Superparamagnetic Iron Oxide-Labeled Schwann Cells and Olfactory Ensheathing Cells Can Be Traced In Vivo by Magnetic Resonance Imaging and Retain Functional Properties after Transplantation into the CNS (original) (raw)
Abstract
Schwann cell (SC) and olfactory ensheathing cell (OEC) transplantation has been shown experimentally to promote CNS axonal regeneration and remyelination. To advance this technique into a clinical setting it is important to be able to follow the fates of transplanted cells by noninvasive imaging. Previous studies, using complex modification processes to enable uptake of contrast agents, have shown that cells labeled in vitro with paramagnetic contrast agents transplanted into rodent CNS can be visualized using magnetic resonance imaging (MRI). Here we show that SCs and OECs efficiently internalize dextran-coated superparamagnetic iron oxide (SPIO) from the culture medium by fluid phase pinocytosis. After transplantation into focal areas of demyelination in adult rat spinal cord both transplanted SPIO-labeled SCs and OECs produce a signal reduction using T2-weighted MRI in anesthetized rats that persists for up to 4 weeks. Although signal reduction was discernable after transplantation of unlabelled cells, this is nevertheless distinguishable from that produced by transplanted labeled cells. The region of signal reduction in SPIO-labeled cell recipients correlates closely with areas of remyelination. Because the retention of functional integrity by labeled cells is paramount, we also show that SPIO-labeled SCs and OECs are able to myelinate normally after transplantation into focal areas of demyelination. These studies demonstrate the feasibility of noninvasive imaging of transplanted SCs and OECs and represent a significant step toward the clinical application of promising experimental approaches.
Keywords: transplantation, Schwann cell, olfactory ensheathing cell, remyelination, magnetic resonance imaging, repair, SPIO
Introduction
Transplantation of Schwann cells (SCs) and olfactory ensheathing cells (OECs) promotes axonal regeneration in experimental models of traumatic spinal cord injury (Xu et al., 1995, 1999; Stichel et al., 1996; Li et al., 1997, 2003; Ramon-Cueto et al., 2000; Lu et al., 2002; Nash et al., 2002) and mediates remyelination in models of primary demyelination (Blakemore, 1977; Duncan et al., 1981; Honmou et al., 1996; Franklin et al., 1996b; Baron-van Evercooren et al., 1997; Imaizumi et al., 1998; Lakatos et al., 2003). For these reasons transplantation of both cell types has a potential therapeutic role for treating patients with spinal cord injury or with nonrepairing demyelinating disease such as multiple sclerosis, a strategy bought closer to realization with data obtained using nonhuman primate and human cells (Avellana-Adalid et al., 1998; Barnett et al., 2000; Kato et al., 2000; Kohama et al., 2001). Fundamental to developing such approaches is the ability to follow the in vivo fate of transplanted cells noninvasively, enabling more accurate interpretation of the functional consequences of transplantation.
Although many approaches have been developed for in vivo tracking of cell fate, at present only magnetic resonance imaging (MRI) has the resolution required to accurately localize transplanted cells in adult mammals. Paramagnetic iron oxides have been used as a cytoplasmic label for tracing cells in vivo using MRI (Yeh et al., 1995; Bulte et al., 2002; Hoehn et al., 2002). The strategy has been used ex vivo to label neurons (Hawrylak et al., 1993), neural stem cells (Bulte et al., 2001, 2003), and progenitors (Bulte et al., 1999; Franklin et al., 1999) before transplantation into the CNS and detection by MRI. Various approaches have been employed to label cells with paramagnetic iron oxides including Sendai viral envelopes, exploitation of transferrin receptors (Bulte et al., 1999), encapsulation within dendrimers (Bulte et al., 2001) or HIV-Tat peptides (Hawrylak et al., 1993), or incubation with paramagnetic iron oxide-containing media (Franklin et al., 1999).
A critical question is whether the labeling technique and/or the presence of cytoplasmic paramagnetic iron oxides interferes with the differentiation and repair capacity of the transplanted cells? In this study we use fluid phase pinocytosis to label SCs and OECs using nanoparticles of superparamagnetic iron oxide (SPIO) and addressed whether these cells can perform the complex process of forming myelin sheaths around demyelinated axons. Although earlier studies have shown myelination within areas receiving a transplanted SPIO-labeled oligodendrocyte progenitor cell line, it was not unequivocally demonstrated that myelination was performed by SPIO-containing cells (Bulte et al., 1999). Here we use a rodent model of persistent demyelination with electron microscopy to demonstrate myelination by SPIO-labeled SCs and OECs that can be identified in magnetic resonance images. Finally, we have obtained magnetic resonance images of transplanted labeled cells within rodent spinal cord. The results obtained in this study provide evidence that two cell types with substantial proregenerative properties can be visualized noninvasively and constitute a significant step toward their therapeutic use in clinical disease.
Materials and Methods
Preparation of primary Schwann cells and olfactory ensheathing cells. Primary SC cultures were generated using a protocol modified from that of Brockes et al. (1979). Briefly, both sciatic nerves were dissected from 2-d-old Fischer rat pups and placed into L-15 medium (Invitrogen, Paisley, UK), where the perineurium and residual blood vessels were removed. The nerves underwent dissociation to single cells using physical disruption and enzymatic digestion. After maceration, enzymatic digestion was performed using Collagenase (ICN Biomedicals, Aurora, OH) followed by 1% Trypsin (bovine pancreas trypsin; Sigma, Poole, UK) at 37°C. The reaction was stopped using a combination of bovine pancreas DNAase (0.04 mg/ml; Sigma), soybean trypsin inhibitor (0.52 mg/ml; Sigma), and bovine serum albumin (Sigma). The tissue was subsequently triturated six to eight times, using a 5 ml ground glass pipette and subsequently through 21 and 23 gauge needles. The cells were resuspended in 10% fetal bovine serum (FBS; Sigma), and plated onto poly-l-lysine (PLL)-coated (13.3 μg/ml; Sigma) 25 cm2 tissue culture flasks (Fisher Scientific, Loughborough, UK). Cytosine arabinoside, 2 × 10-5 M/ml (AraC; Sigma) was added to the media after 24 hr and the cultures left for a period of 72 hr. The cultures were grown in DMEM (Invitrogen) supplemented with 10% FBS (Sigma) (DMEM-FBS) for 48 hr, when AraC was again added for a further 72 hr. The cells were then washed and incubated with Thy1.1 antibody (1:1 supernatant; Serotec, Oxford, UK) and rabbit complement (1:6; Harlan Sera-Lab Ltd, Loughborough, UK) to remove any remaining contaminating fibroblasts. The SCs were then plated onto PLL-coated 25 cm2 flasks supplemented with 10% FBS, supplemented with forskolin (FK; Sigma) and heregulin (Hrg; R & D Systems, Abingdon, Oxon, UK). Fifty percent of the media was changed every 2-3 d during culture. The SC cultures were >98% pure after this procedure.
Primary olfactory bulb cultures were generated from 7-d-old Fischer rat pups using a protocol modified from that reported by Lakatos et al. (2003). Briefly, the outer layers of rostral tip of both olfactory bulbs were removed, and the meninges were dissected free along with any attached blood vessels. Cells were dissociated by tissue maceration followed by enzymatic digestion. Cells were cultured for 24 hr on PLL-coated 75 cm2 flasks in 10% FBS-DMEM, after this AraC was added (10-5 m) for 4 d. The cultures were then washed and fed with 10% FBS-DMEM for 48 hr, before adding AraC for a further 3-4 d. The cultures were enriched for OECs after the second AraC treatment but were further purified using immunopanning for low-affinity nerve growth factor receptor (p75NTR). The cells were briefly exposed to trypsin to detach those with spindle and triangular morphologies. The detached cells were harvested and washed twice with FBS-DMEM before being resuspended in Eagle's minimum essential medium (EMEM)-HEPES (minimum essential medium: 4-[2-hydroxyethyl] piperazine sulfonic acid) (Sigma). The low-affinity nerve growth factor-expressing cells were isolated by immunopanning using an antibody against p75NTR. The cells were then plated onto PLL-coated 25 cm2 flasks in 10% FBS-DMEM with the addition of FK and Hrg. This generated OEC cultures that were >80% pure.
