A direct intracranial record of emotions evoked by subliminal words (original) (raw)
Abstract
A classical but still open issue in cognitive psychology concerns the depth of subliminal processing. Can the meaning of undetected words be accessed in the absence of consciousness? Subliminal priming experiments in normal subjects have revealed only small effects whose interpretation remains controversial. Here, we provide a direct demonstration of semantic access for unseen masked words. In three epileptic patients with intracranial electrodes, we recorded brain potentials from the amygdala, a neural structure that responds to fearful or threatening stimuli presented in various modalities, including written words. We show that the subliminal presentation of emotional words modulates the activity of the amygdala at a long latency (>800 ms). Our result indicates that subliminal words can trigger long-lasting cerebral processes, including semantic access to emotional valence.
Keywords: amygdala, semantic, visual masking
The question of whether a briefly flashed and masked word inaccessible for conscious report can be processed nonconsciously up to an abstract semantic level remains highly debated. In a seminal paper published in 1983, Marcel reported evidence for nonconscious access to word semantics in the masked priming paradigm (1). Typically, nonconscious semantic priming occurs when a subject engaged in a lexical decision task on visible target words responds faster to trials in which the target was immediately preceded by a semantically related masked prime word (e.g., tiger-lion) than to trials with unrelated prime-target pairs. Those effects were interpreted as a demonstration of nonconscious semantic processing of the masked prime words. However, potentially serious flaws in the methodology used to assess the absence of conscious perception of masked words invalidated or qualified most of the first experimental reports (2).
Subsequently, a set of studies reported behavioral and brain-imaging evidence for semantic processing of numbers and words under conditions of demonstrable lack of consciousness of the prime words (3, 4). Again, however, follow-up publications suggested that those nonconscious semantic effects might be entirely accounted for by direct motor specification, i.e., stimulus–response processes bypassing semantic analysis (5, 6). For instance, Abrams and Greenwald (5) asked subjects to evaluate the valence of consciously seen target words as positive or negative. The prior presentation of a masked word, whose valence could be congruent or incongruent with the upcoming target, was shown to facilitate or interfere with the subjects' response. This result seemed to prove that the masked prime was categorized semantically as positive or negative. Crucially, however, Abrams and Greenwald went on to demonstrate that the priming effect was entirely due to the fact that the prime words also were presented as conscious targets in other trials. When they examined generalization to novel primes that were never seen consciously, priming was obtained only inasmuch as some of their letter fragments matched those of a word from the target list. For instance, after repeated conscious classification of the words “smut” and “bile” as negative, the subliminal prime “smile” primed the negative response, not the positive one. This result suggests that the priming effect, in this particular situation, was not due to a subliminal access to semantics. Rather, subjects had learned to respond rapidly to fragments of the target strings with specific left or right key presses, and this sensorimotor learning generalized to other primes made of the same fragments (7).
Currently, the single category of words for which a convincing set of reports demonstrated nonconscious semantic processing, including generalization to novel primes, are number words (8–11). For nonnumerical words, although many important studies have suggested subliminal access to semantics (1, 12, 13), there is yet no uncontroversial evidence that fulfills the two criteria outlined above, namely convincing proof of lack of conscious perception and rejection of the direct sensorimotor specification hypothesis. The interpretation of this absence of positive results remains a matter of debate. Some argue that nonconscious access to quantity, the main semantic attribute of numbers, is the single exception to a general principle stating that semantic representations are necessarily conscious. Others, including ourselves, argue that nonconscious access to word semantics is possible in principle, although it might be difficult to demonstrate with purely behavioral measures because of response variability and the small size of the effects.
In the present study, we had the opportunity to record local responses from the human amygdala, a neural structure that responds to fearful or threatening stimuli presented in various modalities (14), including written words (15, 16). We reasoned that intracranial amygdala recordings might provide a more sensitive measure of subliminal semantic processing than classical behavioral measures. Our rationale proceeded as follows: If neural activity in the amygdala can be modulated by the threatening vs. neutral quality of masked words, then it would prove that semantic attributes of those words have been accessed nonconsciously.
