Virulence of Staphylococcus aureus Small Colony Variants in the Caenorhabditis elegans Infection Model (original) (raw)
Abstract
Small colony variants (SCVs) of Staphylococcus aureus are slow-growing morphological variants that have been implicated in persistent, relapsing, and antibiotic-resistant infections. The altered phenotype of SCVs in most strains has been attributed to defects in electron transport due to mutations in hemin or menadione biosynthesis. The pathogenic capacity of SCVs compared to phenotypically normal strains is variable depending on the attribute examined, with some studies showing reduced virulence of SCVs and others demonstrating normal or heightened virulence. Recently, the nematode Caenorhabditis elegans has been successfully employed as an alternative host to investigate virulence mechanisms of a variety of bacterial pathogens, including S. aureus. In this study, we show that clinical SCVs as well as _hemB_- and _menD_-deficient mutants of S. aureus are greatly reduced in virulence in the C. elegans infection model.
In addition to being a common commensal of humans, Staphylococcus aureus is a remarkably versatile pathogen that can cause a broad range of human infections. Coupled with an extraordinary capacity to develop drug resistance and the emergence of community-circulating, highly virulent strains, S. aureus is a major threat to human health in both the hospital and the community. Past studies have shown that staphylococci have mechanisms for resisting therapy that extend beyond the classic forms of resistance (3, 20). The formation of slow-growing subpopulations of cells that manifest behavior atypical for S. aureus, such as reduced hemolysin production and increased intracellular survival, may represent one such mechanism (14, 21, 22, 31, 33). This subpopulation, designated “small colony variants” (SCVs) due to their fastidious growth characteristics, is being increasingly recovered from clinical specimens, particularly from patients with chronic, persisting, and/or relapsing infections (19, 25). SCVs isolated from these patients are often auxotrophic for hemin or menadione, compounds involved in the synthesis of the electron transport chain components cytochrome and menaquinone, respectively (19, 32, 33). This observation led to the suggestion that the SCV phenotype is associated with genetic changes impairing electron transport, although clinical SCVs may harbor additional mutations. Since variants recovered from clinical specimens may exhibit additional atypical phenotypes, often revert to the wild-type phenotype during cultivation, and are genetically undefined, site-directed mutants in electron transport have also been generated by interrupting the hemin biosynthetic gene, hemB, and the menadione biosynthetic gene, menD, of S. aureus (2, 33). The hemB mutant shows the typical features of SCVs recovered from clinical specimens and is also able to persist intracellularly (3, 24, 30, 33). Intracellular survival is thought to be a critical feature of the ability of SCVs to cause chronic and persistent infections, because the intracellular location may shield these bacteria from host defenses and limit exposure to certain antibiotics (14, 20, 31, 33). Studies have demonstrated that the hemB mutant has a reduced capacity to produce some virulence-associated products (e.g., α-hemolysin, protein A, and thermonuclease) (15, 33) and yet has an increased ability to produce others (e.g., clumping factor and fibronectin-binding protein) (15, 30). The menD mutant also recapitulates the SCV phenotype and is highly resistant to the cationic antimicrobial peptide thrombin-induced platelet microbicidal protein 1 (2).
Recently, we and others have used the nematode Caenorhabditis elegans to model host/pathogen relationships in pathogenic microbes and to assess the contribution of specific gene products to virulence (recently reviewed in reference 26). In this study, the hemB and menD mutants as well as clinical isolates with the SCV phenotype were tested in the C. elegans infection model in order to obtain more information on the pathogenic fitness of these variants and to better characterize C. elegans-S. aureus host/pathogen interactions.
MATERIALS AND METHODS
Caenorhabditis elegans.
Bristol N2 C. elegans nematodes were maintained at 15°C on nematode growth medium plates seeded with Escherichia coli strain OP50 as a food source and were manipulated using established techniques (17).
Bacterial strains.
Bacterial strains used in this study are listed in Table 1. SCVs cultured from clinical specimens were recovered from patients with chronic and recurrent infections such as chronic osteomyelitis (isolates A22616/3 and A22223II). SCVs were identified as previously reported (14, 31). Isolates were confirmed to be S. aureus by testing for the _S. aureus_-specific nuc and coa genes by PCR (7). One SCV isolate (isolate A22616/3) was found to be menadione auxotrophic following testing on chemically defined medium as previously described; the other SCV isolate (isolate A22223II) was hemin auxotrophic. The corresponding isolates with normal phenotypes (isolates A22616/5 and A22223I) were recovered in a parallel or sequential culture from the same patient, respectively. SmaI digests of total bacterial DNA were resolved with the use of pulsed-field gel electrophoresis (PFGE) as previously described (12), demonstrating that the strains with the normal and SCV phenotypes recovered from the same patient were clonal.