Phenotypic characterization of the SCs and OECs. An aliquot of the same cell preparations used for transplantation was plated onto poly-l-lysine-coated 13 mm glass coverslips and cultured for 48 hr. The cells were then washed in PBS, and blocking solution (PBS containing 5% FBS) was added for 15 min. The cells were washed three times with PBS before the addition of antibodies and subsequently before the addition of further reagents. All antibodies were diluted in blocking solution before use; dilutions are given in parenthesis. The cells were characterized by immunofluorescent labeling with p75NTR (1:5; mouse monoclonal hybridoma supernatant, a kind gift from Dr. G. Plant, University of Western Australia, Crawley, Australia) (Yan and Johnson, 1988), Thy1.1 (1:1, mouse anti-rat, Serotec, UK), fibronectin (1:300; rabbit anti-human; Dako, Ely, UK), glial fibrillary acidic protein (GFAP; 1:100; rabbit anti-cow, polyclonal, Dako). All primary and secondary antibodies were incubated for 30 min at room temperature. Secondary antibodies (Jackson ImmunoResearch, Soham, UK) were either conjugated to FITC (green) or Cy3 (red) and were either anti-mouse or anti-rabbit; the FITC was diluted 1:100 and Cy3 1:250. After incubation with primary and secondary antibodies to surface antigens, the cells were fixed with ice-cold methanol and placed at -20°C for 20 min before further cytoplasmic antigens were stained. Nuclei were stained using DAPI contained within the mounting medium (1.5 μg/ml; Vectastain, Peterborough, UK). Before mounting, cells were washed three times in PBS and once in deionized water. Coverslips were mounted face down onto a drop of mounting media and the edges sealed using clear nail varnish.
Production of SPIO label. Dextran-coated SPIO particles were synthesized according to a protocol outlined in the European patent application, International application number PCT/JP93/01092. FeCl3 · 6H2O (37.8 gm) (Sigma) was dissolved in 140 ml of water and deoxygenated by bubbling N2 gas through it for 20 min. After 20 min of deoxygenation, FeCl2 · 4H2O (13.6 gm) (Sigma) was added to the FeCl3 solution and dissolved under nitrogen. Dextran T-10 (105 gm) (Amersham Biosciences, Little Chalfont, UK) was dissolved in 350 ml of water. The FeCl3-FeCl2 solution was added to the dextran solution and mixed. This mixture was heated to 80°C, at which point 242 ml of 3 m NaOH was added continuously over 14 min. The pH of the still hot mixture was altered to 7 and then refluxed for 1 hr. After cooling, the mixture was centrifuged at 2100 × g for 30 min. The supernatant was retained, and 78% of the total supernatant volume of absolute ethanol was added to precipitate a complex. The precipitate was collected by centrifugation and then resuspended in 1 l of water to form a nanocolloid. The nanocolloid was dialyzed in prepared Visking tubing against deionized water and then against 5 l of milliQ water (Millipore, Watford, UK). The dialyzed nanocolloid was purified further by gel filtration (Sephacryl S300; Amersham Biosciences) to remove unbound dextran. The iron concentration of the label was determined by a spectrophotometric assay, and the label was then concentrated to give an iron concentration of 40 mg/ml before use. T2 and molar relaxivity (_R_2) values were obtained at 9.4 T using a Carr-Purcell-Meiboom-Gill pulse sequence. The molar relaxivity at this magnetic field was 427 ± 12.7 mm/sec with respect to Fe concentration. The mean particle diameter, measured by light scattering, was 86 nm.
Labeling of SCs and OECs with SPIO. Cultures of SCs and OEC were passaged at least once before labeling. To determine the optimal labeling concentration of SPIO, the label was added to the cultures at concentrations equivalent to 0.5, 1, 2, 4, 6, and 8 mg/ml of Fe and incubated for 48 hr. Control cultures were incubated without SPIO. These labeling experiments were initially performed in duplicate with the cells being passaged and resuspended in medium containing SPIO or added to cultures that had been established for up to 7 d, to determine the most appropriate method. The results of these experiments showed no difference in the degree of label uptake. As a result of these initial trials, the SPIO was added to established cultures, which prevented unnecessary cell loss during the passaging procedure.
Staining of the cultures with Prussian blue. After incubation with SPIO, a modification of the Perls' Prussian blue (PB) method (Perls' acid ferrocyanide) was used for the detection of iron within the cell cultures. This induces a reaction a reduction of ferric iron to the ferrous state with the formation of a blue precipitate. Cell cultures were washed three times in PBS to remove any free SPIO and fixed for 30 min using 4% glutaraldehyde. The cultures were then washed three times and incubated with a 50:50 mixture of 6% HCl (AnalaR; BDH, Poole, UK) and 2% potassium ferrocyanide (Sigma) for a further 30 min. The cells were washed in PBS and observed using phase microscopy. The fixed cells were then stored in the dark at 4°C to prevent photobleaching.
Electron microscopy of SC cultures after labeling with SPIO. After incubation in 10% FBS-DMEM containing SPIO equivalent to 2 mg/ml of Fe, SCs were extensively washed and detached from the flasks using trypsin. The cells were pelleted and fixed with 4% glutaraldehyde for 30 min before being washed three times in PBS. The pellet was then osmicated for 1 hr before being dehydrated in ascending alcohols and embedded in TAAB resin (TAAB Laboratories, England, UK). Ultrathin sections were mounted onto slot grids for viewing using a Hitachi H600 transmission electron microscope.
Assessment of cell viability. The viability of SCs and OECs after labeling with SPIO was assessed using propidium iodide (PI) exclusion. Cultures were washed three times with PBS to remove residual SPIO and then stained using PI (10 μg/ml; Sigma) and Hoechst (1 μg/ml; Merck, Beeston, Notts, UK). Cells were considered viable if they stained negative for PI but positive for Hoechst, nonviable cells were considered to be those staining positively for both PI and Hoechst.
MRI of SPIO-labeled cells in vitro. SCs were incubated with SPIO at 0.5, 1, 2, and 4 mg/ml for 48 hr. The cultures were washed thoroughly with PBS to ensure complete removal of unbound SPIO before being immobilized in gelatin in 5 mm nuclear magnetic resonance (NMR) tubes, at a concentration of 5 × 104/ml (4% w/v) (Sigma). T2-weighted spin echo images [echo time (TE) = 30 msec; repetition time (TR) = 2 sec], and gradient echo images were acquired from 1 mm transverse slices using a Varian Unity INOVA spectrometer equipped with an Oxford Instruments wide-bore 9.4 T magnet, a Varian unshielded gradient set, and 25 mm diameter 1H imaging probe.
Evaluation of uptake process for SPIO in vitro. SCs were cultured in multiwell plates and preincubated with either colchicine (Sigma), to inhibit pinocytosis (5, 50, and 500 μg/ml), or cytochalasin B (Sigma), to inhibit phagocytosis (10 and 100 μg/ml) for 2 hr (Band et al., 1986; Pratten and Lloyd, 1986; Esteban et al., 1998). After preincubation, the cultures were washed with PBS and incubated with SPIO at a concentration equivalent to 2 mg/ml Fe for 24 hr. The cultures were then fixed and stained for iron using PB. The number of cells detectably and abundantly labeled was determined by counting 10 high-power fields (200×) for each group. Control cultures without preincubation with uptake inhibitors were used with subsequent addition of SPIO.
Animal groups. Male Fischer rats between 180 and 220 gm were used for all experiments. For the SPIO-labeling experiments, animals received X-irradiated ethidium bromide (X-EB) lesions to the dorsal funiculus followed by transplantation of either SPIO-labeled OECs (group 1; n = 10) or SCs (group 2; n = 6), or nonlabeled OEC (group 3; n = 4) or SCs (group 4; n = 3); control groups received X-EB lesions with no transplantation (group 5; n = 4) or ethidium bromide (EB) lesions with no subsequent irradiation or transplantation (group 6; n = 4).
Creation of demyelinated lesions in the spinal cords. Animals were anesthetized using halothane (Fluothane; Schering-Plough Animal Health), perioperative analgesia was provided with Carprofen (subcutaneously, Rimadyl; Pfizer, Tadworth, UK) and Buprenorphine (intramuscularly, Vetergesic; Alstoe Ltd., Animal Health), perioperative antibiosis was provided by means of subcutaneous procaine penicillin (Depocillin; Intervet) along with postoperative intraperitoneal fluid therapy (sodium chloride 0.9%; Aquapharm, Animalcare Ltd., York, UK). A dorsal laminectomy was performed at the level of the L1 vertebra, and a parasagittal incision was made in the meninges to reveal the dorsal funiculus. Using a 10 μl Hamilton syringe held in a micromanipulator, 1 μl of 0.1% ethidium bromide was injected over a period of 20 sec into the dorsal funiculus through the meningeal incision. For X-irradiation, animals were anesthetized 24-48 hr after EB lesions had been created, with an intramuscular injection of a fentanyl citrate-fluanisone combination (Hypnorm; Janssen Pharmaceuticals, High Wycombe, UK) and secured in lateral recumbency. The spine between T11 and L4 was exposed to a single dose of 40 Grays of X-irradiation using a Pantak 255 kV radiotherapy machine. This procedure abolishes the inherent remyelination.
