disease (HD) is a unique lymphoid malignancy characterized by rare neoplastic cells surrounded by abundant reactive cellular infiltrates consisting of cells such as T cells, eosinophils, and plasma cells.1 Based on the characteristics of neoplastic cells and of the reactive cellular background, HD is classified into two major categories termed nodular lymphocyte predominance HD (NLPHD) and classical HD. The latter is further classified into four subtypes: mixed cellularity (MC), nodular sclerosis (NS), lymphocyte-rich, and lymphocyte depletion.1 Although the features of neoplastic cells of NLPHD, known as lymphocytic and histiocytic cells, are relatively homogeneous, those of classical HD, mononucleated Hodgkin’s cells and multinucleated Reed-Sternberg cells (H-RS cells), display a high degree of polymorphism.1 Furthermore, H-RS cells in ∼50% of classical HD cases are infected with Epstein-Barr virus.1 Studies involving single-cell manipulation have revealed that tumor cells of NLPHD and most cases of classical HD represent a monoclonal outgrowth of the B-cell lineage.2–4 Only in rare cases, classical HD can be of the T-cell origin.5,6 Furthermore, tumor cells of NLPHD correspond to antigen-selected germinal center B cells with ongoing somatic mutations.7 This is consistent to other germinal center cell phenotypes present in lymphocytic and histiocytic cells such as their predominant localization within lymphoid follicles, their cytological similarity to centroblasts, and their expression of BCL-6.8–10 On the other hand, H-RS cells of classical HD have crippling mutations of immunoglobulin genes; their rearranged immunoglobulin genes frequently contain stop codons, deletions, and/or frame shifts that disrupt the coding capacity of the immunoglobulin genes.2,3,10 Thus, they are considered to originate from preapoptotic germinal center B cells somehow rescued from apoptotic elimination. However, the precise differentiation stage of H-RS cells remains elusive because of their unusual immunophenotypes.1,10 In this context, Schwering and colleagues11 have recently demonstrated that H-RS cells of classical HD have a fundamental defect in maintaining the B-cell lineage gene expression program, which may have accounted for their escape from apoptosis normally triggered on signaling via the B-cell receptor.
Chemokines are a large group of structurally related cytokines that induce directed migration of specific types of leukocyte through interactions with a group of seven transmembrane G protein-coupled receptors.12 In humans, more than 40 chemokines and 18 functional chemokine receptors have been identified. Based on the arrangement of the conserved cysteine residues in the N–terminal region, chemokines are classified into four subfamilies: CC, CXC, C, and CX3C. Recently, based on the classification of these four subfamilies, the systematic nomenclature system of the chemokine ligands has been formulated.12 A number of studies have documented that classical HD is a neoplasia associated with abnormal production of cytokines and chemokines.4,13,14 This probably accounts for some of the unique features of classical HD such as highly reactive cellular backgrounds and certain systemic symptoms.1 For example, H-RS cells in a large proportion of classical HD have been shown to produce TARC/CCL17 and MDC/CCL22.15–19 These chemokines are known to attract T cells, especially Th2-type memory T cells, via CCR4.12 Consistently, elevated accumulation of CCR4+ T cells as well as Th2 cells has been documented in HD tissues expressing these chemokines.14,15,19,20 H-RS cells, especially of Epstein-Barr virus-associated cases, were also shown to frequently produce MIG/CXCL9 and IP-10/CXCL10.18,19,21 These chemokines are known to attract activated T cells and Th1-type memory cells via CXCR3.12 Selective attraction of CXCR3-expressing T cells by H-RS cells expressing these chemokines has also been demonstrated in HD tissues.19 Furthermore, eotaxin/CCL11 produced by H-RS cells and/or surrounding fibroblasts was reported to be responsible for the frequent accumulation of eosinophils, especially in the NS subtype of classical HD.21,22 So far, however, the mechanism of plasma cell accumulation in HD tissues has not been clarified.
Here we demonstrate that H-RS cells of classical HD frequently express CCL28 and its receptor CCR10.23,24 CCL28 is known to attract eosinophils via CCR3 and plasma cells via CCR10 and CCR3.24–27 We have indeed demonstrated that production of CCL28 by H-RS cells significantly correlates with tissue accumulation of eosinophils and/or plasma cells in classical HD. Furthermore, CCR10 is selectively expressed at the stages of plasmablasts and plasma cells in the B-cell lineage.25–29 Thus, the frequent expression of CCR10 by H-RS cells of classical HD supports their differentiation stage close to that of plasma cells.9
Materials and Methods
Cells and Tissues
L428, L540, HDLM-2, KM-H2, RPMI 6666, and HS445 were all HD-derived cell lines. L428, L540, HDLM-2, and KM-H2 were obtained from Deutsche Sammlung von Migroorganismen und Zellkulturen, Braunschweig, Germany. RPMI 6666 and HS445 were obtained from American Type Culture Collection, Rockville, MD. Paraffin-embedded lymph node specimens from 19 HD cases that were made at diagnostic biopsy and before any treatment were obtained from the tissue specimen files maintained in the Department of Pathology, Kinki University School of Medicine. They were classified according to the World Health Organization classification: 11 cases of MC, 4 cases of NS, 3 cases of lymphocyte-rich, and one case of lymphocyte depletion.1 This work was approved by the ethical committee of Kinki University School of Medicine.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
This was performed as described previously.25 In brief, total RNA was prepared from cells and tissues by using Trizol reagent (Life Technologies, Inc., Gaithersburg, MD). RNA was further purified by using RNeasy (Qiagen, Hilden, Germany). Total RNA (1 μg) was reverse-transcribed using oligo (dT)18 primer and SuperScript II reverse transcriptase (Life Technologies, Inc.). First-strand DNA (20 ng of total RNA equivalent) and original total RNA (20 ng) were amplified in a final volume of 15 μl containing 10 pmol of each primer and 1 U of Ex-Taq polymerase (Takara Shuzo, Kyoto, Japan). Amplification conditions were denaturation at 94°C for 30 seconds (5 minutes at the first cycle), annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds (7 minutes at the last cycle) for 36 cycles for all of the chemokine receptors, 34 cycles for all of the chemokines, and 27 cycles for glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Amplification products (10 μl each) were electrophoretically run on a 1.5% agarose gel and stained with ethidium bromide. The PCR primers used are summarized in Table 1.
Table 1.