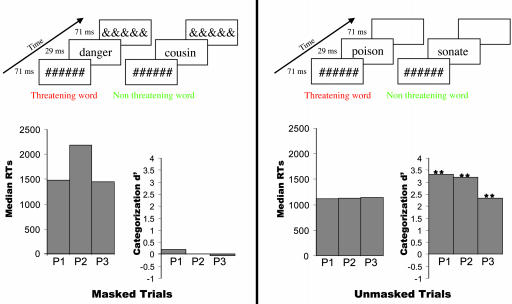
We presented subliminal words by adapting the classical masked priming paradigm. On each trial, a single word was presented briefly (29 ms), preceded and followed by masks consisting of strings of characters (71 ms each; see Fig. 1). To enhance attention and semantic processing, masked trials were randomly intermixed with visible trials in which the poststimulus mask was removed. Furthermore, subjects were engaged in a forced-choice task of categorizing each word as threatening or nonthreatening by pressing response buttons, even on masked trials. To prevent automatic stimulus–response learning, we used two distinct sets of 92 French words each (adapted from ref. 15) for the masked trials and the visible trials, so that masked words were never seen consciously. In each list, half of the words were threatening (e.g., “danger” and “kill”), with variable frequency, length (three to eight letters), and lexical category (verbs and nouns). The other half included nonthreatening, emotionally neutral words (e.g., “cousin” and “see”), matched for frequency, length, and category.
Fig. 1.
Experimental paradigm and behavior. Subjects categorized a 29-ms flashed word as either threatening or neutral. Each word was preceded by a 71-ms mask made of six hash-mark symbols. Masked words were followed by 71-ms ampersands postmask, whereas, on visible trials, no postmask was presented. Reaction times (RTs, in ms) were longer for masked than for unmasked words in the three patients. Although objective word emotional valence categorization assessed by signal detection theory _d_′ was excellent for unmasked words (**, P < 0.01 in χ2 tests), it dropped to chance level for masked words in each of the three patients.
We recorded intracranial local field potentials in three consecutive patients with refractory epilepsy implanted in the amygdalar region from July 2003 to September 2004 in an epileptology unit (Department of Neurology, Hôpital de la Salpêtriere). Patient one (P1) was implanted bilaterally (two electrodes on each side), patient two (P2) had four electrodes in the right amygdala, and patient three (P3) had two electrodes in the left amygdala (see Table 1 and Fig. 1). Clinical exploration, including videoelectroencephalogram recordings, ictal single photon emission computed tomography, and stereoelectroencephalogram data revealed that in the three patients, the implanted amygdalas were not involved in epileptogenic or seizure propagation pathways.
Table 1. Electrodes, anatomical coordinates, and word threat event-related potentials (ERPs) effects.
| Talairach coordinates | P value | |||||
|---|---|---|---|---|---|---|
| Patient | Side | x | y | z | Masked trials | Unmasked trials |
| P3 | Left | -27 | 0 | -32 | 0.028 | 0.006 |
| P1 | Left | -33 | -2 | -29 | 0.008 | 0.019 |
| P2 | Right | 39 | -3 | -30 | 0.004 | 0.016 |
| P2 | Right | 27 | -4 | -31 | 0.037 | <10-4 |
| P2 | Right | 35 | -8 | -25 | 0.008 | <10-4 |
| P1 | Right | 34 | -9 | -24 | 0.050 | 0.269 |
| P2 | Right | 40 | -10 | -22 | 0.064 | <10-4 |
| P3 | Left | -28 | -10 | -26 | 0.625 | 0.999 |
| P1 | Left | -34 | -11 | -23 | 0.014 | 0.0002 |
| P1 | Right | 36 | -17 | -17 | 0.143 | 0.012 |
Materials and Methods
Patients. Experiments were approved by the French ethical committee for biomedical research, and subjects gave informed consent. Three patients suffering from drug-refractory epilepsy were stereotactically implanted as part of a presurgical evaluation with depth electrodes (Ad-Tech Medical Instruments, Racine, WI). The patients were right-handed females (ages 35, 24, and 45 years). Electrodes were 2.3 mm long with 1-mmdiameter cylinders and an interelectrodes distance of 10 mm. The structures to be explored were defined on the basis of ictal manifestations, electroencephalography, and neuroimaging studies. Structural MRI showed the absence of macroscopic lesions in each of these patients. At the time of recordings, patients received antiepileptic therapy. Two epileptogenic foci were found in P1, the most active in the right temporal pole and the second one in the left temporal pole. One focus was found in P2 in the right occipitotemporal cortex. One locus was found in the right medial temporal cortex in P3.