Preparation of cells for transplantation. Cells were detached from the flasks using trypsin in HBSS solution. The cells were then washed, and the pellet was resuspended in EMEM-HEPES. The total number of cells was determined using a hemocytometer. After a further centrifugation, the supernatant was carefully removed, and the cells were resuspended to produce a final concentration of 5 × 104/μl. The cells were then loaded into a 10 μl Hamilton syringe held in a micromanipulator for transplantation. A single microliter of cells was injected over a period of 15-20 sec into the lesion site in the dorsal funiculus, and the needle was left in position for a further 45 sec before being slowly withdrawn.
Histology. Animals were killed by perfusion via the descending aorta under deep pentobarbitone anesthesia with 4% glutaraldehyde or 4% paraformaldehyde in phosphate buffer. The lesion site was identified, and a length of fixed spinal cord was removed extending for 1 cm either side. For resin sectioning, the spinal cord was cut into 1-mm-thick coronal blocks processed to retain their craniocaudal order. The blocks were washed three times in PBS, post-fixed overnight in 2% osmium tetroxide, dehydrated through graded alcohols, and embedded in TAAB resin. Paraformaldehyde-fixed cords were embedded into paraffin wax before sectioning and staining.
Sections of 1 μm were stained with alkaline toluidine blue for light microscopy. Selected blocks were trimmed, and thin sections were cut and stained, using uranyl acetate and lead citrate, for examination using a Hitachi H-600 transmission electron microscope, to assess remyelination by the SPIO-containing transplanted cells. Duplicate thin sections were left unstained to prevent staining artifact from being confused with SPIO inclusions.
Those animals perfused after in vivo MRI scanning had serial 20 μm sections cut throughout the spinal cord at 1 cm on either side of the lesion site. These were stained for the presence of Fe3+ using PB and costained using hematoxylin and eosin.
MRI of ex vivo spinal cords. For ex vivo MRI studies, fixed spinal cords were washed, immersed in PBS, and positioned within 5-mm-diameter NMR tubes. These were then secured within a larger 25 mm tube. T2-weighted images were obtained using a Varian Unity INOVA spectrometer equipped with an Oxford Instruments wide bore 9.4 T magnet, a Varian unshielded gradient set, and 25 mm diameter 1H imaging probe. Spin echo images (TE = 30 msec, TR = 2 sec) were acquired from 1 mm transverse and longitudinal slices centered at the lesion site using 256 phase encode increments, with four transients per increment. The field of view for the majority of the experiment was 25 x 25 mm with an image resolution of 98 × 98 μm. Total image acquisition time was ∼4 hr in each plane. After imaging was completed, the spinal cords were processed for histology, into resin or paraffin as described above.
MRI of transplanted SPIO-labeled cells in vivo. For in vivo MRI studies, animals were anesthetized, using a combination of fentanyl and fluanisone (Hypnorm; Janssen) and Midazolam (Roche, Welwyn Garden City, UK) administered by intraperitoneal injection, 5 d after transplantation and secured within a cradle before being placed within a 45 mm Varian Millipede 1H imaging inside a Magnex shielded gradient set. The above sequences were used to generate T2-weighted spin-echo images. Total acquisition time was ∼34 min for each animal. The animals were then removed from the magnet and allowed to recover. These were then kept for a further 24 d when they were reanesthetized and imaged as before. A group of animals were perfused after the first imaging experiment (n = 4) to assess the distribution of PB-positive cells at the transplant site
Statistical analysis. Statistical analysis was performed using a one-way ANOVA followed by a post hoc comparison using the Bonferroni test. A p value of <0.05 was considered significant.
Results
SCs and olfactory ensheathing cells can be labeled with SPIO in vitro with equal efficiency
Cultures of SCs and OECs prepared by p75NTR immunopanning contained >98 and >80% p75-positive cells, respectively, at the time of transplantation (Fig. 1_A,B,D,E_). Culture purity was assessed by immunocytochemistry, where a p75NTR+ve/GFAP+ve/Thy1.1-ve phenotype identified SCs, a p75NTR+ve/GFAP+ve/Fibronectin-ve (or weakly positive) phenotype identified OECs, and Thy1.1 and fibronectin antibodies were used to identify fibroblast and meningeal cell contaminants in SC and OEC cultures, respectively (Fig. 1_G,H_). To detect uptake of SPIO after incubation in SPIO-containing medium, the cells were stained for iron using the Prussian blue method. Distinctive blue cytoplasmic inclusions, typically clustered around but never entering the nucleus, were only detected in cells incubated with SPIO (Fig. 1_C,F_). To provide an assessment of the comparative in vitro labeling efficiency, cells labeled with SPIO were divided into those that were abundantly or weakly labeled, depending on whether more or <25% of the visible cytoplasmic area contained blue-staining inclusions. As the concentration of SPIO was increased from 0.5 to 8 mg/ml, the proportion of detectably and abundantly labeled cells increased (Fig. 1_I_). However, at 6 mg/ml and above, there was almost complete loss of cells from the cultures, indicating this to be unfavorable for cell survival. To further examine the effect of SPIO labeling on cell viability, a propidium iodide exclusion study was performed, which revealed no significant difference in cell viability in SC cultures incubated with SPIO for up to 48 hr using concentrations up to and including 4 mg/ml of Fe when compared with control SC cultures (control SCs, 96.4 ± 3.2%; 1 mg/ml, 96.3 ± 2.1%; 2 mg/ml, 96.5 ± 3.2%; 4 mg/ml, 96.4 ± 2.8%). Similarly, there was no effect on cell viability in OEC cultures incubated with 2 mg/ml SPIO for 48 hr when compared with control OEC cultures, or SC cultures using an equivalent time point and concentration (control OEC, 97.04 ± 6.62%; SPIO-labeled OEC, 99.6 ± 0.8%). We could detect no uptake of SPIO <0.5 mg/ml after 48 hr. After 48 hr incubation at 2 mg/ml, there was a significantly greater proportion of abundantly labeled cells compared with weakly labeled cells in both populations (p < 0.05 for SC cultures and p < 0.001 for the OEC cultures) (Fig. 1 J). To test the MRI detectability, SCs labeled with SPIO at 0.5-4 mg/ml were immobilized in gelatin (4% w/v). T2-weighted images of a cross section through the gelatin-containing tubes revealed clear differences in signal intensity between labeled and unlabelled cells, and a correlation between the numbers of abundantly labeled cells related well to the increase in MRI signal dropout (Fig. 1 K). On the basis of these results, 2 mg/ml SPIO was chosen as the optimal concentration for cell labeling.
Figure 1.
