Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis (original) (raw)
Abstract
Mucolipidosis type IV (MLIV) is an autosomal recessive lysosomal storage disease characterized by severe psychomotor retardation, achlorhydria, and ophthalmological abnormalities. Cells from several tissues in MLIV patients accumulate large vacuoles that are presumed to be lysosomes, but whose exact nature remains to be determined. Other defects include the deterioration of neuronal integrity in the retina and the cerebellum. MCOLN1, the gene mutated in MLIV patients, encodes a protein called h-mucolipin-1 that has six predicted transmembrane domains and functions as a Ca2+-permeable channel that is modulated by changes in Ca2+ concentration. CUP-5 is the Caenorhabditis elegans functional orthologue of h-mucolipin-1. Mutations in cup-5 result in the accumulation of large vacuoles in several cells, in increased cell death, and in embryonic lethality. We demonstrate here that CUP-5 functions in the biogenesis of lysosomes originating from hybrid organelles. We also show that at least two h-mucolipin family members rescue cup-5 mutant endocytic defects, indicating that there may be functional redundancy among the human proteins. Finally, we propose a model that relates the lysosome biogenesis defect in the absence of CUP-5/h-mucolipin-1 to cellular phenotypes in worms and in humans.
Keywords: mucolipidosis type IV, CUP-5
Endocytosis is the process by which macromolecules and membranes are taken up and processed by cells. There is a basic understanding of the various steps in endocytosis and of the molecules that regulate them (1–3). Internalized molecules are transported through, and sorted by, morphologically and biochemically distinct membrane-bound compartments, called endosomes. Late endosomes/multivesicular bodies, in particular, also receive molecules from the biosynthetic pathway (4). Lysosomes are considered to be the terminal degradative/storage compartments in all cells, with functions that are critical to many cellular processes, including protein turnover, down-regulation of signaling pathways, and release of endocytosed nutrients (5, 6). Several organelles, including the melanosomes in skin melanocytes and the secretory granules in haematopoetic cells, are related to lysosomes and perform specialized functions (5, 6).
Lysosomes are dynamic organelles that can fuse with one another. Recent studies of lysosome biogenesis indicate that they can also fuse with late endosomes, thus creating hybrid organelles. The reformation of lysosomes from the hybrid organelles involves content condensation and the removal of molecules not destined for degradation. Whereas much is now known about the cellular proteins that regulate the fusion reaction, little is known about the molecules required for the reformation/biogenesis of lysosomes (5–9). It is clear however that Ca2+ movement into and out of these compartments is crucial for the fusion and reformation reactions (10).
CUP-5 was originally identified in Caenorhabditis elegans on the basis of a mutation that disrupted endocytosis in scavenger cells called coelomocytes (11, 12). In cup-5 mutants, endocytosed material is not degraded and accumulates in large vacuoles that are heterogeneous in size and in content (12, 13). Preliminary observations indicated that the CUP-5 protein localized to unidentified membrane compartments in WT cells (12). CUP-5 is homologous to the human protein h-mucolipin-1, mutations in which lead to mucolipidosis type IV (MLIV) (12–16). Indeed, expression of h-mucolipin-1 rescues the maternal effect lethality due to loss of function mutations in cup-5 (13). Recent studies have demonstrated that h-mucolipin-1 expressed in Xenopus laevis oocytes localizes to lysosomes, and that it is a Ca2+-permeable channel that is modulated by changes in Ca2+ concentration, and is also permeable to Na+ and K+ (17). There are at least two other proteins that are homologous to h-mucolipin-1 in humans (12). Whereas mutations so far have not been reported for MCOLN2, mutations in MCOLN3 are associated with deafness and pigmentation defects in mice (18). It is not known whether the severity of the symptoms in MLIV, at least in some tissues, might be mitigated due to functional redundancy among the various h-mucolipin proteins or to other genotypic variations.
To determine the cell biological function of CUP-5, we need to understand the exact nature of the cellular defects in its absence. This process includes determining the stage in endocytosis at which a defect is first seen in mutant cells, defining the nature of the large vacuoles that accumulate in mutant cells, and localizing the WT protein to discrete compartments in which it functions during normal endocytosis. We report here that cup-5 mutants are defective in lysosome biogenesis, that a consequence of this defect is the accumulation of aberrant large vacuoles that are hybrids of late endosomes and of lysosomes, and that CUP-5 localizes to presumed sites of lysosome biogenesis and to mature lysosomes. CUP-5/h-mucolipin-1 is therefore an excellent candidate for the channel that regulates Ca2+ levels (and maybe other ions) to promote lysosome reformation/biogenesis.
Materials and Methods
C. elegans Strains and Methods. Standard methods were used for genetic analysis (19). Extrachromosomal arrays were generated by coinjecting restriction-digested plasmids and marker DNA, each at 1–10 μg/ml, along with _Eco_RV-digested genomic DNA at 100 μg/ml into the germ line (20). Several transgenic lines were generated and analyzed from each injection. Markers used: cup-5(ar465) III (12); cup-5(zu223) III (13); and unc-36(e251) III results in the worm having an uncoordinated phenotype (19) and is closely linked to cup-5 (11, 12); qC1 is a balancer chromosome that suppresses recombination between cup-5 and unc-36 (21); _arIs37[p_myo-3::ssGFP] I (11); bIs34[RME-8::GFP] expresses a functional RME-8::GFP fusion protein in several tissues, including coelomocytes (12, 22).
