Activation of Vav/Rho GTPase Signaling by CXCL12 Controls Membrane-Type Matrix Metalloproteinase–Dependent Melanoma Cell Invasion (original) (raw)
. Author manuscript; available in PMC: 2007 Aug 25.
Abstract
Melanoma cells express the chemokine receptor CXCR4, which confers invasive signals on binding to its ligand CXCL12. We show here that knocking down membrane-type matrix metal-loproteinase (MT1-MMP) expression translates into a blockade of invasion across reconstituted basement membranes and type I collagen gels in response to CXCL12, which is the result of lack of MMP-2 activation. Interference with MMP-2 expression further confirms its important role during this invasion. Vav proteins are guanine-nucleotide exchange factors for Rho GTPases that regulate actin dynamics and gene expression. We show that melanoma cells express Vav1 and Vav2, which are activated by CXCL12 involving Jak activity. Blocking Vav expression by RNA interference results in impaired activation of Rac and Rho by CXCL12 and in a remarkable inhibition of CXCL12-promoted invasion. Importantly, up-regulation of MT1-MMP expression by CXCL12, a mechanism contributing to melanoma cell invasion, is blocked by knocking down Vav expression or by inhibiting Jak. Together, these data indicate that activation of Jak/Vav/Rho GTPase pathway by CXCL12 is a key signaling event for MT1-MMP/MMP-2–dependent melanoma cell invasion.
Introduction
Increasing experimental and clinical data are accumulating, which point to the important roles that chemokines and their receptors could play during tumor cell metastasis. Chemokines are a family of small cytokines that promote cell migration and activation, exerting their actions on binding to G protein–coupled receptors (1). CXCR4, the receptor for the chemokine CXCL12 (also named stromal cell–derived factor-1), is expressed in a variety of solid tumor cell types, including melanoma, breast carcinoma, colon carcinoma, prostate cancer, and neuroblastoma (2–7). Importantly, inhibition of CXCL12/CXCR4 interactions impairs metastasis of human breast cancer cells into regional lymph nodes and lung in mice, and expression of CXCR4 on murine B16 melanoma cells correlated with enhanced pulmonary and lymph node metastatic potential (3, 8). Further in vivo studies of tumor cell metastasis in mice together with clinical data indicate that CXCR4 expression conveys tumor cell invasiveness and patient poor prognosis in a variety of solid cancer types (9–14). CXCL12/CXCR4 interaction is likely important not only for tumor cell invasion but also for tumor growth (10, 15). CXCL12 is expressed in lungs, lymph nodes, liver, and bone marrow; therefore, it is reasonable to propose that CXCL12 could guide tumor cells in an organ-selective metastasis; thus, this interaction might represent an important target for antitumor therapeutics (7, 16).
Tumor cell invasion across tissues requires coordinated activation of extracellular matrix (ECM) degradation and cell motility mechanisms. Matrix metalloproteinases (MMP) are multidomain zinc-dependent endopeptidases involved in ECM proteolysis that play key roles in tissue remodeling and tumor invasion (17–19). They can be secreted in a latent form and subsequently processed to active species, but they can a constitute integral membrane proteins, the membrane-type MMPs (MT1-MMP). MT1-MMP is an important component of the pericellular proteolysis machinery involved in the degradation of several ECM proteins, including gelatin, laminin, and fibrillar collagens (20, 21). Furthermore, MT1-MMP is an activator of pro-MMP-2 in coordination with tissue inhibitor of metalloproteinase-2 (TIMP-2), and its proteolytic activity also controls cell adhesion and growth (20, 22). MT1-MMP is expressed in different solid tumor cell types, such as lung, breast, and melanoma, and its expression often correlates with tumor invasiveness across tissue barriers (23–28). Notably, transgenic mice for MT1-MMP display tumor promotion in mammary gland (29), and conditional expression of this MMP confers tumorigenicity and invasion on normal epithelial cells (28). MT1-MMP and MMP-2 have been found in malignant melanoma specimen often associated to the invading tumor front (30–32), suggesting that their proteolytic activity could be involved in melanoma cell dissemination.
Rho GTPases, such as Rho, Rac, and Cdc42, are key regulators of cell motility (33, 34), whose activation is controlled by guanine-nucleotide exchange factors (GEF), which stimulate the exchange of GDP for GTP on Rho proteins (35). Active Rho GTPases can then interact with downstream targets and produce different biological responses. Although abundant evidence indicates that activation of Rho GTPases plays important roles during tumor cell invasion (36), limited information is available on the GEFs that activate these GTPases and that therefore constitute central molecules regulating invasion (37, 38).
Vav proteins are GEFs that catalyze the activation of Rac and Rho and regulate cell morphology and motility as well as gene expression (39–41). Three Vav family members have been described: Vav1 is predominantly expressed on hematopoietic cells, whereas Vav2 and Vav3 have a broad expression pattern. Vav proteins contain distinct domains, including CH, Ac, DH, PH, ZF, PR, SH3, and SH2, which have the potential to participate in different interactions (39, 40). Activation of Vav GEF activity requires phosphorylation at tyrosine residues located in the Ac domain (42, 43). The DH domain binds to Rho GTPases and is responsible for GEF activity, whereas deletion of domains CH and Ac generates a Vav form displaying constitutive GEF activity (39, 42, 44). On the other hand, the SH2 and SH3 domains interact with autophosphorylated tyrosine kinases and with several adaptor proteins (39–41). Little is known on Vav protein expression on solid tumor cells and whether they play a role in tumorigenesis. Vav1 was found earlier in neuroblastoma cells (45), and a more recent report described its ectopic expression in pancreatic cancer cells and an important role in the control of their proliferation (46).
We described previously that expression of CXCR4 on melanoma cells enables in vitro migration, invasion, and activation of these cells in response to CXCL12 (2, 47). Invasion across reconstituted basement membranes promoted by CXCL12 was dependent on activation of MT1-MMP and Rho GTPase functions. Furthermore, we showed that CXCL12-triggered up-regulation of MT1-MMP expression and function on these cells contributed to increase in invasion and that Rac and Rho controlled this up-regulation. Importantly, CXCR4 expression on malignant melanoma predicts poor prognosis (11, 14). The present study addressed several important points for a better characterization of signaling events and MMP activities that are involved in CXCL12-promoted melanoma cell invasion. First, we investigated Vav protein expression on melanoma cells and their potential involvement in invasion. We have determined MMP-2 role in this invasion and have finally analyzed Vav functional relations with Rho GTPases and MT1-MMP during this invasion.
Materials and Methods
Cells and antibodies
The human melanoma cells lines BLM, Mel 57, and MeWo were cultured as reported previously (47). Samples of human melanoma tissues were obtained from patients with metastatic lesions undergoing surgical treatment (s.c. tissue, lymph node, and lung metastasis), in agreement with the Medical Ethics Committee at the Hospital General Universitario Gregorio Marañón (Madrid, Spain; no. SAF2002-04615). All patients gave written informed consent to use their samples for research purposes. Histopathologic diagnosis was confirmed for each specimen. Melanoma cells were obtained from specimens with high tumor volume, processed, cultured, and used for expression analyses as described previously (47). The human multiple myeloma cell line NCI-H929 was cultured in RPMI 1640 supplemented with 10% fetal bovine serum. The monoclonal antibody (mAb) anti-MT1-MMP LEM-2/15 was provided by Dr. Alicia G. Arroyo (Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain). Anti-Vav mAb was from Santa Cruz Biotechnology (Santa Cruz, CA), whereas polyclonal anti-DH and anti-SH2 Vav1 antibodies were generated and described in a previous work (48). Rabbit antibodies against Vav2 were generated by immunization with synthetic peptides corresponding to Vav2 acidic region, and immunoprecipitation and immunoblotting analyses indicated that they did not recognize Vav1 or Vav34. Control P3X63 and anti-integrin β1 TS2/16 mAb were gifts from Dr. Francisco Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain), mAbs to CXCR4 and MMP-9 were from R&D Systems (Minneapolis, MN), anti-MMP-2 was from Neomarkers (Fremont, CA), anti-Rac and anti-paxillin were from BD Biosciences (San Diego, CA), anti-Rho and anti-phosphotyrosine PY20 were from Santa Cruz Biotechnology, anti–green fluorescent protein (GFP) was from Molecular Probes (Eugene, OR), and anti-Akt and anti-phospho-Akt (Ser473) antibodies were from Cell Signaling (Beverly, MA).