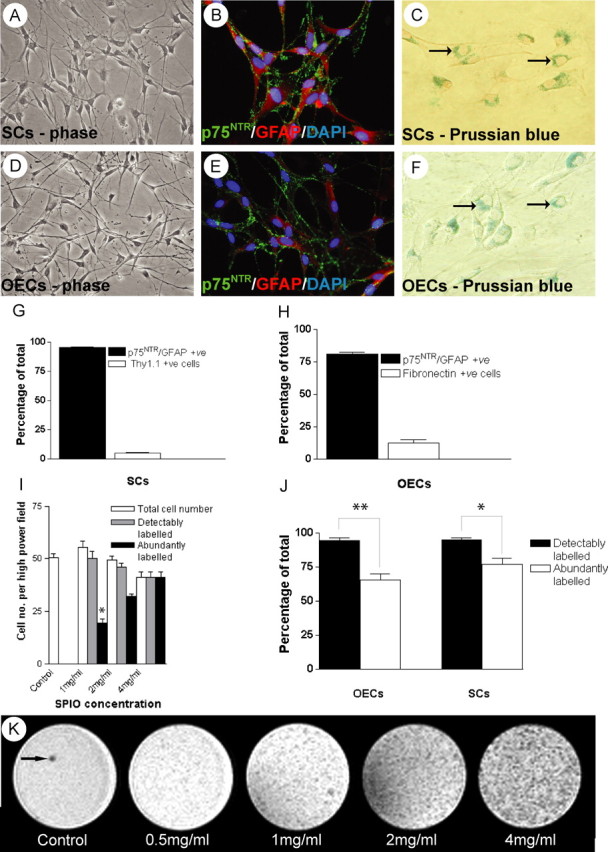
In vitro appearance of SCs and OECs labeled with SPIO. A, Phase micrograph showing SCs in vitro after purification and amplification for 4 weeks. B, sample of the SCs shown in A, immunolabeled for p75NTR (green) and GFAP (red) and nuclei stained with DAPI (blue). C, PB-stained Schwann cells after incubation for 48 hr with SPIO (2 mg/ml equivalent of Fe). Blue intracellular inclusions containing SPIO can be identified (arrows). D, Phase micrograph of OECs in vitro after purification using immunopanning for p75NTR and subsequent amplification for 4 weeks. E, OECs immunolabeled in vitro for p75NTR (green) and GFAP (red) nuclei stained with DAPI (blue). F, PB-stained SCs after incubation for 48 hr with SPIO (2 mg/ml equivalent of Fe); arrows indicate blue intracellular inclusions. G, Graph showing the proportion of p75NTR-positive SCs in cultures from B, stained for p75NTR, GFAP, and Thy1.1. H, Graph showing the proportion of p75-positive OECs in cultures from E, when stained for p75NTR, GFAP, and fibronectin. I, Graph showing the effect of increasing the concentration of SPIO (0-4 mg/ml Fe equivalent) on the labeling intensity of cultured SCs. The proportions of abundantly labeled cells increases relative to those detectably labeled with increasing concentrations of SPIO. At >2 mg/ml this increase is no longer significant. J, Graph showing the comparative labeling intensities between SCs and OECs after 48 hr incubation with SPIO at a concentration equivalent to 2 mg/ml Fe. There is no significant difference between the degree of detectably or abundantly labeled cells between SCs and OECs. There is a significant difference between those cells detectably and abundantly labeled within each cell type (p < 0.05 for SCs and p < 0.001 for OECs). K, T2-weighted MR images through a series of gelatin phantoms (4% w/v) containing SPIO-labeled SCs at increasing concentrations of SPIO (0.5-4 mg/ml, cell concentration 5 × 104/ml). The labeled cells embedded within gelatin are increasingly apparent as black (hypointense) pixels as the concentration of SPIO is increased. Note the presence of a hypointense air bubble within the control gelatin phantom (arrow).
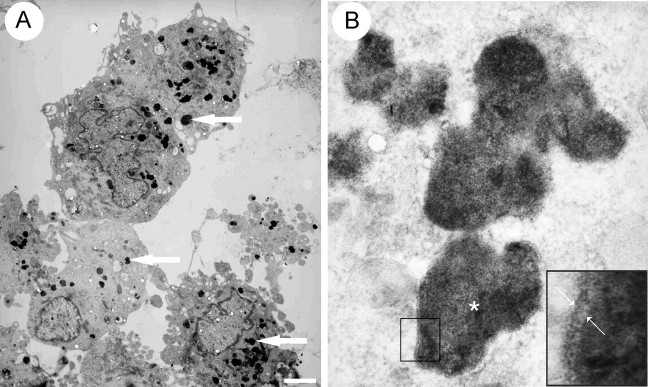
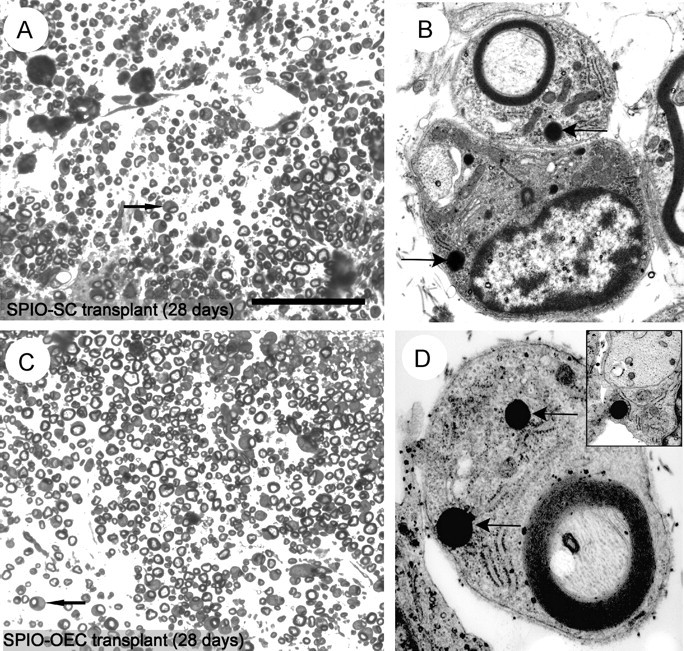
Finally, the electron microscopic (EM) appearance of labeled SCs in vitro was examined to enable verification of labeled cells in vivo. This revealed multiple dark electron-dense cytoplasmic inclusions (Fig. 2_A_) (mean particle size 0.45 μm ± 0.147; range 0.24-1 μm), which at high power appeared granular (Fig. 2_B_) and were occasionally surrounded by an identifiable membrane (Fig. 2_B_, inset), a feature consistent with active uptake. This appearance was similar to that previously reported after SPIO labeling of an oligodendrocyte progenitor cell line (Franklin et al., 1999). These inclusions were always cytoplasmic and were never seen in unlabelled control cells (data not shown).
Figure 2.
Electron microscopic in vitro appearance of SCs labeled with SPIO. A, SCs labeled with SPIO equivalent to 2 mg/ml Fe for 48 hr. Multiple electron-dense intracytoplasmic SPIO inclusions are present within each cell (arrows). B, Higher-power image showing a small number of the SPIO inclusions present in A. Inset shows the margin of the inclusion marked with an asterisk, demonstrating the SPIO inclusion enclosed within a membrane (arrows) and residing in an endocytic compartment. Scale bar: A, 1 μm; B, 0.1 μm.
In vitro cell labeling with SPIO is predominantly by pinocytosis
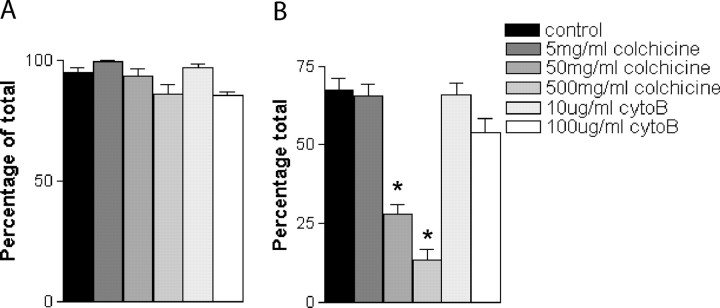
To determine the mechanism by which cells became labeled with SPIO, primary SCs were SPIO-labeled in the presence of either colchicine, an inhibitor of fluid phase pinocytosis, or cytochalasin B, an inhibitor of phagocytosis. No significant changes in the proportion of cells that were SPIO-labeled were evident after cytochalasin B treatment, at either concentration tested, compared with controls (Fig. 3). However, colchicine treatment resulted in a significant decrease in SPIO uptake at labeling concentrations of 50 and 500 μg/ml (p < 0.001) (Fig. 3), indicating that cell labeling with SPIO occurs predominantly by pinocytosis rather than phagocytosis.
Figure 3.
Effects of inhibitors of phagocytosis and pinocytosis on the uptake of SPIO in vitro. SC cultures were preincubated for 2 hr with colchicine or cytochalasin B before incubation with SPIO for a further 24 hr. A, Graph demonstrating the proportion of cells detectably labeled for each culture condition. There is no significant difference between any of the groups. B, Graph demonstrating the proportion of cells abundantly labeled for each culture condition; there is a significant reduction in the degree of labeling in the SC cultures incubated with 50 and 500 mg/ml of colchicine, when compared with the other culture conditions (*p < 0.001). There was no significant effect of cytochalasin B at any of the concentrations used.