Controls. Twelve right-handed healthy subjects (10 females; age 21–48 years; mean age = 30) received the same behavioral testing as the epileptic patients. They gave their informed consent. Educational level ranged from 0 to 10 years after high school (mean = 4 years).
Procedure. Patients were presented with several blocks of randomized trials sharing the same elementary structure (92 masked words and 92 unmasked words). P1 and P3 received three blocks, and P2 received five blocks. The lists used in the masked and unmasked trials were inverted between P1 and P2. For each patient, the hands assigned to the threatening and nonthreatening responses were inverted halfway through the experiment.
ERPs. The local field potential was digitized at 400 Hz from intracerebral electrodes referenced to the vertex (Nicolet). Epochs were then extracted (–500 ms plus 1,000 ms from word onset), submitted to automatic artifact rejection (±300-μV threshold), visually inspected, low-pass-filtered (70 Hz), and notch-filtered (50 Hz) by using eeglab software (17). Baseline correction (from –500 to 0 ms before word onset) was applied, and potentials were averaged.
Statistics. Experimental conditions were compared by using sample-by-sample t tests, with a criterion of P < 0.05 for a minimum of 30 consecutive samples. We further checked the statistical significance of this criterion through Monte Carlo permutations. This procedure is particularly relevant to estimate the statistical significance of effects observed with a signal of unknown distribution (18). For each patient and for each electrode showing a significant effect, we computed 1,000 random permutations of the observed trials in two surrogate conditions; for each permutation, we then counted the number of surrogate effects satisfying our criterion (a minimum of 30 consecutive samples with t test P < 0.05) anywhere in the ERP time window from 200 ms (earliest ERP effects of lexicality, for printed words vs. pseudowords or consonants strings; see ref. 19) to 900 ms (latest observed effect on our data set). We then computed the observed probability of this criterion (number of surrogate effects per 1,000) and used this proportion as an estimate of the first-order α risk. For each patient and each electrode, this P value was <0.05. Once a main effect of emotional words was observed and validated with the permutation procedure, the presence of additional effects (e.g., word frequency and word length) on the same electrode site and time window was assessed by using ANOVA performed on the potential averaged over the 40-ms window centered on this effect.
