Small Conductance Ca2+-Activated K+ Channel Knock-Out Mice Reveal the Identity of Calcium-Dependent Afterhyperpolarization Currents (original) (raw)
Abstract
Action potentials in many central neurons are followed by a prolonged afterhyperpolarization (AHP) that influences firing frequency and affects neuronal integration. In hippocampal CA1 pyramidal neurons, the current ascribed to the AHP (IAHP) has three kinetic components. The IfastAHP is predominantly attributable to voltage-dependent K+ channels, whereas Ca2+-dependent and voltage-independent K+channels contribute to the ImediumAHP (ImAHP) and IslowAHP (IsAHP). Apamin, which selectively suppresses a component of the mAHP, increases neuronal excitability and facilitates the induction of synaptic plasticity at Schaffer collateral synapses and hippocampal-dependent learning. The Ca2+-dependent components of the AHP have been attributed to the activity of small conductance Ca2+-activated K+ (SK) channels. Examination of transgenic mice, each lacking one of the three SK channel genes expressed in the CNS, reveals that mice without the SK2 subunit completely lack the apamin-sensitive component of the ImAHP in CA1 neurons, whereas the IsAHP is not different in any of the SK transgenic mice. In each of the transgenic lines, the expression levels of the remaining SK genes are not changed. The results demonstrate that only SK2 channels are necessary for the ImAHP, and none of the SK channels underlie the IsAHP.
Keywords: SK channels, apamin, CA1 neurons, ImAHP, transgenic mice, knock-out
Introduction
A principal determinant of neuronal excitability is the afterhyperpolarization (AHP) that follows an action potential. In hippocampal CA1 pyramidal cells and many other neurons, three overlapping kinetic components of the AHP, frequently recorded as the current ascribed to the AHP (IAHP) in voltage clamp, have been distinguished. Reports using pharmacological agents to examine the underlying channels have suggested that BK channels (large conductance voltage- and Ca2+-dependent K+ channels) contribute to the IfastAHP (IfAHP) with time constants on the order of 50 msec (Lancaster and Adams, 1986; Shao et al., 1999; Sah and Faber, 2002). A component of the ImediumAHP (ImAHP) is eliminated by apamin, a selective blocker of small conductance Ca2+-activated K+ (SK) channels, and the apamin-sensitive current decays with time constants of ∼200 msec (Stocker et al., 1999; Gerlach et al., 2004). BK channels, KCNQ (_I_M) and HCN (_I_h), also contribute to the medium component (Storm, 1989). The channels underlying the slow component, IslowAHP (IsAHP), are K+ selective, voltage independent, and require Ca2+ influx through voltage-dependent Ca2+ channels for activation (Lancaster and Adams, 1986). These characteristics have suggested that SK channels are responsible for the sAHP, although the IsAHP is not blocked by apamin (Stocker et al., 1999; Bowden et al., 2001; Gerlach et al., 2004).
Application of apamin to CA1 neurons increases excitability, which can be seen as a shortened interspike interval, especially early in a burst of action potentials, and increases the number of action potentials for a given current injection. The action potential duration and the resting membrane potential do not appear altered. Thus, the apamin-sensitive mAHP in CA1 neurons principally affects the instantaneous firing rate and sets the duration of the interspike interval within a train of action potentials (Stocker et al., 1999). The IsAHP demonstrates different kinetics, with a prominent rising phase after a voltage command with a τ rise of ∼500 msec; the IsAHP decays slowly over several seconds. Suppressing the sAHP with activators of protein kinase A blocks spike-frequency adaptation and bursting behavior, resulting in tonic firing (Madison and Nicoll, 1982; Pedarzani and Storm, 1993; Stocker et al., 1999).
In addition to contributing to the IAHP, apamin-sensitive channels in CA1 neurons also affect the induction of synaptic plasticity and memory encoding. Apamin shifts the threshold for induction of NMDA receptor-dependent synaptic plasticity to lower stimulus frequencies and enhances hippocampal-dependent spatial and nonspatial memory encoding (Stackman et al., 2002).
Four SK channel family genes have been cloned, and three of them are expressed in the CNS: SK1, 2, and 3 (Köhler et al., 1996; Ishii et al., 1997b; Joiner et al., 1997; Stocker and Pedarzani, 2000). SK channels are molecular complexes of four poreforming subunits with four molecules of constitutively associated calmodulin (CaM). Binding of Ca2+ to the N-terminal E-F hands of CaM triggers channel gating (Xia et al., 1998; Keen et al., 1999). SK1, 2, and 3 are apamin sensitive, although their affinities vary. Moreover, when heterologously coexpressed, SK subunits are capable of forming heteromeric channels, although it is not clear that they do so in vivo (Ishii et al., 1997a; Sailer et al., 2002; Benton et al., 2003; Monaghan et al., 2003). Apamin dose-response from CA1 in hippocampal brain slice recordings revealed an IC50 of 480 pm for the apamin-sensitive component of the ImAHP. Although solution exchange in the slice is not optimal, the data support a major contribution of SK2 to the ImAHP (Stocker et al., 1999). To determine the contribution of each of the SK channels to the IAHP in CA1 neurons, transgenic mouse lines were constructed, each lacking one of the SK channel genes, and brain slice recordings were conducted.