TABLE 1.
Strains and plasmid used in this study
| Strain or plasmid | Genotype and/or phenotypea | Source or reference |
|---|---|---|
| Strains | ||
| C. elegans | ||
| Bristol N2 | Wild-type nematode strain | Caenorhabditis Genetics Center (http://www.sanger.ac.uk) |
| E. coli | ||
| OP50 | Uracil auxotrophy | 8 |
| S. aureus | ||
| A22223I | Clinical isolate, normal phenotype | This study |
| A22223II | Hemin-auxotrophic SCV, isolated from the same patient as A22223I, from a fistula tract originating from chronic osteomyelitis, clonal by PFGE | This study |
| A22616/5 | Clinical isolate, normal phenotype | 3 |
| A22616/3 | Menadione-auxotrophic SCV, isolated from the same patient as A22616/5, clonal by PFGE | 3 |
| Newman | Wild-type strain, ATCC 25904 | 10 |
| III33 | Newman hemB::ermB, SCV | 13 |
| KM2 | Newman hemB::ermB, pCE12 | 13 |
| COL | Prototypic MRSA strain | http://www.tigr.org |
| Ia48 | COL hemB::ermB, SCV | 30 |
| KM1 | COL hemB::ermB, pCE12 | This study |
| COL menD | COL menD::ermC | 34 |
| 8325-4 | Prophage-cured derivative of wild-type strain NCTC 8325, rsbU mutant | http://www.genome.ou.edu |
| DB24 | 8325-4 menD::ermC | 2 |
| DB25 | DB24 with menD restored by allelic exchange | 2 |
| Plasmid | ||
| pCE12 | pCX19::hemB | 33 |
The construction of hemB mutants by allelic replacement of S. aureus strains Newman and COL, named III33 and Ia48, respectively, has been previously described (13, 30). Corresponding plasmid-complemented strains of mutant III33 (designated KM2) and Ia48 (designated KM1) were constructed using plasmid pCE12, which contains the PCR-amplified hemB gene cloned into the staphylococcal expression vector pCX19 (reference 30 and this study). Transformation of the auxotrophic mutants with pCE12 resulted in normal growth of each mutant on both liquid and solid media (data not shown).
The construction of the menD mutant DB24 and repaired menD mutant DB25 in S. aureus strain 8325-4 by allelic exchange has been described elsewhere previously (2). Phage transduction of the menD mutation into S. aureus strain COL has been previously reported (34).
Bacterial strains were maintained at −70°C in tryptic soy (TS) broth containing 15% glycerol. S. aureus strains were grown at 37°C with aeration in TS broth that was supplemented with 2.5 μg/ml erythromycin or 10 μg/ml chloramphenicol, if appropriate.
C. elegans survival assays.
C. elegans killing assays were performed as previously described (27), with the following modifications. For standard assay plates, 10 μl of a culture grown overnight was spread onto 3.5-cm-diameter plates containing TS agar supplemented with 5 μg/ml nalidixic acid and additional antibiotics, as appropriate, and incubated at 37°C for 6 h. For “spotted-lawn” assay plates, 3 ml (for SCVs) or 1 ml (for wild-type or complemented/repaired strains) of a 24-h culture was pelleted by centrifugation, decanted of the supernatant, and resuspended in 100 μl TS medium. Fifty microliters of the concentrated bacteria was then spotted onto a TS agar plate supplemented with nalidixic acid and additional antibiotics, as appropriate. The spotted-lawn assay plates were used for nematode survival assays once the lawns had dried, usually within 30 to 60 min.
Hermaphrodite nematodes of the 4th larval (L4) stage were transferred from their normal food source to the tested strain, and their survival was monitored over time at 25°C. Approximately 20 to 25 nematodes were transferred to each plate, and all experiments were conducted in triplicate and repeated at least three times. Nematodes were classified as dead when they failed to respond to touch and pharyngeal pumping was no longer observed. Worms that died as a result of crawling off the plate were censored from the analysis. For each killing assay, nematode survival was calculated by the Kaplan-Meier method, and survival differences were tested for significance by use of the log rank test (GraphPad Prism, version 3.0). P values of <0.05 were considered statistically significant. Nematode alimentary tracts were examined by differential interference contrast microscopy with a standard Axioplan2 microscope fitted with Normarski optics (Zeiss) using established methodologies (29).