Molecular Methods. Standard methods were used for the manipulation of recombinant DNA (23). All enzymes were from New England Biolabs (Beverly, MA), unless otherwise indicated.
Plasmids. To express GFP::CUP-5 in coelomocytes, we introduced GFP::CUP-5 coding sequences in front of a coelomocyte-specific promoter (12). The LMP-1::GFP plasmid expresses a translational fusion of the C. elegans LAMP/CD68 protein LMP-1 (24), including its own promoter but lacking a stop codon, to a flexible Gly-Ala linker and to GFP (25). The GFP::RAB-5 plasmid expresses a translational fusion of GFP to the C. elegans RAB-5 (worm ORF F26H9.6) (26). To express mannosidase II::GFP in coelomocytes, we subcloned a published C. elegans mannosidase II::GFP fusion construct (27) in front of the coelomocyte-specific promoter (12). To express TRAM::GFP in coelomocytes, we subcloned a published C. elegans TRAM::GFP fusion construct (27) in front of the coelomocyte-specific promoter (12). To make an RME-8::mRFP1 fusion construct, we replaced GFP with monomeric red fluorescent protein 1 (mRFP1) (28), a monomeric form of DsRed, in the published RME-8::GFP plasmid (22). MCOLN1 (human) and MCOLN3 (human) cDNAs were amplified by PCR and were subcloned in front of a coelomocyte-specific promoter (12). GFP::CUP-5 and RME-8::mRFP1 plasmids express functional fusion proteins because they rescue the coelomocyte endocytosis defect in worms bearing mutations in cup-5 and in rme-8, respectively. In the absence of corresponding mutations, we determined that the rest of the plasmids did not induce a coelomocyte endocytosis defect when introduced into p_myo-3_::ssGFP worms. Details of plasmid constructions are available on request.
BSA Conjugated to Rhodamine (BSA-Rhod) Endocytosis Assay. As described (12, 22), BSA-Rhod (Sigma) dissolved at 1 mg/ml in water was injected into the body cavity of young adult hermaphrodites at 20°C. At various times, the worms were mounted in a drop of ice-cold 1% formaldehyde, and slides were kept on ice until viewing. cup-5(zu223) worms exhibit maternal effect lethality. To assay these worms, we injected the uncoordinated progeny of cup-5(zu223) unc-36(e251)/qC1; bIs34[RME-8::GFP] worms.
Microscopy. Worms were fixed in 1% formaldehyde. Deconvolution images were recorded as digital images (with Z series containing 0.15-μm sections) by using a Series 300 charge-coupled device camera (Photometrics, Tucson, AZ) and were deconvolved by using deltavision software (Applied Precision, Issaquah, WA). Confocal images were taken with a Nikon PCM 2000, by using HeNe 543 excitation for the red dye and argon 488 for the green dye.
Results
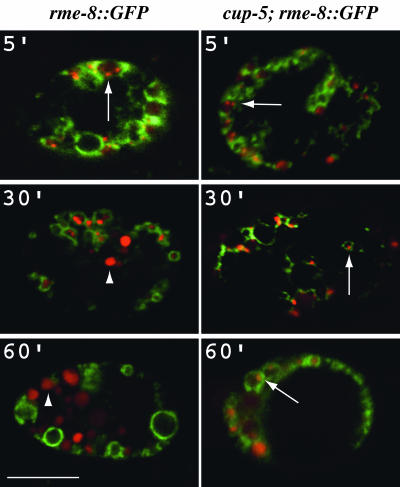
Endocytosis Is Normal Until Lysosome Biogenesis in cup-5 Mutant Coelomocytes. To detect the stage in endocytosis at which a defect is first observed, we injected BSA-Rhod into the body cavity of worms and monitored its uptake and trafficking through endocytic compartments in coelomocytes expressing the late endosomal marker RME-8::GFP (22). By using such an assay, we had previously determined that early stages of endocytosis were normal in coelomocytes of the hypomorphic allele, cup-5(ar465) (12). We describe here a more comprehensive analysis in the null allele, cup-5(zu223) (13). After injection of BSA-Rhod into the body cavity of WT rme-8::GFP worms, we observe three distinct and sequential morphological events: (i) the BSA-Rhod appears in RME-8::GFP-labeled late endosomes; (ii) BSA-Rhod is condensed in small compartments that are “budding” from the RME-8::GFP-labeled endosomes; which are presumed to be nascent lysosomes forming from late endosomal compartments (large arrows in Fig. 1); and (iii) by 30 min, the BSA-Rhod appears in distinct compartments, lysosomes, that are not labeled with RME-8::GFP but can be labeled with a lysosomal marker LMP-1::GFP (see Fig. 3). BSA-Rhod remains localized to these lysosomes for the duration of the experiment (arrowheads in Fig. 1).