Summary of the Primers Used for RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Chemokine receptor | ||
| CXCR1 | 5′-GGCTGCTGGGGACTGTCTATGAAT-3′ | 5′-GCCCGGCCGATGTTGTTG-3′ |
| CXCR2 | 5′-CCGCCCCATGTGAACCAGAA-3′ | 5′-AGGGCCAGGAGCAAGGACAGAC-3′ |
| CXCR3 | 5′-CAACGCCACCCACTGCCAATACAA-3′ | 5′-CAGGCGCAAGAGCAGCATCCACA-3′ |
| CXCR4 | 5′-ATCTTCCTGCCCACCATCTACTCCATCATC-3′ | 5′-ATCCAGACGCCAACATAGACCACCTTTTCA-3′ |
| CXCR5 | 5′-AACTACCCGCTAACGCTGGAAATGGAC-3′ | 5′-CACGGCAAAGGGCAAGATGAAGACC-3′ |
| CXCR6 | 5′-ATGGCAATGTCTTTAATCTCGACAA-3′ | 5′-TGAAAGCTGGTCATGGCATAGTATT-3′ |
| CCR1 | 5′-CAACTCCGTGCCAGAAGGTGAA-3′ | 5′-GCCAGGGCCCAAATGATGAT-3′ |
| CCR2 | 5′-CCAACGAGAGCGGTGAAGAAGTC-3′ | 5′-TCCGCCAAAATAACCGATGTGAT-3′ |
| CCR3 | 5′-GAGCCCGGACTGTCACTTTTG-3′ | 5′-CAGATGCTTGCTCCGCTCACAG-3′ |
| CCR4 | 5′-AAGAAGAACAAGGCGGTGAAGATG-3′ | 5′-AGGCCCCTGCAGGTTTTGAAG-3′ |
| CCR5 | 5′-CTGGCCATCTCTGACCTGTTTTTC-3′ | 5′-CAGCCCTGTGCCTCTTCTTCTCAT-3′ |
| CCR6 | 5′-CCTGGGGAATATTCTGGTGGTGA-3′ | 5′-CATCGCTGCCTTGGGTGTTGTAT-3′ |
| CCR7 | 5′-GTGCCCGCGTCCTTCTCATCAG-3′ | 5′-GGCCAGGACCACCCCATTGTAG-3′ |
| CCR8 | 5′-GGCCCTGTCTGACCTGCTTTTT-3′ | 5′-ATGGCCTTGGTCTTGTTGTGGTT-3′ |
| CCR9 | 5′-CACTGTCCTGACCGTCTTTGTCT-3′ | 5′-CTTCAAGCTTCCCTCTCTCCTTG-3′ |
| CCR10 | 5′-TGCTGGATACTGCCGATCTACTG-3′ | 5′-TCTAGATTCGCAGCCCTAGTTGTC-3′ |
| XCR1 | 5′-TGACCATCCACCGCTACC-3′ | 5′-ATCTGGGTCCGAAACAGC-3′ |
| CX3CR1 | 5′-TGGCCTTGTCTGATCTGCTGTTTG-3′ | 5′-ATGGCTTTGGCTTTCTTGTGGTTC-3′ |
| Chemokine | ||
| CXCL8/IL-8 | 5′-GTGGCTCTCTTGGCAGCCTTCCTGAT-3′ | 5′-TCTCCACAACCCTCTGCACCCAGTTT-3′ |
| CXCL9/Mig | 5′-CATGCTGGTGAGCCAAGCAGTTTGAA-3′ | 5′-CACTTCTGTGGGGTGTTGGGGACAAG-3′ |
| CXCL10/IP-10 | 5′-TGCAAGCCAATTTTGTCCACGTGTTG-3′ | 5′-GCAGCTGATTTGGTGACCATCATTGG-3′ |
| CXCL12/SDF-1 | 5′-CCCTCTGTGAGATCCGTCTTTGGCCT-3′ | 5′-TCTGATTGGAACCTGAACCCCTGCTG-3′ |
| CXCL13/BLC | 5′-TGATCGAATTCAAATCTTGCCCCGTG-3′ | 5′-AAGCTTGAGTTTGCCCCATCAGCTCC-3′ |
| CXCL16 | 5′-ACTACACGAGGTTCCAGCTCC-3′ | 5′-CTTTGTCCGAGGACAGTGATC-3′ |
| CCL1/I-309 | 5′-AAGGCCCAAGCCAGACCAGAAGACAT-3′ | 5′-ATGTGGTTTCCAGAGCCCACAATGGA-3′ |
| CCL2/MCP-1 | 5′-CAAACTGAAGCTCGCACTCTCGCCTC-3′ | 5′-AGCACAGATCTCCTTGGCCACAATGG-3′ |
| CCL3/MIP-1α | 5′-GCCCGGTGTCATCTTCCTAACCAAGC-3′ | 5′-AGGGGACAGGGGAACTCTCAGAGCAA-3′ |
| CCL5/RANTES | 5′-CCCCGTGCCCACATCAAGGAGTATTT-3′ | 5′-CGTCCAGCCTGGGGAAGGTTTTTGTA-3′ |
| CCL11/Eotaxin | 5′-ACCACCTCTCACGCCAAAGCTCACAC-3′ | 5′-CGGCACAGATATCCTTGGCCAGTTTG-3′ |
| CCL17/TARC | 5′-ACTGCTCCAGGGATGCCATCGTTTTT-3′ | 5′-ACAAGGGGATGGGATCTCCCTCACTG-3′ |
| CCL19/ELC | 5′-CCAGCCTCACATCACTCACACCTTTGC-3′ | 5′-TGTGGTGAACACTACAGCAGGCACCC-3′ |
| CCL20/LARC | 5′-TACTCCACCTCTGCGGCGAATCAGAA-3′ | 5′-GTGAAACCTCCAACCCCAGCAAGGTT-3′ |
| CCL21/SLC | 5′-AACCAAGCTTAGGCTGCTCCATCCCA-3′ | 5′-TATGGCCCTTTAGGGGTCTGTGACCG-3′ |
| CCL22/MDC | 5′-AGGACAGAGCATGGCTCGCCTACAGA-3′ | 5′-TAATGGCAGGGAGGTAGGGCTCCTGA-3′ |
| CCL25/TECK | 5′-CCAAGGTGTCTTTGAGGACTGCTGCC-3′ | 5′-GGGAGACATTCCTCTTGCTGCTGCTG-3′ |
| CCL27/ILC | 5′-CTACAGCAGCATTCCTACTGC-3′ | 5′-ATGGAGCTTTCTCTCTTGGTG-3′ |
| CCL28/MEC | 5′-AGAAGCCATACTTCCCATTGC-3′ | 5′-AGCTTGCACTTTCATCCACTG-3′ |
| XCL1/Ltn | 5′-ACACCATCACGGAAGGCTCCTTGAGA-3′ | 5′-AGTGAAATGAGCTGGCTGGCTGGAGA-3′ |
| CX3CL1/Fkn | 5′-GCCATGTTCACCTACCAGAGCCTCCA-3′ | 5′-TGGAAGGTGGAGAATGGTCAAGGCTG-3′ |
| GAPDH | 5′-GCCAAGGTCATCCATGACAACTTTGG-3′ | 5′-GCCTGCTTCACCACCTTCTTGATGTC-3′ |
Flow Cytometric Analysis
Cells were washed twice with phosphate-buffered saline containing 3% fetal bovine serum and incubated at 4°C for 30 minutes with the following monoclonal or polyclonal antibodies; mouse anti-human CXCR4 (12G5; DAKO Japan, Kyoto, Japan), mouse anti-human CXCR5 (51505.111; DAKO Japan), mouse anti-human CXCR6 (56811.111; R&D Systems, Minneapolis, MN), mouse anti-human CCR4 (53103.111; BD Biosciences, Mountain View, CA), mouse anti-human CCR6 (53103.111; R&D Systems), mouse anti-human CCR7 (2H7; BD Biosciences), rat anti-human CCR3 (61828; R&D Systems), goat anti-human CCR8 (Alexis Biochemicals, San Diego, CA), and