RESULTS
We have previously shown that C. elegans nematodes that feed on nonpathogenic bacteria such as the auxotrophic E. coli strain OP50 or Bacillus subtilis live an average of 2 to 3 weeks, whereas nematodes fed most S. aureus strains die over the course of several days (11, 27). To investigate the virulence potential of S. aureus SCVs in the C. elegans model system, worms were fed clinical S. aureus SCVs and their matched parental isolates. The SCV strains have been shown to be auxotrophic for hemin or menadione and are clonal with phenotypically normal S. aureus isolates that were simultaneously or consecutively recovered from the same patient, as determined by pulsed-field gel electrophoresis analysis. Using standard nematode killing assays, strains A22223II, a hemin-auxotrophic SCV, and A22616/3, a menadione-auxotrophic SCV, were significantly less virulent for nematodes than their parental isolates, strains A22223I and A22616/5, respectively (data not shown).
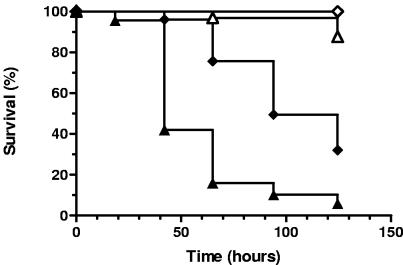
We were concerned that slow growth of the SCVs on standard assay plates might bias the results towards an attenuated phenotype due to the thin lawns produced on these plates. It was conceivable that increased worm survival on the SCV plates could be the result of decreased exposure to the pathogen. Therefore, nematode survival assays were performed with plates that were seeded with concentrated bacteria to insure that differences in nematode survival were not simply due to limited exposure to pathogenic bacteria on the SCV plates (see Materials and Methods). Using this modified “spotted-lawn” nematode survival assay, the clinical SCV strains A22223II and A22616/3 remained significantly less virulent for nematodes than parental strains A22223I and A22616/5, respectively (Fig. 1). Of note, several (usually 5 to 20) large colony revertants would typically arise per plate on the SCV lawns during the course of each experiment.
FIG. 1.
Survival of C. elegans on clinically isolated S. aureus SCVs. Survival of nematodes fed S. aureus A22223I (clinical wild-type isolate; closed triangles; n = 69) versus A22223II (hemin-auxotrophic SCV; open triangles; n = 65) and A22616/5 (clinical wild-type isolate; closed diamonds; n = 55) versus A22616/3 (menadione-auxotrophic SCV; open diamonds; n = 56) is shown. Survival was plotted using the Kaplan-Meier method, and pairwise comparisons using the log rank test between the SCV and matched wild-type isolates were significant at a P value of <0.0001.
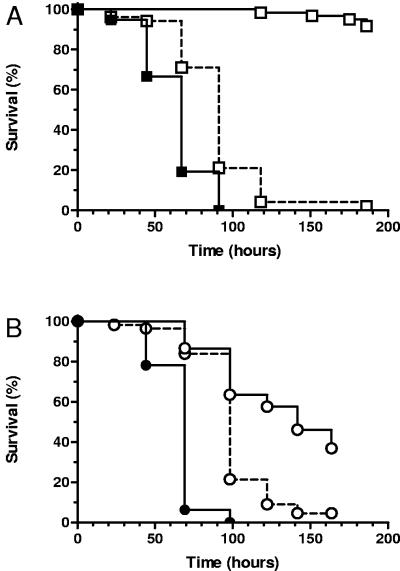
To further investigate the importance of bacterial respiration in S. aureus infection of nematodes, the nematocidal activity of _hemB_-deficient mutants was evaluated in the spotted-lawn C. elegans survival assay. As shown in Fig. 2A, the hemB mutant strain III33 was avirulent in the C. elegans infection assay compared to the isogenic parental strain, Newman. To confirm that the reduced virulence of III33 was due to the interruption of hemB, the mutation was complemented using plasmid pCE12, which contains the PCR-amplified hemB gene driven by the xlyA promoter. Previous studies have shown that complementation with pCE12 in strain KM2 restores hemolytic activity and normal growth characteristics, even in the absence of xylose (33). As shown in Fig. 2A, the virulence of KM2 was restored to near-wild-type levels in the C. elegans infection assay.
