Reexamination of the Role of Ubiquitin-Like Modifier ISG15 in the Phenotype of UBP43-Deficient Mice (original) (raw)
Abstract
UBP43/USP18 was described as a specific protease that removes conjugated ubiquitin-like modifier ISG15 from target proteins. The severe phenotype of UBP43−/− mice characterized by premature death, brain cell injury, and deregulated STAT1 signaling was ascribed to an enhanced conjugation of ISG15. In contrast, no phenotypic changes were detected in ISG15−/− mice. To verify the role of ISG15 in the phenotype of UBP43−/− mice, we employed mice deficient for both ISG15 and UBP43. Here, we show that the phenotype of UBP43−/− mice was not rescued by the absence of ISG15, as evident from unchanged mortality, neurological symptoms, and occurrence of hydrocephalus. Also, the reported hypersensitivity of UBP43−/− mice to an interferon inducer, poly(I · C), was ISG15 independent. Furthermore, no evidence for a role of ISG15 in the modulation of STAT1 signaling or in the resistance against lymphocytic choriomeningitis virus and vesicular stomatitis virus was found. Presented results clearly demonstrate that the phenotypic alterations of UBP43−/− mice are not caused by the lack of ISG15 deconjugation and must be due to another, non-ISG15-mediated molecular mechanism.
Postranslational modification of proteins by covalently linked ubiquitin and ubiquitin-like modifiers (UBLs) is a rapid and versatile regulatory mechanism involved in many cellular processes (5, 14). ISG15 is a 15-kDa interferon (IFN)-induced UBL which bears two ubiquitin-like domains, each about 30% homologous to ubiquitin (1, 3, 6, 12). Analogous to ubiquitin and other UBLs, ISG15 is conjugated by an isopeptide bond to target proteins (7). Correspondingly, an ISG15-specific enzymatic cascade that consists of an activating UBE1L (E1) and a conjugating UbcH8 (E2) enzyme was recently identified (18, 19). Several ISG15-modified substrates such as serpin 2a (4), JAK1, STAT1, phospholipase Cγ1, and ERK1 (8) were reported, but rigorous evidence for an ISG15 covalently linked to these proteins, with the exception of serpin 2a, has not yet been found. Most recently, a large number (158) of ISG15 target proteins that function in diverse cellular pathways were identified by mass spectroscopy (20).
Previous work from several laboratories implicated ISG15 and ISGylation in diverse biological activities, ranging from a role in pregnancy to antiviral and antitumor defense (11, 17). Speculations on the biological significance of ISG15 were further amplified by analyses of mice lacking UBP43/USP18 (16), a protease that removes conjugated ISG15 from substrate proteins (9). Mice with a targeted deletion of UBP43 (UBP43−/−) have elevated levels of ISG15 conjugates and develop brain injury due to necrosis of ependymal cells, accompanied by hydrocephalus and early death (16). Investigators reported a prolonged STAT1 phosphorylation in the absence of UBP43 and claimed that ISG15 modification is an important factor in the regulation of the JAK-STAT pathway and IFN signaling (10, 17). Most recently, some evidence was presented for a role of UBP43 and possibly ISGylation in innate immunity against viral infection (15).
We have questioned the previously postulated biological activities of ISG15, since ISG15−/− mice are healthy, without any obvious defect, and analysis of ISG15-/- did not reveal any evidence for defective STAT1 and IFN signaling (13). In order to elucidate the role of ISG15 in the phenotype of UBP43−/− mice and to dissect ISG15-dependent and ISG15-independent effects, we generated mice deficient for both ISG15 and UBP43. Thus, if the severe consequences of UBP43 deletion are due to enhanced ISG15 conjugation (16), they should be rescued in the double knockouts. The analyses of ISG15−/− UBP43−/− mice presented here unequivocally demonstrate that phenotypic alterations in UBP43−/− mice are caused by an ISG15-independent mechanism.