Fig. 1.
Time course of uptake of BSA-Rhod by WT or by cup-5(zu223) coelomocytes expressing RME-8::GFP. Confocal images of unc-36(e251); rme-8::GFP (Left) or cup-5(zu223) unc-36(e251); rme-8::GFP (Right) coelomocytes at the indicated times after BSA-Rhod injection into the body cavity of the respective worms. RME-8::GFP is green, and the BSA-Rhod is red. Large arrows indicate concentrations of the BSA-Rhod in compartments labeled with RME-8::GFP. Arrowheads indicate lysosomes that are not labeled with RME-8::GFP. (Bar, 5 μm in all images.)
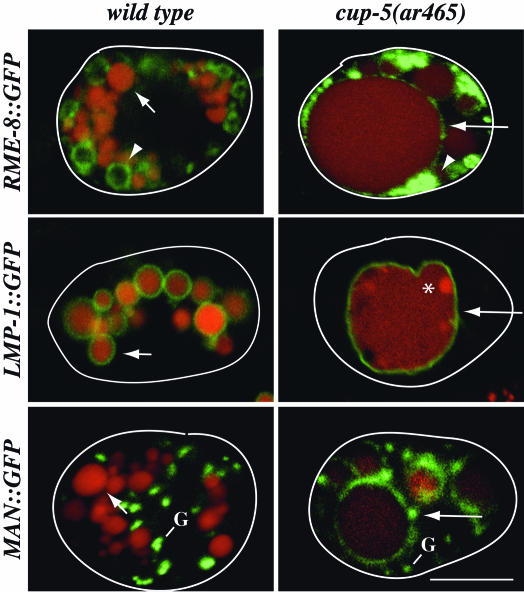
Fig. 3.
Morphological characterization of large vacuoles in coelomocytes of cup-5(ar465) worms. Shown are confocal images of WT or of cup-5(ar465) coelomocytes expressing the indicated GFP fusions (green) 24 h after BSA-Rhod (red) injection into the body cavity. Large arrows indicate large vacuoles, small arrows indicate lysosomes, arrowheads indicate late endosomes, the asterisk indicates heterogeneity in BSA-Rhod staining within the large vacuoles, and the small compartments stained by mannosidase II::GFP (MAN::GFP) are indicated with a G. Coelomocytes are outlined. (Bar, 5 μm in all images.)
Although the early stages of endocytosis are normal in cup-5(zu223) coelomocytes, we do not see a similar appearance of the dye in a distinct lysosomal compartment. Indeed, in cup-5 mutant coelomocytes, the BSA-Rhod remains concentrated in the budding regions of the late endosomal compartments after 60 min (large arrows in Fig. 1) and even after 120 min (data not shown), indicating that CUP-5 is required for the exit of endocytosed material from the late endosomal compartment. We observed similar results in the independently derived cup-5(ar465) mutant (12). Our results indicate that the coelomocyte defect in the viable allele ar465 is as pronounced as in the maternal-effect-lethal allele zu223 (H.F., unpublished results).
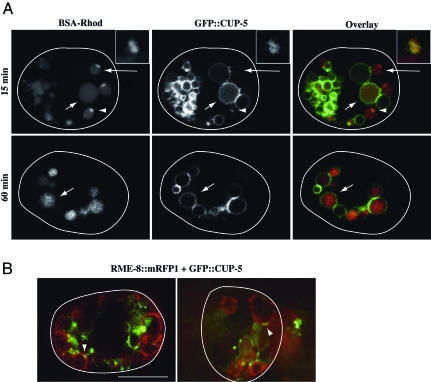
CUP-5 Localizes Strongly to Mature Lysosomes and also to Nascent Lysosomes Budding from RME-8-Labeled Organelles in WT Coelomocytes. Based on our functional observations indicating a requirement for CUP-5 in lysosome formation, we predicted that CUP-5 would normally localize to late endosomes, to lysosomes, or to both compartments in WT cells. To identify the CUP-5-positive compartment(s), we repeated the time course of BSA-Rhod uptake in coelomocytes expressing a rescuing GFP::CUP-5 fusion protein. After 15 min of uptake, most of the BSA-Rhod is in compartments that mostly lacked GFP::CUP-5 (large arrow in Fig. 2_A_). These late endosomes are the ones that are normally labeled with RME-8::GFP (see Fig. 1). However, GFP::CUP-5 colocalized with 30% (n = 30) of the BSA-Rhod budding from these compartments (arrowhead in Fig. 2 A); indeed, GFP::CUP-5 localized to the limiting membrane of these nascent lysosomes (Fig. 2 A Inset). Almost all of the internalized BSA-Rhod colocalized with the GFP::CUP-5 on mature lysosomes 60 min (90%, n = 100; small arrow in Fig. 2 A) or 24 h (100%; n = 100; H.F., unpublished results) after the start of the uptake assay. These results indicate that CUP-5 localizes to nascent and mature lysosomes.
Fig. 2.