RNA interference, reverse transcription-PCR, and transfections
Small interfering RNA (siRNA) duplexes were designed to human MT1-MMP, Vav1, and Vav2 and shown on Table 1. In addition, we used a control siRNA (Table 1) as well as validated siRNA for CXCR4 and MMP-2. siRNA duplexes were purchased from Dharmacon (Lafayette, CO) and Ambion (Austin, TX). All 21-nucleotide siRNA duplexes were verified to be specific for their targets by BLAST search against the human genome. For reverse transcription-PCR (RT-PCR), cells were lysed in TriReagent (Sigma-Aldrich, St. Louis, MO), and RNA was extracted and reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Amplification of human Vav1 was done by PCR using primers 5′-TGCCTATGCAGCGAGTTCTC-3′and 5′-CCCTGCGATGTAGTTTGTCC-3′ and Taq DNA polymerase (Invitrogen Corp., Carlsbad, CA). For Vav2, the primers were 5′-TCAAAGAAGCACTGGAAGCC-3′and 5′-CAGAAAAACTG-GAAGCCCTG-3′. The PCR profile for both Vav1 and Vav2 was done as described (49). Amplification of human CXCR4 was done using primers 5′-TTCTACCCCAATGACTTGTG-3′and 5′-ATGTAGTAAGGCAGCCAACA-3′. Following 1-minute initial denaturation, profile was 40 cycles of 30-second denaturation at 94°C, 30-second annealing at 55°C, and 45-second polymerization at 72°C. Amplification of MT1-MMP cDNA was done with primers and profile conditions as reported previously (50). As cDNA loading control, aliquots of each sample were amplified with human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (47). For transfections, BLM cells were transiently transfected with expression vectors coding for GFP alone (pEGFP-C1), GFP-fused wild-type (WT) MT1-MMP (51), GFP-fused Vav1 and Vav2 WT, and the mutant forms Vav1 SH3-SH2-SH3 and Vav1 and Vav2 ΔCH+Ac (52). GFP vectors (1.5 μg) or siRNA (100 nmol/L) were transfected using LipofectAMINE reagent (Invitrogen) according to the described method (47).
Table 1.
siRNA duplexes used in this work
| Name | Target bases | Sense strand | Antisense strand |
|---|---|---|---|
| MT1-MMP (2) | 215-235 | UGUUCGAAGGAAGCGCUACdTdT | GUAGCGCUUCCUUCGAACAdTdT |
| MT1-MMP (3) | 2,105-2,125 | UUGGCAGCCUCUCACUACUdTdT | AGUAGUGAGAGGCUGCCAAdTdT |
| Vav1 (3) | 2,133-2,153 | CGUCGAGGUCAAGCACAUUdTdT | AAUGUGCUUGACCUCGACGdTdT |
| Vav2 (2) | 1,220-1,240 | AGUCCGGUCCAUAGUCAACdTdT | GUUGACUAUGGACCGGACUdTdT |
| Vav2 (3) | 1,370-1,390 | CAACAAGGACGUCAAGAAGdTdT | CUUCUUGACGUCCUUGUUGdTdT |
| Control | — | AUUGUAUGCGAUCGCAGACdTdT | GUCUGCGAUCGCAUACAAUdTdT |
Invasion and zymography assays
Invasions were done as established earlier (47). Briefly, BLM or transfected BLM cells resuspended in invasion medium were loaded on filters coated with Matrigel (BD Biosciences, Bedford, MA) or with type I collagen-rich solution (MP Biomedicals, Aurora, OH) in Transwell (BD Falcon, Franklin Lakes, NJ). The lower compartments of invasion chambers were filled with invasion medium with or without CXCL12 (300 ng/mL; R&D Systems). For zymography, conditioned medium was resolved under nonreducing conditions on SDS-PAGE gels embedded with 1 mg/mL gelatin (Sigma-Aldrich). Gels were rinsed with 2.5% Triton X-100 followed by incubation in reaction buffer as described (47) and finally stained with Coomassie blue. Areas of gelatinolytic activity were visualized as transparent bands.
Confocal microscopy and immunohistochemistry
For confocal microscopy, melanoma cells attached to fibronectin were fixed with paraformaldehyde 4% in PBS, incubated with primary antibodies followed by incubation with fluorochrome-conjugated secondary antibodies, and mounted with Mowiol. Images were captured using a Leica TCS-SP2-AOBS-UV confocal microscope with ×63 oil immersion objective. For staining of F-actin, cells were fixed as above and permeabilized with 0.5% Triton X-100 in PBS. Cells were subsequently incubated with Alexa 633-phalloidin (Molecular Probes) and observed with the confocal microscopy. Images displayed were captured at the same section in the different samples. For immunohistochemistry, punch biopsies were immediately frozen, and acetone-fixed 4-μm-thick sections of cryopreserved tissue were first incubated with 10% nonimmune goat serum (Zymed, South San Francisco, CA). Samples were then incubated for 1 hour at 23°C with rabbit anti-human Vav1 or Vav2, HMB-45 (DAKO, Glostrup, Denmark), rabbit anti-S-100 (Novocastra Laboratories, Newcastle, United Kingdom), or isotype-matched control antibodies. All antibodies were used at dilutions of 1 to 5 μg/mL and anti-Vav antibodies were first tested for reactivity on lymphoid tissue. Immunoenzymatic staining was done using DAKO LSAB 2 biotinylated link anti-mouse and anti-rabbit immunoglobulins and streptavidin labeled with alkaline phosphatase (DAKO). Alkaline phospha-tase activity was detected by fuchsin (Fuchsin Substrate-Chromogen System; DAKO), and hematoxylin was used for counterstaining. The slides were mounted using an aqueous permanent mounting medium (DAKO). Vav expression by tumor cells was detected based on melanoma cell morphology and expression of markers of melanoma/melanocyte lineage (HMB-45/S-100) in correlative histologic sections. Samples were considered positive for Vav expression when at least 30% of the malignant cells were positively stained. Other Vav-positive cells present in the tissue sections were identified as HMB-45-negative lymphocytes that were not taken into account.
Immunoprecipitation, Western blotting, and GTPase assays
For immunoprecipitation, melanoma cell lines or melanoma cells from lymph node metastasis preincubated with or without CXCL12 were washed in ice-cold stop buffer and lysed as described (53). Lysates were precleared with protein A-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden), and supernatants were incubated with antibodies followed by specific coupling to protein A-Sepharose beads. Proteins were eluted in Laemmli buffer, resolved by SDS-PAGE, and transferred to polyvinylidene fluoride (PVDF) membranes (Amersham Pharmacia Biotech). Membranes were incubated with antibodies followed by washing and incubation with horseradish peroxidase–conjugated secondary antibodies. Proteins were visualized using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). After stripping and blocking, the blots were reprobed with control antibodies to test for total protein content or with antibodies to detect coimmunopre-cipitation. GTPase activity assays were done as reported previously (47). In brief, starved BLM cells were resuspended in invasion medium and incubated at 37°C with or without CXCL12 (150 ng/mL). Cells were lysed and aliquots from extracts kept for total lysate controls, and the remaining volume was mixed with glutathione _S_-transferase (GST)-PAK-CD ( for Rac) or glutathione _S_-transferase (GST)-C21 ( for RhoA) fusion proteins (54) in the presence of glutathione-agarose beads. Following incubation, bound proteins were eluted in Laemmli buffer, resolved by SDS-PAGE, and analyzed by Western blotting.