goat anti-human CXCL16 (R&D Systems). As negative controls, isotype-matched control immunoglobulins obtained from DAKO Japan or BD Biosciences were used. After washing, cells were incubated at 4°C for 30 minutes with phycoerythrin-conjugated goat anti-mouse IgG, fluorescein isothiocyanate-conjugated goat anti-mouse IgM, phycoerythrin-conjugated goat anti-rat IgG, or biotinylated rabbit anti-goat IgG. In the case of biotinylated secondary antibody, cells were further incubated with phycoerythrin-labeled streptavidin at 4°C for 30 minutes in the third staining step. For detection of surface CCR10, we used CCL27-Fc chimera protein as described previously.26 After staining, cells were immediately analyzed on FACSCalibur on gating viable cells (BD Biosciences).
Chemotaxis Assay
Recombinant human CXCL16 and CCL27 were purchased from R&D Systems. Migration assays of HD-derived cell lines were performed using Transwell plates with 5-μm pore size (Corning, Corning, NY) as described previously.25 In brief, HD cell lines were suspended at 2 × 106/ml in RPMI 1640 containing 0.5% bovine serum albumin and 20 mmol/L HEPES, pH 7.4. Cells were added to upper chambers in a final volume of 100 μl, while chemokines were added to lower chambers in a final volume of 600 μl. After 4 hours at 37°C, cells migrated into lower chambers were enumerated. All assays were done in duplicate.
Enzyme-Linked Immunosorbent Assay
Cells were seeded in a 24-well plate at a density of 1 × 106 cells/well and cultured for 3 days. After collecting culture supernatants, MEC/CCL28 was measured using an enzyme-linked immunosorbent assay kit purchased from R&D Systems. For standardization of assay, serially diluted recombinant CCL28 was always included in each enzyme-linked immunosorbent assay plate. The detection limits were routinely 20 pg/ml.
Immunohistochemistry
Tissue sections were made from formalin-fixed and paraffin-embedded tissue blocks, and treated with microwave for 5 minutes for three times in Target Retrieval Solution (DAKO, Carpinteria, CA). Sections were then incubated at 4°C overnight with monoclonal mouse anti-LMP-1 (CS1-4; DAKO Japan), goat anti-TARC/CCL17 (Genzyme/Techne, Cambridge, MA), rabbit anti-MDC/CCL22 (Peprotech EC, London, England), mouse anti-eotaxin/CCL11 (43911.11; R&D), goat anti-MEC/CCL28 (DAKO Japan), mouse anti-CCR4 (KM-2160; Kyowa Hakko, Tokyo, Japan), rabbit anti-CCR10 (Biocarta, San Diego, CA), or anti-CD138/syndecan-1 (B-B4; Immunotech, Marseille, France). Isotype-matched immunoglobulins obtained from DAKO Japan were used as negative controls. Sections were next incubated with appropriate biotin-labeled second antibodies (Vector Laboratories, Burlingame, CA). Finally, sections were treated with the Vectastain ABC/HRP kit (Vector) according to the manufacturer’s instructions. Peroxidase enzymatic development was performed using diaminobenzidine and H2O2, resulting in dark brown products in positive cells. Sections were counterstained with hematoxylin before dehydration and mounted in nonaqueous mounting medium (Muto Pure Chemicals, Ltd., Tokyo, Japan). To determine the numbers of eosinophils, plasma cells, and CCR10+ cells in HD tissues, four of us (HH, HM, ST, and YT) separately counted their numbers in at least 10 separate high-power fields (HPFs) for each tissue specimen after appropriate staining. The results were averaged and scored as follows: 0, 0 to 5 cells/HPF; 1, 5 to 10 cells/HPF; 2, 10 to 20 cells/HPF; 3, >20 cells/HPF.
In Situ Hybridization
Expression of CCL28 mRNA was examined by in situ hybridization using DAKO’s Gene Point System (DAKO Japan). Based on the cDNA sequence of human CCL28,23,24 synthetic oligonucleotide probes were ordered from Greiner Japan (Tokyo, Japan). The sequence of anti-sense probe was 5′-CACTTTCATCCACTGCTTAACAGTATGGTTGT-3′.
Statistical Analysis
Statistical significance was evaluated by using the Fisher’s exact probability test.