Both SCs and OECs labeled with SPIO can be detected by MRI after transplantation into demyelinating lesions in rat spinal cord
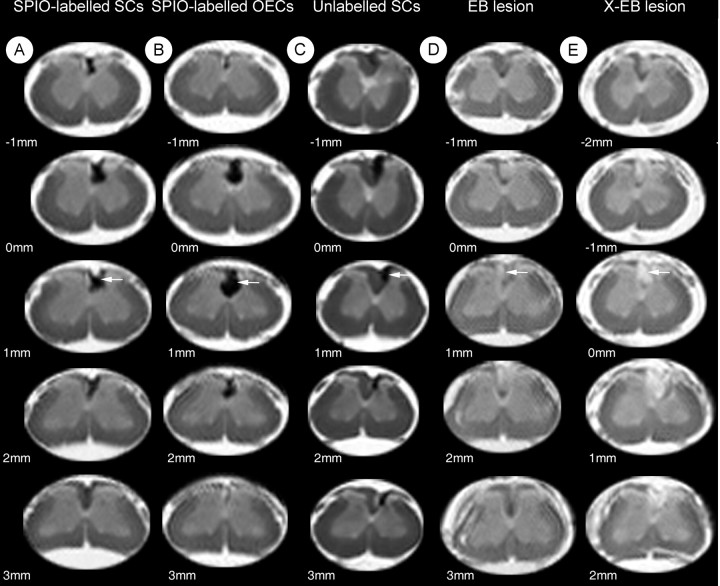
Having established an efficient labeling protocol for the two populations, we next addressed whether transplanted cells could be localized using anesthetized animals that were subsequently allowed to recover after imaging. Animals received transplants of SPIO-labeled and unlabelled SCs or OECs into X-EB lesions. Five days later, they were reanesthetized for MRI. The laminectomy through which cells were transplanted into the spinal cord was readily identifiable on T2-weighted images and indicated the site of transplantation. This was also confirmed by attaching a water-filled glass bulb to the skin of the animal above the laminectomy site. The transplant site showed a marked reduction in signal intensity (black on T2-weighted images) in animals receiving SPIO-labeled cells that was detectable for ∼2-3 mm around the point of transplantation (Fig. 4_A,B_, arrows). A slight reduction in signal intensity was also apparent in control animals receiving unlabelled cells at the transplant site. This was variable, and the signal reduction was consistently smaller than in those animals receiving SPIO-labeled cells (Fig. 4_C_).
Figure 4.
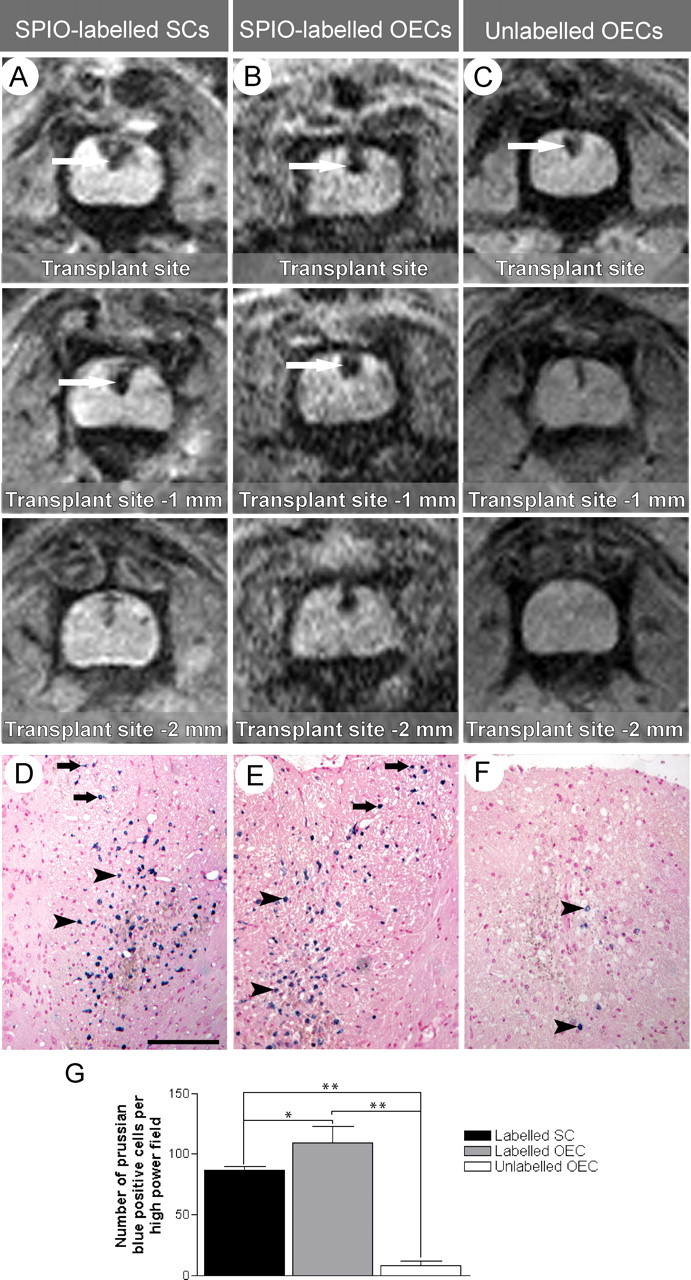
In vivo MR images 5 d after transplantation of SPIO-labeled SCs (A) and OECs (B) and unlabelled glial cells (C) into focal areas of demyelination in the dorsal funiculus of the spinal cord. Images are from 1-mm-thick slices and progress in a caudal direction beginning at the transplant site (0 mm). A and B show a large reduction in signal intensity (black) in the region of the transplant site because of presence of SPIO-labeled SCs and OECs, demonstrating the bloom effect, in which the distortion of the magnetic field is over a greater area than the likely distribution of SPIO (arrows). The images from a recipient of unlabelled OECs (C) shows a smaller reduction in signal intensity with no bloom effect (arrow) when compared with A and B. Hematoxylin and eosin sections costained with PB, 5 d after transplantation of SPIO-labeled SCs (D), SPIO-labeled OECs (E), and unlabelled OECs (F), into X-EB lesions in the dorsal funiculus. PB-positive cells are visible in D-F (arrowheads), although in control animals (F) the numbers are substantially lower. PB-positive cells are present along the injection tract in recipients of SPIO-labeled cells only (D, E, black arrows). G, There is a significant difference in the mean numbers of PB-positive cells per high-power field from animals receiving SPIO-labeled cells compared with controls (**p < 0.001). There is also a significant difference in PB-positive cells, between the SPIO labeled SCs and SPIO labeled OECs at the transplant site (*p < 0.05). Scale bar: D—F, 150 μm.
After this first imaging procedure, a group of animals was killed after imaging at 5 d, to determine the abundance and distribution of SPIO-labeled cells within the spinal cord. PB-positive cells were present in all animals examined (Fig. 4_D-F_), within the dorsal funiculus and around the site of injection in animals receiving SPIO-labeled cells (Fig. 4_D,E_). Small numbers of PB-positive cells were also observed in the spinal cord from animals receiving unlabelled cells (Fig. 4_F_), although these were significantly fewer and less widespread than those in animals receiving SPIO-labeled cells (Fig. 4_G_). These results suggested that the differences in the signal hypointensity after transplantation of labeled and unlabeled cells were attributable to the SPIO labeling.
A second group of animals were allowed to recover after the first imaging procedure and were reanesthetized 23 d later for reimaging using the same parameters as previously. The signal reduction in the region of the transplant site extended for a further 1-2 mm craniocaudally, compared with the images from 5 d, indicating dispersal of labeled cells during the intervening period (Fig. 5_A,B_). After this second imaging procedure, these animals were perfused, and the spinal cords were reimaged ex vivo using the same parameters. This revealed a strong correlation between obtained in vivo and those obtained ex vivo, with comparable size and shape (Fig. 5_B,C_). A similar observation occurred in the unlabelled OEC-transplant group (Fig. 5_E,F_). There was no craniocaudal spread of signal reduction in spinal cords from control animals beyond that seen after 5 d (Fig. 5_D,F_).
Figure 5.