Results
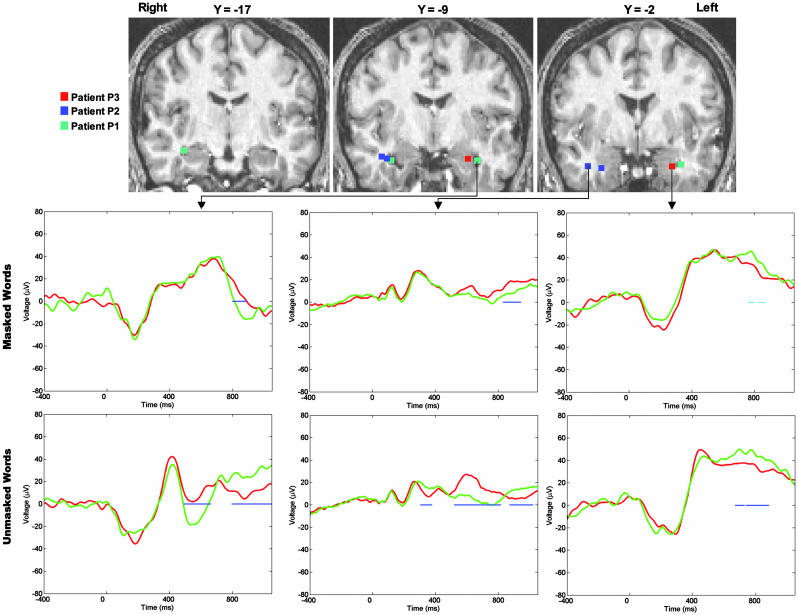
Averaged stimulus-locked local field potentials of the masked words trials revealed a robust subliminal influence of emotional content in each of the three patients. The effect was seen in 5 of the 10 amygdala electrodes, the two left amygdala electrodes in P1, the two right amygdala electrodes in P2, and the most anterior left amygdala electrode in P3, which showed a weaker effect. The difference between threatening and neutral words first became significant ≈800 ms and peaked ≈870 ms after word onset (Fig. 2). Although late relative to stimulus onset, this effect occurred much earlier than the behavioral motor response (median RTs were 1,494 ms, 1,986 ms, and 1,446 ms for P1, P2, and P3, respectively). A similar but nonsignificant trend was also observed on four of the five remaining amygdalar electrodes within the same time window.
Fig. 2.
Effects of threat recorded in the amygdala for nonconscious and conscious words. (Top) Three coronal slices of P1 normalized brain with the locations of the 10 electrodes used to record intracranial local field potential. (Middle and Bottom) For each patient, one electrode is selected (arrows), and the corresponding ERPs are shown for threatening (red) and nonthreatening (green) words in the masked (Middle) and unmasked (Bottom) conditions. Significant differences are indicated by blue (30 successive samples with P < 0.05 in a bilateral t test) or cyan (15 successive samples with P < 0.05 in a bilateral t test) horizontal bars. In the three patients, a significant difference between threatening and nonthreatening masked words was observed ≈870 ms after word presentation. A polarity inversion for the most internal and anterior electrode (rightmost panels) tentatively suggests a generator located within the lateral amygdalar nucleus. In the three patients, unmasked words elicited earlier, more ample, and sustained responses within the same electrodes.
For each of the 10 amygdalar electrodes, the average potential across a 40-ms time window centered on the peak of the effect (850–890 ms for P1, 870–910 ms for P2, and 840–880 ms for P3) was submitted to an ANOVA with word frequency (below vs. above median), word length (three to five vs. six to eight letters), and emotional content (threatening vs. neutral) as factors. A main effect of emotional content was now observed on seven electrodes (P < 0.05; see Table 1). No main effect of nonsemantic parameters was found, nor any interaction of those factors with threat (_P_ > 0.5). Additional analyses showed that response codes (left vs. right hand for the response to threatening words) and response validity (error vs. correct response) did not affect the semantic effect. Interestingly, although six electrodes showed the same electrical polarity, with threatening words eliciting a more positive response than neutral words, a polarity inversion was observed in the most anterior and internal electrode (see P3 in Fig. 2). This inversion suggests that the effect originated from the lateral nucleus of the amygdala.