FIG. 2.
Survival of C. elegans on SCV hemB mutants. (A) Survival of nematodes fed S. aureus Newman (wild type; closed squares; n = 57), III33 (hemB SCV; open squares with a solid line; n = 61), and KM2 (_hemB_-complemented III33; open squares with a dashed line; n = 52). (B) Survival of nematodes fed S. aureus COL (wild type; closed circles; n = 55), Ia48 (hemB SCV; open circles with a solid line; n = 56), and KM1 (_hemB_-complemented Ia48; open circles with a dashed line; n = 61). Survival was plotted using the Kaplan-Meier method. Pairwise comparisons using the log rank test by strain were as follows: Newman versus III33, III33 versus KM2, COL versus Ia48, and Ia48 versus KM1 (all P < 0.0001).
Several recent reports have shown that the importance of particular genes to S. aureus virulence traits can be strain specific (4, 6, 23). To determine if the importance of hemin auxotrophy for S. aureus pathogenesis was strain specific, we evaluated the contribution of hemB to virulence in the prototypic methicillin-resistant S. aureus strain COL. As shown in Fig. 2B, hemB mutant strain Ia48 was significantly attenuated in C. elegans killing compared to COL, while pCE12 complementation in strain KM1 partially restored virulence. The degree of attenuation of Ia48 was not as large as that of the Newman hemB mutant III33. The difference in attenuation between the hemB mutants may reflect differences in how oxidative phosphorylation contributes to the virulence capacity of COL and Newman or alternatively may reflect other genetic and metabolic differences between the two strains.
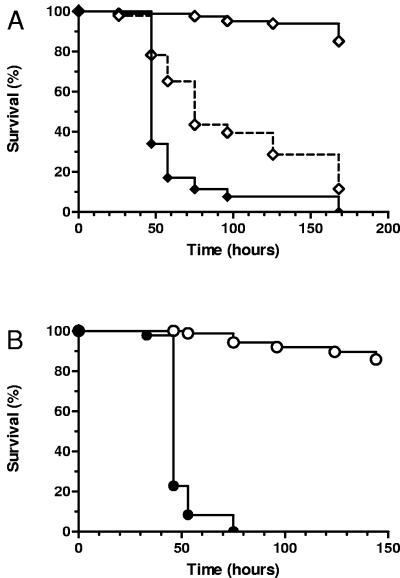
Next, we sought to ascertain whether genetically defined menadione-auxotrophic SCV mutants are attenuated for virulence in worm killing, similar to clinical SCV strain A22616/3. As shown in Fig. 3A, menD mutant strain DB24 was highly attenuated in worm killing compared to the parental S. aureus strain 8325-4, while the _menD_-repaired strain DB25 was partially restored in virulence. The reduced virulence of menD SCVs was confirmed in S. aureus strain COL, as shown in Fig. 3B. Of note, no large colony revertants were observed on the lawns of the site-directed hemB and menD mutants.
FIG. 3.
Survival of C. elegans on SCV menD mutants. (A) Survival of nematodes fed S. aureus 8325-4 (wild type; closed diamonds; n = 91), DB24 (menD SCV; open diamonds with a solid line; n = 83), and DB25 (_menD_-repaired DB24; open diamonds with a dashed line; n = 92). (B) Survival of nematodes fed S. aureus COL (wild type; closed circles; n = 92) and COL menD (menD SCV; open circles; n = 93). Survival was plotted using the Kaplan-Meier method. Pairwise comparisons using the log rank test by strain were as follows: 8325-4 versus DB24, DB24 versus DB24, and COL versus COL menD (all P < 0.0001).
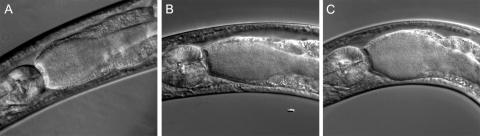
Examination of the C. elegans intestinal tracts using differential interference contrast microscopy revealed no appreciable differences in S. aureus accumulation at 8, 20, and 48 h of exposure to a wild-type strain or SCVs. After 8 h of feeding, the digestive tracts of roughly one-half of the surveyed nematodes contained tightly packed cocci, predominantly posterior to the terminal bulb and anterior to the rectum. After 24 h of feeding, the digestive tracts of all surveyed worms were colonized with S. aureus to a qualitatively similar degree (Fig. 4). No qualitative difference in S. aureus accumulation was observed in worms fed SCV and parental strains throughout the course of infection.