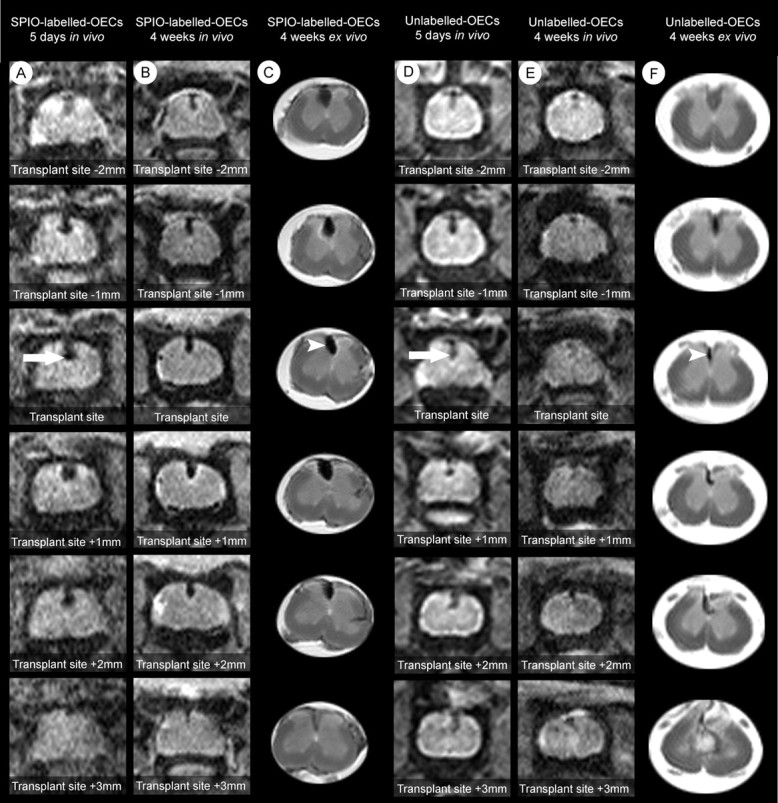
MR images showing the appearance of transplanted cells in vivo and ex vivo within the same animals. All panels show T2-weighted MR images of 1 mm slice thickness progressing in a rostral (negative) to caudal (positive) direction, where 0 represents the transplant site. MR images from a recipient of SPIO-labeled OECs (A-C) and unlabelled OECs (D-F) transplanted into an X-EB lesion in the dorsal funiculus are shown. A, Serial 1 mm images beginning 2-3 mm rostral to the transplant site 5 d after transplantation. The hypointense region (black) in the dorsal funiculus (arrow) can be seen for up to 5 mm around the transplant site. B shows subsequent images 4 weeks after transplantation, of the region of spinal cord shown in A. The hypointense area is still present within the dorsal funiculus and now extends for >5 mm. C shows the ex vivo appearance of the spinal cord shown in B, after fixation. D, At 5 d a smaller hypointense region is present in association with transplantation of unlabelled OECs (arrow). E, Images of the region of spinal cord shown in D at 4 weeks after transplantation. A hypointense area remains present but without further rostrocaudal spread. F, The ex vivo appearance of the spinal cord shown in E, after fixation. The ex vivo images are sharper, because of lower signal-to-noise ratios. However, there is a close correlation between the region of hypointensity in the dorsal funiculus obtained in vivo and that seen within the fixed cord (compare B, C, and E, F).
To confirm that the substantial signal reduction was caused by the presence of SPIO-labeled cells and not by lesion induction or a result of remyelination (and the sequestration of endogenous iron during this process), three groups of controls were used: first, animals receiving unlabelled cell transplants into X-EB lesions (Fig. 6_C_), second, animals injected with EB but without either X-irradiation or cell transplantation (these lesions undergo spontaneous remyelination) (Fig. 6_D_); third, animals with nontransplanted X-EB lesions that do not undergo endogenous remyelination and remain persistently demyelinated (Fig. 6_E_). Spinal cords from those animals receiving unlabelled cell transplants showed a slight reduction in signal intensity after 4 weeks at the site of transplantation (Fig. 6_C_). However, this was consistently less than in animals receiving SPIO-labeled cell transplants (Fig. 6_A,B_). Those animals that received an EB injection alone showed no detectable signal reduction on T2-weighted scans either at the lesion site or in any of the sections of the dorsal funiculus. Instead there was a slight increase in signal intensity occurring as a result of demyelination and the remyelination process, two processes that cannot be distinguished reliably using T2-weighted MRI (Fig. 6_D,E_). Spinal cords of animals with nontransplanted X-EB lesions also showed a clear increase in signal intensity in the region of the lesion site that contrasted with the surrounding white matter, consistent with the presence of demyelinated axons (Fig. 6_E_). These observations indicate that the signal reduction seen in the animals receiving SPIO-labeled cells is attributable to the presence of labeled cells.
Figure 6.
Ex vivo T2-weighted MR images 4 weeks after transplantation of SPIO-labeled SCs and OECs and unlabelled SCs compared with spontaneously remyelinating EB lesions and nonremyelinating X-EB lesions. Images are of 1-mm-thick slices, where 0 represents the transplant site with rostral (negative) and caudal images (positive) indicated. The presence of SPIO within labeled cells SCs (A) and OECs (B) shortens the T2 of the surrounding water protons and producing a signal void at the transplant site (arrow). A bloom effect can be seen in 1 mm serial slices for at least 4 mm. The cord receiving unlabeled SCs (C), has a smaller hypointense region than that present in A and B (arrow). The series of images shown here represent the maximal detected signal reduction caused by transplantation of unlabelled cells; in many animals there was no apparent signal reduction. D, T2-weighted MR images 4 weeks after creation of an EB lesion in the dorsal funiculus. The lesion site is hyperintense relative to the normal dorsal funiculus rostral and caudal to the lesion. The persisting hyperintensity (arrow) in these spontaneously remyelinating lesions indicates that remyelination per se is not responsible for the hypointensity seen in the cell transplant recipients. E, T2-weighted MR images 4 weeks after creation of an X-EB lesion in the dorsal funiculus. The persistently demyelinated region can be seen (arrow) is hyperintense relative to the normal dorsal funiculus. It is not possible to distinguish between this persistently demyelinated and the remyelinated dorsal funiculus shown in D.
SPIO-labeled SCs and OECs retain the ability to myelinate after transplantation into areas of persistent demyelination
To determine the ability of the labeled cells to differentiate and participate in repair, we performed electron microscopic studies on the lesioned and transplanted spinal cords that had been imaged ex vivo 4 weeks after transplantation. Selected areas containing remyelinated axons were examined by EM, with and without uranyl acetate and lead citrate staining to prevent confusion of SPIO with staining artifact (Fig. 7_A,C_). Numerous cells clearly associated with myelin sheaths and containing electron-dense cytoplasmic inclusions, similar to those seen in labeled cells in vitro, were identified (Fig. 7_B,D_, arrows). Transplanted SPIO-labeled SCs and OECs were also identified associating with demyelinated axons by extending thin cytoplasmic processes around them but without forming compacted myelin sheaths (Fig. 7_D_, inset). These observations clearly indicate that SPIO-labeled SCs and OECs retain functional integrity. However, in both transplant groups small numbers of large debris-filled macrophages were also identified on the basis of their characteristic ultrastructural features, which contained SPIO inclusions. Their presence indicated that dead cells containing SPIO may be engulfed by host phagocytic cells or that they may take up SPIO released into the extracellular environment. Their overall contribution to the MRI signal remains to be clarified.
Figure 7.
Remyelination by transplanted SPIO-labeled SCs and SPIO-labeled OECs. A, Light micrograph of a toluidine blue-stained resin section from the transplant site in the dorsal funiculus 4 weeks after transplantation of SPIO-labeled SCs. Peripheral type myelination with characteristic signet ring appearance (arrow) can be seen around most of the axons (there is no remyelination in the absence of cell transplantation in the X-EB lesion). B, Electron micrograph of transplanted SCs from the area shown in A. The top cell has produced a myelin sheath and contains electron-dense cytoplasmic SPIO inclusions (arrow). The bottom cell is extending processes around a demyelinated axon and contains SPIO inclusions (black arrow). C, Light micrograph of a toluidine blue-stained resin section from the transplant site in the dorsal funiculus, 4 weeks after transplantation of SPIO-labeled OECs. As with SC transplantation, peripheral type myelination with characteristic signet ring appearance (black arrow) can be seen around many of the axons. D, Electron micrograph showing two SPIO inclusions (black arrows) within a myelinating OEC. Inset, A transplanted OEC containing a SPIO inclusion extending processes around a demyelinated axon. Scale bar: A, C, 50 μm; B, 0.75 μm; D, 0.25 μm; D, inset, 0.3 μm.
Having established that labeled cells retain the ability to myelinate, we next compared the signal changes detectable by MRI with the histological changes evident by light microscopy. At 28 d after transplantation, the contour of the remyelination boundary correlated with the hypointense region on the ex vivo MRI scans in both SC and OEC recipients, with no remaining demyelinated or normal tissue contributing to the hypointensity (Fig. 8_A-F_). In control animals receiving unlabelled cell transplants, the hypointense region seen on the ex vivo MR images correlated with areas of macrophage infiltration rather than of remyelination (Fig. 8_G-I_). In animals injected with EB, only remyelination was widespread throughout the lesion area (Fig. 8_K,L_) and correlated with the generally hyperintense (white) signal seen by MRI. These data support the view that the MRI signal hypointensity relates predominantly to the presence of SPIO-labeled cells in the transplanted animals. However, in animals receiving unlabelled cells, a reduction in signal may occur in areas of significant macrophage infiltration. This macrophage signal is likely to be caused by the sequestration of iron from hemoglobin associated with hemorrhage.
Figure 8.