MATERIALS AND METHODS
Mice.
Double-deficient ISG15−/− UBP43−/− mice were obtained from a successive breading of homozygous ISG15−/− mice (13) and heterozygous UBP43+/− mice (16). The UBP43+/− mice were as previously described, except that the pGK-neo cassette was removed (kindly provided by Dong-Er Zhang, Scripps Research Institute, La Jolla, Calif.). Both parental strains were on a mixed genetic background (129, C57BL/6, and Swiss Webster). The mice were kept under standard pathogen-free conditions.
Cells.
Primary mouse embryonic fibroblasts (MEF) were derived by cutting embryonic day 13.5 embryos without head and inner organs into pieces. Material from each embryo was incubated in 2 ml of trypsin-EDTA solution (200 mg/liter EDTA, 500 mg/liter trypsin; BioWhittaker) for 20 min and then passed several times through a 1-ml automatic pipette to obtain cell suspensions. Upon inactivation of trypsin by the addition of medium containing fetal calf serum, cells were plated and used up to a maximum passage number of six.
Histology.
Mice were euthanized, and brains were removed and fixed in 4% buffered formalin. Dissected brains were embedded in paraffin before staining with hematoxylin-eosin for the assessment of hydrocephalus.
Poly(I · C) injections.
Seven-day-old mice were injected intraperitoneally with poly(I · C) (Sigma) at a concentration of 50 μg/g of body weight. The sensitivity of mice to poly(I · C) treatment was assessed by their survival.
Western blotting.
Cells in 6-cm dishes were treated with IFN-β as indicated and lysed at different time points with radioimmunoprecipitation assay buffer containing protease inhibitors (Boehringer). Proteins (60 μg/lane) were loaded on 7.5% sodium dodecyl sulfate-containing polyacrylamide gels and, upon separation, were blotted to nitrocellulose, using standard techniques. STAT1 (mouse monoclonal) and STATp Tyr701 (rabbit) antibody were purchased from Cell Signaling; antiactin antisera (goat) were from Santa Cruz. Western blots were incubated with primary antibodies according to the manufacturer's protocol, and secondary antibodies were coupled to horseradish peroxidase. Blots were developed using ECL reagent (Amersham).
Activation of IFN-inducible genes and Northern blot analysis.
MEF were treated with recombinant IFN-β (100 U/ml) (Calbiochem). After stimulation, cells were lysed and RNA was isolated using TRI-Reagent (Sigma) according to the manufacturer's protocol. A total of 5 to 10 μg/lane RNA was separated on 1% formaldehyde-agarose gels and blotted to positively charged nylon membrane. Probes were radioactively labeled with Rediprime (Amersham) and hybridized using Express-Hyb-Solution (Clontech) according to the manufacturer's protocol.
Viral infections and assay of antiviral activity.
Wild-type, ISG15−/−, UBP43−/−, and ISG15−/− UBP43−/− mice were intracerebrally inoculated with 105 infectious units (IU) of lymphocytic choriomeningitis virus (LCMV) in 50 μl phosphate-buffered saline. The WE strain LCMV was produced and titrated in L929 cells as PFU (2).
For the infection of cells, 1 day prior to infection, 4 × 105 MEF per well were seeded in six-well plates, and 100 U/ml of recombinant murine IFN-β was added to half of the wells. After 24 h cells were infected with LCMV (0.1 PFU/cell). Cell supernatants were harvested on days 1, 2, and 3 after infection; they were centrifuged and stored at −70°C until virus titration. The cells were washed, and RNA was isolated using Trizol reagent according to the manufacturer's protocol (Invitrogen). The RNA was separated on 1.2% agarose gels containing formaldehyde. Upon capillary transfer to positively charged nylon membrane, blots were hybridized using a probe against LCMV 5S RNA.