Statistical analyses
Data were analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparisons. In both analyses, the minimum acceptable level of significance was P < 0.05.
Results
Role of MT1-MMP and MMP-2 on CXCL12-promoted mela-noma cell invasion
Transfection into BLM melanoma cells of siRNA for CXCR4 or MT1-MMP, or overexpression of a GFP-fused WT MT1-MMP form (MT1-GFP), confirmed the key role of MT1-MMP in melanoma cell invasion toward CXCL12 (47; Fig. 1_A_ and B; see also Supplementary Fig. S1).
Figure 1.
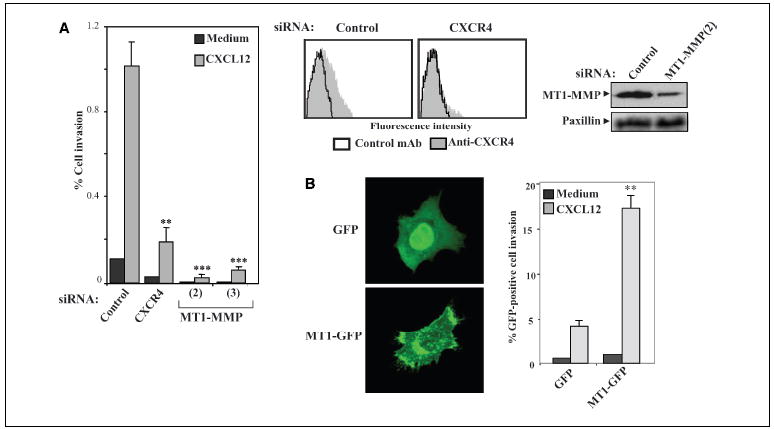
CXCR4 and MT1-MMP requirement for CXCL12-promoted melanoma cell invasion. A, BLM melanoma cells were transfected with siRNA for CXCR4 or with two different siRNAs for MT1-MMP (2 and 3), and transfectants were subjected to invasion assays across Matrigel toward CXCL12 or invasion medium alone. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. ***, P < 0.001; **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test). Middle and right, expression of CXCR4 and MT1-MMP on BLM siRNA transfectants was analyzed by flow cytometry and Western blotting. Blots were reprobed with anti-paxillin mAb to check for total protein content. B, BLM cells were transfected with pEGFP (GFP) or GFP-fused WT MT1-MMP (MT1-GFP) expression vectors and analyzed by confocal microscopy (left) or subjected to invasion assays toward CXCL12 ( right). Columns, mean % invasive GFP-positive cells of two independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly stimulated with respect to GFP transfectants (one-way ANOVA test).
As MT1-MMP is an activator of pro-MMP-2 processing in coordination with TIMP-2, and both MT1-MMP and MMP-2 have been found in malignant melanoma specimen (30, 32), we explored MMP-2 involvement in CXCL12-promoted melanoma cell invasion. BLM cells transfected with MT1-MMP (2) siRNA exhibited a blockade of pro-MMP-2 processing to the mature form compared with control siRNA transfectants as shown by gelatin zymography of their supernatant cultures (Fig. 2_A_). Blocking of invasion to CXCL12 shown by MT1-MMP siRNA transfectants as well as inhibition in their proMMP-2 processing raised the possibility that impairment in invasion could be the result of decreased MMP-2 activity. Therefore, we transfected siRNA specific for MMP-2 in BLM melanoma cells and did invasion assays with transfectants. Both expression and gelatinolytic activity of MMP-2 were drastically reduced in MMP-2 siRNA transfectants compared with control counterparts, whereas MMP-9 expression was unaltered (Fig. 2_B_). Invasion assays across Matrigel revealed that CXCL12-promoted invasion of MMP-2 siRNA transfectants was substantially inhibited in comparison with control siRNA transfectants (Fig. 2_C_, left).
Figure 2.
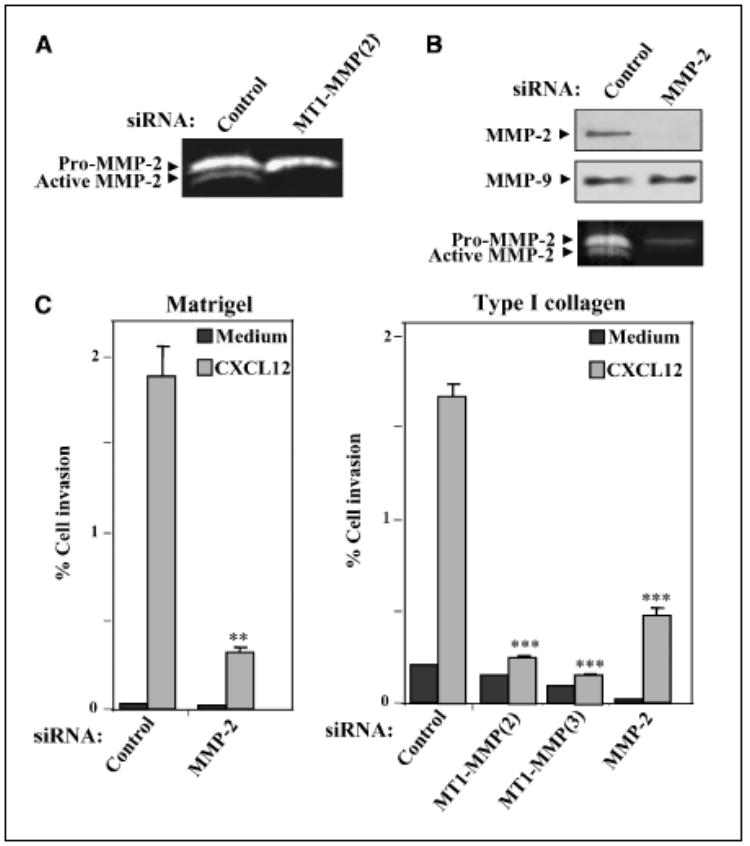
Role of MMP-2 on CXCL12-promoted melanoma cell invasion. A, supernatants from BLM cells transfected with control or MT1-MMP (2) siRNA were analyzed by gelatin zymography. B, BLM cells were transfected with MMP-2 or control siRNA, and transfectant lysates were subjected to Western blotting using anti-MMP-2 mAb followed by reprobing membranes with anti-MMP-9 antibodies (top and middle). Supernatants from transfected cells were assayed by gelatin zymography to detect MMP-2 (bottom). C, control, MT1-MMP, or MMP-2 siRNA BLM transfectants were subjected to invasion assays across Matrigel (left) or type I collagen gels (right) toward CXCL12 or medium alone. Columns, mean % cell invasion of three (B) or two (C) independent experiments done in duplicate; bars, SD. ***, P < 0.001; **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test).
To further characterize MT1-MMP and MMP-2 involvement in melanoma cell invasion to CXCL12, we did invasion assays across type I collagen-rich gel preparations. BLM cells transfected with siRNA for MT1-MMP displayed a large decrease in their invasion across type I collagen gels in response to CXCL12 (Fig. 2_C_, right). MMP-2 siRNA transfectants exhibited a notable impairment in their CXCL12-promoted invasion although consistently of lower magnitude compared with MT1-MMP siRNA transfectants (Fig. 2_C_, right). These results indicate that melanoma cells can use their MT1-MMP and MMP-2 proteolytic activities to advance through basement membranes and type I collagen-rich gels.