Results
RT-PCR Analysis on Chemokine Receptor Expression in HD-Derived Cell Lines
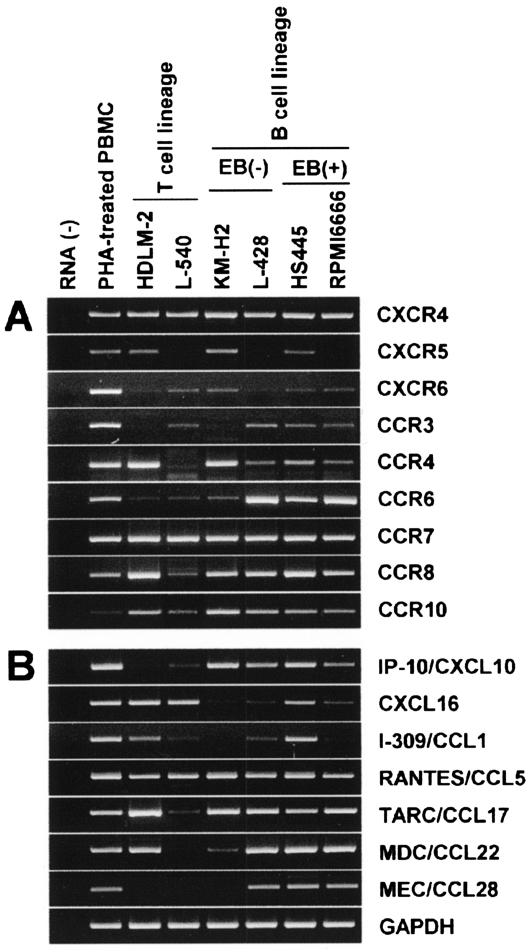
It is now known that H-RS cells of classical HD are mostly of the B cell lineage and derived from preapoptotic germinal center B cells.2–4 However, the exact differentiation stage of H-RS cells is still controversial. Because chemokine receptors are valuable markers for lymphocyte differentiation,12 we wished to determine the expression profile of chemokine receptors in H-RS cells. To begin with, we performed a systematic RT-PCR analysis on expression of all 18 chemokine receptors in a panel of six HD-derived cell lines. HDLM-2 and L540 were positive for T-cell markers, whereas KM-H2, L428, HS445, and RPMI 6666 were positive for B-cell markers. HS445 and RPMI 6666 were also infected with Epstein-Barr virus. Figure 1A shows the highlights of the analysis. All six HD-derived cell lines expressed CXCR4, CCR7, and CCR8. Some cell lines also expressed CXCR5, CCR3, and/or CCR4. These results were partly consistent to previous reports.30,31 Furthermore, we newly found that HD-derived cell lines consistently expressed CXCR6 and CCR10. Importantly, these receptors are known to be selectively expressed by plasma cells in the B-cell lineage.25 Among other chemokine receptors, CXCR2 and CCR1 were positive in a fraction of B-cell lineage HD cell lines; KM-H2, HS445, and RPMI 6666 expressed CXCR2, whereas L-428, HS445, and RPMI 6666 expressed CCR1 (data not shown).
Figure 1.
RT-PCR analysis on expression of chemokine receptors and chemokines in HD-derived cell lines. Total RNA was prepared from PBMCs stimulated with phytohemagglutinin for 3 days (positive control) and six HD-derived cell lines. RT-PCR was performed to examine the expression of 18 chemokine receptors (A) and 21 chemokines (B) (see Table 1 for primers). For details, see Materials and Methods. This figure shows the highlights of the representative results from at least two experiments.
Flow Cytometric Analysis on Chemokine Receptor Expression in HD-Derived Cell Lines
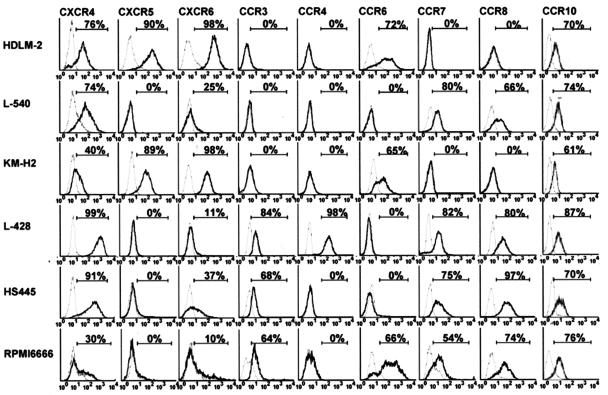
We further examined surface expression of various chemokine receptors in HD-derived cell lines by flow cytometry. The results are shown in Figure 2. Overall, surface expression of the chemokine receptors was consistent with their mRNA expression. However, there were also some discrepancies between the levels of mRNA expression and those of surface expression in some HD cell lines. For example, despite the relatively strong mRNA expression of CCR4 and CCR7 (Figure 1A), HDLM-2 and KM-H2 were virtually negative for surface expression of CCR4 and CCR7. These results may indicate a posttranscriptional regulation or an abortive mutation of such chemokine receptors. Notably, CXCR6 and CCR10 were consistently detected on the surface of HD-derived cell lines.
Figure 2.
Flow cytometric analysis on surface expression of chemokine receptors on HD-derived cell lines. Six HD-derived cell lines were stained for indicated chemokine receptors by using specific antibodies or CCL27-Fc chimera protein. Isotype-matched IgG or Fc protein served as negative controls. For details, see Materials and Methods. Representative results from at least two separate experiments are shown. Percentages of positive cells are indicated.
Chemotactic Response of HD-Derived Cell Lines
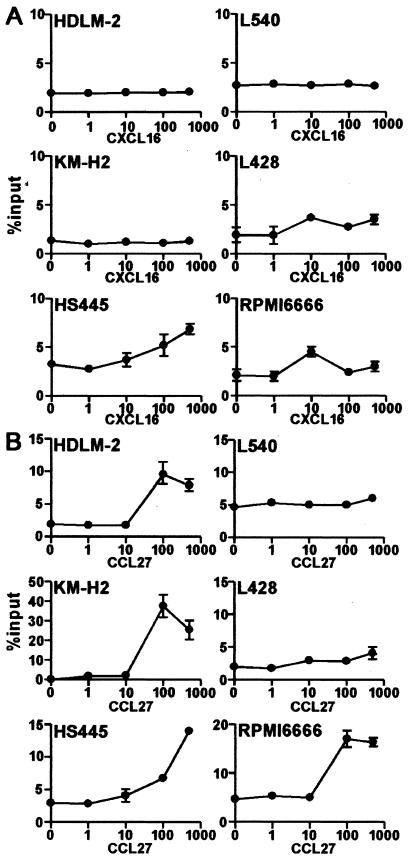
To explore the functional relevance of CXCR6 and CCR10 expression on HD-derived cell lines, we examined their chemotactic responses to the soluble form of CXCL16, a transmembrane-type chemokine and the specific ligand for CXCR6,32,33 and CCL27, the monospecific ligand for CCR10.34,35 CXCL16 induced relatively weak but significant migration in three HD-derived cell lines (L428, HS445, and RPMI 6666) (Figure 3A). On the other hand, CCL27 induced much more vigorous migration in four HD-derived cell lines (HDLM-2, KM-H2, HS445, and RPMI 6666). These results confirmed that CXCR6 and CCR10 expressed on HD-derived cell lines were functional at least in a faction of these cell lines.
Figure 3.
Chemotactic response of HD-derived cell lines to CXCL16 and CCL27. Indicated HD-derived cell lines were examined for chemotactic responses to CXCL16, the ligand for CXCR6 (A), or to CCL27, the monospecific ligand for CCR10 (B), both at nM. For details, see Materials and Methods. Representative results from at least two separate assays are shown.