The antiviral state of IFN-treated MEF against vesicular stomatitis virus (VSV) infection was determined using a cytopathic effect assay. Briefly, MEF were seeded into 24-well plates at a density of 1 × 105 cells per well and incubated with serial dilutions of recombinant murine IFN-β (Calbiochem) as indicated. After 24 h, cells were incubated with different doses of VSV (Indiana strain) ranging from 104 PFU/well to 108 PFU/well. Cell viability was determined by crystal violet staining 24 h after infection.
RESULTS AND DISCUSSION
Mortality of UBP43−/− ISG15−/− mice.
The severe phenotype and the early death of UBP43−/− mice were interpreted as evidence indicating that the enhanced conjugation and accumulation of ISG15 conjugates is not tolerated in vivo (16). If this assumption is correct, then deleting ISG15 should rescue the defect of UBP43−/− mice. As we have shown previously, the ISG15−/− mice are healthy and without any obvious defect (13).
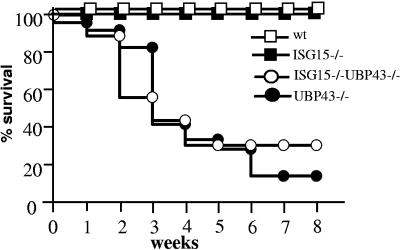
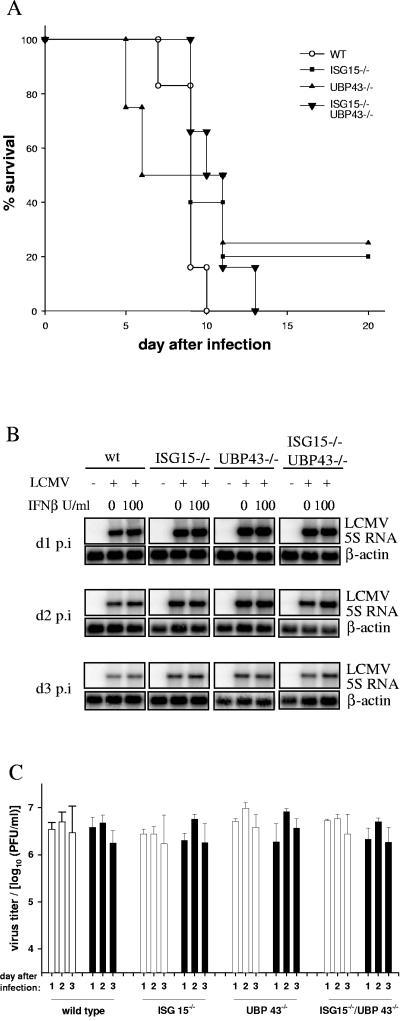
Double-deficient ISG15−/− UBP43−/− mice were obtained by breeding ISG15−/− with UBP43+/− mice, as described in Materials and Methods. As shown in Fig. 1, the mortality of double-deficient UBP43−/− ISG15−/− mice compared with that of UBP43−/− mice was not significantly different, with 50% of both types of mice dying at about 4 weeks of age. This result clearly indicates that the enhanced conjugation and accumulation of ISG15 conjugates is not responsible for the early death of UBP43−/− mice.
FIG. 1.
Mortality of ISG15−/− UBP43−/− and UBP43−/− mice. Survival of wild-type (n = 25), ISG15−/− (n = 25), UBP43−/− (n = 36), and ISG15−/− UBP43−/− (n = 24) mice was monitored for a time period of 8 weeks. wt, wild type.
Neuropathological symptoms and hydrocephalus.
Increased levels of conjugated ISG15 in UBP43−/− mice were also taken as the cause of ependymal cell necrosis, aqueductal stenosis, and hydrocephalus. It was assumed that the resulting compression of vital central nervous system structures leads to the premature death of UBP43−/− mice (16).