Melanoma cells express Vav proteins: role in invasion promoted by CXCL12
The Rho GTPases Rac and RhoA are activated by CXCL12 on melanoma cells, and expression of dominant-negative mutant forms of these GTPases inhibit cell invasion in response to CXCL12 (47). Vav proteins are GEFs that interact with and activate Rac and Rho, promoting reorganization of actin cytoskeleton and regulation of gene expression (39, 40). Expression of Vav on melanoma cells isolated from lymph node metastases and on melanoma cell lines was analyzed at RNA and protein levels. RT-PCR experiments revealed that Vav1 and Vav2 were expressed on BLM melanoma and metastatic melanoma cells as well as on the myeloma cell line NCI-H929 that was used as a positive control (Fig. 3_A_, left). Vav protein expression on melanoma cells could only be detected by immunoprecipitation followed by Western blotting using anti-Vav1 and anti-Vav2 antibodies (Fig. 3_A_, middle and right). Instead, Vav1 protein expression was readily detected on T and B lymphocyte cell lines already by Western blotting (data not shown). These results suggested that melanoma cells express low levels of Vav proteins.
Figure 3.
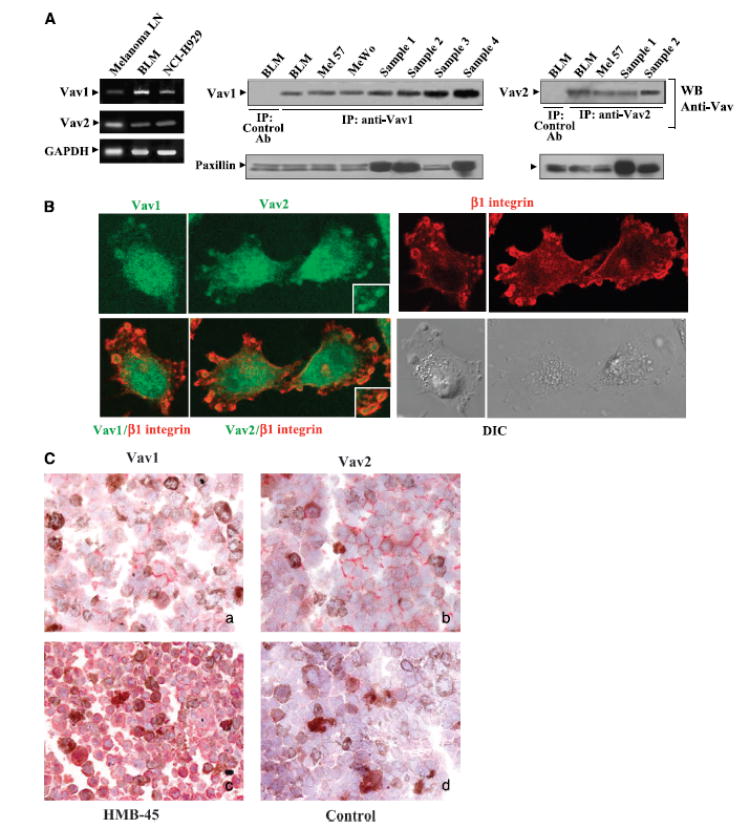
Expression of Vav proteins on melanoma cells. A, lysates from metastatic melanoma cells isolated from infiltrated lymph nodes (Melanoma LN) and BLM melanoma cells were analyzed by RT-PCR using Vav1- or Vav2-specific primers. Lysates from NCI-H929 myeloma cells were used as positive controls. Control amplification using GAPDH-specific primers (left). Cell lysates from the melanoma cell lines BLM, Mel 57, and MeWo and from four different metastatic melanoma samples (Sample 1-Sample 4) were subjected to immunoprecipitation followed by Western blotting using Vav1, Vav2, or control antibodies. Aliquots from cell lysates were kept aside for subsequent loading control in Western blotting using anti-paxillin antibodies (middle and right). B, BLM cells were subjected to confocal microscopy using anti-Vav1, anti-Vav2, or anti-integrin β1 antibodies followed by FITC- or Texas red–conjugated secondary antibodies. Merged Vav1/β1 and Vav2/β1 as well as 5-(3,3-dimethyl-triazeno) imidazole-4-carboxamide differential interference contrast (DIC) microscopy images are also shown. Insets, enlargements of membrane protrusion structures. Control antibodies gave no staining (data not shown). C, immunohistochemical analysis of Vav1 and Vav2 expression on melanoma lymph node metastasis. Original magnification, x400. a, anti-Vav1; b, anti-Vav2; c, anti-HMB-45; d, control rabbit serum. Note in (c) the predominant red staining of melanoma cells with the selective anti-HMB-45 antibody as well as red positive staining of tumor cells by Vav1 (a) and Vav2 (b) antibodies. Brown color is due to the melanin content of tumor cells.
Expression of Vav in BLM melanoma cells was confirmed immunofluorescence and confocal microscopy using anti-antibodies. Analyses of Vav1 and Vav2 subcellular localization revealed that, in addition to a diffuse cytoplasmic distribution, they were also localized near the leading edge of spreading cells in bleb-like protrusions that were surrounded by β1 integrins (Fig. 3_B_). Control experiments indicated that cells containing bleb-like protrusions were negative for the apoptotic marker active caspase-34. Vav protein expression on tumor tissue was analyzed by immunohistochemistry of metastatic melanoma lymph node sections using antibodies to Vav1 or Vav2. Specimen analyzed corresponded to massively infiltrated lymph nodes where histopathologic analyses unequivocally identified as melanoma cells that were strongly stained with the melanoma-specific marker HMB-45. The results revealed low expression levels of both Vav1 and Vav2 on the melanoma cell periphery near the plasma membrane (Fig. 3_C_). Six of seven samples were positive for Vav2 expression by tumor cells, whereas four of seven gave clearly detectable staining for Vav1, although in all cases lower than Vav2. In addition to tumor cells, other cells, such as lymphocytes, displayed the same pattern of Vav localization along the cell periphery (nontumoral areas of lymph nodes and tonsils; data not shown).
Activation of Vav GEF activity requires phosphorylation at tyrosine residues located on its Ac domain (42, 43). CXCL12 promoted time-dependent phosphorylation of Vav1 and Vav2 in BLM cells (Fig. 4_A_, left and right). Furthermore, Vav1 phosphorylation induced by CXCL12 correlated with an increase in the amounts of Rac and, to a lesser extent of RhoA, in Vav1 immunoprecipitates as detected by Western blotting using antibodies against these GTPases. Instead, similar levels of Rac and RhoA were found in Vav2 immunoprecipitates following stimulation with CXCL12. These data indicate that CXCL12 promotes activation of Vav proteins in melanoma cells and suggest that active Vav interact with Rac and RhoA.
Figure 4.