Expression of Chemokines by HD-Derived Cell Lines
It is now known that H-RS cells produce a number of chemokines, which may explain the extensive background accumulation of various leukocytes in classical HD.4,14 Therefore, we also systematically examined the expression of various chemokines in the HD-derived cell lines. Figure 1B shows the highlights of the results. As reported previously,15–19 most HD-derived cell lines strongly expressed IP-10/CXCL10, RANTES/CCL5, TARC/CCL17, and MDC/CCL22. Some cell lines also expressed I-309/CCL1. Here we newly found consistent expression of CXCL16, a transmembrane-type CXC chemokine and the ligand of CXCR6,32,33 in the HD-derived cell lines, even though the expression levels were relatively low in some cell lines. Furthermore, we newly found strong expression of MEC/CCL28, a chemokine selectively expressed by certain mucosal epithelial cells and the ligand for CCR10 and CCR3,23,24 in three B-lineage HD cell lines. Among other chemokines examined, SDF-1/CXCL12 was expressed by HDLM-2 and KM-H2, MIP-1α/CCL3 was expressed by L428, HS445, and RPMI 6666, LARC/CCL20 was expressed by KM-H2 and RPMI 6666, and lymphotactin/XCL1 was expressed by HS445 (data not shown).
Surface Expression of CXCL16 and Secretion of MEC/CCL28 by HD-Derived Cell Lines
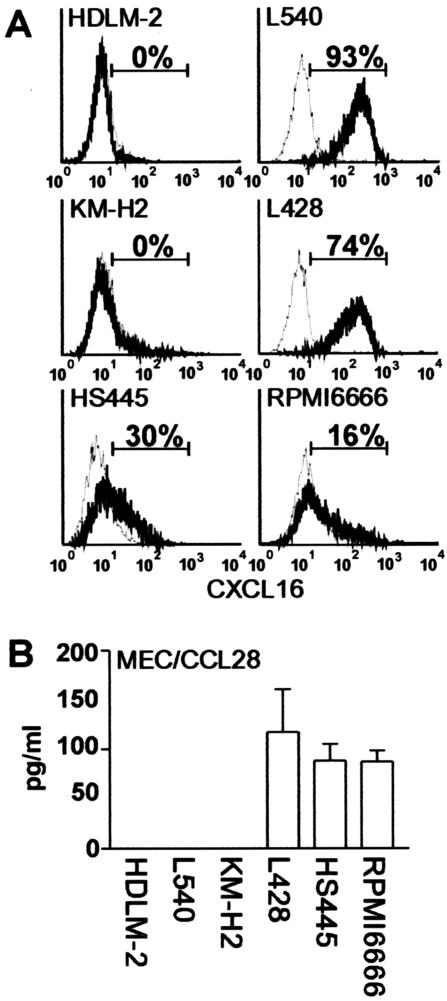
On the RT-PCR results, we further examined surface expression of CXCL16, a transmembrane-type CXC chemokine,32,33 on HD-derived cell lines. As shown in Figure 4A, four HD-derived cell lines (L-540, L428, HS445, and RPMI 6666) were confirmed to express CXCL16 on their cell surface. We also examined secretion of MEC/CCL28 by HD-derived cell lines. As shown in Figure 4B, the three cell lines (L428, HS445, and RPMI 6666) that were positive for MEC/CCL28 mRNA (Figure 1B) were confirmed to secrete MEC/CCL28 protein into culture supernatant.
Figure 4.
Surface expression of CXCL16 and secretion of CCL28 by HD-derived cell lines. A: Six HD-derived cell lines were stained for surface CXCL16 by using goat anti-CXCL16. Isotype-matched goat IgG served as negative control. For details, see Materials and Methods. Representative results from at least two separate experiments are shown. Percentages of positive cells are indicated. B: Cells from indicated HD-derived cell lines were seeded in a 24-well plate at a density of 1 × 106 cells/well and cultured for 3 days. CCL28 secreted in culture supernatants was measured with enzyme-linked immunosorbent assay. All assays were done in triplicate. For details, see Materials and Methods. Representative results from two separate experiments are shown.
Immunohistochemical Studies on HD Tissues
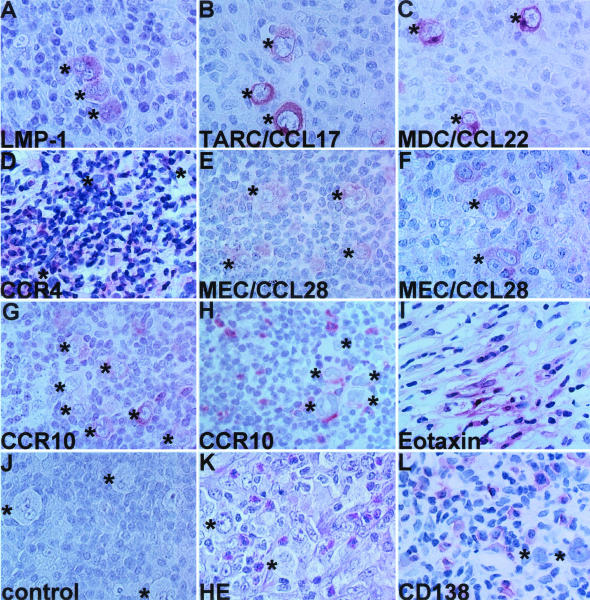
To extend the results obtained from HD-derived cell lines, we decided to focus on CCR10 and CCL28 and studied their expression in classical HD cases. Compared to Western countries, HD is relatively rare in Japan and the MC subtype is predominant. Here, we managed to examine a total of 19 classical HD cases consisting of 11 cases of MC, 4 cases of NS, 3 cases of lymphocyte-rich, and 1 case of lymphocyte depletion. Figure 5 shows the representative results of immunohistochemistry, and Table 2 summarizes the whole results. H-RS cells from 12 of 19 cases were positive for LMP-1 (Figure 5A, Table 2). In accordance with previous studies,15–19 H-RS cells from 12 of 19 cases were positive for TARC/CCL17 (Figure 5B, Table 2), and those from 12 of 16 cases were positive for MDC/CCL22 (Figure 5C, Table 2). In such cases, background accumulation of CCR4+ cells was also frequently observed (Figure 5D). Importantly, we observed staining of MEC/CCL28 in H-RS cells from 15 of 19 cases (Figure 5, E and F; Table 2). Staining of MEC/CCL28 in H-RS cells was, however, relatively weak in comparison to those of TARC/CCL17 and MDC/CCL22. Occasionally, cells in the background were also weakly positive for MEC/CCL28 (not shown). Furthermore, we observed expression of CCR10 in H-RS cells in 7 of 19 cases (Figure 5G, Table 2). Notably, these cases were all positive for MEC/CCL28, suggesting a potential autocrine role (Table 2). We also observed frequent background accumulation of CCR10+ cells in MEC/CCL28-positive cases (Figure 5H, Table 2). In the case of eotaxin/CCL11, we observed its production only in NS cases and by surrounding fibroblasts (Figure 5I, Table 2). We confirmed no staining with isotype controls (Figure 5J).