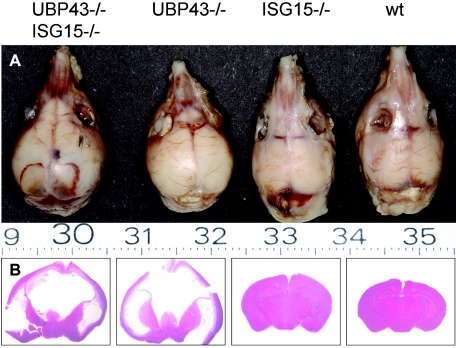
We have observed similar neurological symptoms, like convulsions, tremor, and loss of balance in both UBP43−/− mice and in the double-knockout UBP43−/− ISG15−/− mice. Both types of mice developed hydrocephalus to similar extents, as documented in Fig. 2.
FIG. 2.
Hydrocephalus in UBP43−/− and UBP43−/− ISG15−/− mice. Macroscopic images of sculls (A) and hematoxylin-eosin stained coronal sections (B) of brains derived from 8-week-old mice of the indicated genotypes. wt, wild type.
The fact that the brain injury was manifested also in the absence of ISG15 indisputably rules out any role of ISG15 in the neuropathological alterations described previously in UBP43−/− mice (16).
Hypersensitivity to poly(I · C).
The survival rate of UBP43−/−mice treated with the IFN-inducer poly(I · C) was shown to be drastically reduced (10). These observations were interpreted as a hypersensitivity to IFN, due to an enhanced ISGylation and prolonged IFN signaling in UBP43−/− cells (10, 17).
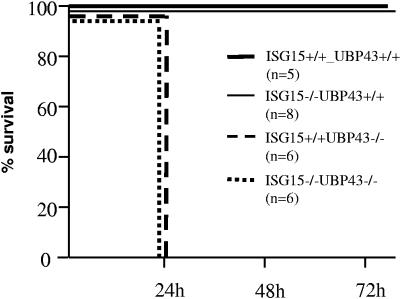
Here, we have examined the effect of poly(I · C) on the survival of mice, using methods similar to those described by Malakhova et al. (10). Figure 3 shows that as expected, wild-type and ISG15−/− UBP43+/+ mice were not affected by this treatment. On the contrary, both types of mice lacking UBP43, (UBP43−/− ISG15 +/+ and UBP43−/− ISG15−/− mice) died within 24 h after poly(I · C) treatment. The fact that mice lacking both ISG15 and UBP43 were also poly(I · C) hypersensitive rules out the involvement of ISG15 in this phenomenon.
FIG. 3.
Hypersensitivity to poly(I · C). Sensitivity of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− mice to poly(I · C) treatment was assessed by intraperitoneal injection of mice with poly(I · C) (50 mg/g of body weight).
STAT1 signaling and expression of IFN-induced genes.
It was reported that protein ISGylation modulates the JAK-STAT signaling pathway and enhances IFN signaling. UBP43-deficient cells revealed a prolonged STAT1 tyrosine phosphorylation and an extended induction of IFN target genes upon IFN stimulation (10, 17). In contrast, the STAT1 phosphorylation as well as the induction of STAT1 target genes was not affected by the lack of ISG15 (13).
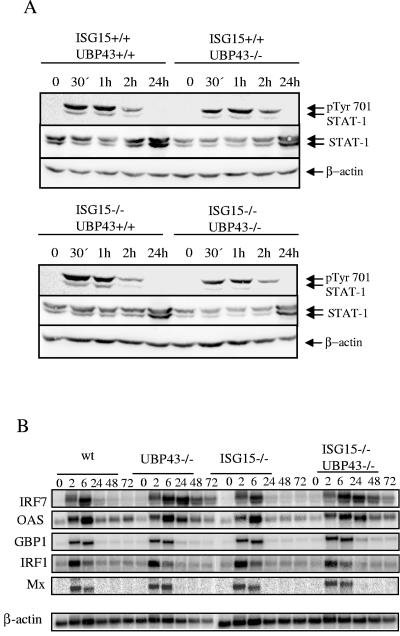
Therefore, it was interesting to compare the kinetics of STAT1 (Y701) phosphorylation after IFN treatment of MEF derived from ISG15−/− UBP43−/−, UBP43−/−, ISG15−/−, and wild-type mice. As shown in Fig. 4A, STAT1 phosphorylation was induced and terminated with the same kinetics in all four types of cells. The same results were obtained also with bone marrow cells (data not shown).