Materials and Methods
Transgenic mice SK1
Δ_/_Δ mice. A 6.6 kb genomic DNA fragment encompassing exon 1 [5′ untranslated region (UTR)] to the intron between exons 6 (pore) and 7 was isolated from a 129/Sv mouse genomic library. A single loxP site was inserted into the 5′UTR 40 nucleotides 5′ of the initiator methionine codon. A cassette consisting of the neomycin resistance gene flanked by loxP sites and the coding sequence for enhanced green fluorescent protein (GFP) was inserted into a unique _Hpa_I site in the intron between exons 5 and 6. Recombination across loxP sites results in the loss of exons 3, 4, and 5 [encoding initiation of translation through transmembrane (TM) five]. The targeting construct was electroporated into embryonic stem (ES) cells (University of Cincinnati ES Core, Cincinnati, OH), and G418-resistant colonies were analyzed for homologous recombination by PCR and genomic Southern blot. One positive clone, when injected into C57Bl/6 blastocysts, yielded two chimeras that transmitted the targeted allele via the germline. Animals heterozygous for the floxed allele were bred to the Cre-deleter mouse that expresses Cre recombinase ubiquitously from the two-cell stage (Schwenk et al., 1995). Offspring heterozygous for the recombined allele (+/Δ) were bred to yield homozygous-deleted SK1 animals (Δ/Δ).
SK2 Δ_/_Δ mice. A single loxP site was introduced into the 5′UTR 300 nucleotides 5′ of the initiator methionine and a cassette consisting of the neomycin-resistance gene flanked by loxP sites, and the coding sequence for enhanced GFP was inserted into an _Eco_RV site in intron 2, ∼2.3 kb from the end of exon 2. Recombination at the loxP sites yields an allele that is deleted for SK2 exons 1 and 2. The targeting construct was electroporated into ES cells, and several properly recombined ES cell clones were injected into C57Bl/6 blastocysts. One chimera gave germline transmission of the targeted allele. Crosses between heterozygous-floxed SK2 and the Cre deleter mouse yielded offspring that are heterozygous for the SK2_-deleted allele (+/Δ). Crosses of SK2 +/Δ mice yielded SK2 Δ/_Δ mice.
SK3 tTA mice. The SK3 mice used in this study have been reported previously (Bond et al., 2000). The SK3 gene was altered by homologous recombination, inserting a doxycycline (dox)-sensitive gene switch into the exon encoding the 5′UTR. Using this strategy, temporal and spatial expression patterns are preserved, whereas gene expression is controlled by dietary dox. The SK3 tTA mice have approximately threefold basal overexpression of SK3, and SK3 expression is effectively eliminated by dietary dox from conception.
Western blotting
Mouse brains were homogenized in 320 mm sucrose, 10 mm HEPES, 1 mm EGTA, pH 7.4 (HS), supplemented with a protease inhibitor mixture. Nuclear material was removed by centrifugation at 900 × g. Crude synaptosomal membranes were recovered in the pellet after centrifugation at 10,000 × g, washed once, and lysed in hypotonic solution containing 10 mm HEPES, 1 mm EGTA, pH 7.4 (HE). The lysed synaptotomsal membranes were collected by centrifugation at 25,000 × g, resuspended in HS, and layered onto a sucrose step-gradient (1.2, 1.0, and 0.8 m sucrose in 10 mm HEPES, 1 mm EGTA). After centrifugation at 150,000 × g for 90 min, membranes banding at the 1.0/1.2 m sucrose interface were recovered, diluted in HE, and collected by centrifugation for 30 min at 150,000 × g. These membranes were stored in aliquots in HS at -80°C. Thirty micrograms of membrane protein was separated on 8% acrylamide-SDS gels and transferred to nitrocellulose. Primary antibody dilutions were as follows: affinity purified rabbit anti-SK2, 2 μg/ml; anti-SK3, 1:1000 (raised against DTSGHFHDSGVGDLDEDPKCP in the N-terminal domain; Alomone Labs, Jerusalem, Israel); anti-Na+K+ATPase, 1:25,000 (courtesy of Dr. Svetlana Lutsenko, Oregon Health and Science University, Portland, OR). Protein-antibody complexes were visualized using Picosignal ECL reagents (Pierce, Rockford, IL). The polyclonal anti-SK2 antiserum was raised in rabbits against a sequence (ETQMENYDKHVTYNAE) in the C-terminal domain (Global Peptide Service, Fort Collins, CO). Anti-SK2 antibodies were purified using the peptide antigen immobilized on Sulfo-Link resin (Pierce).