CUP-5 localization to nascent and to mature lysosomes. (A) Confocal images of individual coelomocytes expressing GFP::CUP-5 (green) after injection of BSA-Rhod (red) into the body cavity. Images were collected at the 15- or 60-min time points. Large arrows indicate late endosomes, small arrows indicate lysosomes, and arrowheads indicate overlap in staining on nascent lysosomes. (Inset) A higher-magnification view of an en face view of nascent lysosomes budding from a late endosome in another coelomocyte. (B) Confocal (Left) and deconvolution (Right) images of coelomocytes coexpressing RME-8::mRFP1 (red) and GFP::CUP-5 (green). Arrowheads indicate localization of CUP-5 to a microdomain on RME-8-labeled compartments. Coelomocytes are outlined. (Bar, 5 μm in all images.)
We performed further experiments to determine whether the late endosomal marker RME-8, tagged with mRFP1, ever colocalized with CUP-5::GFP in WT coelomocytes. In agreement with the BSA-Rhod time courses in both RME-8::GFP and GFP::CUP-5 expressing coelomocytes, most of the RME-8 and CUP-5 proteins localize to different compartments. However, we consistently observed small discrete domains of CUP-5 localization on RME-8-labeled compartments (arrowheads in Fig. 2_B_).
These results indicate that CUP-5 is found on subdomains of the late endosomes. Experiments above indicated that this CUP-5-positive subdomain is where lysosomally-destined endocytic cargo accumulates in buds. Taken together, these experiments indicate that CUP-5 is normally located in a position to directly participate in the exit of lysosomally-directed cargo from late endosomes.
The Large Vacuoles in cup-5 Mutant Coelomocytes Display Properties of Aberrant Late Endosome/Lysosome Hybrid Organelles. One consequence of a defect in lysosome biogenesis in cup-5 mutant coelomocytes is that by 24 h, the BSA-Rhod-positive compartments become grossly enlarged, most likely because they continue to receive fluid and membrane from other compartments in the cell, but are defective in export from this compartment (12). The BSA-Rhod staining within the large vacuoles is not homogenous, indicating the presence of subendosomal structures (asterisk in Fig. 3).
We had previously reported (12) that the late endosomal marker RME-8::GFP does not localize to the large vacuoles in cup-5 mutant coelomocytes. After closer examination by using longer exposures, we now see that RME-8::GFP is present on the large abnormal vacuoles (large arrow in Fig. 3). Its staining is much less pronounced than on normal late endosomal compartments (arrowhead in Fig. 3), most likely due to the aberrant molecular composition of these large vacuoles. Note that the RME-8::GFP localization to large vacuoles is not apparent at standard exposures that reveal the staining around late endosomes (Fig. 3), and that we never see RME-8::GFP around lysosomes in WT cells at any exposure (H.F., unpublished data). The surprising localization of RME-8 to these abnormal vacuoles indicates that the vacuoles maintain late endosomal character even many hours after they receive endocytic cargo.
Further experiments indicated that the abnormal vacuoles contain molecular markers for lysosomes as well. We established a GFP-tagged marker for the C. elegans orthologue of LAMP/ CD68, LMP-1 (24), a standard marker for mammalian lysosomes, and showed that this protein is expressed in coelomocytes. BSA-Rhod pulse–chase analysis established that this marker normally resides in the limiting membrane of lysosomes, the terminal endocytic compartment in coelomocytes (small arrow in Fig. 3). Interestingly, we observed that LMP-1::GFP localizes to the limiting membrane of the large abnormal vacuoles of cup-5 mutant coelomocytes (large arrow in Fig. 3).
We examined the localization of a Golgi marker in cup-5 mutant coelomocytes. We reasoned that Golgi resident proteins that are transiently transported to normal late endosomes, either directly or after transport to the plasma membrane and subsequent endocytosis, and are then either recycled back to the Golgi or are sent to lysosomes for degradation might get trapped in the aberrant large vacuoles. Mannosidase II::GFP, a Golgi-resident protein, shows a punctate pattern of staining in coelomocytes (G in Fig. 3) and in muscle cells (27). In cup-5 mutant coelomocytes, the mannosidase II::GFP is partially mislocalized to the membrane of the large vacuoles (large arrow in Fig. 3).
Finally, we examined the localization of an early endosome marker (RAB-5) and of an endoplasmic reticulum marker (TRAM) (27, 29). Neither marker localized to the large vacuoles (Fig. 6, which is published as supporting information on the PNAS web site). These large vacuoles therefore constitute aberrant hybrid organelles that have characteristics of both late endosomes and lysosomes.