Figure 5.
Immunohistochemical analysis of HD tissues. Classical HD tissue specimens were examined with the following reagents: A, anti-LMP-1 (case 6); B, anti-TARC/CCL17 (case 6); C, anti-MDC/CCL22 (case 6); D, anti-CCR4 (case 13); E, anti-MEC/CCL28 (case 7); F, anti-MEC/CCL28 (case 5); G, anti-CCR10 (case 7); H, anti-CCR10 (case 6); I, anti-eotaxin/CCL11 (case 13); J, a representative isotype control (case 5); K, H&E staining (case 5); L, anti-CD138/syndecan-1 (case 6). For details, see Materials and Methods. All experiments were done at least twice and representative results are shown. Asterisks indicate H-RS cells. Original magnifications, ×1000.
Table 2.
Results of Immunohistochemical Staining of Classical HD Tissue Specimens
| Case | Age | Sex | Type | Stage | H-RS cells (% positive cells) | Infiltrating cells (score) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMP-1 | CCR10 | CCL28 | CCL17 | CCL22 | CCL11 | Eosinophils | Plasma cells | CCR10+ | |||||
| 1 | 44 | F | MC | 3 | 50–70 | 0 | 0 | <5 | 30–50 | 0 | 0 | 0 | 0 |
| 2 | 71 | M | MC | 2 | 0 | 0 | 0 | 0 | 30–50 | 0 | 0 | 0 | 0 |
| 3 | 66 | M | MC | 2 | >70 | 30–50 | >70 | >70 | >70 | 0 | 3 | 0 | 0 |
| 4 | 59 | F | MC | 1 | 0 | 30–50 | 50–70 | <5 | 0 | 0 | 0 | 3 | 0 |
| 5 | 67 | M | MC | 2 | >70 | 0 | >70 | 50–70 | >70 | 0 | 3 | 3 | 3 |
| 6 | 68 | M | MC | 3 | >70 | 30–50 | >70 | >70 | 50–70 | 0 | 2 | 3 | 3 |
| 7 | 30 | M | MC | 1 | 0 | >70 | >70 | 0 | 0 | 0 | 0 | 3 | 1 |
| 8 | 52 | M | MC | 4 | >70 | 0 | >70 | 0 | 0 | 0 | 0 | 2 | 0 |
| 9 | 55 | M | MC | 2 | >70 | 0 | 50–70 | 0 | 0 | 0 | 3 | 2 | 2 |
| 10 | 18 | F | MC | 2 | 50–70 | 0 | 50–70 | >70 | ND | ND | 3 | 1 | 2 |
| 11 | 65 | M | MC | 2 | 30–50 | 0 | >70 | >70 | ND | ND | 3 | 2 | 2 |
| 12 | 34 | M | NS | 4 | 0 | 50–70 | >70 | >70 | >70 | +* | 3 | 0 | 1 |
| 13 | 47 | M | NS | 2 | 0 | >70 | >70 | >70 | >70 | +* | 3 | 0 | 1 |
| 14 | 13 | M | NS | 1 | 30–50 | 0 | 50–70 | 0 | ND | ND | 0 | 2 | 0 |
| 15 | 31 | F | NS | 2 | 0 | 0 | >70 | >70 | 50–70 | +* | 3 | 3 | 0 |
| 16 | 33 | M | LR | 2 | 50–70 | 30–50 | 30–50 | 5–30 | 50–70 | 0 | 0 | 3 | 3 |
| 17 | 51 | M | LR | 1 | 0 | 0 | 0 | 0 | 30–50 | 0 | 0 | 0 | 0 |
| 18 | 59 | M | LR | 1 | 30–50 | 0 | 30–50 | 50–70 | 30–50 | 0 | 3 | 3 | 3 |
| 19 | 66 | F | LD | 3 | >70 | 0 | 0 | 0 | >70 | 0 | 0 | 0 | 0 |
In Situ Hybridization for MEC/CCL28
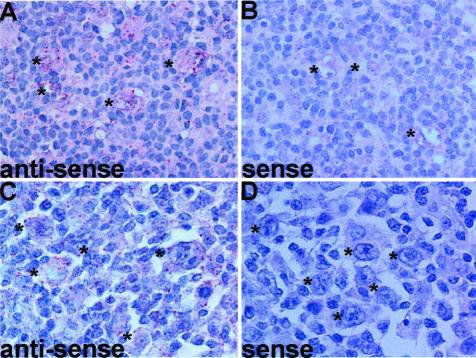
To confirm the expression of MEC/CCL28 mRNA by H-RS cells, we further performed in situ hybridization for MEC/CCL28. By using an anti-sense probe for MEC/CCL28, we examined five tissue specimens (cases 2, 3, 4, 5, and 7). H-RS cells in four tissue specimens (cases 3, 4, 5, and 7) showed positive hybridization signals (Figure 6, A and C). These results were consistent with those of immunohistochemistry (Table 2). On the other hand, no such hybridization signals were seen with a sense probe (Figure 6, B and D). These results confirmed the expression of MEC/CCL28 mRNA by H-RS cells.
Figure 6.
In situ hybridization for MEC/CCL28. Expression of CCL28 mRNA in HD tissues was examined by in situ hybridization using DAKO’s Gene Point System (DAKO Japan). For details, see Materials and Methods. Representative results from at least two separate experiments are shown. A: Hybridization with anti-sense (case 7). B: Hybridization with sense probe (case 7), hybridization with anti-sense probe (case 4), hybridization with sense probe (case 4). Asterisks indicate H-RS cells. Original magnifications, ×1000.