Real-time PCR
Whole-brain total RNA or total hippocampal RNA was isolated using Tri-reagent according to the protocol of the manufacturer. Total RNA was reverse-transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) in the presence of random hexamers but without dithiothriotol. Real-time PCRs were performed in triplicate for each SK transcript in each genotype, and expression levels were determined by comparison with 18S ribosomal RNA (rRNA). The amplicon for 18S was 76 bp (primers: CCGCAGCTAGGAATAATGGA, CCCTCTTAATCATGGCCTCA); for SK1, 118 bp (primers: GCTCTTTTGCTCTGAAATGCC, CAGTCGTCGGCACCATTGTCC); for SK2, 151 bp (primers: GTCGCTGTATTCTTTAGCTCTG, ACGCTCATAAGTCATGGC); for SK3, 148 bp (primers: GCTCTGATTTTTGGGATGTTTG, CGATGATCAAACCAAGCAGG ATGA). All SK amplicons span an intron. The efficiencies of the primer pairs were tested in a validation experiment using serial dilutions of a wild-type cDNA [slope of ΔCt (SKCt-18SCt) < 0.1; data not shown). The threshold cycle (Ct) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold. The reaction master mix, consisting of 10× buffer, Mg (_C_f = 4 mm), deoxyribonucleotide triphosphates (_C_f = 200 μm), Platinum taq polymerase (Invitrogen; 0.6 U/20 μl reaction), and SYBR Green (Molecular Probes, Eugene, OR; 0.5× recommended concentration of the manufacturer), was aliquoted, the cDNA substrates added, and then further aliquoted and primers added (_C_f = 200 nm). Reactions were then split into triplicates for amplification in an MJ Research (Watertown, MA) Opticon DNA Engine with cycling parameters 95°C one time for 2 min, 95°C for 30 sec/64°C for 45 sec, with fluorescence read at 78°C for 40 cycles. A melting curve and gel electrophoresis analysis verified that a single product was amplified in all reactions.
Analysis
For each run, the relative mRNA level was determined by the expression 2-ΔΔCt (ΔCt (SKCt-18SCt) within each genotype, ΔΔCt (ΔCtSK transgene-ΔCtwild type) (ABI Prism 7700 Sequence Detection System, user bulletin 2; Applied Biosystems, Foster City, CA). The mean and SE of the value 2-ΔΔCt for each SK mRNA in each genotype, across all runs, were plotted. Statistical significance was determined by one-way ANOVA of ΔCt values across all genotypes followed by Bonferroni t test.
Preparation of hippocampal slices
Mice (16-20 d of age) were used for all studies in accordance with guidelines approved by the department of animal care at Oregon Health and Science University. Mice were first sedated by intraperitoneal injection of a ketamine/xylazine mixture and then perfused through the left ventricle with ice-cold oxygenated artificial CSF (ACSF) solution described below. After decapitation, the hippocampus was removed, and transverse slices (350 μm thick) were cut with a vibratome (Leica VT 1000S; Leica, Nussloch, Germany) in ACSF. Slices were subsequently incubated at 35°C for 30 min in ACSF and allowed to recover at room temperature for 30 min before recording. All recordings were performed at room temperature. The ACSF solution contained (in mm): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, saturated with 95% O2/5% CO2, pH 7.35.
Electrophysiology
CA1 neurons were visualized with a microscope equipped with infrared-differential interference contrast optics (Leica DMLFS). Whole-cell recording pipettes were fabricated from thin-wall borosilicate glass having resistances of 1.5-3 MΩ. Pipettes were filled with an intracellular solution containing (in mm): 140 KMeSO4, 8 NaCl, 1 MgCl2, 10 HEPES, 2 Mg-ATP, 0.4 Na2-GTP, 20 μm EGTA, pH 7.3 (290 mOsm). Slices were continuously superfused with ACSF saturated with 95% O2/5% CO2. Whole-cell patch-clamp currents were recorded with a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA), digitized using an ITC-16 analog-to-digital converter (InstruTech, Greatneck, NY), and acquired onto computer using Pulse software (Heka Elektronik, Lambrecht, Germany).