FIG. 4.
Colonization of the C. elegans digestive tract. Differential interference contrast photomicrographs of the terminal bulb and anterior intestinal tract of nematodes after feeding for 20 h on lawns of (A) COL, (B) Ia48, or (C) KM1 are shown.
DISCUSSION
Many reports as well as prospective studies have demonstrated poor clinical and microbiologic responses to even prolonged antimicrobial therapy in patients infected with S. aureus SCVs (19, 25). Variants recovered from these patients with chronic and/or relapsing infections are often auxotrophic for hemin or menadione. However, the study of clinical SCVs can be problematic, because they may also carry mutations in other determinants that impact pathogenic fitness, especially since these isolates typically display multiple phenotypic changes compared to parental strains. These problems were circumvented here by examining strains carrying specific disruptions of the hemin biosynthetic gene hemB, which encodes aminolevulinic acid dehydratase, and the menadione biosynthetic gene menD, which encodes the putative bifunctional enzyme 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase-2-oxoglutarate decarboxylase. Importantly, hemB and menD mutants recapitulate critical features of the SCV phenotype, including reduced hemolysin production, increased intracellular persistence in nonprofessional phagocytes, and increased susceptibility to platelet-secreted cationic antimicrobial peptides (e.g., thrombin-induced platelet microbicidal protein 1) (2, 16, 30, 33). The hemB mutant has previously been tested in two animal models of S. aureus infection. In a murine model of septic arthritis, NMRI mice inoculated with a hemB mutant displayed a higher frequency and severity of arthritis than mice inoculated with the parent strain exhibiting the normal phenotype (13). Interestingly, the mice inoculated with the hemB mutant also demonstrated reduced bacterial burden in their kidneys and joints compared with mice exposed to the isogenic parental strain. It was hypothesized that SCVs may be more virulent on a per-organism basis than their parental isolates, perhaps due to the ability of SCVs to produce high amounts of destructive proteases. In another study, the hemB and menD mutants of S. aureus strain 8325-4 were compared to the parental and complemented strains in a rabbit endocarditis model (2). The hemB mutant was found to be equally virulent to wild-type and _hemB_-complemented strains, as measured by vegetation bacterial densities, dissemination to the liver and spleen, and sensitivity to oxacillin therapy. In contrast, the menD mutant demonstrated reduced colonization levels in the liver and spleen, and disseminated foci of infection were less responsive to oxacillin therapy than wild-type and _menB_-complemented strains. The differences between these SCV mutants were thought to be related to the fact that each organ is probably replete with hemin derived from embolic infarcts that occur during the course of experimental endocarditis. Thus, hemin might circumvent the hemB mutation-induced defect in the cytochrome system but not the menD mutation defect in the menaquinone system (2).
While it may not be feasible or accurate to test clinically derived isolates with SCV phenotypes in traditional animal models due to their inherent phenotypic instability and lack of genetic definition, the C. elegans infection model offers a simple and rapid means to evaluate the virulence potential of clinical SCVs and to compare these isolates with hemB and menD mutants mimicking the SCV phenotype. The SCV phenotype can easily be tracked during the assay, and thus, the influence of reversion to the normal phenotype during the course of the experiment can be monitored. In fact, some reversion to large colony growth did occur during the later stages of the assays with clinical SCV isolates in the present study, but this reversion did not appear to significantly impact the assay results, as judged by parallel assays using engineered hemB and menD mutants. In addition, virulence may be assessed without the external influence of hemin on hemin-auxotrophic SCVs, in contrast to the many mammalian infection models.
We found that clinical SCVs that were auxotrophic for hemin or menadione were less virulent in this invertebrate infection model and that the reduced virulence could not be ascribed to reduced exposure to the pathogen. Moreover, hemB and menD mutants were similarly less virulent than isogenic parental and complemented strains, confirming the importance of bacterial respiration for virulence in this infection model system. Reduced virulence of the SCV strains was not the result of an impaired ability to colonize the nematode digestive tract. We have previously observed that other S. aureus virulence-attenuated mutants demonstrate comparable levels of colonization of the nematode digestive tract (5, 27). In contrast, we have recently noted that impaired biofilm formation reduces the ability of Staphylococcus epidermidis to colonize the nematode digestive tract (J. Begun, S. Calderwood, F. Ausubel, and C. Sifri, unpublished observations).
The reduced virulence of the hemB mutants observed in the C. elegans infection model stands in marked contrast with observations made with the hemB SCVs in experimental septic arthritis and endocarditis. However, this reduced virulence capacity is congruent with the clinical characteristics of SCV infection since these organisms are typically recovered in patients with chronic, indolent, and/or relapsing disease. The reduced virulence of the menD SCV in experimental endocarditis suggests that endogenous hemin does not complement the SCV defect of this mutant in this experimental model, in contrast to the hemB mutant. We and others have previously observed that S. aureus tricarboxylic acid (TCA) cycle mutants are attenuated for virulence in _C. elegans_-based and other in vivo systems (1, 5, 9, 18). In S. aureus, the TCA cycle is repressed during exponential growth, leading to the accumulation of acetyl coenzyme A (acetyl-CoA). Depletion of glucose via glycolysis during exponential growth triggers entry into post-exponential-phase growth and acetyl-CoA catabolism. Therefore, flux of acetyl-CoA through the TCA cycle serves as the primary source of energy for the production of secreted virulence factors (28). The loss of a functional electron transport system in hemin- and menadione-auxotrophic SCVs also results in greatly reduced extracellular protein production, including α-hemolysin (15, 33). The genes for several S. aureus exoproteins, such as hla (α-hemolysin), have been shown to be important for nematode-mediated killing (27). Therefore, we hypothesize that the reduced production of α-hemolysin and perhaps other virulence products in the SCV strains due to the loss of oxidative phosphorylation leads to reduced virulence in nematodes.
Another notable difference between nematode and mammalian infection models is the importance of cell surface adhesins. In contrast to disease in vertebrates, at least 10 different staphylococcal surface proteins and sortase A (srtA), a gene required for their proper display, do not appear to contribute to nematode colonization or disease (1). Interestingly, hemB mutants exhibit increased expression of surface adhesins such as clumping factor and fibronectin-binding proteins (30). Thus, increased adhesion production in hemB and conceivably other SCV mutants may mitigate the effect of reduced production of other virulence factors in some mammalian infection models but would not be predicted to alter disease in the nematode.
This is the first in vivo study to assess the virulence capacity of clinical S. aureus SCV isolates as well as hemB and menD mutants that recapitulate the SCV phenotype. We conclude that clinical SCVs and hemB and menD mutants are less virulent in this simple invertebrate model of acute S. aureus infection. The reduced virulence may be a reflection of reduced exoprotein production due to defects in oxidative phosphorylation. Inhibition of bacterial respiration as a virulence-inhibiting mechanism will be the subject of further research.
Acknowledgments
We thank Arnold Bayer (Harbor-UCLA) and Richard Proctor (University of Wisconsin) for providing strains.
This work was supported by NIAID grant K08 AI053677 and by a Harvard Medical School Center for AIDS Research Feasibility Project grant to C.D.S., by a research grant from Aventis Pharmaceuticals to S.B.C., and by a research grant from BMBF (Pathogenomic) to C.V.E.
REFERENCES
- 1.Bae, T., A. K. Banger, A. Wallace, E. M. Glass, F. Aslund, O. Schneewind, and D. M. Missiakas. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA 101**:**12312-12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, D. M., C. von Eiff, P. J. McNamara, G. Peters, M. R. Yeaman, A. S. Bayer, and R. A. Proctor. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J. Infect. Dis. 187**:**1654-1661. [DOI] [PubMed] [Google Scholar]
- 3.Baumert, N., C. von Eiff, F. Schaaff, G. Peters, R. A. Proctor, and H. G. Sahl. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb. Drug Resist. 8**:**253-260. [DOI] [PubMed] [Google Scholar]
- 4.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71**:**4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begun, J., C. D. Sifri, S. Goldman, S. B. Calderwood, and F. M. Ausubel. 2005. Staphylococcus aureus virulence factors identified by using a high-throughput _Caenorhabditis elegans_-killing model. Infect. Immun. 73**:**872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70**:**470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brakstad, O. G., K. Aasbakk, and J. A. Maeland. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30**:**1654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77**:**71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulter, S. N., W. R. Schwan, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. R. Folger, A. S. Bayer, and C. K. Stover. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30**:**393-404. [DOI] [PubMed] [Google Scholar]
- 10.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6**:**95-107. [DOI] [PubMed] [Google Scholar]
- 11.Garsin, D. A., J. M. Villanueva, J. Begun, D. H. Kim, C. D. Sifri, S. B. Calderwood, G. Ruvkun, and F. M. Ausubel. 2003. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300**:**1921. [DOI] [PubMed] [Google Scholar]
- 12.Ichiyama, S., M. Ohta, K. Shimokata, N. Kato, and J. Takeuchi. 1991. Genomic DNA fingerprinting by pulsed-field gel electrophoresis as an epidemiological marker for study of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 29**:**2690-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson, I. M., C. von Eiff, R. A. Proctor, G. Peters, C. Ryden, and A. Tarkowski. 2003. Virulence of a hemB mutant displaying the phenotype of a Staphylococcus aureus small colony variant in a murine model of septic arthritis. Microb. Pathog. 34**:**73-79. [DOI] [PubMed] [Google Scholar]
- 14.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177**:**1023-1029. [DOI] [PubMed] [Google Scholar]
- 15.Kohler, C., C. von Eiff, G. Peters, R. A. Proctor, M. Hecker, and S. Engelmann. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 185**:**6928-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo, S. P., M. R. Yeaman, and A. S. Bayer. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect. Immun. 64**:**3758-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, J. A., and J. T. Fleming. 1995. Basic culture methods, p. 3-29. In H. F. Epstein and D. C. Shakes (ed.), Caenorhabditis elegans: modern biological analysis of an organism, vol. 48. Academic Press, San Diego, Calif. [Google Scholar]
- 18.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26**:**399-407. [DOI] [PubMed] [Google Scholar]
- 19.Proctor, R. A., D. M. Bates, and P. J. McNamara. 2001. Electron transport-deficient Staphylococcus aureus small-colony variants as emerging pathogens, p. 95-110. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections 5. ASM Press, Washington, D.C.
- 20.Proctor, R. A., B. Kahl, C. von Eiff, P. E. Vaudaux, D. P. Lew, and G. Peters. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl. 1)**:**S68-S74. [DOI] [PubMed] [Google Scholar]
- 21.Proctor, R. A., and G. Peters. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27**:**419-422. [DOI] [PubMed] [Google Scholar]
- 22.Proctor, R. A., P. van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20**:**95-102. [DOI] [PubMed] [Google Scholar]
- 23.Rigoulay, C., J. M. Entenza, D. Halpern, E. Widmer, P. Moreillon, I. Poquet, and A. Gruss. 2005. Comparative analysis of the roles of HtrA-like surface proteases in two virulent Staphylococcus aureus strains. Infect. Immun. 73**:**563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadowska, B., A. Bonar, C. von Eiff, R. A. Proctor, M. Chmiela, W. Rudnicka, and B. Rozalska. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32**:**191-197. [DOI] [PubMed] [Google Scholar]
- 25.Seifert, H., H. Wisplinghoff, P. Schnabel, and C. von Eiff. 2003. Small colony variants of Staphylococcus aureus and pacemaker-related infection. Emerg. Infect. Dis. 9**:**1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sifri, C. D., J. Begun, and F. M. Ausubel. 2005. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13**:**119-127. [DOI] [PubMed] [Google Scholar]
- 27.Sifri, C. D., J. Begun, F. M. Ausubel, and S. B. Calderwood. 2003. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71**:**2208-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 70**:**6373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulston, J., and J. Hodgkin. 1998. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Vaudaux, P., P. Francois, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, R. A. Proctor, P. J. McNamara, G. Peters, and C. Von Eiff. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70**:**5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32**:**1643-1647. [DOI] [PubMed] [Google Scholar]
- 32.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25**:**1250-1251. [DOI] [PubMed] [Google Scholar]
- 33.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Gotz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179**:**4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Eiff, C., P. J. McNamara, K. Becker, D. Bates, X.-H. Lei, M. Ziman, B. R. Bochner, G. Peters, and R. A. Proctor. 2006. Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with small colony variant phenotype. J. Bacteriol., 188**:**687-693. [DOI] [PMC free article] [PubMed]