Correlation between MEC/CCL28 and Infiltration of Eosinophils and/or Plasma Cells
MEC/CCL28 is a shared ligand of CCR3 and CCR10.23,24 CCR3 is selectively expressed on eosinophils,12 whereas CCR10 was originally reported to be expressed by cutaneous lymphocyte antigen-expressing skin-homing memory T cells.34,35 Recently, however, we and others have shown that plasma cells selectively express CCR10 in the B-cell lineage.25–27 Thus, the highly frequent production of MEC/CCL28 by H-RS cells may be partly responsible for the frequent accumulation of eosinophils and/or plasma cells in HD tissues. To test this hypothesis, we analyzed the correlations between the production of MEC/CCL28 by H-RS cells and background accumulation of eosinophils and plasma cells. Eosinophils were enumerated on hematoxylin and eosin staining (Figure 5K), whereas plasma cells were enumerated after staining with anti-CD138 (Figure 5L). As summarized in Table 2, essentially all HD cases that showed accumulation of eosinophils and/or plasma cells were positive for MEC/CCL28. The correlation was: MEC/CCL28 versus eosinophils, P = 0.0325; MEC/CCL28 versus plasma cells, P = 0.009; MEC/CCL28 versus eosinophils + plasma cells, P = 0.0001. The positivity of MEC/CCL28 in H-RS cells also correlated with the background accumulation of CCR10+ cells (P = 0.0325). On the other hand, the positivity of MEC/CCL28 in H-RS cells did not significantly correlate with the positivity of LMP-1 (Epstein-Barr virus infection) (P = 0.6027), TARC/CCL17 (P = 0.1174), or MDC/CCL22 (P = 1). Even though TARC/CCL17 is not directly chemotactic for eosinophils,12 immunoreactivity of TARC/CCL17 in H-RS cells was also significantly correlated with eosinophil infiltration (P = 0.0198). In contrast to a previous report,17 however, the positivity of MDC/CCL22 in H-RS was not significantly correlated with eosinophil accumulation in our HD cases (P = 0.2821).
Discussion
Classical HD is histologically characterized by the abundant background cellular infiltrates consisting of cells such as T cells, eosinophils, and plasma cells.1 Recent studies have shown that various chemokines produced by H-RS cells are likely to account for such reactive cellular infiltrates.4,14 Here we newly found that HD-derived cell lines frequently express CXCL16, a transmembrane type chemokine,32,33 and MEC/CCL28, a chemokine selectively expressed by certain mucosal epithelial cells (Figures 1 and 4).23,24 Because MEC/CCL28 is capable of attracting eosinophils via CCR3 and plasma cells via CCR10 and CCR3,24–27 we examined its expression in classical HD cases. We indeed demonstrated that H-RS cells of a large proportion of HD cases were positive for MEC/CCL28 (Figure 5, Table 2). We confirmed de novo expression of MEC/CCL28 mRNA in H-RS cells by in situ hybridization (Figure 6). Importantly, the MEC/CCL28 positivity in H-RS cells showed a significant correlation with the background accumulation of eosinophils, plasma cells, and CCR10+ cells (Table 2). Thus, MEC/CCL28 may play an important role in tissue accumulation of eosinophils and/or plasma cells in classical HD of especially MS subtype. It remains to be seen whether CXCL16 is also expressed by H-RS cells in HD tissues and plays a role in HD pathogenesis.
There were, however, some discrepancies between MEC/CCL28 production by H-RS cells and tissue accumulation of eosinophilia and/or plasma cells (Table 2). For example, even though MEC/CCL28 can attract both eosinophils and plasma cells, many HD cases show selective accumulation of either eosinophils or plasma cells. Thus, besides MEC/CCL28, there must be other factors that are also required for efficient infiltration of eosinophils or plasma cells in classical HD tissues. For example, besides CCR10, plasma cells express CXCR4, CXCR6, and weakly CCR3.25 Thus, the chemokines acting on these other chemokine receptors may also be involved in tissue accumulation of plasma cells.36 There were also some discrepancies between infiltration of plasma cells and that of CCR10+ cells (Table 2). Such discrepancies may be explained in part by a low-level expression of CCR10 on infiltrating plasma cells or by infiltration of CCR10+ T cells instead of plasma cells.34,35 As for eosinophils, Teruya-Feldstein and colleagues21 previously reported a direct correlation between production of eotaxin/CCL11 by H-RS cells of especially NS subtype and the extent of tissue eosinophilia. On the other hand, Jundt and colleagues22 reported that, in HD tissues, fibroblasts but not H-RS cells express eotaxin/CCL11 most probably on stimulation with tumor necrosis factor-α released by the latter. In consistency with the latter report, we observed production of eotaxin/CCL11 only in NS cases and only by surrounding fibroblasts (Table 2). Thus, at least in MC subtype, MEC/CCL28 produced by H-RS cells may play a major role in tissue accumulation of eosinophils. Furthermore, there are many other chemokines that are capable of attracting eosinophils via CCR3 such as RANTES/CCL5 (Figure 1A).12 Thus, chemokines other than MEC/CCL28 and eotaxin/CCL11 may also be involved in tissue accumulation of eosinophils in classical HD.
We also newly found consistent expression of CXCR6, the receptor for CXCL16,32,33 and CCR10, the receptor for CCL27 and CCL28,23,24,34,35 in HD-derived cell lines. We indeed demonstrated frequent expression of CCR10 in H-RS cells in tissue specimens, albeit at a varying degree of cellular expression (Figure 5, Table 2). Because CXCR6 and CCR10 are known to be selectively expressed by plasma cells in the B-cell lineage,25–27 this may indicate that H-RS cells of at least a fraction of classical HD were derived from cells at the terminal stages of B-cell differentiation. Previously, Carbone and colleagues9 demonstrated that H-RS cells of all cases of classical HD expressed CD138/syndecan-1, a proteoglycan associated with postgerminal center terminal B-cell differentiation, albeit at a varying degree of cellular expression. Together, these observations support that the differentiation stages of classical HD are closely related to that of plasma cells. Furthermore, CCR10+ HD cases were all MEC/CCL28+ (Table 2). Thus, MEC/CCL28 and CCR10 might have an autocrine role in such cases. It remains to be seen whether there are any phenotypic differences between CCR10+ and CCR10− HD cases. It also remains to be seen whether CXCR6 is indeed expressed by H-RS cells in HD tissues.
In conclusion, the expression of MEC/CCL28 by H-RS cells may define a major subtype of classical HD, which is frequently accompanied by tissue infiltration of eosinophils and/or plasma cells. H-RS cells of at least a fraction of classical HDs express CCR10, supporting their differentiation stage close to that of plasma cells. The present study has thus revealed yet another important association of the chemokine system with classical HD, providing a further insight into the origin and pathophysiology of classical HD.
Acknowledgments
We thank Dr. Yasuhiro Maeda and Dr. Takahiro Shimada for valuable advice.
Footnotes
Address reprint requests to Osamu Yoshie, M.D., Department of Microbiology, Kinki University School of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan. E-mail: o.yoshie@med.kindai.ac.jp.
Supported in part by a High-Tech Research Center Grant from the Ministry of Education, Culture, Sports, and Technology, Japan; and by Solution Oriented Research for Science and Technology of the Japan Science and Technology Corporation.