Our results suggest that the amygdala is automatically involved in the coding of a semantic dimension of words, threat, regardless of their category, frequency, or length. To further demonstrate that this semantic access occurred subliminally, we used both subjective and objective measures of consciousness. During the experiment, objective discrimination (_d_′) estimated by detection measures was excellent for unmasked words (Fig. 1) All _d_′ values were >2.3, as calculated on the basis of 276 trials for P1 and P3 and 460 trials for P2. In contrast, performance dropped to chance level (null _d_′) for masked words. The values of _d_′ were 0.2, 0.0, and –0.05 for P1, P2, and P3, respectively, with P > 0.2 in χ2 tests, as calculated on the same number of trials as for unmasked words. At the end of the experimental trials, patients were presented with each of the 184 words in an explicit recognition test. None of the masked words was recognized, whereas almost all unmasked words were recognized as presented during the experiment (recognition rates > 89%). In an additional block of 92 trials, we also collected subjective verbal reports on each trial. In the three subjects, none of the masked words was reported and none elicited the feeling of having seen a word. The validity of the masking procedure was further strengthened by replicating these measures on a group of 12 control subjects. On the set of 276 masked words trials, _d_′ values were at chance level (mean, _d_′= 0.03; range, –0.24 to 0.27; P > 0.5 in a t test). In the explicit recognition test, none of the masked words was recognized in the 12 subjects tested. We also analyzed RTs recorded in the main categorization task. A strong masking effect was observed, with faster RTs for unmasked words (mean RT = 839 ms) than for masked words [mean RT = 1,251 ms; F(1, 11) = 11.56; P = 0.006]. A main effect of threat was observed with faster RTs for threatening words than for neutral words [F(1, 11) = 53.71; P < 10–4; effect size = 98 ms]. Crucially, a strong interaction was observed between masking and threat [_F_(1, 11) = 8.03; _P_ = 0.0162]. Planned contrasts showed that the effect of threat was present only for unmasked words (_P_ < 10–4) but not for masked words (_P_ > 0.9). The same general pattern was observed on individual data for the three patients [masking by threat interaction, F(1, 2) = 16, 69; P = 0.05]. This qualitative difference between masked and unmasked trials was not a consequence of the overall RT difference between the masked and unmasked conditions. To demonstrate this point, control RTs were categorized as faster or slower than the individual median, separately for masked and unmasked trials. The masking × threat interaction did not interact with response speed (P > 0.9), indicating that threat modulated only unmasked trials, independent of absolute RT speed. Finally, threat effect was absent even on masked trials with fast RTs (P > 0.9). Taken together, behavioral data collected in the three patients and in controls confirm the absence of conscious perception of the masked words.
Our paradigm also allowed us to probe the impact of consciously seen emotional words. Whenever the postmask was removed, subjects easily perceived and categorized words as neutral or threatening (93%, 92%, and 81% hits, respectively). In 7 of the 10 amygdalar electrodes, we observed a difference between consciously seen threatening and neutral words, similar to that seen with subliminal words (Fig. 2). The conscious effect peaked around 600–650 ms after word onset, i.e., earlier than the subliminal effect. This effect showed the same polarity, and the seven electrodes where it was observed included the five electrodes that showed a subliminal effect. ANOVA performed on voltages averaged across a 40-ms window (570–610 ms for P1, 590–630 ms for P2, and 650–690 ms for P3) revealed an effect of emotional content in 8 of 10 electrodes. In addition to this effect, an even earlier effect of word threat was observed in four electrodes (one in P1 and three in P2) ≈350 ms from word onset, and a late sustained effect was observed in three electrodes (one in P1, two in P2, and two in P3) ≈850–900 ms.
Discussion
In summary, our results indicate that the emotional content of subliminal words modulates amygdala activity within the same regions that are also involved in the conscious evaluation of emotional words. Our study concerns only a limited number of epileptic patients receiving antiepileptic drugs. Thus, the results may not be easily generalized to normal subjects. However, in all three patients, seizures did not involve the amygdala region, suggesting that our records may reflect essentially normal processing.