For measurement of the IAHP, CA1 neurons were held at -55 mV, and IAHP currents were evoked by depolarizing voltage commands to 20 mV for 100 msec followed by a return to -55 mV for 10 sec. All cells had a resting membrane potential more hyperpolarized than -55 mV and input resistances of 150-350 MΩ. Access resistance was stable at <20 MΩ and was 80% compensated. IAHP recordings were filtered at 1 kHz and digitized at a sampling frequency of 1 kHz; the records were filtered further off-line with a 300 Hz Gaussian filter (for one pass). Data were analyzed in Igor Pro (Wavemetrics, Lake Oswego, OR). Data are expressed as mean ± SEM. Statistical significance was tested using ANOVA where appropriate, and p < 0.01 was considered significant. Apamin was obtained from Calbiochem (La Jolla, CA).
Results
SK transgenic mice
Three SK channel genes, SK1, SK2, and SK3, are expressed in the CNS, and each gene shows a distinct tissue and cell type expression profile (Köhler et al., 1996; Stocker and Pedarzani, 2000).
The mRNAs for each of the SK genes have been detected in CA1 neurons by in situ hybridization (Stocker et al., 1999). To determine the contributions of each of the genes to the IAHP in CA1 neurons, conditional knock-out alleles were constructed by homologous recombination (Fig. 1_A_). For SK1 and SK2, flanking coding exons with loxP sites (floxing) created conditional alleles. For SK1, loxP sites were inserted within exon 3 and after exon 5, encompassing the sequences that encode the initiator methionine through TM domain five. For SK2, loxP sites flanked exons encoding the intracellular N-terminal domain and TMs one and two. Excision of the SK coding exons in all cell types, including germ cells, was accomplished by crossing animals harboring the floxed allele with a Cre-deleter mouse (Schwenk et al., 1995), which ubiquitously expresses Cre recombinase very early after conception. Crossing heterozygous animals yielded homozygous-null offspring for either the SK1 or SK2 gene. SK3 knockout mice have been reported previously and were achieved by insertion of a tetracycline-regulated gene switch into the exon encoding the 5′ UTR. SK3 expression is abolished by dietary dox (Bond et al., 2000) (see below). Homozygous female transgenic mice lacking any one of the SK genes were viable and reproductively competent.
Figure 1.
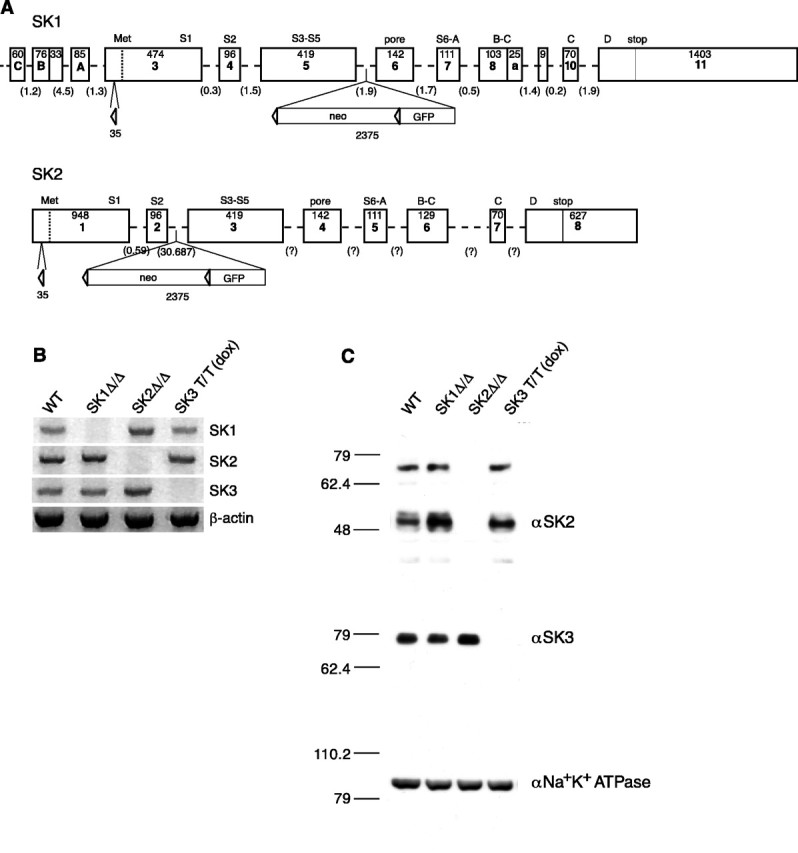
SK transgenic mice. A, Schematic representations of the SK1 and SK2 transgene loci; SK1 flox (top) and SK2 flox (bottom) alleles. The sizes of the exons (in base pairs) and the channel domains they encode are presented, and the sizes of the introns (in kilobase pairs) are given for those that have been determined. The inserted elements (triangles represent loxP sites), their sizes, and positions in the targeting constructs are shown below the exon-intron mosaic. B, RT-PCR analysis using total brain cDNA shows the loss of each SK mRNA in the respective knock-out mice. β-actin was amplified to approximate the relative amounts of cDNA from the different genotypes. C, Western blots of brain proteins for SK2 and SK3. The same samples (30 μg) were loaded in each lane for each of the blots. The top blot was probed with an affinity-purified polyclonal SK2 antiserum and shows the loss of SK2 in SK2 Δ_/_Δ mice. The middle blot was probed with a polyclonal SK3 antiserum and shows the loss of SK3 protein in SK3 T/T mice that had been administered dietary doxycycline (Bond et al., 2000) (see Materials and Methods). The bottom blot was probed with a polyclonal Na+-K+ ATPase antiserum and shows the relative sample loading across all lanes.