References
- Weiss LM, Chan JKC, MacLennan K, Warnke RA. Pathology of classical Hodgkin’s disease. Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM, editors. Philadelphia: Lippincott, Williams, and Wilkins,; Hodgkin’s Disease. 1999:pp 101–120. [Google Scholar]
- Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Falini B, Delsol G, Isaacson PG, Pileri S, Stein H. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med. 1997;337:453–458. doi: 10.1056/NEJM199708143370703. [DOI] [PubMed] [Google Scholar]
- Staudt LM. The molecular and cellular origins of Hodgkin’s disease. J Exp Med. 2000;191:207–212. doi: 10.1084/jem.191.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueschen M, Rajewsky K, Baeuninger A, Baur AS, Oudejans JJ, Roers A, Hansmann M-L, Kueppers R. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma. J Exp Med. 2000;191:387–394. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz V, Hummel M, Marafioti T, Anagnostopoulos I, Assaf C, Stein H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95:3020–3024. [PubMed] [Google Scholar]
- Braeuninger A, Kueppers R, Strickler JG, Wacker H-H, Rajewsky K, Hansmann M-L. Hodgkin and Reed-Sternberg cells in lymphocyte predominant Hodgkin disease represent clonal populations of germinal center-derived tumor B cells. Proc Natl Acad Sci USA. 1997;94:9337–9342. doi: 10.1073/pnas.94.17.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B, Bigerna B, Pasqualucci L, Fizzotti M, Martelli MF, Pileri S, Pinto A, Carbone A, Venturi S, Pacini R, Cattoretti G, Pescarmona E, Lo Coco F, Pelicci P-G, Anagnastopoulos I, Dalla-Favera R, Flenghi L. Distinctive expression pattern of the BCL-6 protein in nodular lymphocyte predominance of Hodgkin’s disease. Blood. 1996;87:465–471. [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Gaidano G, Franceschi S, Capello D, Drexler HG, Falini B, Dalla-Favera R. Expression status of BCL-6 and syndecan-1 identifies distinct histogenic subtypes of Hodgkin’s disease. Blood. 1998;92:2220–2228. [PubMed] [Google Scholar]
- Stein H, Diehl V, Marafioti T, Jox A, Wolf J, Hummel M. The nature of Reed-Sternberg cells, lymphocytic and histiocytic cells and their molecular biology in Hodgkin’s disease. Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM, editors. Philadelphia: Lippincott, Williams, and Wilkins,; Hodgkin’s Disease. 1999:pp 121–138. [Google Scholar]
- Schwering I, Braeuninger A, Klein U, Jungnickel B, Tinguely M, Diehl V, Hansmann M-L, Dalla-Favera R, Rajewsky K, Kueppers R. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101:1505–1512. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- Kapp U, Yeh W-C, Patterson B, Elia AJ, Kaegi D, Ho A, Hessel A, Tipsword M, Williams A, Mirtsos C, Itie A, Moyle M, Mak TW. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med. 1999;189:1930–1945. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S, van den Berg A. Interaction between host T cells and Reed-Sternberg cells in Hodgkin lymphomas. Semin Cancer Biol. 2000;10:345–350. doi: 10.1006/scbi.2000.0327. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells: a possible explanation for the characteristic T-cell infiltrate in Hodgkin’s lymphoma. Am J Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J, Annunziata CM, Barash S, Staudt L, Dillon P, He W-W, Ricciardi-Castagnoli P, Rosen CA, Carter KC. Reed-Sternberg cell genome expression supports a B-cell lineage. Blood. 1999;94:411–416. [PubMed] [Google Scholar]
- Hedvat CV, Jaffe ES, Qin J, Fillippa DA, Cordon-Cardo C, Tosato G, Nimer SD, Teruya-Feldstein J. Macrophage-derived chemokine expression in classical Hodgkin’s lymphoma: application of tissue microarrays. Mod Pathol. 2001;14:1270–1276. doi: 10.1038/modpathol.3880473. [DOI] [PubMed] [Google Scholar]
- Maggio EM, Van Den Berg A, Visser L, Diepstra A, Kluiver J, Emmens R, Poppema S. Common and differential chemokine expression patterns in RS cells of NLP, EBV positive and negative classical Hodgkin lymphomas. Int J Cancer. 2002;99:665–672. doi: 10.1002/ijc.10399. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Tutiya T, Yamaguchi T, Suzuki K, Suzumiya J, Kawasaki C, Haraoka S, Kikuchi M. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: relation with expression of CXC and CC chemokines on H&RS cells. Int J Cancer. 2002;98:567–572. doi: 10.1002/ijc.10218. [DOI] [PubMed] [Google Scholar]
- Vermeer MH, Dukers DF, ten Berge RL, Bloemena E, Wu L, Vos W, de Vries E, Tensen CP, Meijer CJ, Willemze R. Differential expression of thymus and activation regulated chemokine and its receptor CCR4 in nodal and cutaneous anaplastic large-cell lymphomas and Hodgkin’s disease. Mod Pathol. 2002;15:838–844. doi: 10.1097/01.MP.0000021006.53593.B0. [DOI] [PubMed] [Google Scholar]
- Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin’s disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999;93:2463–2470. [PubMed] [Google Scholar]
- Jundt F, Anagnostopoulos I, Bommert K, Emmerich F, Mueller G, Foss H-D, Royer H-D, Stein H, Doerken B. Hodgkin/Reed-Sternberg cells induce fibroblasts to secrete eotaxin, a potent chemoattractant for T cells and eosinophils. Blood. 1999;94:2065–2071. [PubMed] [Google Scholar]
- Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, Abrams J, Kershenovich D, Smith K, McClanahan T, Vicari AP, Zlotnik A. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem. 2000;275:22313–22323. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–1317. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Fujisawa R, Izawa D, Hieshima K, Takada K, Yoshie O. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J Virol. 2002;76:3072–3077. doi: 10.1128/JVI.76.6.3072-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueppers R, Klein U, Schwering I, Distler V, Braeuninger A, Cattoretti G, Tu Y, Stolovitzky GA, Califano A, Hansmann M-L, Dalla-Favera R. Identification of Hodgkin and Reed-Sternberg cell-specific genes by gene expression profiling. J Clin Invest. 2003;111:529–537. doi: 10.1172/JCI16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepken UE, Foss H-D, Meyer D, Hinz M, Leder K, Stein H, Lipp M. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood. 2002;99:1109–1116. doi: 10.1182/blood.v99.4.1109. [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;4:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460–3464. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou Y-R, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movement. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]