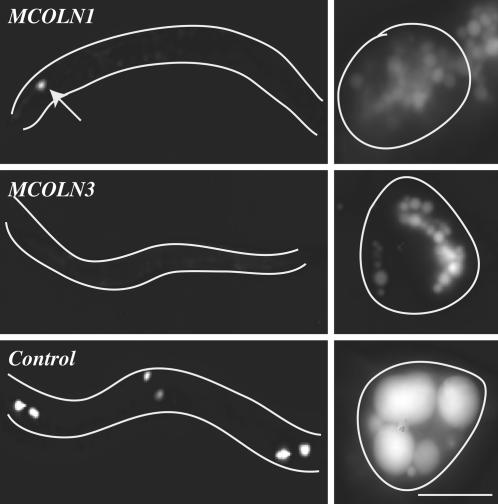
Expression of Human MCOLN1 or MCOLN3 in Worms Rescues the cup-5 Coelomocyte Endocytosis Defect. Expression of MCOLN1 in worms has been previously shown to partially rescue the maternal effect lethality of cup-5(zu223) worms (13). To assay rescue of the coelomocyte lysosome biogenesis defect, we subcloned the human MCOLN1 and MCOLN3 into a coelomocyte-specific expression vector and introduced these plasmids into cup-5(ar465); pmyo-3::ssGFP worms. All of the coelomocytes in this mutant strain accumulate abnormally large and bright GFP-positive vacuoles (12). Suppression of this mutant phenotype is seen as a reduction in the size and brightness of the vesicles in coelomocytes as they appear in WT worms. Expression of MCOLN1 and MCOLN3 almost completely suppressed (> 80%, n = 100) the cup-5 coelomocyte phenotype (Fig. 4). This finding indicates that CUP-5 is a functional orthologue of h-mucolipin-1 and h-mucolipin-3, and possibly other mucolipin proteins as well. These data support the idea that CUP-5 and human mucolipin proteins perform the same biological function within cells.
Fig. 4.
Rescue of the cup-5 coelomocyte endocytosis defect by expression of human homologues. Shown are epifluorescence images of cup-5(ar465); p_myo-3_::ssGFP worms expressing the indicated human genes in their coelomocytes. (Left) Low-magnification images showing whole worms (outlined in white). (Right) High-magnification images showing individual coelomocytes (outlined in white). GFP staining in rescued/WT coelomocytes is not obvious at this magnification in whole worms, whereas mutant coelomocytes are very bright. For comparison, we show a worm expressing human MCOLN1 with one of its coelomocytes that is not fully rescued (arrow). Due to their brightness, the mutant/nonrescued coelomocytes of control worms were photographed by using 1/10th the exposure time as the WT/rescued coelomocytes. (Bar, 5 μm in high-magnification images.)
Discussion
CUP-5 Function in the Biogenesis of Lysosomes. In all fixed worms, and by using the appropriate markers, we consistently observe condensation of lysosomal content in structures within late endosomes of coelomocytes. This observation suggests that lysosome biogenesis occurs at a very high rate in these cells, which makes them ideal for continued studies of this process.
In WT cells, BSA-Rhod first appears in RME-8-labeled late endosomes. Subsequently, the BSA-Rhod condenses in buds containing CUP-5 but not RME-8. Our observation that only 30% of these buds contain CUP-5 is misleading because the concentration of CUP-5 in these structures is very low, making it very hard to consistently detect the protein on these structures. Similarly, the relatively weak expression of LMP-1::GFP in coelomocytes precluded us from observing LMP-1 on these buds. This finding is in contrast to mature lysosomes where CUP-5 and LMP-1 accumulate and are easily detected.
Given that the size of these buds/vesicles is 0.3–0.4 μm (12, 22), they most likely represent nascent lysosomes rather than transport vesicles, which are typically 50–100 nm in diameter, destined for lysosomes. As such, the late endosomal compartments that contain these budding vesicles are reminiscent of hybrid organelles that originate from the fusion of late endosomes and lysosomes that have been described in mammalian cells (7, 30). It has been shown that members of the CUP-5/h-mucolipin-1 protein family can function as ion channels. Furthermore, it has been demonstrated that the resolution of hybrid organelles into lysosomes and late endosomes requires intraor-ganellar Ca2+ (10). Our studies implicate CUP-5/h-mucolipin-1 in this process and indicate them as excellent candidates for the essential Ca2+ channel. In addition to their channel function, CUP-5/h-mucolipin-1 may use other unidentified molecular mechanisms to regulate lysosome biogenesis, lysosome function, and other cellular processes.
In cup-5 mutants, the condensation of lysosomal content into nascent lysosomes proceeds normally. However, the complete maturation of these structures into discrete lysosomes is blocked/delayed, ultimately resulting in the accumulation of large vacuoles that display some of the characteristics of both late endosomes and of lysosomes (Fig. 5). The appearance of the large vacuoles indicates that the “arrested” hybrid organelles in cup-5 mutants are still competent to receive fluid and membrane and/or to fuse with other compartments. Indeed, we have previously shown that BSA-Rhod endocytosed by cup-5(ar465); pmyo-3::ssGFP mutant coelomocytes accumulates in the same large vacuoles containing previously endocytosed GFP (12). Given the early defect in lysosome biogenesis, potential roles for CUP-5 in mature lysosomes cannot be assayed in cup-5 mutant cells.
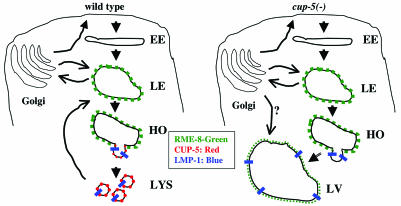
Fig. 5.