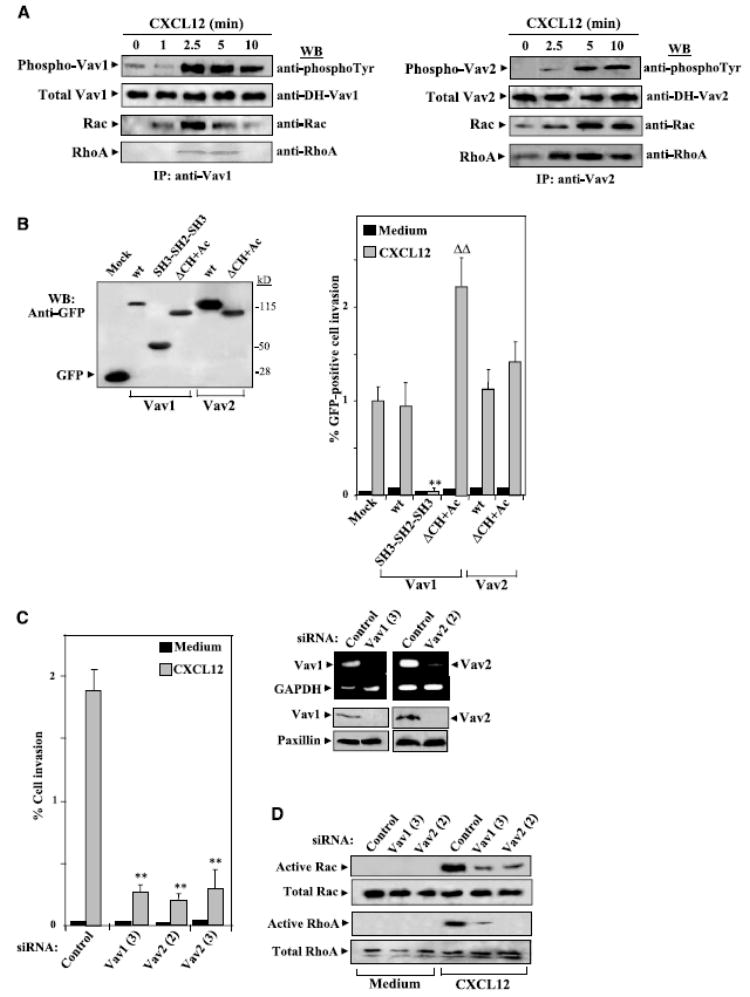
Role of Vav proteins on CXCL12-promoted melanoma cell invasion. A, BLM cells were incubated for the indicated times with CXCL12 followed by cell lysis and immunoprecipitation with anti-Vav1 or Vav-2 antibodies. Immunoprecipitates were resolved by SDS-PAGE and proteins were electrotransferred to PVDF membranes that were incubated sequentially with anti-phosphotyrosine, anti-Vav1 or anti-Vav-2, anti-Rac, and anti-RhoA antibodies. B, BLM cells were transfected with expression vectors coding for GFP-fused WT or the indicated mutant Vav1 or Vav2 or GFP alone (Mock), and trans-fectants were lysed and subjected to Western blotting using anti-GFP antibodies (left) or to Matrigel invasion assays in response to CXCL12 (right). C, left, BLM cells were transfected with control, Vav1, or Vav2 siRNA, and transfectants were tested in Matrigel invasion assays to CXCL12; right, BLM siRNA transfectants were analyzed by RT-PCR and Western blotting using Vav1- or Vav2-specific reagents. D, BLM siRNA transfectants were incubated with or without CXCL12 and subjected to GTPase assays to detect active Rac and RhoA. Columns, mean % cell invasion of at least three independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test); ΔΔ, P < 0.01, invasion was significantly increased in comparison with invasion displayed by Vav1 WT transfectants incubated with CXCL12.
To study the role of Vav proteins on CXCL12-promoted melanoma cell invasion, we followed two different approaches. First, we transfected BLM melanoma cells with vectors coding for GFP-fused WT and mutant forms of Vav and did invasion assays with transfectants. For mutant Vav, we used a truncated form that only contains the COOH-terminal SH3-SH2-SH3 region (Vav1 SH3-SH2-SH3; ref. 48), a domain highly homologous between Vav1 and Vav2 that interacts with tyrosine kinases responsible for Vav1 phosphorylation. Thus, this mutant should interfere with the activation of endogenous Vav by sequestering kinases important for its phosphorylation, therefore acting as a putative dominant negative. In addition, we used mutant Vav1 and Vav2 lacking the CH and acidic regions (Vav1 ΔCH+Ac) that display constitutive GEF activity toward Rho GTPases (44). Expression of the different GFP-Vav forms in transfectants was monitored by Western blotting using anti-GFP antibodies (Fig. 4_B_, left). Invasion assays revealed that SH3-SH2-SH3 Vav transfectants displayed a large impairment in invasion across Matrigel in response to CXCL12 compared with Vav1 and Vav2 WT transfectants (Fig. 4_B_, right). Furthermore, ΔCH+Ac Vav1 transfectants showed a remarkable further increase in invasion toward CXCL12, but their basal invasion did not augment in relation to WT transfectant basal invasion. Instead, we were unable to detect up-regulation of CXCL12-promoted invasion of ΔCH+Ac Vav2 transfectants, although expression levels of GFP-Vav1 ΔCH+Ac and GFP-Vav2 ΔCH+Ac were similar. These results suggest that activation of Vav plays an important role during melanoma cell invasion in response to CXCL12.
To more directly determine Vav involvement in this invasion, we transfected siRNA for Vav1 and Vav2 in BLM cells followed by testing transfectant invasion across basement membranes Vav1 (3), Vav2 (2), and Vav2 (3) siRNA transfectants displayed a remarkable impairment in invasion toward CXCL12 compared with control siRNA transfectants (Fig. 4_C_, left). Interference with Vav1 and Vav2 expression in BLM cells by transfection of their siRNA was confirmed by RT-PCR and Western blotting (Fig. 4_C_, right). Importantly, knocking down Vav1 and Vav2 expression substantially impaired CXCL12-promoted Rac and Rho activation in BLM transfectants (Fig. 4_D_). Together, these results show that CXCL12 stimulate Vav proteins in melanoma cells, which then interact with and activate Rac and Rho, and indicate that activation of the Vav-Rho GTPase pathway is required for invasion toward CXCL12.
Previous reports established that Jak2 stimulates Vav1 (55) and that CXCL12 activates the Jak/Stat pathway (56). We used the Jak inhibitor AG490 to determine Jak involvement in CXCL12-promoted melanoma cell invasion and Vav activation. AG490 at 25 to 100 μmol/L remarkably inhibited the invasion of BLM cells across reconstituted basement membranes as well as the phosphorylation of Vav1 and Vav2 in response to CXCL12 (Fig. 5_A_ and B). These data indicate that Jak activation by CXCL12 is an upstream event that leads to Vav protein activation and that it is required for efficient melanoma cell invasion to this chemokine. Control cell cycle analyses by flow cytometry indicated that treatment with AG490 did not affect BLM cell viability (Supplementary Fig. S2).
Figure 5.