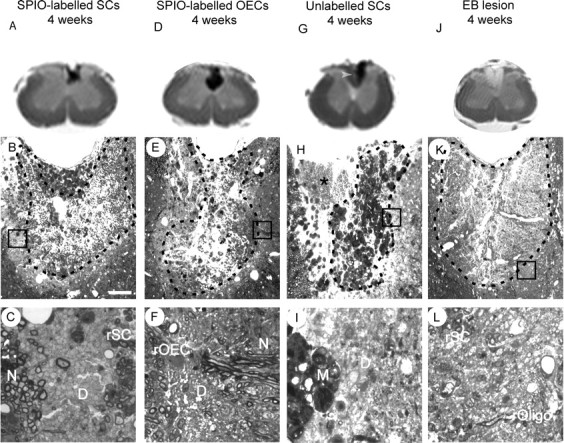
Comparison of ex vivo MR images with the light micrographs from the respective spinal cord sections 4 weeks after transplantation of SPIO-labeled SCs and OECs and unlabelled SCs into X-EB lesions; a nontransplanted EB lesion after 4 weeks is also shown. A, D, MR images from a recipient of transplanted SPIO-labeled SCs and OECs, respectively; B and E show the corresponding light micrographs. The arrows delineate the perimeter of the remyelinated areas in the dorsal funiculus and demonstrate a good correlation with the outline of the hypointense regions on the MR images. C, F, High-power views from the boxed areas in B and E, highlighting axons remyelinated by transplanted SCs (rSC) or OECs (rOEC). Regions containing demyelinated axons (D) and normally myelinated axons (N) lie outside the hypointense regions on the MR images. G, MR image from a recipient of transplanted unlabelled SCs. The region of hypointensity in the dorsolateral part of the dorsal funiculus is less extensive than that seen in A and D. H, The corresponding light micrograph from the MR image shown in G. The cluster of macrophages on the top left of the lesion site (arrowheads) correlates with the region of hypointensity seen on MR image. The region of remyelinated dorsal funiculus (*) does not correspond with the hypointensity on the corresponding MR image, but instead relates to an area of hyperintensity adjacent to the hypointense region (white arrow). I, A high-power view of the boxed area in H; debris-filled macrophages (M) and demyelinated axons (D) are present. J, MR image showing the hyperintense appearance of the epicenter of a spontaneously remyelinating EB lesion. K, The corresponding light micrograph from MR image shown in J. The endogenous remyelination is mediated by both oligodendrocytes and SCs. L, A high-power view from the lesion in K indicated by the box, demonstrating axons remyelinated by SCs (rSC) or oligodendrocytes (rOligo). Scale bar: C, F, I, L, 12 μm; B, E, H, 50 μm; K, 100 μm.
Discussion
As the experimental basis of cell-based therapies for neurological disease becomes ever more extensive, attention is being increasingly directed toward clinical implementation. Several recent studies have addressed the issue of how the fate of cells transplanted into damaged CNS might be followed by noninvasive imaging techniques in both experimental and clinical settings. The use of paramagnetic iron oxides have emerged as contrast agents of considerable promise that can be preloaded into transplanted cells and that allow the cells to be subsequently imaged within tissue by MRI (Hawrylak et al., 1993; Yeh et al., 1995; Bulte et al., 1999, 2001, 2003; Franklin et al., 1999; Lewin et al., 2000; Hoehn et al., 2002). In this study we use this approach to establish the feasibility of noninvasive imaging of transplanted SCs and OECs, two cell types that both support axon regeneration and are able to remyelinate demyelinated axons, thereby making them potential candidates for transplant-based therapies for spinal cord injury or chronic demyelinating disease (Kocsis, 1999; Franklin and Barnett, 2000; Bunge, 2002). In the first part of this study we show that cultured SCs and OECs will readily take up SPIO particles from a suspension, giving high levels of cell labeling. We also provide clear evidence that the means of uptake is by pinocytosis. This process results in SPIO particles residing in the cytoplasm within membrane-bound vesicles that have a distinctive electron-dense appearance, allowing labeled cells to be readily identified by electron microscopy. This straightforward means of cell labeling is similar to our experience with labeling CG4 cells, an oligodendrocyte progenitor cell line, and in our hands we have not found it necessary to use labeling enhancement strategies to achieve efficient labeling (Hawrylak et al., 1993; Bulte et al., 1999, 2001; Lewin et al., 2000).
We next showed, first with fixed cords removed from perfused recipient animals (ex vivo imaging) and subsequently with live, anesthetized animals allowed to recover after imaging (in vivo imaging), that SPIO-labeled SCs and OECs gave contrast in the MR images that was substantially larger than those obtained in any of the control groups. The preparation of SPIO used in these experiments exerts predominantly T2 effects that manifest as a reduction in signal intensity compared with the surrounding white matter. From this we inferred that the reduced signal was attributable to the presence of SPIO-labeled cells, the presence of which was confirmed histologically after imaging. These results provide clear proof-of-principle that transplanted SPIO-labeled SCs and OECs can be imaged within CNS lesions, opening up the possibility of developing the technique as a means of following the fate of either cell type in a clinical context. This would be especially useful for interpreting the outcome of cell delivery; for example, should the therapy prove less effective than expected, the distribution and number of transplanted cells detectable by MRI would indicate whether additional cell delivery was warranted.
Although a clear signal was obtained from spinal cord into which labeled SCs and OECs had been transplanted, nevertheless, a smaller signal was also obtained after transplantation of unlabelled cells. Histological analysis of this tissue, with Prussian blue stain to detect ferric iron, revealed positive staining immediately associated with the point of injection. The injection procedure, even with fine glass micropipettes, induces small focal areas of hemorrhage. The most likely explanation for the Prussian blue staining in this tissue is the presence of iron-containing hemosiderin and deoxyhaemoglobin, which are associated with hemorrhage and are both paramagnetic (Lee et al., 2004). Because the dimensions of the signal in the control animals was considerably less than the large “bloom” effect associated with SPIO, where the distortion of the magnetic field is over a greater area than the distribution of the contrast agent, we are confident that the signal we obtained in the latter case was primarily attributable to the presence of SPIO. However, our results reveal the possibility of hemorrhage, confounding the interpretation of SPIO-labeled cell grafts and raise this as an issue that will need to be considered with further developments of this approach. This is likely to be a short-term complication, disappearing as the hemorrhage resolves and is cleared by macrophages. Despite this, it is feasible that newer agents having greater T2-shortening effects may reduce the significance of confounding factors of endogenous agents that shorten T2 (Hinds et al., 2003).
Finally, we showed that the high SPIO content within the labeled cells does not impair cell function. This is an important observation, given the toxic effects that can be mediated by high iron exposure (De Freitas and Meneghini, 2001; Kress et al., 2002; Patel et al., 2002). Clearly, if this were to occur, then the clinical applications of this technique would be limited, despite the advantages gained by being able to visualize transplanted cells in vivo. In previous imaging studies, the issue of retention cell function has either not been addressed (Franklin et al., 1999; Hoehn et al., 2002), has been demonstrated in vitro but not in vivo (Bulte et al., 2001, 2003), or has been inferred from the coincidence of labeled cells and evidence of functional integrity without clearly demonstrating a direct relationship between the two (Bulte et al., 1999). Both transplanted SCs and OECs will engage demyelinated axons and extend sheet-like processes around them that eventually compact to form myelin sheaths (Blakemore, 1977; Duncan et al., 1981; Franklin et al., 1996b; Imaizumi et al., 1998). This is a highly specialized and complex cell process involving a coordinated expression of numerous genes, many specifically associated with myelination (Lemke, 1988; Topilko and Meijer, 2001). Such a function might therefore be expected to expose functional impairment in labeled cells. However, we were able to identify myelinating cells of both types that contained SPIO particles within their cytoplasm. The identification of SPIO-containing cells in the process of myelination indicated that SPIO did not preclude the process of myelination and that labeled myelinating cells were unlikely to have obtained SPIO after having myelinated. The possibility that cells might obtain label after transplantation was raised by the presence of macrophages containing SPIO particles. Macrophages are a prominent cell type within demyelinating lesions, recruited from both the resident microglial population and from circulating monocytes in response to the myelin debris generated in abundance during demyelination (Ousman and David, 2000). These highly phagocytic cells are likely to have obtained SPIO from the extracellular environment, probably present as a consequence of death of transplanted labeled cells rather than release of SPIO from healthy surviving cells. The possibility of transfer of label to nontransplanted cells, especially to robustly phagocytic cells such as macrophages, where the particles may become concentrated and give rise to a MRI signal disproportionate to the number of labeled cells, is a potential limitation of the technique that will require further analysis, particularly at longer survival times.