FIG. 4.
STAT1 responses of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− MEF. (A) MEF were stimulated with 100 U/ml of IFN-β for the times indicated. Western blots were incubated with anti-pTyr701 antibody to detect STAT1 phosphorylation and with anti-STAT1 and β-actin as loading controls. (B) MEF were stimulated with 100 U/ml IFN-β for the times indicated. Upon RNA isolation, Northern blots were hybridized with probes specific for the STAT-1 target genes IRF7, 2′,5′ oligoadenylate synthetase (OAS), guanylate-binding protein-1 (GBP1), IRF1, and Mx.1, with β-actin as a loading control. wt, wild type.
Despite the unchanged STAT1 phosphorylation, the expression of two IFN-stimulated genes, IRF7 and 2′,5′ oligoadenylate synthetase, was slightly prolonged in cells lacking either UBP43 or UBP43 and ISG15, while the expression of three other IFN-induced genes, IRF1, GBP1, and Mx.1, remained unaffected (Fig. 4B). Whether the extended expression of IRF7 and GBP1 represents a UBP43-specific effect remains to be investigated. Nevertheless, our results do not support the conclusions of Malakhova et al. (10) on the role of ISG15 in JAK-STAT and IFN signaling.
Antiviral activity.
Recently, Ritchie et al. reported that the ISG15 protease UBP43 and possibly protein ISGylation do have a role in innate immunity against viral infection (15). Mice deficient for UBP43 were resistant to fatal intracranial infection with LCMV or VSV (15). Our attempt to reproduce this experiment was unsuccessful. All four types of animals, ISG15−/− UBP43−/−, UBP43−/−, ISG15−/−, and wild-type mice succumbed rapidly to the intracranial LCMV infections (Fig. 5A). This is not an unexpected finding, as due to the developing hydrocephalus (16), already uninfected UBP43−/− mice have an overall reduced fitness and survival rate (Fig. 1).
FIG. 5.
Viral infections. (A) Wild-type (n = 6), UBP43−/− (n = 4), ISG15−/− (n = 5), and ISG15−/− UBP43−/− (n = 6) mice were infected intracranially with 105 IU of LCMV, and survival was monitored daily. (B) RNA from MEF of the indicated genotypes pretreated with the indicated amounts of IFN-β was isolated 1, 2, or 3 days postinfection (d1 p.i., d2 p.i., and d3 p.i., respectively) with LCMV, as described in Materials and Methods. RNA was separated, and Northern blots were hybridized using a probe specific for 5S LCMV and for actin as a loading control, respectively. (C) Virus replication in wild-type, UBP43−/−, ISG15−/−, and ISG15−/− UBP43−/− MEF either untreated (open bars) or treated with 100 U/ml IFN-β (filled bars) 24 h prior to infection with LCMV was assessed by measuring viral titers in the supernatant 1, 2, or 3 days after infection as described in Materials and Methods. wt, wild type.
Additional evidence for the antiviral activity was drawn from the resistance of IFN-treated UBP43-deficient cells against VSV and LCMV (15). Therefore, we asked whether the lack of ISG15 would affect the antiviral state of UBP43-deficient MEF. IFN-treated and untreated MEF from wild-type, ISG15−/−, UBP43−/−, and ISG15−/− UBP43−/− mice were infected with LCMV, and the amounts of viral RNA and virus titers were determined at days 1, 2, and 3 postinfection. As documented in Fig. 5B and C, LCMV replication was not affected by the lack of UBP43, ISG15, or both genes.