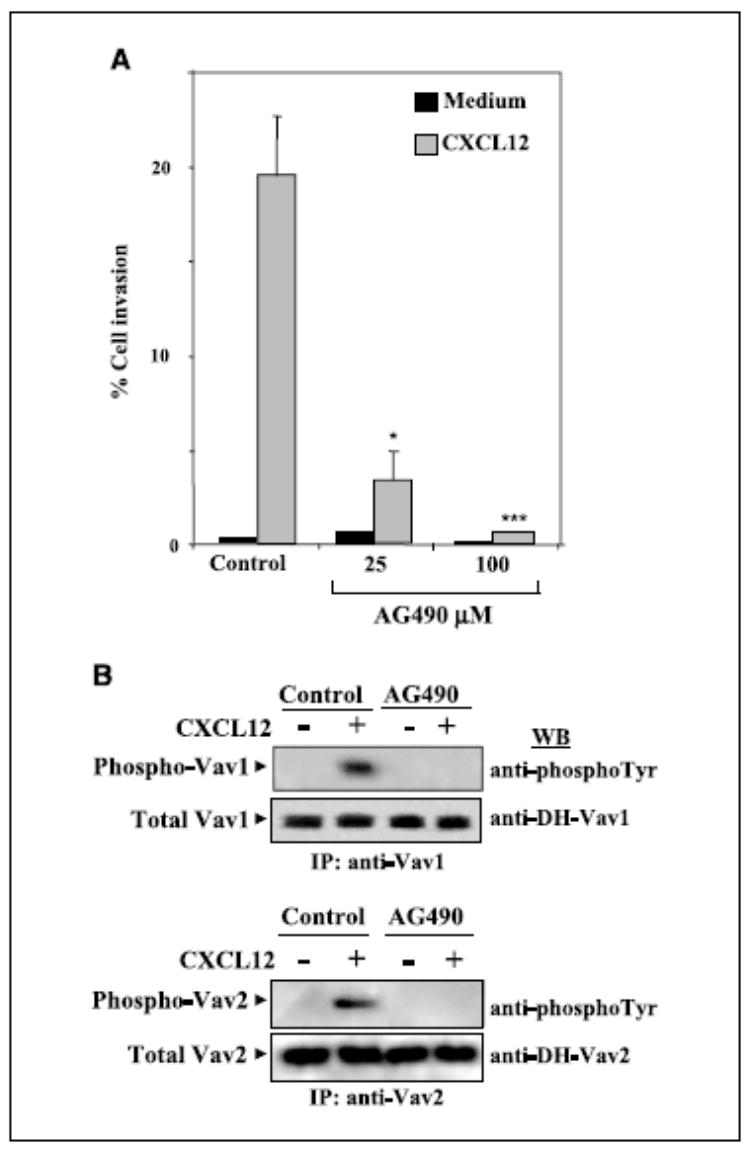
Role of Jak on CXCL12-promoted melanoma cell invasion and Vav activation. A, BLM cells were subjected to Matrigel invasion assays to CXCL12 in the absence or presence of the indicated concentrations of AG490. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. ***, P < 0.001; *, P < 0.05, invasion was significantly inhibited (one-way ANOVA test). B, BLM cells treated without (Control) or with AG490 (2 hours, 25 μmol/L) were incubated for 5 minutes in the absence or presence of CXCL12 (150 ng/mL) and subjected to analyses of Vav1 or Vav2 phosphorylation as described in Fig. 4.
Up-regulation of MT1-MMP expression by CXCL12 depends on Vav function
We described earlier that CXCL12 triggers an increase in MT1-MMP expression in BLM melanoma cells that was impaired by expression of dominant-negative mutant forms of Rac and Rho (47). To study the role of Vav proteins in CXCL12-triggered up-regulation of MT1-MMP expression, we transfected BLM cells with Vav1 and Vav2 siRNA and did Western blotting with transfectants exposed to CXCL12. Enhancement in MT1-MMP expression in response to CXCL12 was strongly inhibited in BLM cells transfected with Vav1 and Vav2 siRNA compared with up-regulated expression exhibited by control siRNA transfectants (Fig. 6_A_). Furthermore, increase in MT1-MMP expression in BLM cells was blocked by cell treatment with AG490 (Fig. 6_B_). These results indicate that activation of Vav proteins by CXCL12 represents an important control point for subsequent regulation of MT1-MMP expression and therefore for CXCL12-promoted melanoma cell invasion across basement membranes.
Figure 6.
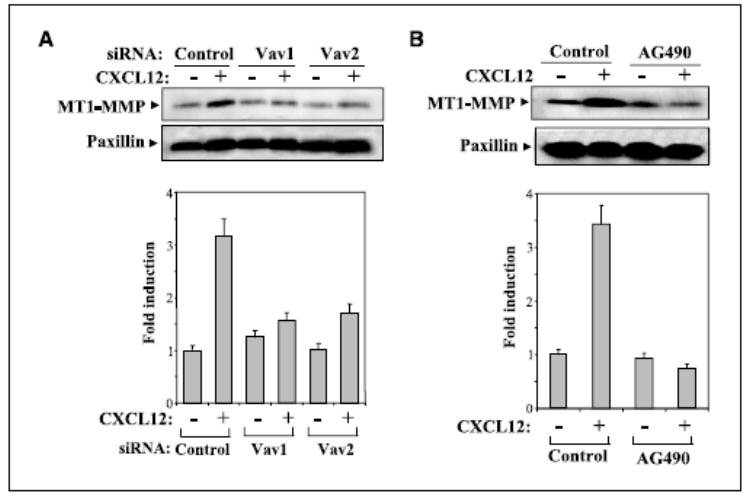
Vav controls up-regulation of MT1-MMP expression in response to CXCL12. BLM cells transfected with Vav1 (3), Vav2 (2), or control siRNA (A) or untransfected BLM cells treated with or without AG490 (B) were incubated for 24 hours with or without CXCL12, and following solubilization, cell lysates were analyzed by Western blotting using anti-MT1-MMP mAb. Blots were reprobed with anti-paxillin mAb to test for sample protein content. Bottom, densitometer analysis in arbitrary units showing fold induction of MT1-MMP expression from above gels.
Role of phosphatidylinositol 3-kinase in CXCL12-promoted melanoma cell invasion
Phosphatidylinositol 3-kinases (PI3K) are involved in the control of cell migration and survival (57). To get insights into the potential involvement of PI3K in CXCL12-promoted melanoma cell invasion, we first assayed whether this chemokine was capable of activating Akt, a downstream PI3K effector. BLM cell exposure to CXCL12 resulted in a transient Akt phosphorylation, indicating that CXCL12 activated PI3K (Fig. 7_A_). We used LY294002 and wortmannin at concentrations reported to inhibit PI3K (58) to investigate whether PI3K activation was implicated in BLM cell invasion toward CXCL12. Both inhibitors impaired the invasion, but inhibition was partial ranging from 50% to 65% (Fig. 7_B_). Cell cycle analysis revealed that neither LY294002 nor wortmannin significantly affected melanoma cell viability (Supplementary Fig. S2). Remarkably and distinct from inhibition by AG490, up-regulation of MT1-MMP expression by CXCL12 in BLM cells was not affected by LY294002 or wortmannin (Fig. 7_C_). Furthermore, transfection of Vav1 or Vav2 siRNA did not alter CXCL12-triggered Akt phosphorylation (Fig. 7_D_). Therefore, these data indicate that PI3K activity contributes to CXCL12-promoted melanoma cell invasion across basement membranes independently of enhancement in MT1-MMP expression.
Figure 7.
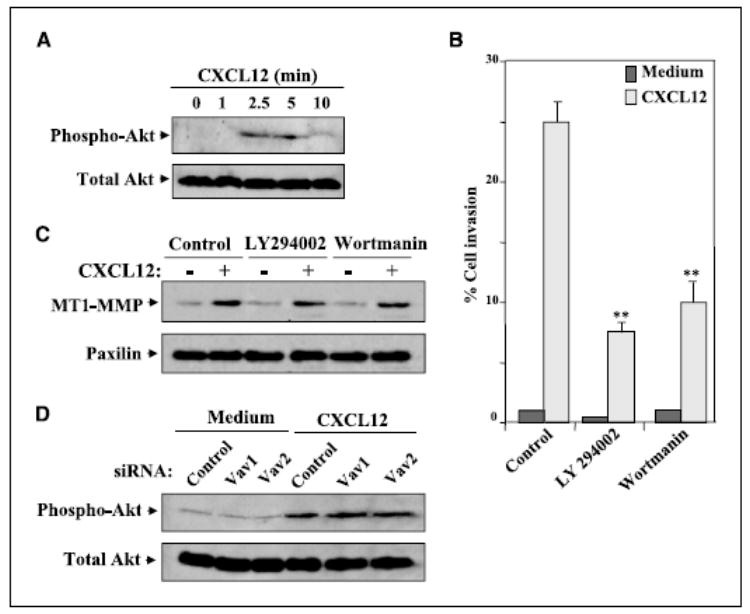
Role of PI3K in CXCL12-promoted melanoma cell invasion. A, BLM cells were incubated with CXCL12 (150 ng/mL) for the indicated times, and following solubilization, cell lysates were analyzed by Western blotting using anti-phospho-Akt followed by reprobing with anti-Akt antibodies. B, BLM cells were subjected to Matrigel invasion assays toward CXCL12 in the absence (Control) or in the presence of 25 μmol/L LY294002 or 100 nmol/L wortmannin. Columns, mean % cell invasion of three independent experiments done in duplicate; bars, SD. **, P < 0.01, invasion was significantly inhibited (one-way ANOVA test). C, BLM cells were incubated with or without CXCL12 in the absence or presence of LY294002 or wortmannin. Following solubilization, cell lysates were analyzed by Western blotting using anti-MT1-MMP mAb. Blots were reprobed with anti-paxillin mAb to test for protein content. D, control, Vav1 (3), or Vav2 (2) siRNA BLM transfectants were incubated for 5 minutes with or without CXCL12 and subjected to Western blot analyses of phospho-Akt and total Akt expression as above.