This study describes the development of an experimental approach for studying the behavior of SCs and OECs transplanted into the CNS, which has clear potential clinical applications. We have shown that both cell types can readily be labeled in vitro, that labeled cells retain their functional properties and can be detected by MRI, and that in vivo imaging of anesthetized animals offers the possibility of tracking the fate of labeled cells on multiple occasions within the same individual. At present the level of resolution is only sufficient to provide information about the location of large groups of cells and cannot detect the distribution of single separated cells that may migrate away from the bulk of transplanted cells. Even so, detectable cells may be confined to a relatively small area; the transverse dimension of the rat dorsal funiculus within which labeled cells were detected is <1 mm. In the in vivo imaging study, in which animals were imaged 5 d after transplantation, the signal reduction occurred over a longitudinal distance of 3-5 mm, whereas at 28 d in vivo and ex vivo, the maximal longitudinal length of signal reduction had increased to 5-6 mm. We know from earlier studies that the acute distribution of a 1 μl suspension of cells injected into rat dorsal funiculus is in the region of 2 mm (Lipsitz et al., 1995; Franklin et al., 1996a). The images therefore reveal progressive spread of cells away from the point of injection. In addition to cell migration, this spread is likely to involve cell proliferation, a process that will dilute the amount of label within individual cells. This means that detectable signal reduction can still occur despite proliferation of transplanted cells. As such, these results constitute a significant development in noninvasive imaging of cells transplanted into the CNS and establish a powerful tool for bridging the gap between experimental and clinical sciences.
Footnotes
This work was funded by the International Spinal Research Trust. We thank Mike Peacock for his assistance.
Correspondence should be addressed to Dr. Robin Franklin, Neuroregeneration Laboratory, School of Veterinary Medicine, University of Cambridge, Madingley Road, Cambridge CB3 0ES, UK. E-mail: rjf1000@cam.ac.uk.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249799-12$15.00/0
References
- Avellana-Adalid V, Bachelin C, Lachapelle F, Escriou C, Ratzkin B, Baron-Van Evercooren A (1998) In vitro and in vivo behaviour of NDF-expanded monkey Schwann cells. Eur J Neurosci 10: 291-300. [DOI] [PubMed] [Google Scholar]
- Band H, Bhattacharya A, Talwar GP (1986) Mechanism of phagocytosis by Schwann cells. J Neurol Sci 75: 113-119. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, Dunn LT, Papanastassiou V, Kennedy PG, Franklin RJ (2000) Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain 123: 1581-1588. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Avellana-Adalid V, Lachapelle F, Liblau R (1997) Schwann cell transplantation and myelin repair of the CNS. Mult Scler 3: 157-161. [DOI] [PubMed] [Google Scholar]
- Blakemore WF (1977) Remyelination of CNS axons by Schwann cells transplanted from the sciatic nerve. Nature 266: 68-69. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Field KL, Raff MC (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res 165: 105-118. [DOI] [PubMed] [Google Scholar]
- Bulte JWM, Zhang SC, Van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA (1999) Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA 96: 15256-15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, Van Gelderen P, Moskowitz BM, Duncan ID, Frank JA (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19: 1141-1147. [DOI] [PubMed] [Google Scholar]
- Bulte JW, van Zijl PC, Mori S (2002) Magnetic resonance microscopy and histology of the CNS. Trends Biotechnol 20: S24-S28. [Google Scholar]
- Bulte JW, Ben Hur T, Miller BR, Mizrachi-Kol R, Einstein O, Reinhartz E, Zywicke HA, Douglas T, Frank JA (2003) MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med 50: 201-205. [DOI] [PubMed] [Google Scholar]
- Bunge MB (2002) Bridging the transected or contused adult rat spinal cord with Schwann cell and olfactory ensheathing glia transplants. Prog Brain Res 137: 275-282. [DOI] [PubMed] [Google Scholar]
- De Freitas JM, Meneghini R (2001) Iron and its sensitive balance in the cell. Mutat Res 475: 153-159. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Aguayo AJ, Bunge RP, Wood PM (1981) Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J Neurol Sci 49: 241-252. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Mulero V, Munoz J, Meseguer J (1998) Methodological aspects of assessing phagocytosis of Vibrio anguillarum by leucocytes of gilthead seabream (Sparus aurata L) by flow cytometry and electron microscopy. Cell Tissue Res 293: 133-141. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Barnett SC (2000) Olfactory ensheathing cells and CNS regeneration: the sweet smell of success? Neuron 28: 15-18. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Bayley SA, Blakemore WF (1996a) Transplanted CG4 cells (an oligodendrocyte progenitor cell line) survive, migrate, and contribute to repair of areas of demyelination in X-irradiated spinal cord but not in normal spinal cord. Exp Neurol 137: 263-276. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Gilson JM, Franceschini IA, Barnett SC (1996b) Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia 17: 217-224. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, Blaschuk KL, Bearchell M, Presoz LC, Setzu A, Brindle K, ffrench-Constant C (1999) Magnetic resonance imaging of transplanted oligodendrocyte precursors in the rat brain. NeuroReport 10: 3961-3965. [DOI] [PubMed] [Google Scholar]
- Hawrylak N, Ghosh P, Broadus J, Schlueter C, Greenough WT, Lauterbur PC (1993) Nuclear magnetic resonance (NMR) imaging of iron oxide-labeled neural transplants. Exp Neurol 121: 181-192. [DOI] [PubMed] [Google Scholar]
- Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE (2003) Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102: 867-872. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Focking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Buhrle C (2002) Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA 99: 16267-16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD (1996) Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci 16: 3199-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD (1998) Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci 18: 6176-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD (2000) Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia 30: 209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD (1999) Restoration of function by glial cell transplantation into demyelinated spinal cord. J Neurotrauma 16: 695-703. [DOI] [PubMed] [Google Scholar]
- Kohama I, Lankford KL, Preiningerova J, White FA, Vollmer TL, Kocsis JD (2001) Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J Neurosci 21: 944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dineley KE, Reynolds IJ (2002) The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci 22: 5848-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Smith PM, Barnett SC, Franklin RJM (2003) Meningeal cells enhance limited CNS remyelination by transplanted olfactory ensheathing cells. Brain 126: 598-609. [DOI] [PubMed] [Google Scholar]
- Lee IH, Bulte JW, Schweinhardt P, Douglas T, Trifunovski A, Hofstetter C, Olson L, Spenger C (2004) In vivo magnetic resonance tracking of olfactory ensheathing glia grafted into the rat spinal cord. Exp Neurol 187: 509-516. [DOI] [PubMed] [Google Scholar]
- Lemke G (1988) Unwrapping the genes of myelin. Neuron 1: 535-543. [DOI] [PubMed] [Google Scholar]
- Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18: 410-414. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G (1997) Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277: 2000-2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G (2003) Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci 23: 727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz D, Archer DR, Duncan ID (1995) Acute dispersion pf glial cells following transplantation into the myelin deficient rat spinal cord. Glia 14: 237-242. [DOI] [PubMed] [Google Scholar]
- Lu J, Feron F, Mackay-Sim A, Waite PME (2002) Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 125: 14-21. [DOI] [PubMed] [Google Scholar]
- Nash HH, Borke RC, Anders JJ (2002) Ensheathing cells and methylprednisolone promote axonal regeneration and functional recovery in the lesioned adult rat spinal cord. J Neurosci 22: 7111-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, David S (2000) Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 30: 92-104. [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S (2002) Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci 22: 6578-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratten MK, Lloyd JB (1986) Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro. Biochim Biophys Acta 307-313. [DOI] [PubMed]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J (2000) Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25: 425-435. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Lips K, Wunderlich G, Müller HW (1996) Reconstruction of transected postcommissural fornix in adult rat by Schwann cell suspension grafts. Exp Neurol 140: 21-36. [DOI] [PubMed] [Google Scholar]
- Topilko P, Meijer D (2001) Transcription factors that control Schwann cell development and myelination. In: Glial cell development (Jessen KR, Richardson WD, eds), pp 223-244. Oxford: Oxford UP.
- Xu XM, Guénard V, Kleitman N, Bunge MB (1995) Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol 351: 145-160. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang SX, Li HY, Aebischer P, Bunge MB (1999) Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci 11: 1723-1740. [DOI] [PubMed] [Google Scholar]
- Yan Q, Johnson EM (1988) An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci 8: 3481-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TC, Zhang W, Ildstad ST, Ho C (1995) In vivo dynamic MRI tracking of rat T-cells labeled with superparamagnetic iron-oxide particles. Magn Reson Med 33: 200-208. [DOI] [PubMed] [Google Scholar]