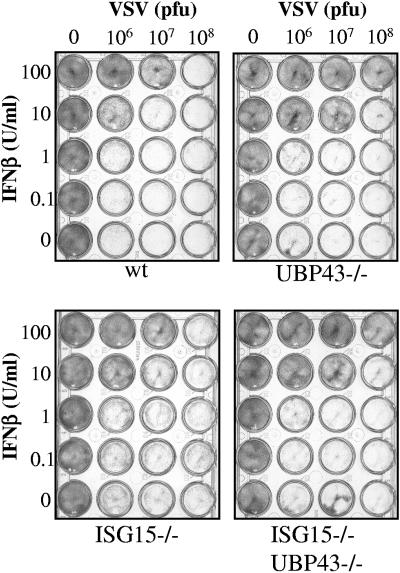
In order to test whether the enhanced resistance of UBP43−/− cells against the VSV cytopathic effect (15) is ISG15 dependent, MEF from wild-type, ISG15−/−, UBP43−/−, and ISG15−/− UBP43−/− mice were treated with IFN and infected with VSV, and cell viability was assessed (Fig. 6). A slight degree of protection against VSV cytopathic effect was consistently observed in both types of cells lacking UBP43. Currently, we cannot rule out whether the lack of UBP43 is responsible for the slightly enhanced resistance of MEF against VSV. Nevertheless, our results show that this effect is ISG15 independent. As discussed above, in vivo infections with VSV could not be performed, due to the high mortality and reduced fitness of UBP43−/− mice.
FIG. 6.
Antiviral response of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− cells upon VSV infection. MEF were incubated with serial dilutions of recombinant murine IFN-β as indicated. After 24 h, cell cultures were infected with VSV and cell viability was monitored 24 h later. wt, wild type.
In summary, our results do not support the notion (15) of a possible role of ISGylation in innate immunity against infection with LCMV and VSV.
Concluding remarks.
In this report, analyses of double-deficient UBP43−/− ISG15−/− mice were used to reexamine the conclusions from experiments obtained with UBP43−/− mice (10, 16) that were erroneously interpreted as a consequence of defective ISG15 deconjugation. We have shown here that the increased mortality, occurrence of hydrocephalus, and poly(I · C)hypersensitivity of UBP43−/− mice are not related to ISG15.
Other previously reported findings, the extended tyrosine phosphorylation of STAT1 and the enhanced antiviral resistance of UBP43-deficient mice (10, 15), were not confirmed in our hands.
Neither the lack of ISG15 (13) nor enhanced ISGylation (this report) was shown to be associated with a distinct phenotypic alteration in mice, and consequently, the question of the biological meaning of ISG15 remains to be solved.
The fact that the phenotypic alterations seen in UBP43−/− mice are not caused by the lack of ISG15 deconjugation suggests another, non-ISG15-mediated, molecular mechanism. Thus, UBP43 protease activity might not be as specific for ISG15 conjugates as assumed by Malakhov et al. (9), and it could also cleave other ubiquitin or UBL conjugates in vivo. Alternatively, UBP43 might have still another unknown biological function.
Acknowledgments
This research was supported by Deutsche Forschungsgemeinschaft grants Ho 493/12 and KN 590/1.
We thank Dong Er-Zhang for UBP43+/- mice, Thomas Meyer for advice in VSV assays, and Marcus Wietstruk, Claudia Pallasch, and Stefanie Zischkau for excellent technical assistance.