Model of CUP-5 function in coelomocytes. In WT, solutes and membrane are internalized and delivered to the early endosome (EE). Molecules from the endocytic and biosynthetic pathways are then delivered to late endosomes (LE). Lysosomal hydrolases and the solutes and internal vesicles destined for degradation are condensed in membrane structures budding off from these late endosomes. We refer to these structures as hybrid organelles (HO). The scission of the budding vesicles yields primary lysosomes (LYS), which, based on data from mammalian cells, can fuse with late endosomes or with each other. RME-8 (green) localizes to late endosomes. CUP-5 (red) and LMP-1 (blue) localize to sites of lysosome biogenesis on late endosomes. In cup-5 mutants, the maturation/scission of lysosomes budding from hybrid organelles is defective. These endosomes continue to receive membrane and solutes from the endocytic and biosynthetic pathways and hence increase in size. Due to their aberrant molecular composition, these large vacuoles (LV) contain less RME-8::GFP than late endosomes or hybrid organelles in WT cells. We do not know whether there is direct transport of biosynthetic material from the Golgi to these large vacuoles.
Cells in various tissues from MLIV patients display large heterogeneous vacuoles similar to those found in cup-5 mutant C. elegans coelomocytes; such structures do not have the characteristic morphology of dense core lysosomes (31–34). The large vacuoles in cells from MLIV patients contain functional lysosomal hydrolases (35) that could start to degrade the internalized membranes, thus contributing to the heterogeneity in content of the large vacuoles. The aberrant biochemical composition of the large vacuoles is consistent with a block or delay in sorting of molecules from these structures to other compartments. This kind of phenotype is also consistent with the observed delay in the metabolism of lipids that accumulate in aberrant vacuoles in MLIV cells (36–39).
Other Phenotypes of cup-5 Mutants/MLIV Patients. We had previously described preliminary evidence indicating that a second defect in cup-5 mutant coelomocytes is an increase in fluid phase uptake (12). The present study does not address this defect and concentrates on the lysosome biogenesis defect. It should be noted that the possible increased uptake makes our observation that the primary defect is in the scission of nascent lysosomes from late endosomes more striking, because it is based on morphological comparisons between WT and mutant cells at different times during the processing of endocytosed material.
cup-5(zu223) completely eliminates CUP-5 activity resulting in a lysosome biogenesis defect in coelomocytes, in maternal effect embryonic lethality, and in increased cell death (13). cup-5(ar465) is a hypomorphic mutation that only exhibits the coelomocyte defect, most likely because reduced CUP-5 activity is only limiting in coelomocytes that exhibit very high rates of endocytosis (12). Analogous to the cup-5 phenotypes in worms, the absence of h-mucolipin-1 causes a severe MLIV developmental phenotype partly caused by the degeneration of some tissues (40), and the accumulation of large vacuoles in some tissues that are associated with defects in cellular functions, whereas a milder point mutation that does not eliminate the protein only affects certain cellular functions (41).
It is still not known whether the more severe phenotypes in worms and in humans are the response of specific cell types to perturbations in endocytosis or whether they are due to a function of CUP-5/h-mucolipin-1 in cells. It is clear that blocking apoptosis in worms does not rescue the endocytic defect of cup-5 null worms and only partially rescues the maternal effect lethality, indicating that the embryonic lethality is at least partly due to additional unspecified defects in cell function (13).
We propose the following model for the observed phenotypes. Loss of function of CUP-5 results in a lysosome biogenesis defect and the accumulation of large vacuoles in most cells. Either directly or indirectly, this results in a rise in Ca2+ concentration in the cytoplasm. It is relevant in this context that inducing swelling of lysosomes in Madin–Darby canine kidney cells results in a transient release of the Ca2+ stores from these compartments (42). Furthermore, it has been shown that elevating cytosolic Ca2+ concentration can trigger fusions of lysosomes with each other and with other compartments (43, 44). This finding establishes a positive feedback loop leading to the appearance of the extremely large vacuoles in cup-5 and MLIV mutant cells.
What are the effectors of the cell death/degeneration in cells lacking CUP-5 or h-mucolipin-1? Recent evidence (45) indicates that cellular Ca2+ overload, or perturbation of intracellular Ca2+ compartmentalization, can cause cytotoxicity, and trigger either apoptotic or necrotic cell death. Indeed, it has been determined that necrotic cell death in C. elegans is inhibited by reducing the function of regulators of Ca2+ release from the endoplasmic reticulum (46). Another likely possibility is that the large vacuoles in cup-5 mutant cells might be fragile or leaky and may release lysosomal hydrolases into the cytoplasm. Indeed, the aberrant localization of cathepsin D, a lysosomal aspartic protease, to the cytoplasm, either through ectopic expression or by permeabilization of lysosomal membranes, can induce cell death (47, 48).