Analysis by reverse transcription (RT)-PCR and Western blotting verified effective genetic manipulations for each SK subunit. Brain cDNAs from SK1 Δ_/Δ, SK2 Δ/_Δ, and SK3 T/T(dox) mice or wild-type controls were used for PCRs specific for each of the SK mRNAs (Fig. 1_B_, left). The results showed the loss of SK1, SK2, or SK3 mRNA expression in the respective mice. Western blotting was performed with brain membrane proteins using SK2- or SK3-specific polyclonal antibodies. The results showed an absence of the respective SK protein.
SK2 channels underlie the apamin-sensitive current in CA1 neurons
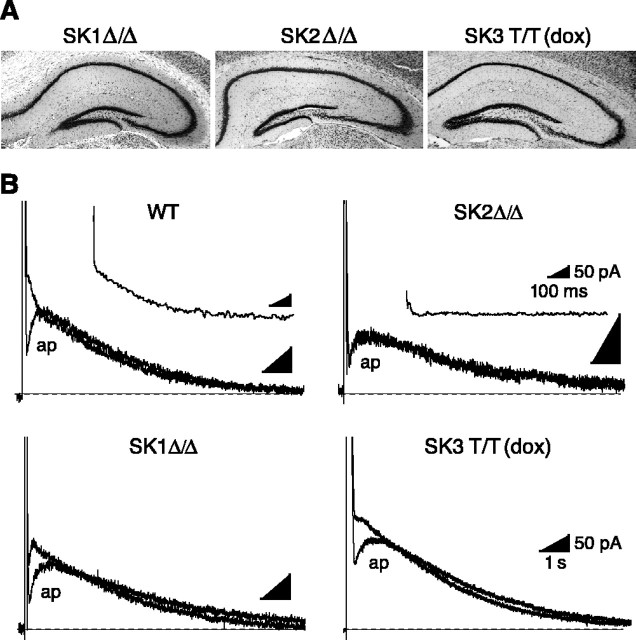
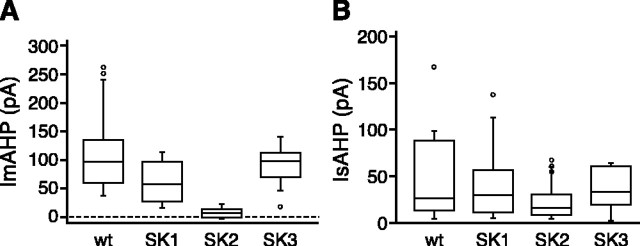
To determine the contributions of each of the SK subunits to the IAHP in CA1 neurons, whole-cell voltage-clamp recordings were performed in hippocampal slices from the different SK transgenic mice. Anatomically, there were no obvious abnormalities in the hippocampi of any of the SK transgenic mice, and the CA1 region was not different from wild type (Fig. 2_A_). The IAHP was measured at -55 mV after a 100 msec depolarization to 20 mV (Fig. 2_B_). In slices from wild-type mice, apamin blocked an early component of the IAHP and the apamin-sensitive component of the ImAHP, obtained as the difference between the IAHP in control and after apamin application, decayed with a time constant of 192 ± 11 msec (n = 15) (Fig. 2_B_, inset; Table 1). Apamin also blocked an initial component in each genotype except SK2 Δ_/Δ, in which the difference trace was essentially flat. To quantify the apamin-sensitive component of the ImAHP and the IsAHP in each of the transgenic mice, the amplitude of the apamin-sensitive current was measured at 100 msec after the depolarizing pulse, whereas the IsAHP was measured at 1 sec in the presence of apamin. The results show that in CA1 neurons, the amplitude of the apamin-sensitive component was not significantly different in mice lacking SK1 or SK3. In contrast, SK2 Δ/Δ mice completely lacked the apamin-sensitive component of the ImAHP (Fig. 3). The time constant of decay of the apamin-sensitive component of the ImAHP was not different from wild type in SK1 Δ/Δ or SK3 T/T(dox) mice. Although the amplitude of the IsAHP was more variable, the IsAHP amplitude in any of the transgenic lines was not different from wild type, showing that no member of the SK channel family is required for the IsAHP (Fig. 3). The time constants for the decay of the apamin-sensitive component of the ImAHP and IsAHP, as well as the half-rise time of the IsAHP, are summarized in Table 1 and show that except for SK2 Δ/_Δ mice that lack the apamin-sensitive component of the ImAHP, these kinetic parameters, as well as membrane capacitance, were not different in any of the transgenic mice compared with wild-type mice.