REFERENCES
- 1.Farrell, P. J., R. J. Broeze, and P. Lengyel. 1979. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature 279**:**523-525. [DOI] [PubMed] [Google Scholar]
- 2.Gegin, C., and F. Lehmann-Grube. 1992. Control of acute infection with lymphocytic choriomeningitis virus in mice that cannot present an immunodominant viral cytotoxic T lymphocyte epitope. J. Immunol. 149**:**3331-3338. [PubMed] [Google Scholar]
- 3.Haas, A. L., P. Ahrens, P. M. Bright, and H. Ankel. 1987. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J. Biol. Chem. 262**:**11315-11323. [PubMed] [Google Scholar]
- 4.Hamerman, J. A., F. Hayashi, L. A. Schroeder, S. P. Gygi, A. L. Haas, L. Hampson, P. Coughlin, R. Aebersold, and A. Aderem. 2002. Serpin 2a is induced in activated macrophages and conjugates to a ubiquitin homolog. J. Immunol. 168**:**2415-2423. [DOI] [PubMed] [Google Scholar]
- 5.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67**:**425-479. [DOI] [PubMed] [Google Scholar]
- 6.Korant, B. D., D. C. Blomstrom, G. J. Jonak, and E. Knight, Jr. 1984. Interferon-induced proteins. Purification and characterization of a 15,000-dalton protein from human and bovine cells induced by interferon. J. Biol. Chem. 259**:**14835-14839. [PubMed] [Google Scholar]
- 7.Loeb, K. R., and A. L. Haas. 1992. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J. Biol. Chem. 267**:**7806-7813. [PubMed] [Google Scholar]
- 8.Malakhov, M. P., K. I. Kim, O. A. Malakhova, B. S. Jacobs, E. C. Borden, and D. E. Zhang. 2003. High-throughput immunoblotting. Ubiquitin-like protein ISG15 modifies key regulators of signal transduction. J. Biol. Chem. 278**:**16608-16613. [DOI] [PubMed] [Google Scholar]
- 9.Malakhov, M. P., O. A. Malakhova, K. I. Kim, K. J. Ritchie, and D. E. Zhang. 2002. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 277**:**9976-9981. [DOI] [PubMed] [Google Scholar]
- 10.Malakhova, O. A., M. Yan, M. P. Malakhov, Y. Yuan, K. J. Ritchie, K. I. Kim, L. F. Peterson, K. Shuai, and D. E. Zhang. 2003. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 17**:**455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martensen, P. M., and J. Justesen. 2004. Small ISGs coming forward. J. Interferon Cytokine Res. 24**:**1-19. [DOI] [PubMed] [Google Scholar]
- 12.Narasimhan, J., J. L. Potter, and A. L. Haas. 1996. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J. Biol. Chem. 271**:**324-330. [DOI] [PubMed] [Google Scholar]
- 13.Osiak, A., O. Utermöhlen, S. Niendorf, I. Horak, and K.-P. Knobeloch. 2005. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningits virus. Mol. Cell. Biol. 25**:**6338-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickart, C. M. 2004. Back to the future with ubiquitin. Cell 116**:**181-190. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie, K. J., C. S. Hahn, K. I. Kim, M. Yan, D. Rosario, L. Li, J. C. de la Torre, and D. E. Zhang. 2004. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 10**:**1374-1378. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie, K. J., M. P. Malakhov, C. J. Hetherington, L. Zhou, M. T. Little, O. A. Malakhova, J. C. Sipe, S. H. Orkin, and D. E. Zhang. 2002. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 16**:**2207-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie, K. J., and D. E. Zhang. 2004. ISG15: the immunological kin of ubiquitin. Semin. Cell Dev. Biol. 15**:**237-246. [DOI] [PubMed] [Google Scholar]
- 18.Yuan, W., and R. M. Krug. 2001. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 20**:**362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao, C., S. L. Beaudenon, M. L. Kelley, M. B. Waddell, W. Yuan, B. A. Schulman, J. M. Huibregtse, and R. M. Krug. 2004. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc. Natl. Acad. Sci. USA 101**:**7578-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, C., C. Denison, J. M. Huibregtse, S. Gygi, and R. M. Krug. 2005. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA 102**:**10200-10205. [DOI] [PMC free article] [PubMed] [Google Scholar]