Functional Redundancy in the h-mucolipin Family. Both h-mucolipin-1 and h-mucolipin-3 can substitute for CUP-5 function in lysosome biogenesis in coelomocytes, indicating functional redundancy among the human proteins. However, the symptoms seen in MLIV patients lacking MCOLN1 and in mice lacking MCOLN3 do not overlap. It remains to be determined whether this is due to tissue-specific expression of the two proteins, or whether, if ubiquitously expressed, they have some unique functions in cellular pathways. Based on the rescue of the cup-5 defect and the high sequence similarity between h-mucolipin-1 and h-mucolipin-3, we would predict that both proteins are involved in aspects of lysosome biogenesis and/or function. We speculate that at least in some tissues, h-mucolipin-3 can replace h-mucolipin-1 because, in contrast to their other severe defects, MLIV patients have no hearing problems despite the fact that h-mucolipin-1 is highly expressed in inner ears (E.G., unpublished results). Finally, given the functional conservation between many worm and human genes and pathways, insights into the functions of worm proteins that modulate CUP-5 activity or processes are immediately translatable to humans and can be easily verified.
Supplementary Material
Supporting Figure
Acknowledgments
We thank Hope Dang, John Kennedy, John Falardeau, and Paul Fonarev for technical assistance and Julie Donaldson and Danny Brower for critical reading of the manuscript. This work was supported by a National Institute of General Medical Sciences grant (to H.F.) and a March of Dimes grant (to B.D.G.). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Abbreviations: BSA-Rhod, BSA conjugated to rhodamine; MLIV, mucolipidosis type IV; mRFP1, monomeric red fluorescent protein 1.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. NM_020533 (MCOLN1) and AF475085 (MCOLN3)].
References
- 1.Clague, M. J. (1998) Biochem. J. 336**,** 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Hondt, K., Heese-Peck, A. & Riezman, H. (2000) Annu. Rev. Genet. 34**,** 255–295. [DOI] [PubMed] [Google Scholar]
- 3.Sorkin, A. (2000) J. Cell Sci. 113**,** 4375–4376. [DOI] [PubMed] [Google Scholar]
- 4.Lemmon, S. K. & Traub, L. M. (2000) Curr. Opin. Cell Biol. 12**,** 457–466. [DOI] [PubMed] [Google Scholar]
- 5.Luzio, J. P., Poupon, V., Lindsay, M. R., Mullock, B. M., Piper, R. C. & Pryor, P. R. (2003) Mol. Membr. Biol. 20**,** 141–154. [DOI] [PubMed] [Google Scholar]
- 6.Mullins, C. & Bonifacino, J. S. (2001) BioEssays 23**,** 333–343. [DOI] [PubMed] [Google Scholar]
- 7.Luzio, J. P., Mullock, B. M., Pryor, P. R., Lindsay, M. R., James, D. E. & Piper, R. C. (2001) Biochem. Soc. Trans. 29**,** 476–480. [DOI] [PubMed] [Google Scholar]
- 8.Luzio, J. P., Rous, B. A., Bright, N. A., Pryor, P. R., Mullock, B. M. & Piper, R. C. (2000) J. Cell Sci. 113**,** 1515–1524. [DOI] [PubMed] [Google Scholar]
- 9.van Deurs, B., Holm, P. K., Kayser, L. & Sandvig, K. (1995) Eur. J. Cell Biol. 66**,** 309–323. [PubMed] [Google Scholar]
- 10.Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, S. R. & Luzio, J. P. (2000) J. Cell Biol. 149**,** 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fares, H. & Greenwald, I. (2001) Genetics 159**,** 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fares, H. & Greenwald, I. (2001) Nat. Genet. 28**,** 64–68. [DOI] [PubMed] [Google Scholar]
- 13.Hersh, B. M., Hartwieg, E. & Horvitz, H. R. (2002) Proc. Natl. Acad. Sci. USA 99**,** 4355–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun, M., Goldin, E., Stahl, S., Falardeau, J. L., Kennedy, J. C., Acierno, J. S., Jr., Bove, C., Kaneski, C. R., Nagle, J., Bromley, M. C., et al. (2000) Hum. Mol. Genet. 9**,** 2471–2478. [DOI] [PubMed] [Google Scholar]
- 15.Bassi, M. T., Manzoni, M., Monti, E., Pizzo, M. T., Ballabio, A. & Borsani, G. (2000) Am. J. Hum. Genet. 67**,** 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargal, R., Avidan, N., Ben-Asher, E., Olender, Z., Zeigler, M., Frumkin, A., Raas-Rothschild, A., Glusman, G., Lancet, D. & Bach, G. (2000) Nat. Genet. 26**,** 118–123. [DOI] [PubMed] [Google Scholar]
- 17.LaPlante, J. M., Falardeau, J., Sun, M., Kanazirska, M., Brown, E. M., Slaugenhaupt, S. A. & Vassilev, P. M. (2002) FEBS Lett. 532**,** 183–187. [DOI] [PubMed] [Google Scholar]
- 18.Di Palma, F., Belyantseva, I. A., Kim, H. J., Vogt, T. F., Kachar, B. & Noben-Trauth, K. (2002) Proc. Natl. Acad. Sci. USA 99**,** 14994–14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner, S. (1974) Genetics 77**,** 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mello, C. & Fire, A. (1995) Methods Cell Biol. 48**,** 451–482. [PubMed] [Google Scholar]
- 21.Graham, P. L. & Kimble, J. (1993) Genetics 133**,** 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y., Grant, B. & Hirsh, D. (2001) Mol. Biol. Cell 12**,** 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., Fritch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd. Ed.