Figure 2.
Voltage-clamp analysis of the IAHP. A, Thionin-stained hippocampal sections from SK knock-out mice. No obvious anatomical abnormalities were observed in any of the genotypes. B, Voltage-clamp recordings of mouse CA1 neurons after a 100 msec depolarizing pulse to +20 mV. Apamin (ap) blocks an initial component of the IAHP in all of the genotypes except SK2 Δ_/Δ mice, which lack the apamin-sensitive current. Subtraction of the traces before and after apamin application yielded the ImAHP (shown for wild type and SK2 Δ/Δ). Scale bars for full traces correspond to those for SK3 T/T (dox); those for the insets correspond to the values shown for SK2 Δ/_Δ.
Table 1.
Cell parameters for different transgenic mice
| Genotype | Cap, pF ± SEM (n) | Rinp, MΩ ± SEM (n) | τ ImAHP, msec ± SEM (n) | T1/2 IsAHP, msec ± SEM (n) | τ IsAHP, sec ± SEM (n) |
|---|---|---|---|---|---|
| WT | 120.1 ± 15.4 (15) | 257.6 ± 21.7 (15) | 192.4 ± 10.9 (15) | 183.0 ± 12.4 (3) | 2.9 ± 0.2 (7) |
| SK2 Δ/Δ[ρ] | 130.5 ± 14.0 (15) | 270.0 ± 25.5 (15) | 244.2 ± 98.9 (3) | 3.2 ± 0.2 (9) | |
| SK1 Δ/Δ[ρ] | 123.0 ± 20.7 (8) | 219.5 ± 18.1 (8) | 200.2 ± 26.0 (7) | 163.7 ± 52.9 (2) | 3.3 ± 0.3 (5) |
| SK3 T/T (dox) | 137.5 ± 8.5 (12) | 232.5 ± 12.6 (13) | 197.0 ± 15.7 (11) | 135.2 ± 11.9 (2) | 2.7 ± 0.1 (8) |
Figure 3.
SK2 channels underlie the apamin-sensitive component of the ImAHP, whereas the SK channels do not contribute to the IsAHP. Left, Box plot of the amplitudes of the apamin-sensitive component of the ImAHP from the different genotypes measured 100 msec after the pulse. Mice lacking SK2 channels do not express an apamin-sensitive ImAHP. Statistical differences were determined by ANOVA, yielding p values <0.001 for _SK2_ Δ_/_Δ, 0.23 for _SK1_ Δ_/_Δ, 0.92 for _SK3 T/T_ (dox) mice compared with wild type. Right, Box plot of IsAHP amplitudes. The IsAHP was measured in the presence of apamin as the current amplitude at 1 sec after the end of the pulse. Statistical differences were determined by ANOVA, yielding _p_ values of 0.26 for SK2 Δ/Δ, 1.00 for _SK1_ Δ_/_Δ, 0.90 for _SK3 T/T_ (dox) mice compared with wild type. There were no significant differences between groups (_n_ > 7), showing that none of the SK channels contribute to the IsAHP.
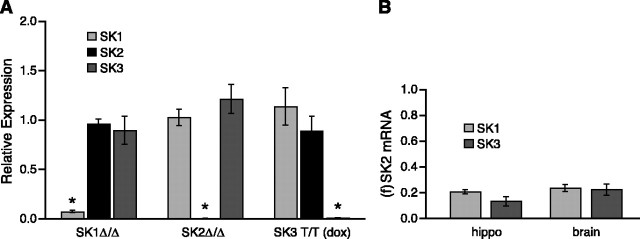
To evaluate whether there are changes in the steady-state mRNA expression levels of the remaining SK channel genes in each of the null mice, mouse brain cDNA was used for quantitative RT-PCR (see Materials and Methods). The values for each of the SK amplicons were normalized to those obtained from wild-type animals; 18S rRNA was used as the internal reference. As shown in Figure 4, mRNA levels for the two remaining intact SK genes were not significantly altered in any of the knock-out animals.
Figure 4.
Quantitative analysis of SK mRNA levels in SK transgenic mice. A, Real-time PCR performed with whole-brain RNA from the different SK transgenic mice was used to evaluate the levels of each of the SKm RNAs, normalized to the levels determined for wild type (n = 5 for each genotype; see Materials and Methods). 18S ribosomal RNA was used as the internal reference. B, Comparison of levels of SK1 and SK3 mRNAs to SK2 mRNA in whole brain and hippocampus of wild-type mice by real-time PCR. 18S rRNA was used as the internal reference. Relative expression compared with wild type was calculated as the mean of 2-ΔΔCt ± SEM and shows that mRNA levels derived from the unmodified SK genes are not significantly altered in any of the transgenic mice. Statistically significant differences (p < 0.05) in expression levels compared with wild type are indicated by an asterisk.
Although not significant, there was a clear trend to lower apamin-sensitive component ImAHP amplitudes in the SK1 Δ_/_Δ mice (Table 2). Recent reports show that heterologous coexpression of SK1 with either SK2 or SK3 results in heteromeric channels and suggest that rodent SK1 subunits, incapable of forming functional homomeric channels themselves, may contribute to the levels of channels containing SK2 or SK3. To investigate the potential contribution of SK1, the levels of SK1 and SK3 mRNAs, normalized to SK2 mRNA levels, were determined from wild-type whole brain or hippocampus. The results show that SK1 mRNA levels are ∼20% of SK2 levels in either whole brain or hippocampus. SK3 levels are ∼20% in whole brain but reduced to ∼10% relative to SK2 mRNA levels in hippocampus (Fig. 4).
Table 2.
AHP current amplitudes
| Genotype | ImAHP (pA) | p value | IsAHP (pA) | p value |
|---|---|---|---|---|
| WT | 116.7 ± 68.2 (15) | 46.6 ± 46.8 (15) | ||
| SK2 Δ/Δ | 0.9 ± 8.4 (16) | <0.01 | 22.6 ± 19.0 (16) | 0.26 |
| SK1 Δ/Δ | 63.0 ± 38.5 (8) | 0.23 | 45.0 ± 44.8 (8) | 1.00 |
| SK3T/T dox | 98.4 ± 31.9 (12) | 0.92 | 35.4 ± 25.3 (12) | 0.90 |
Discussion
The results show that SK2 subunits are necessary for the apamin-sensitive component of the ImAHP. In SK1 Δ_/_Δ or SK3 T/T(dox) mice, the apamin-sensitive component of the ImAHP was not significantly different from control, there were no significant changes in the levels of SK2 mRNA, and SK2 protein levels were not obviously upregulated, suggesting that only SK2 is necessary for the apamin-sensitive component of the ImAHP. This is consistent with previous suggestions based on the pharmacological profile of the current and the high specificity of apamin (Stocker et al., 1999; Sailer et al., 2002).
In heterologous expression studies, mouse and rat SK1 subunits, different from human SK1 subunits, do not form functional homomeric channels in the plasma membrane, but they are incorporated into heteromeric channels with SK2 or SK3 subunits (Benton et al., 2003; Monaghan et al., 2003). These data suggest that SK1 subunits may contribute to the number of functional SK2-containing channels, although the stoichiometry of SK1/SK2 heteromeric channels is not known. Quantitative PCR showed that in hippocampus, SK1 mRNA is present at levels that are ∼20% of SK2. Also, the SK1 mRNA population contains multiple splice variants (Shmukler et al., 2001). Only two of these coassemble with SK2 into plasma membrane channels, and only one of those two splice forms, comprising ∼40% of the SK1 transcripts, is expressed in the brain (our unpublished observations). The native levels of the different SK subunit mRNAs are in contrast to heterologous expression studies in which both subunit types are robustly coexpressed. Therefore, it is probable that in native neurons, only a small percentage of heteromeric SK1/SK2 channels would form. If all of these heteromeric channels were absent in the SK1 Δ_/_Δ mice, it is unlikely that more than a 10% reduction of the apamin-sensitive ImAHP would be seen. Nevertheless, we cannot unequivocally exclude a contribution of SK1 subunits.
In situ hybridization results show that in CA1 neurons, SK2 mRNA is the most highly expressed of the SK channel mRNAs and SK1 is also abundant, whereas SK3 is lower but detectable (Stocker et al., 1999; Stocker and Pedarzani, 2000). The relative transcript levels detected by in situ hybridization are consistent with the quantitative PCR results presented here. Immunohistochemical studies showed SK1 and SK2 immunoreactivity in CA3 neurons, only SK2 immunoreactivity was prominent in CA1 neurons; SK3 was excluded from the pyramidal and granule cell layers of the hippocampus (Sailer et al., 2002; Obermair et al., 2003). Interestingly, SK3 immunoreactivity was found predominantly in the terminal field of the mossy fibers and in fine varicose fibers, and in hippocampal cultures, SK3 was colocalized with presynaptic markers (Obermair et al., 2003).
Indeed, the biophysical properties of the heterologously expressed SK channels are quite comparable, exhibiting essentially identical EC50 values for Ca2+ gating and similar gating kinetics. It is likely that different functional roles for the channels are endowed by distinct subcellular localizations and interactions with different microdomain components. It is interesting that SK2 channels, required for the apamin-sensitive ImAHP, may also be responsible for the effects of apamin on the induction of synaptic plasticity in CA1 neurons. These dual roles may reflect spatially and functionally distinct subpopulations of SK2 channels within the same CA1 neuron.
Like the SK channels that contribute to the ImAHP, the channels underlying the IsAHP are voltage independent and K+ selective, and they require Ca2+ influx for activation (Lancaster and Adams, 1986; Sah, 1996). Indeed, SK channels have been suggested to underlie the sAHP (Bowden et al., 2001). However, the results show that the cloned SK channels are not necessary for the IsAHP that is responsible for spike-frequency adaptation. In particular, the IsAHP in mice lacking the least apamin-sensitive SK subunit, SK1, is similar to wild type and is not apamin sensitive. In addition, the Ca2+ dependence of the channels underlying the IsAHP may not be mediated by the same calmodulin-dependent gating mechanism that is the molecular hallmark of SK channels (Xia et al., 1998; Sah and Clements, 1999; Vogalis et al., 2003; Gerlach et al., 2004). Based on the present results, it seems likely that the IsAHP channels have not yet been identified molecularly and that the channels underlying the sAHP are distinct from SK channels.
Footnotes
This work was supported by National Institutes of Health Grant NS38880 to J.P.A. and J.M. We thank the staff of the University of Cincinnati Transgenic Core Facility and Zhongying Yang for her technical excellence. We also thank Lori Vaskalis for her patience and graphics support.
Correspondence should be addressed to Dr. John P. Adelman, Vollum Institute, Oregon Health and Science University, 3181 Southwest Sam Jackson Park Road, Portland, OR 97239.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245301-06$15.00/0
References
- Benton DC, Monaghan AS, Hosseini R, Bahia PK, Haylett DG, Moss GW (2003) Small conductance Ca2+-activated K+ channels formed by the expression of rat SK1 and SK2 genes in HEK 293 cells. J Physiol (Lond) 553: 13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP (2000) Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science 289: 1942-1946. [DOI] [PubMed] [Google Scholar]
- Bowden SE, Fletcher S, Loane DJ, Marrion NV (2001) Somatic colocalization of rat SK1 and D class (Ca(v)1.2) L-type calcium channels in rat CA1 hippocampal pyramidal neurons. J Neurosci 21: RC175(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach AC, Maylie J, Adelman JP (2004) Activation kinetics of the slow afterhyperpolarization in hippocampal CA1 neurons. Pflügers Arch 448: 187-196. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Maylie J, Adelman JP (1997a) Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem 272: 23195-23200. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J (1997b) A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651-11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang L-Y, Tang MD, Kaczmarek LK (1997) hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA 94: 11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J (1999) Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci 19: 8830-8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP (1996) Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709-1714. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR (1986) Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55: 1268-1282. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA (1982) Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature 299: 636-638. [DOI] [PubMed] [Google Scholar]
- Monaghan AS, Benton DC, Bahia PK, Hosseini R, Shah YA, Haylett DG, Moss GW (2003) The SK3 subunit of small conductance Ca2+-activated K+ channels interacts with both SK1 and SK2 subunits in a heterologous expression system. J Biol Chem 279: 1003-1009. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Kaufmann WA, Knaus HG, Flucher BE (2003) The small conductance Ca2+-activated K+ channel SK3 is localized in nerve terminals of excitatory synapses of cultured mouse hippocampal neurons. Eur J Neurosci 17: 721-731. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF (1993) PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11: 1023-1035. [DOI] [PubMed] [Google Scholar]
- Sah P (1996) Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150-154. [DOI] [PubMed] [Google Scholar]
- Sah P, Clements JD (1999) Photolytic manipulation of [Ca2+]i reveals slow kinetics of potassium channels underlying the afterhyperpolarization in hippocampal pyramidal neurons. J Neurosci 19: 3657-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES (2002) Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345-353. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG (2002) Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci 22: 9698-9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K (1995) A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF (1999) The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol (Lond) 521: 135-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmukler BE, Bond CT, Wilhelm S, Bruening-Wright A, Maylie J, Adelman JP, Alper SL (2001) Structure and complex transcription pattern of the mouse SK1 K(Ca) channel gene, KCNN1. Biochim Biophys Acta 1518: 36-46. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T (2002) Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22: 10163-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P (2000) Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15: 476-493. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P (1999) An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramdial neurons. Proc Natl Acad Sci USA 96: 4662-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF (1989) An afterhyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol (Lond) 409: 171-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Storm JF, Lancaster B (2003) SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci 18: 3155-3166. [DOI] [PubMed] [Google Scholar]
- Xia X-M, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503-507. [DOI] [PubMed] [Google Scholar]