Discussion
Invasion of melanoma cells across basement membranes in response to CXCL12 requires functional interplay between GTPases Rac and Rho and MT1-MMP activities (47). Activation of Rho GTPases is dependent on their interaction with GEFs, which catalyze the exchange of bound GDP by GTP on the GTPases (35). Therefore, characterization of GEFs that activate Rac and Rho during CXCL12-promoted melanoma cell invasion, as well as identification of upstream and downstream molecules participating in this signaling pathway, is of key importance to identify mechanisms controlling invasion. In the present study, we show that melanoma cells express the GEFs Vav1 and Vav2 and that Vav activation by CXCL12 is needed for subsequent Rac and Rho activation and for invasion across Matrigel basement membranes. Importantly, we provide evidence that up-regulation by CXCL12 of MT1-MMP expression requires activation of Vav-Rho GTPase signaling pathway. Finally, we show that MT1-MMP plays a crucial role on CXCL12-promoted melanoma cell invasion by activating pro-MMP-2 processing to mature MMP-2, which in turn is a main MMP facilitating the invasion across basement membranes and type I collagen in response to this chemokine.
Expression of Vav1 and Vav2 was found on melanoma cells isolated from lymph node metastases as well as on several melanoma cell lines, including highly metastatic BLM cells. The levels of Vav proteins found on melanoma cells were consistently low as detected by immunoprecipitation, immunofluorescence confocal microscopy, and immunohistochemistry. Remarkably, Vav proteins were localized at submembrane areas both in BLM cells and in metastatic melanoma tissue sections. Vav-containing bleb-like protrusions surrounded by β1 integrins that were located near the leading lamellae on BLM cells may be related to similar structures defined on melanoma tumor cells invading three-dimensional Matrigel (59). Although much of reported work on Vav proteins concerns cells of the hematopoietic lineage, very little is known on Vav expression on solid tumor cells, and to our knowledge, this is the first description of Vav expression and function on melanoma cells. Several previous works also reported Vav expression on neuroblastoma and pancreatic tumor cells (45, 46).
Phosphorylation of Vav proteins is a required step for the stimulation of their GEF activity on Rho GTPases (42, 43). We found that CXCL12 efficiently phosphorylated both Vav1 and Vav2 on BLM melanoma cells. Once phosphorylated, Vav1 predominantly interacted with Rac and, to a lesser extent, with RhoA in BLM cells, similarly to what has been reported in Vav1-Rho GTPase interactions on immune cells (39–41). Instead, phosphorylated Vav2 showed similar tendency to bind both Rac and RhoA. Preliminary confocal microscopy experiments revealed that if there was a Vav preferential localization at plasma membrane on cell stimulation with CXCL12 this was too subtle to be detected using this technique5.
Importantly, transfection of dominant-negative Vav forms or knocking down Vav1 and Vav2 expression by transfection of their siRNA resulted in a remarkable impairment in CXCL12-promoted Rac and Rho activation as well as invasion of melanoma cells toward CXCL12, indicating that Vav GEF activity on Rac and Rho is a key step controlling this invasion. Thus, even if Vav proteins are expressed at low levels on melanoma cells, their activity is crucial for efficient invasion of these cells in response to CXCL12. Still, impairment in CXCL12-promoted Rho GTPase activation and invasion in response to CXCL12 in Vav siRNA transfectants was not complete and revealed functional differences between Vav1 and Vav2 in terms of specificity of Rho GTPase activation. These data suggest that additional GEF activities other than Vav proteins participate in the activation. Further support for the importance of Vav activation in this invasion came from results obtained with BLM transfectants expressing constitutive active forms of Vav1, which displayed a notable increased invasion to CXCL12 compared with WT transfectants. At present, we do not know the mechanisms underlying the lack of induced invasion observed with transfectants expressing constitutive active Vav2. Different functional roles have been reported earlier for Vav1 and Vav2 (60, 61), which could underlie some of the differences observed here.
Further characterization of pathways involved in delivering intracellular activating signals for melanoma cell invasion in response to CXCL12 revealed that blocking Jak activity with AG490 resulted in inhibition of Vav1 and Vav2 phosphorylation, Rac activation and in substantial impairment of invasion in BLM cells toward this chemokine. Therefore, Jak kinases, which are targets of CXCL12 activation (56) and have shown earlier to interact with Vav (55), represent upstream molecules that regulate CXCL12-promoted Vav phosphorylation and subsequent melanoma cell invasion. Whether Jak proteins are directly involved in CXCL12-promoted phosphorylation of Vav or indirectly stimulate this phosphorylation is not known at present.
Activation of PI3K by CXCL12 has been shown earlier on carcinoma cells (62). We found that CXCL12 promoted the phosphorylation of Akt on BLM melanoma cells, suggesting an upstream activation of PI3K. In addition, PI3K-dependent downstream signaling mediated a portion of the invasion of these cells in response to CXCL12 as seen by the partial inhibition exerted by PI3K inhibitors in this process.
MT1-MMP plays a key role during melanoma cell invasion toward CXCL12, as both blocking its expression by RNA interference or inhibiting its activity with anti-MT1-MMP mAb abolished this invasion (ref. 47; this work). Furthermore, increase in MT1-MMP expression by CXCL12 represents a final event contributing to the invasion of these cells. Enhanced MT1-MMP expression was found earlier to depend on Rac and Rho activation by CXCL12 (47). Here, we show that knocking down Vav1 and Vav2 expression by RNA interference in melanoma cells results in a remarkable reduction in up-regulation of MT1-MMP expression by CXCL12. Moreover, treatment with AG490 similarly impaired the increase in MT1-MMP expression due to this chemokine. Instead, inhibition of PI3K-dependent signaling did not affect the enhancement in the expression of this metalloproteinase, suggesting that the activity of this kinase is important during MT1-MMP-independent molecular events controlling the invasion. Therefore, these results identify the pathway linking Jak, Vav, and Rho GTPases whose activation is crucial for subsequent up-regulation of MT1-MMP expression and melanoma cell invasion in response to CXCL12. Characterization of downstream mechanisms involved in increase in MT1-MMP expression, including transcriptional and post-transcriptional events, is an important issue of study. In this regard, nuclear factor of activated T cells and nuclear factor-nB are known transcription factors mediating Vav-dependent regulation of gene expression (63–65). The promoter for MT1-MMP contains binding sites for both factors (66, 67), raising the possibility that they might constitute key mediators of CXCR4-promoted increase in MT1-MMP expression in melanoma cells.
Finally, invasion assays using BLM cells transfected with siRNA for MT1-MMP or MMP-2 revealed that MT1-MMP-dependent MMP-2 activation was required for efficient melanoma cell invasion to CXCL12. The results also indicated that MMP-2 was found to be the predominant metalloproteinase whose activity was necessary for the invasion across Matrigel as well as through type I collagen gels. However, data also suggested that direct MT1-MMP activity on type I collagen could also contribute to this invasion, in line with its reported capacity to directly degrade this ECM protein (68). Both MT1-MMP and MMP-2 have been found in the front of metastasizing melanoma cells, and their activities are important for tumor invasion and growth (30, 31). Our present results indicate that CXCL12 can be a trigger of these activities and that coordinated activation by CXCL12 of Vav-Rho GTPase pathway leading to MT1-MMP and MMP-2 stimulation is necessary for efficient invasion.
Knowledge on CXCR4 expression and function on solid tumor cells is rapidly expanding and, together with the clinical relevance of its expression and the responsiveness of these cells to tumor stroma CXCL12, makes the CXCL12/CXCR4 interaction an attractive target for cancer therapy (7, 16). The results from this work shed important information on intracellular pathways activated during invasion of melanoma cells in response to CXCL12. The identification of Vav expression and function in melanoma cells and the characterization of the functional interdependence between Vav-Rho GTPases and MT1-MMP during invasion to CXCL12 highlight the importance of the activation of cell motility and ECM degradation mechanisms during this invasion. Our data open up further studies that could provide potentially useful information for therapeutic intervention aimed to inhibit melanoma cell metastasis.
Supplementary Material
Acknowledgments
Grant support: Ministerio de Educación y Ciencia grant SAF2002-00207, Fundación de Investigación Médica Mutua Madrileña (J. Teixidó), and grants SAF2003-00028 (X. Bustelo) and SAF2002-04615-C02-02 (P. Sánchez-Mateos).
We thank Drs. Goos N.P. van Muijen, Alicia G. Arroyo, and Francisco Sánchez-Madrid for the reagents, María T. Seisdedos and Isabel Treviño for their help in confocal microscopy and immunohistochemistry, and Julia Villarejo for melanoma cell processing and culture.
Footnotes
4
N. Movilla and X.R. Bustelo, unpublished results.
5
I. Molina-Ortiz and J. Teixidó, unpublished results.
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 3.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 4.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. [PubMed] [Google Scholar]
- 5.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 6.Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–57. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 8.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–34. [PubMed] [Google Scholar]
- 9.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 10.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 11.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–41. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 12.Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–50. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 14.Longo-Imedio MI, Longo N, Trevino I, Lazaro P, Sanchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–5. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- 15.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Epstein RJ. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4:901–9. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- 17.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 18.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 20.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–32. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 21.Seiki M, Mori H, Kajita M, Uekita T, Itoh Y. Membrane-type 1 matrix metalloproteinase and cell migration. Biochem Soc Symp. 2003;70:253–62. doi: 10.1042/bss0700253. [DOI] [PubMed] [Google Scholar]
- 22.Seiki M, Koshikawa N, Yana I. Role of pericellular proteolysis by membrane-type 1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Metastasis Rev. 2003;22:129–43. doi: 10.1023/a:1023087113214. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 24.Ueno H, Nakamura H, Inoue M, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–60. [PubMed] [Google Scholar]
- 25.Tsunezuka Y, Kinoh H, Takino T, et al. Expression of membrane-type matrix metalloproteinase 1 (MT1-MMP) in tumor cells enhances pulmonary metastasis in an experimental metastasis assay. Cancer Res. 1996;56:5678–83. [PubMed] [Google Scholar]
- 26.Seftor RE, Seftor EA, Koshikawa N, et al. Cooperative interactions of laminin 5 g2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–7. [PubMed] [Google Scholar]
- 27.Kurschat P, Zigrino P, Nischt R, et al. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–62. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- 28.Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha HY, Moon HB, Nam MS, et al. Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adeno-carcinoma in transgenic mice. Cancer Res. 2001;61:984–90. [PubMed] [Google Scholar]
- 30.Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metallo-proteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000;191:245–56. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH632>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Kurschat P, Wickenhauser C, Groth W, Krieg T, Mauch C. Identification of activated matrix metalloproteinase-2 (MMP-2) as the main gelatinolytic enzyme in malignant melanoma by in situ zymography. J Pathol. 2002;197:179–87. doi: 10.1002/path.1080. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–44. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 34.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 36.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 37.Habets GG, van der Kammen RA, Stam JC, Michiels F, Collard JG. Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene. 1995;10:1371–6. [PubMed] [Google Scholar]
- 38.Minard ME, Herynk MH, Collard JG, Gallick GE. The guanine nucleotide exchange factor Tiam1 increases colon carcinoma growth at metastatic sites in an orthotopic nude mouse model. Oncogene. 2005;24:2568–73. doi: 10.1038/sj.onc.1208503. [DOI] [PubMed] [Google Scholar]
- 39.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–77. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–86. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 41.Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Vav1: a key signal transducer downstream of the TCR. Immunol Rev. 2003;192:42–52. doi: 10.1034/j.1600-065x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 42.Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–33. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 43.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–72. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 44.Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–21. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S. The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol. 2003;199:526–33. doi: 10.1002/path.1314. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, et al. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Bartolome RA, Galvez BG, Longo N, et al. Stromal cell-derived factor-1a promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 2004;64:2534–43. doi: 10.1158/0008-5472.can-03-3398. [DOI] [PubMed] [Google Scholar]
- 48.Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR. Structural determinants for the biological activity of Vav proteins. J Biol Chem. 2002;277:45377–92. doi: 10.1074/jbc.M208039200. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Bernal D, Wright N, Sotillo-Mallo E, et al. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin α4β1. Mol Biol Cell. 2005;16:3223–35. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin α(v) β(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metal-loproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87:12–9. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 51.Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–21. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol Cell Biol. 2000;20:1678–91. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ticchioni M, Charvet C, Noraz N, et al. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood. 2002;99:3111–8. doi: 10.1182/blood.v99.9.3111. [DOI] [PubMed] [Google Scholar]
- 54.Sander EE, van Delft S, ten Klooster JP, et al. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–98. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuguchi T, Inhorn RC, Carlesso N, Xu G, Druker B, Griffin JD. Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J. 1995;14:257–65. doi: 10.1002/j.1460-2075.1995.tb06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1a triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–710. [PubMed] [Google Scholar]
- 57.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 58.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 59.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 60.Pearce AC, Wilde JI, Doody GM, et al. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood. 2002;100:3561–9. doi: 10.1182/blood.V100.10.3561. [DOI] [PubMed] [Google Scholar]
- 61.Fujikawa K, Miletic AV, Alt FW, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankowski K, Kucia M, Wysoczynski M, et al. Janowska-Wieczorek A, Ratajczak MZ. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyo-sarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63:7926–35. [PubMed] [Google Scholar]
- 63.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–46. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costello PS, Walters AE, Mee PJ, et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, NF-nB pathways. Proc Natl Acad Sci U S A. 1999;96:3035–40. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doody GM, Billadeau DD, Clayton E, et al. Vav-2 controls NFAT-dependent transcription in B- but not T-lymphocytes. EMBO J. 2000;19:6173–84. doi: 10.1093/emboj/19.22.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor- nB-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926–35. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 67.Alfonso-Jaume MA, Mahimkar R, Lovett DH. Cooperative interactions between NFAT (nuclear factor of activated T cells) c1 and the zinc finger transcription factors Sp1/Sp3 and Egr-1 regulate MT1-MMP (membrane type 1 matrix metalloproteinase) transcription by glomerular mesangial cells. Biochem J. 2004;380:735–47. doi: 10.1042/BJ20031281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–23. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.