Furthermore, our results mesh with previous neuroimaging studies in normal subjects, which demonstrated that faces (20), sounds (21), and even abstract stimuli such as threatening words can activate the amygdala (15). Masked pictures of fearful faces (22) or of aversively conditioned faces (23) also can trigger an amygdala response in the absence of conscious reportability of the stimuli. Our work supplements those previous results by demonstrating that subliminal amygdala activation can occur with a long latency and for abstract stimuli such as words. It was previously thought that automatic amygdala responses were confined to a fast and coarse stimulus analysis mediated largely by dedicated subcortical routes (24). Yet we did not find a subliminal effect of emotional content for several hundred milliseconds, a late latency that contrasts with earlier emotional intracranial ERP effects elicited by nonlinguistic stimuli such as facial expressions (25). This aspect of our results is suggestive of the existence of an upstream series of visual word recognition, lexical access, and semantic processing stages, which are all logically necessary before the extraction of emotional meaning. Indeed, by using a large number of different words and by never presenting the same words in the masked and visible conditions, our design excluded the possible contribution of a nonsemantic mapping between stimuli and emotional responses based on a shallow analysis of fragments of stimuli, an interpretation that has been proposed for previous experiments in normal subjects (5). It could be argued that the late latency of the amygdala modulation for masked words reflected a slow-arising conscious activation associated with the difficulty of perceiving the stimuli. However, we could not find any evidence of conscious access to masked words, as illustrated by null _d_′, absence of explicit recognition, and absence of threat effect on RTs for masked trials. We tentatively suggest that in such a task subjects use a top-down amplification to introspect the content of emotional networks. As observed in other masking paradigms (26), a top-down influence may amplify the unconscious activation of the amygdala but still fail to make it accessible to conscious report.
So far, nonconscious semantic processing of masked word was demonstrated only for the attribute of quantity associated with masked numbers (8–10). Although several studies suggested that subliminal semantic access also was possible for other types of words (1, 12, 13), this possibility remained hotly debated (2, 5). We suggest that intracranial recordings, together with other localized brain imaging techniques such as functional MRI (27), may contribute importantly to this debate by providing direct evidence that brain regions involved in semantic level analysis can be activated by subliminal primes. As argued by Sternberg (28), such local brain signals may provide more sensitive measures of processing at specific cognitive stages than the composite behavioral measure provided by response times.
In conclusion, our results add to previous subliminal priming experiments suggesting that a extended stream of word processing stages can unfold in the absence of consciousness (4). The exact pathways mediating subliminal semantic access remain to be identified but may include the left fusiform gyrus, which is involved in the visual analysis of subliminal words (29), and the left middle temporal cortex, which was recently shown to exhibit subliminal priming for semantically related words (27). The left anterior temporal lobe also is a likely generator of the N400 semantic violation effects that have been shown to resist under conditions of reduced consciousness (13, 30). The more general question of assessing which of the multiple cortical representations contributing to conscious word semantics (31) are at work in the nonconscious semantic access revealed here constitutes an exciting challenge open to experimental inquiry.
Acknowledgments
We thank the three patients for their constant collaboration and the member editor and the two anonymous expert referees for their insightful and constructive remarks.
Author contributions: L.N., R.G., M.B., S.D., and L.C. designed research; L.N., R.G., C.A., and S.C. performed research; L.N., R.G., C.A., D.H., S.D., and L.C. analyzed data; and L.N., R.G., M.B., S.D., and L.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RT, reaction time; ERP, event-related potential; P_n_, patient n; _d_′, discrimination.
References
- 1.Marcel, A. J. (1983) Cognit. Psychol. 15**,** 197–237. [DOI] [PubMed] [Google Scholar]
- 2.Holender, D. (1986) Behav. Brain Sci. 9**,** 1–23. [Google Scholar]
- 3.Greenwald, A. G. (1996) Science 273**,** 1699–702. [DOI] [PubMed] [Google Scholar]
- 4.Dehaene, S., Naccache, L., Le Clec, H. G., Koechlin, E., Mueller, M., Dehaene-Lambertz, G., van de Moortele, P. F. & Le Bihan, D. (1998) Nature 395**,** 597–600. [DOI] [PubMed] [Google Scholar]
- 5.Abrams, R. L. & Greenwald, A. G. (2000) Psychol. Sci. 11**,** 118–124. [DOI] [PubMed] [Google Scholar]
- 6.Damian, M. F. (2001) J. Exp. Psychol. Hum. Percept. Perform. 27**,** 154–165. [DOI] [PubMed] [Google Scholar]
- 7.Neumann, O. (1990) Psychol. Res. 52**,** 207–215. [DOI] [PubMed] [Google Scholar]
- 8.Naccache, L. & Dehaene, S. (2001) Cognition 80**,** 215–229. [DOI] [PubMed] [Google Scholar]
- 9.Naccache, L. & Dehaene, S. (2001) Cereb. Cortex 11**,** 966–974. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald, A. G., Abrams, R. L., Naccache, L. & Dehaene, S. (2003) J. Exp. Psychol. Learn Mem. Cognit. 29**,** 235–247. [DOI] [PubMed] [Google Scholar]
- 11.Reynvoet, B., Brysbaert, M. & Fias, W. (2002) Q. J. Exp. Psychol. A 55**,** 1127–1139. [DOI] [PubMed] [Google Scholar]
- 12.Perea, M. & Gotor, A. (1997) Cognition 62**,** 223–240. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer, M. (2002) Brain Res. Cogn. Brain Res. 13**,** 27–39. [DOI] [PubMed] [Google Scholar]
- 14.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23**,** 155–184. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg, N., Silbersweig, D., Engelien, A., Emmerich, S., Malavade, K., Beattie, B., Leon, A. C. & Stern, E. (1999) Proc. Natl. Acad. Sci. USA 96**,** 10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson, A. K. & Phelps, E. A. (2001) Nature 411**,** 305–309. [DOI] [PubMed] [Google Scholar]
- 17.Delorme, A. & Makeig, S. (2004) J. Neurosci. Methods 15**,** 9–21. [DOI] [PubMed] [Google Scholar]
- 18.Manly, B. F. J. (1997) Randomization, Bootstrap, and Monte Carlo Methods in Biology (Chapman & Hall/CRC, Boca Raton, FL), 2nd Ed.
- 19.Cohen, L., Dehaene, S., Naccache, L., Lehericy, S., Dehaene-Lambertz, G., Henaff, M. A. & Michel, F. (2000) Brain 123**,** 291–307. [DOI] [PubMed] [Google Scholar]
- 20.Breiter, H. C., Etcoff, N. L., Whalen, P. J., Kennedy, W. A., Rauch, S. L., Buckner, R. L., Strauss, M. M., Hyman, S. E. & Rosen, B. R. (1996) Neuron 17**,** 875–887. [DOI] [PubMed] [Google Scholar]
- 21.Zald, D. H. & Pardo, J. V. (2002) Neuroimage 16**,** 746–753. [DOI] [PubMed] [Google Scholar]
- 22.Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B. & Jenike, M. A. (1998) J. Neurosci. 18**,** 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, J. S., Ohman, A. & Dolan, R. J. (1998) Nature 393**,** 467–470. [DOI] [PubMed] [Google Scholar]
- 24.Vuilleumier, P., Armony, J. L., Driver, J. & Dolan, R. J. (2001) Neuron 30**,** 829–841. [DOI] [PubMed] [Google Scholar]
- 25.Krolak-Salmon, P., Henaff, M. A., Vighetto, A., Bertrand, O. & Mauguiere, F. (2004) Neuron 42**,** 665–676. [DOI] [PubMed] [Google Scholar]
- 26.Naccache, L., Blandin, E. & Dehaene, S. (2002) Psychol. Sci. 13**,** 416–424. [DOI] [PubMed] [Google Scholar]
- 27.Devlin, J. T., Jamison, H. L., Matthews, P. M. & Gonnerman, L. M. (2004) Proc. Natl. Acad. Sci. USA 101**,** 14984–14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sternberg, S. (2001) Acta Psychol. (Amsterdam) 106**,** 147–246. [DOI] [PubMed] [Google Scholar]
- 29.Dehaene, S., Naccache, L., Cohen, L., Bihan, D. L., Mangin, J. F., Poline, J. B. & Riviere, D. (2001) Nat. Neurosci. 4**,** 752–758. [DOI] [PubMed] [Google Scholar]
- 30.Luck, S. J., Vogel, E. K. & Shapiro, K. L. (1996) Nature 383**,** 616–618. [DOI] [PubMed] [Google Scholar]
- 31.Bookheimer, S. (2002) Annu. Rev. Neurosci. 25**,** 151–188. [DOI] [PubMed] [Google Scholar]