- 24.Kostich, M., Fire, A. & Fambrough, D. M. (2000) J. Cell Sci. 113**,** 2595–2606. [DOI] [PubMed] [Google Scholar]
- 25.Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C. (1994) Science 263**,** 802–805. [DOI] [PubMed] [Google Scholar]
- 26.Pereira-Leal, J. B. & Seabra, M. C. (2001) J. Mol. Biol. 313**,** 889–901. [DOI] [PubMed] [Google Scholar]
- 27.Rolls, M. M., Hall, D. H., Victor, M., Stelzer, E. H. & Rapoport, T. A. (2002) Mol. Biol. Cell 13**,** 1778–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A. & Tsien, R. Y. (2002) Proc. Natl. Acad. Sci. USA 99**,** 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucci, C., Parton, R. G., Mather, I. H., Stunnenberg, H., Simons, K., Hoflack, B. & Zerial, M. (1992) Cell 70**,** 715–728. [DOI] [PubMed] [Google Scholar]
- 30.Mullock, B. M., Bright, N. A., Fearon, C. W., Gray, S. R. & Luzio, J. P. (1998) J. Cell Biol. 140**,** 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman, E. R., Livni, N., Shapira, E., Merin, S. & Levij, I. S. (1974) J. Pediatr. (Berlin) 84**,** 519–526. [DOI] [PubMed] [Google Scholar]
- 32.Goebel, H. H., Kohlschutter, A. & Lenard, H. G. (1982) Clin. Neuropathol. 1**,** 73–82. [PubMed] [Google Scholar]
- 33.Lubensky, I. A., Schiffmann, R., Goldin, E. & Tsokos, M. (1999) Am. J. Surg. Pathol. 23**,** 1527–1531. [DOI] [PubMed] [Google Scholar]
- 34.Merin, S., Livni, N., Berman, E. R. & Yatziv, S. (1975) Invest. Ophthalmol. 14**,** 437–448. [PubMed] [Google Scholar]
- 35.Bach, G., Chen, C. S. & Pagano, R. E. (1999) Clin. Chim. Acta 280**,** 173–179. [DOI] [PubMed] [Google Scholar]
- 36.Bargal, R. & Bach, G. (1997) J. Inherited Metab. Dis. 20**,** 625–632. [DOI] [PubMed] [Google Scholar]
- 37.Chen, C. S., Bach, G. & Pagano, R. E. (1998) Proc. Natl. Acad. Sci. USA 95**,** 6373–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeigler, M., Bargal, R., Suri, V., Meidan, B. & Bach, G. (1992) Prenatal Diagn. 12**,** 1037–1042. [DOI] [PubMed] [Google Scholar]
- 39.Jansen, S. M., Groener, J. E., Bax, W. & Poorthuis, B. J. (2001) J. Inherited Metab. Dis. 24**,** 577–586. [DOI] [PubMed] [Google Scholar]
- 40.Altarescu, G., Sun, M., Moore, D. F., Smith, J. A., Wiggs, E. A., Solomon, B. I., Patronas, N. J., Frei, K. P., Gupta, S., Kaneski, C. R., et al. (2002) Neurology 59**,** 306–313. [DOI] [PubMed] [Google Scholar]
- 41.Bach, G. (2001) Mol. Genet. Metab. 73**,** 197–203. [DOI] [PubMed] [Google Scholar]
- 42.Haller, T., Volkl, H., Deetjen, P. & Dietl, P. (1996) Biochem J. 319**,** 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaconi, M. E., Lew, D. P., Carpentier, J. L., Magnusson, K. E., Sjogren, M. & Stendahl, O. (1990) J. Cell Biol. 110**,** 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakker, A. C., Webster, P., Jacob, W. A. & Andrews, N. W. (1997) J. Cell Sci. 110**,** 2227–2238. [DOI] [PubMed] [Google Scholar]
- 45.Orrenius, S., Zhivotovsky, B. & Nicotera, P. (2003) Nat. Rev. Mol. Cell Biol. 4**,** 552–565. [DOI] [PubMed] [Google Scholar]
- 46.Xu, K., Tavernarakis, N. & Driscoll, M. (2001) Neuron 31**,** 957–971. [DOI] [PubMed] [Google Scholar]
- 47.Deiss, L. P., Galinka, H., Berissi, H., Cohen, O. & Kimchi, A. (1996) EMBO J. 15**,** 3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 48.Boya, P., Andreau, K., Poncet, D., Zamzami, N., Perfettini, J. L., Metivier, D., Ojcius, D. M., Jaattela, M. & Kroemer, G. (2003) J. Exp. Med. 197**,** 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure