Down-Regulation of the kps Region 1 Capsular Assembly Operon following Attachment of Escherichia coli Type 1 Fimbriae to d-Mannose Receptors (original) (raw)
Abstract
A differential-display PCR procedure identified the capsular assembly gene kpsD after Escherichia coli type 1 fimbrial binding to mannose-coated Sepharose beads. Limiting-dilution reverse-transcribed PCRs confirmed down-regulation of the kpsD gene, and Northern blot and lacZ fusion analyses showed down-regulation of the kpsFEDUCS region 1 operon. KpsD protein levels fell, and an agglutination test showed less K capsular antigen on the surface following the bacterial ligand-receptor interaction. These data show that binding of type 1 fimbriae (pili) to d-mannose receptors triggers a cross talk that leads to down-regulation of the capsule assembly region 1 operon in uropathogenic E. coli.
Uropathogenic Escherichia coli (UPEC) strains are the primary causes of urinary tract infections in humans (14). A variety of virulence factors have been shown to be important for UPEC pathogenicity, including adhesins, hemolysins, capsules, and iron-acquiring proteins (35). Adherence to uroepithelial cells is a critical first step in the pathogenesis of UPEC strains. Both P and type 1 fimbriae (pili) are the most frequently observed pilus structures on E. coli cells isolated from the urinary tracts of patients (18, 25). Type 1 fimbriae bind to mannose-containing receptors found in the urinary tracts of mice and humans (12, 21). The ability to attach to these mannose receptors enables the bacteria to gain a foothold in the urinary tract that may lead to invasion into the bladder epithelium (20).
Several techniques have been used to try to elucidate what might be occurring within the bacterial cell following bacterial attachment or what may be important for in vivo survival of UPEC within a human host (3, 4, 34, 37). In this study, a differential-display PCR (DDPCR) was applied to determining what genes might be up- or down-regulated following binding of type 1 fimbriae to mannose receptors to obtain clues as to what might be occurring within the bacteria following entry and initiation of an infection within a human urinary tract.
The NU149 uropathogenic strain of E. coli (28) was grown in Luria broth as previously described (15) to allow for optimal expression of type 1 fimbriae, which was confirmed by an enzyme immunoassay with anti-149 pilus antiserum (30; data not shown). This culture was divided into two parts. One aliquot was reacted with Sepharose 4L beads (Sigma Chemical Co., St. Louis, Mo.), whereas the other aliquot was reacted with d-mannose-coated Sepharose beads (17). The interaction between strain NU149 cells and d-mannose-coated Sepharose beads resulted in 67% of the population binding to the beads, whereas NU149 cells mixed with plain Sepharose beads led to only 21% of the population either binding nonspecifically or being trapped by the beads. After 1.5 h, total RNAs were isolated from both populations by using a hot phenol extraction procedure (30) and treated twice with RNase-free DNase (Boehringer-Mannheim). A DDPCR that was previously described was performed, utilizing the PLCA2 primer to run the amplification (32). The DDPCR products were separated on 5% sequencing gels, and the numbers and intensities of bands were compared for the lane containing plain Sepharose versus that containing the d-mannose-coated Sepharose. Several bands were either missing in one lane compared to the other or had reduced intensity (data not shown). Each band was processed as previously described (32), using the PLCA2 primer to reamplify the DNAs. The resulting PCR products were ligated to pTZ18R plasmid DNA cut with SmaI (27). After verification that there was an insert (data not shown), each recombinant plasmid was sequenced, using M13 forward and reverse primers and the Sequenase 2.0 kit (USB, Cleveland, Ohio). One of the cloned DDPCR DNA products showed extensive homology with the E. coli kpsD gene (24). The DDPCR indicated that the kpsD transcript level was lower in the lane that represented binding to mannose-coated Sepharose beads (data not shown).
The kpsD gene is part of the kpsFEDUCS operon, also named capsule region 1, involved in assembly of capsular subunits that comprise the K antigen of E. coli (36). A single promoter drives transcription of the polycistronic kpsFEDUCS transcript. Strain NU149 appears to have a group II capsule gene locus structure organized into three regions. Regions 1 (kpsFEDUCS) and 3 (kpsMT) are very conserved at the genetic level and are involved in the assembly and transport of the capsular material. The final region, region 2 (kfiABCD), is unique to each serotype and is directly involved in the biosynthesis of the capsular material (36).
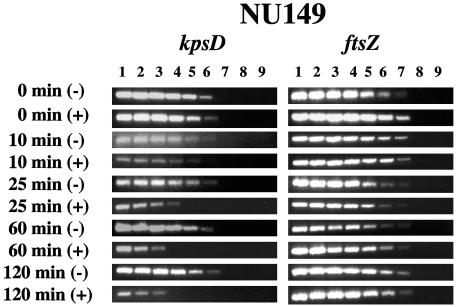
To verify that there was a down-regulation of the kpsD gene following binding to the d-mannose-coated Sepharose beads, the NU149 strain was grown in Luria broth and divided into aliquots: one was reacted with the plain Sepharose beads, and the other was reacted with d-mannose-coated Sepharose beads. After 0, 10, 25, 60, and 120 min, total RNAs were extracted from both sets of cultures and converted into cDNAs as noted above. With these cDNAs, limiting-dilution reverse-transcribed PCRs (LD-RT-PCRs) were performed with the KpsD1 (5′-AACGGACAGAAGTCGGATACG-3′) and KpsD2 (5′-TGTAATAAGGAGGCGTAGACG-3′) primer pair, synthesized by Integrated DNA Technologies (Coralville, Iowa). The LD-RT-PCR conditions were as follows: an initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and elongation at 72°C for 1 min, ending with a final elongation at 72°C for 7 min at the end of the last cycle. A 367-bp product was amplified. Each cDNA population was diluted twofold up through 1:256, and each dilution was PCR amplified. Amplification products were analyzed on 1.5% agarose gels, comparing the populations reacted with plain Sepharose versus those reacted with mannose-coated Sepharose beads. As a control, amplifications of each cDNA population were done using a primer pair specific for the ftsZ gene of E. coli that has been used previously (33). The results indicated that at time zero there was no difference between the E. coli cell populations. However, beginning at 10 min and proceeding through 120 min, there was a gradual decline in the level of kpsD transcripts in the mannose-coated Sepharose population compared to that in the plain Sepharose population that culminated in an eightfold decline after 120 min (Fig. 1). The level of ftsZ transcripts remained unchanged throughout the time course for both populations. This suggested that the ligand-receptor interaction between type 1 fimbriae and the mannose receptors led to the down-regulation of kpsD transcription.
FIG. 1.
Quantitative determination of kpsD and ftsZ transcript levels by LD-RT-PCR analysis. The cDNAs generated from strain NU149 cells mixed with plain Sepharose or d-mannose-coated Sepharose beads were examined at 0, 10, 25, 60, and 120 min. The KpsD1-KpsD2 and ECFtsZ1-ECFtsZ2 primer pairs were used to amplify serially twofold-diluted cDNAs targeting kpsD (367-bp product) and ftsZ (302-bp product) transcripts, respectively. The PCR amplifications were done a minimum of three times. All PCR products were electrophoresed on 1.5% agarose gels. The following dilutions of cDNAs were used: undiluted (lane 1), 1:2 (lane 2), 1:4 (lane 3), 1:8 (lane 4), 1:16 (lane 5), 1:32 (lane 6), 1:64 (lane 7), 1:128 (lane 8), and 1:256 (lane 9).
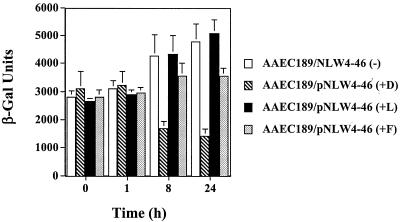
To further substantiate that the binding of type 1 fimbriae to mannose receptors had an effect on capsule region 1 transcription in E. coli cells over time, the single promoter for the kpsFEDUCS gene cluster (region 1), which gives rise to an approximately 7.9-kb polycistronic transcript (36), was fused to lacZ by using plasmid pUJ9 (9). This was then moved to the single-copy-number plasmid pPP2-6 (33), resulting in plasmid pNLW4-46. E. coli AAEC189 cells (Δ_fim_ Δ_lac_ [5]) were cotransformed with pNLW4-46, containing the kps-lacZ reporter fusion, and pWRS1-17, which has the entire fim operon encoded on it (30). The recombinant AAEC189 cells were reacted with plain Sepharose beads, d-mannose-coated Sepharose beads, l-mannose-coated Sepharose beads, and plain Sepharose plus 50 mM free d-mannose. When tested for β-galactosidase levels at 0 and 1 h, all of the populations looked fairly similar, but at 8 h and then ultimately at 24 h there was more than a threefold difference in β-galactosidase units in the population that was mixed with d-mannose-coated Sepharose compared to that mixed with plain Sepharose (Fig. 2). The kps levels increased slightly in the population mixed with plain Sepharose and l-mannose-coated beads after a 24-h exposure. Although the kinetics of the down-regulation of the gene cluster and hence kpsD were different compared to the results of the LD-RT-PCR analysis, this can be explained by the need for transcription and translation of lacZ in a β-galactosidase assay as opposed to merely transcription in the PCR-based assay. Moreover, mixing the bacterial cells with l-mannose-coated Sepharose did not affect the level of kpsFEDUCS operon (region 1) expression. On the other hand, the addition of 50 mM free d-mannose to the population mixed with plain Sepharose did affect region 1 expression, suggesting that merely having the interaction with d-mannose, regardless of whether it is bound to a bead or free, was affecting this expression.
FIG. 2.
Regulation of the kpsFEDUCS promoter following attachment to plain Sepharose (−), l-mannose-coated Sepharose (+L), d-mannose-coated Sepharose (+D), or plain Sepharose plus 50 mM free d-mannose (+F). The assays were done using a kpsFEDUCS promoter fused to lacZYA as a transcriptional fusion on a single-copy-number plasmid placed in strain AAEC189. The β-galactosidase (β-Gal) activity is expressed in Miller units; means ± standard deviations are indicated from three separate runs.
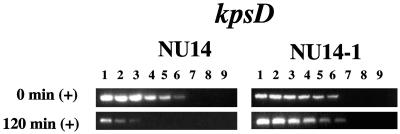
To assess whether the attachment of the type 1 fimbrial adhesin to its receptor might be involved in the down-regulation of kpsD, wild-type strain NU14 cells (15) were compared to cells of strain NU14-1 (19), an fimH mutant strain missing the FimH adhesin, after mixing each bacterial population with d-mannose-coated Sepharose beads. Upon performing LD-RT-PCR analyses, the wild-type strain, NU14, had the same level of down-regulation of kpsD as strain NU149 had at the same time point (Fig. 3). However, the NU14-1 strain appeared to display the same level of kpsD expression at both the 0- and 120-min time points. Transcription of kpsD appears to be negatively regulated by the ligand-receptor binding between type 1 fimbriae and d-mannose.
FIG. 3.
Quantitative determination of kpsD transcript levels in strains NU14 (wild type) and NU14-1 (fimH mutant) by LD-RT-PCR analysis. The cDNAs generated from strain NU14 or NU14-1 cells mixed with d-mannose-coated Sepharose beads at 0 and 120 min were examined. The KpsD1-KpsD2 primer pair was used to amplify serially twofold-diluted cDNAs targeting kpsD (367-bp product) transcripts. The PCR amplifications were done a minimum of three times. All PCR products were electrophoresed on 1.5% agarose gels. The following dilutions of cDNAs were used: undiluted (lane 1), 1:2 (lane 2), 1:4 (lane 3), 1:8 (lane 4), 1:16 (lane 5), 1:32 (lane 6), 1:64 (lane 7), 1:128 (lane 8), and 1:256 (lane 9).
Northern blot hybridizations were then performed at two time points, 0 and 2 h, following the interaction of strain NU149 cells with either plain or d-mannose-coated beads. Total RNAs were isolated from the four samples as previously described. Ten micrograms of total RNA from each population was loaded on a 1% denaturing agarose gel, processed, and hybridized with the radiolabeled kpsD PCR product as described previously (30). Following a 6-day exposure on a phosphorimager screen (Amersham Biosciences, Piscataway, N.J.), the results with ImageQuant 5.2 software indicated a 6.9-fold difference in the kpsFEDUCS 7.9-kb transcript level after 2 h of mixing with d-mannose-coated beads compared to mixing with plain beads (Fig. 4). The level of kpsFEDUCS transcripts in the 2 h (−) lane increased by 1.8-fold, which was consistent with an increase observed in the β-galactosidase measurements (Fig. 2). Thus, the entire kpsFEDUCS operon is down-regulated following type 1 fimbrial binding to d-mannose residues, confirming the lacZ fusion results.
FIG. 4.
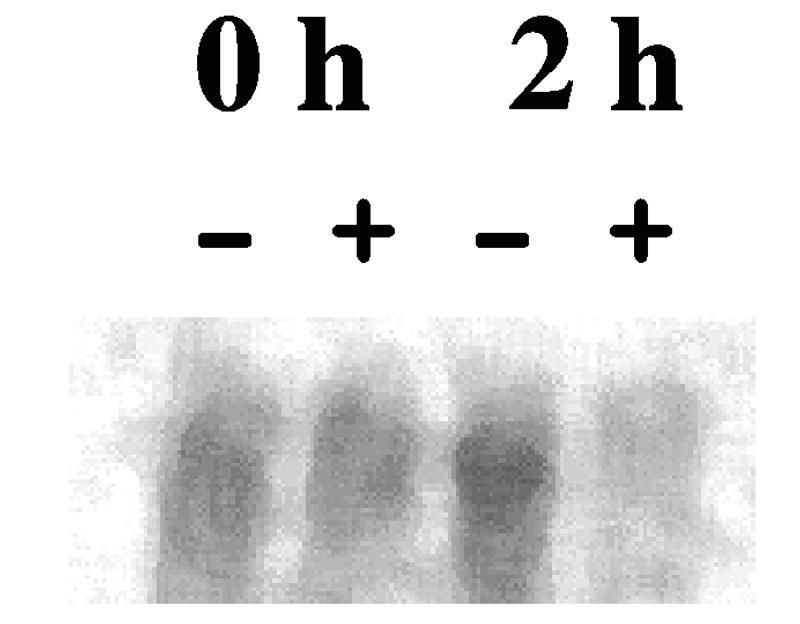
Northern hybridization analysis of transcription of the kpsFEDUCS operon from total RNAs isolated from NU149 cells mixed with plain Sepharose (−) or d-mannose-coated Sepharose (+) beads after 0 and 2 h of exposure. Ten micrograms of total RNA from NU149 cells mixed with plain Sepharose beads or d-mannose-coated Sepharose beads was probed with a kpsD DNA fragment. After 6 days, the blot was developed with a phosphorimager and the amounts of kpsFEDUCS RNA (approximate size, 7.9 kb) were compared between lanes by using ImageQuant 5.2 software.
An examination of KpsD protein levels was then performed to determine if the transcriptional down-regulation carried over to the protein level. Again, two populations were examined: one reacted with d-mannose-coated beads and one reacted with plain Sepharose beads. The bacteria were lysed with lysozyme and sodium dodecyl sulfate, and total protein determinations were made for each group, using the Bio-Rad protein assay (Bio-Rad, Hercules, Calif.). Ninety-six-well microtiter plates were coated with 20 mg of total protein lysate in bicarbonate buffer as previously described (31). To measure KpsD protein levels, a rabbit anti-KpsD antibody (provided by Ian Roberts, University of Manchester) (2) was used at a concentration of 1:3,000 as a primary antibody in an enzyme-linked immunosorbent assay. Anti-rabbit immunoglobulin G labeled with alkaline phosphatase (Sigma) at a dilution of 1:3,000 was used as a secondary antibody, and the wells were developed with the substrate _p_-nitrophenylphosphate (Sigma) as previously described (31). Three time points were tested, 0, 8, and 24 h, comparing NU149 cells mixed with d-mannose-coated versus plain Sepharose at each time point. The results showed no difference in KpsD protein levels for both populations at time point zero but a twofold decline in KpsD after 8 h in the population reacted with d-mannose-coated beads and a further decline after 24 h in this population (Fig. 5). This confirmed not only that kpsD transcript levels dropped but that KpsD protein levels were also lower.
FIG. 5.
Enzyme-linked immunosorbent assay analysis of the KpsD protein from strain NU149 cells mixed with plain or d-mannose-coated Sepharose beads for 0, 8, and 24 h. Optical densities at 405 nm (O.D.405) were determined, and means ± standard deviations are indicated from three runs. (−), plain Sepharose; (+), d-mannose-coated Sepharose.
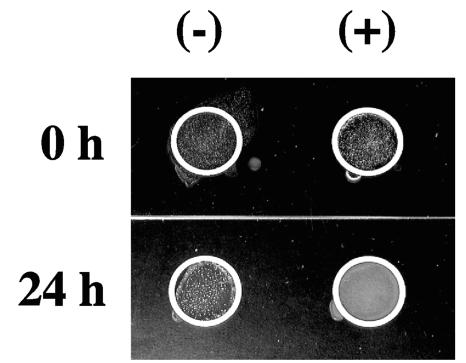
Although KpsD levels fell following adherence by type 1 fimbriated E. coli to mannose receptors, this alone did not confirm that capsular material was reduced on the surface of the bacterial cell. To address the potential role that this physiological change may have on the bacteria, the level of K capsular antigen was then assessed. Since the role of KpsD protein is to assemble capsule subunits onto the surface of the bacterial cell (36), a reduction in the assembly protein may affect the distribution of capsular antigen on the outer surface of the E. coli cells, which in turn could affect the association between the bacterial cell and the host cell that it is attached to in vivo. A past study that compared a kpsD mutant strain to the wild-type strain demonstrated a decline in capsular presentation on the exterior of the E. coli cells (6), so one would predict, since KpsD levels fell after type 1 fimbrial binding to mannose receptors, that less capsular antigen would reach the surface of the E. coli cells. E. coli strain NU14 has been typed as having the K1 antigen (16), but the serotype for strain NU149 is not known. A Directagen agglutination kit (Becton Dickinson, Sparks, Md.) was used to assay for K1 antigen on NU14 cells mixed with d-mannose-coated Sepharose or plain Sepharose at time zero and after 24 h. The time zero results demonstrated equal agglutination reactions when the population mixed with d-mannose-coated beads was compared to the population mixed with plain Sepharose (Fig. 6). However, the population mixed with d-mannose-coated beads displayed less agglutination (white clumps) after 24 h compared to the group mixed with plain Sepharose, demonstrating that less K1 antigen appeared to assemble on the surface of the E. coli cells because of the ligand-receptor interchange between the type 1 fimbriae and the mannose residues.
FIG. 6.
Directagen analysis of K1 capsular expression in strain NU14. Bacterial cells were mixed with plain Sepharose (−) or d-mannose-coated Sepharose (+) beads and assayed after 0 and 24 h. White clumps of bacteria indicate a positive response.
However, one could not rule out that the other biosynthesis genes involved in E. coli K-antigen production in region 2 (i.e., kfiA, kfiB, and kfiD) might be responsible rather than the effect from a reduction in KpsD levels. To address this point, an LD-RT-PCR procedure was then done using primers specific for the kfiC gene, found in region 2, involved in the biosynthesis of group II capsular antigen (36). The LD-RT-PCRs were performed with cDNAs from strain NU14 and the KfiC1 (5′-TCTTGCATATCATCGTTTGC-3′) and KfiC2 (5′-CAGCTCAGAATTCTGGCAA-3′) primer pair (Integrated DNA Technologies). The LD-RT-PCR amplification conditions were the same as those noted for amplification of kpsD. There was no difference in kfiC transcription between the cell population mixed with d-mannose-coated Sepharose compared to the population mixed with plain Sepharose beads at either 0 or 2 h (data not shown). Thus, the reduction in capsular antigen on the surface appears to be the result of lower KpsD levels and not a consequence of transcriptional regulation of at least one structural gene for capsules.
This study is the first to use a DDPCR technique to characterize changes in UPEC following the binding of the type 1 fimbrial adhesin FimH to its mannose receptor. DDPCR has been previously used to examine host cell responses following binding by bacteria (32) and to assess changes in the bacteria themselves (1). Our results show that a capsular assembly gene operon, region 1, containing the kpsFEDUCS gene cluster, is quickly down-regulated at the transcriptional level following bacterial binding, a reduction in the KpsD protein occurs, and less capsular polysaccharide is deposited on the surface of the E. coli cells. Although the data generated with the fimH mutant strain suggest that FimH contact with d-mannose receptors might be regulating kps gene cluster expression, we do not have enough information yet to prove a direct linkage. Certainly, we do not know the chain of events that ultimately leads to the down-regulation of the kps gene cluster following binding of type 1 fimbriae to d-mannose receptors.
UPEC strains adhere to bladder epithelial cells via the type 1 fimbrial adhesin FimH. The FimH adhesin is also responsible for the invasion of the bacteria into the bladder epithelium (20). The presence of a thick capsule surrounding the bacterial cells may hinder both tight adherence by the E. coli cells and the invasion process itself. Certainly, extracellular polysaccharide is important for the in vivo survival of the bacteria, exemplified by the recent signature-tagged mutagenesis study that indicated a pivotal role for the capsule in E. coli residing in the urinary tract (4). Although the presence of a capsule seems to be critical for long-term persistence within the urinary tract of man and mouse, it may also serve to hinder other key steps in the pathogenesis of UPEC within the urinary tract. A recent study suggests that the bacterial capsule may block intimate attachment mediated by protein antigen 43, found on the surface of UPEC strains (29). Previous studies with Klebsiella pneumoniae have indicated that the bacterial capsule impedes close adherence and invasion into epithelial cells (11, 26). Moreover, a bacterial capsule also negatively affects tight adherence and invasion by Neisseria meningitidis (10, 13).
Adherence of E. coli to a host cell through ligand-receptor binding does engender a cross talk between the bacterial cell and the host cell that is likely to result in greater fitness for the bacteria as a result of regulation of specific genes. Zhang and Normark (37) examined binding of P fimbriae to a receptor and identified a sensor-regulator gene essential for the bacterial iron starvation response. The gene was transcriptionally activated by the ligand-receptor interaction. Attached E. coli cells have significantly less OmpX outer membrane protein (23), which in turn can affect type 1 fimbriated E. coli attachment to abiotic surfaces (22). Certainly, other genes must be affected as well by ligand-receptor binding.
One can envision that E. coli cells entering the urinary tract from the outside require the capsule initially. Once the bacteria have bound loosely to bladder epithelial cells via type 1 fimbriae, the capsule becomes a steric hindrance for tight adherence by the bacteria and subsequent invasion. Down-regulation of the region 1 capsular operon, which includes kpsD, occurs quickly, which in turn leads to less capsular material distributed on the exterior of the bacterial cells. This might be advantageous to the bacteria because capsular subunits may accumulate in the cytoplasm as intermediates and be available for quick assembly if the conditions change. Mutations in region 1 capsule genes have previously resulted in such an accumulation of intermediate products (7, 8). The loss of capsular material would facilitate tight adherence and invasion by the bacteria to escape the immune system.
Acknowledgments
We thank Ian Roberts and Carlos Arrecubieta for the antiserum to the KpsD protein as well as Greg Anderson for critical reading of the paper.
This study was funded by a UW-L Graduate Student Research Grant to N.L.W. and NIH grant 1R15AI47801-01A2 to W.R.S.
REFERENCES
- 1.Abu Kwaik, Y., and L. L. Pederson. 1996. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol. Microbiol. 21**:**543-556. [DOI] [PubMed] [Google Scholar]
- 2.Arrecubieta, C., T. C. Hammarton, B. Barrett, S. Chareonsudjai, N. Hodson, D. Rainey, and I. S. Roberts. 2001. The transport of group 2 capsular polysaccharides across the periplasmic space in Escherichia coli. J. Biol. Chem. 276**:**4245-4250. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani-Mougeot, F. K., S. Pancholi, M. Daoust, and M. S. Donnenberg. 2001. Identification of putative urovirulence genes by subtractive cloning. J. Infect. Dis. 182**:**S21-S23. [DOI] [PubMed] [Google Scholar]
- 4.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45**:**1079-1093. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., M. S. McClain, J. A Prine, P. J. Calie, and B. I. Eisenstein. 1991. Type1 fimbriation and fimE mutants of Escherichia coli K-12. J. Bacteriol. 173**:**5298-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner, D., V. Sieberth, C. Pazzani, I. S. Roberts, G. J. Boulnois, B. Jann, and K. Jann. 1993. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J. Bacteriol. 175**:**5984-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cieslewicz, M., and E. Vimr. 1996. Thermoregulation of kpsF, the first region 1 gene in the kps locus for polysialic acid biosynthesis in Escherichia coli K1. J. Bacteriol. 178**:**3212-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieslewicz, M., and E. Vimr. 1997. Reduced polysialic acid capsule expression in Escherichia coli K1 mutants with chromosomal defects in kpsF. Mol. Microbiol. 26**:**237-249. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn_5_ transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172**:**6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries, F. P., A. van der Ende, J. P. M. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64**:**2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favre-Bonte, S., B. Joly, and C. Forestier. 1999. Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect. Immun. 67**:**554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita, K., T. Yamamato, T. Yokota, and R. Kitagawa. 1989. In vitro adherence of type 1-fimbriated uropathogenic Escherichia coli to human ureteral mucosa. Infect. Immun. 57**:**2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy, S. J., M. Christodoulides, R. O. Weller, and J. E. Heckels. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36**:**817-829. [DOI] [PubMed] [Google Scholar]
- 14.Hooton, T. M., and W. E. Stamm. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. N. Am. 11**:**551-581. [DOI] [PubMed] [Google Scholar]
- 15.Hultgren, S. J., W. R. Schwan, A. J. Schaeffer, and J. L. Duncan. 1986. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect. Immun. 54**:**613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, J. R., S. J. Weissman, A. L. Stell, E. Trintchina, D. E. Dykhuizen, and E. V. Sokurenko. 2001. Clonal and pathotypic analysis of archetypal Escherichia coli cystitis isolate NU14. J. Infect. Dis. 184**:**1556-1565. [DOI] [PubMed] [Google Scholar]
- 17.Jones, C. H., J. S. Pinkner, A. V. Nicholes, L. N. Slonim, S. N. Abraham, and S. J. Hultgren. 1993. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc. Natl. Acad. Sci. USA 90**:**8397-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisielius, P. V., W. R. Schwan, S. K. Amundsen, J. L. Duncan, and A. J. Schaeffer. 1989. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect. Immun. 57**:**1656-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276**:**607-611. [DOI] [PubMed] [Google Scholar]
- 20.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282**:**1494-1497. [DOI] [PubMed] [Google Scholar]
- 21.O'Hanley, P., D. Lark, S. Falkow, and G. Schoolnik. 1985. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. J. Clin. Investig. 75**:**347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto, K., and M. Hermansson. 2004. Inactivation of ompX causes increased interactions of type 1 fimbriated Escherichia coli with abiotic surfaces. J. Bacteriol. 186**:**226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto, K., J. Norbeck, T. Larsson, K.-A. Karlsson, and M. Hermansson. 2001. Adhesion of Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. J. Bacteriol. 183**:**2445-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazzani, C., C. Rosenow, G. J. Boulnois, D. Bronner, K. Jann, and I. S. Roberts. 1993. Molecular analysis of region 1 of the Escherichia coli K5 antigen gene cluster: a region encoding proteins involved in cell surface expression of capsular polysaccharide. J. Bacteriol. 175**:**5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pere, A., B. Nowicki, H. Saxen, A. Siitonen, and T. K. Korhonen. 1987. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156**:**567-574. [DOI] [PubMed] [Google Scholar]
- 26.Sahly, H., R. Podschun, T. A. Oelschlaeger, M. Greiwe, H. Parolis, D. Hasty, J. Kekow, U. Ullmann, I. Ofek, and S. Sela. 2000. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 68**:**6744-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd. ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Schaeffer, A. J., W. R. Schwan, S. J. Hultgren, and J. L. Duncan. 1987. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55**:**373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schembri, M. A., D. Dalsgaard, and P. Klemm. 2004. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 186**:**1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan, W. R., H. S. Seifert, and J. L. Duncan. 1992. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J. Bacteriol. 174**:**2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwan, W. R., C. Waltenbaugh, and J. L. Duncan. 1990. Bacteria as solid phase in a concentration fluorescence immunoassay analysis of antibodies to surface antigens. J. Immunol. Methods 126**:**247-252. [DOI] [PubMed] [Google Scholar]
- 32.Schwan, W. R., S. Kugler, S. Schuller, D. J. Kopecko, and W. Goebel. 1996. Detection and characterization by differential PCR of host eukaryotic cell genes differentially transcribed following uptake of intracellular bacteria. Infect. Immun. 64**:**91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwan, W. R., J. L. Lee, F. A. Lenard, B. T. Matthews, and M. T. Beck. 2002. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect. Immun. 70**:**1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorsa, L. J., S. Dufke, and S. Schubert. 2004. Identification of novel virulence-associated loci in uropathogenic Escherichia coli by suppression subtractive hybridization. FEMS Microbiol. Lett. 230**:**203-208. [DOI] [PubMed] [Google Scholar]
- 35.Svanborg, C., and G. Godaly. 1997. Bacterial virulence in urinary tract infection. Infect. Dis. Clin. N. Am. 11**:**513-529. [DOI] [PubMed] [Google Scholar]
- 36.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31**:**1307-1319. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, J. P., and S. Normark. 1996. Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science 273**:**1234-1236. [DOI] [PubMed] [Google Scholar]