The distribution of intermediate-conductance, calcium-activated, potassium (IK) channels in epithelial cells (original) (raw)
Abstract
Intermediate-conductance, calcium-activated, potassium (IK) channels were first identified by their roles in cell volume regulation, and were later shown to be involved in control of proliferation of lymphocytes and to provide a K+ current for epithelial secretory activity. Until now, there has been no systematic investigation of IK channel localization within different epithelia. IK channel immunoreactivity was present in most epithelia, where it occurred in surface membranes of epithelial cells. It was found in all stratified epithelia, including skin, cornea, oral mucosa, vaginal mucosa, urothelium and the oesophageal lining. It occurred in the ducts of fluid-secreting glands, the salivary glands, lacrimal glands and pancreas, and in the respiratory epithelium. A low level of expression was seen in serous acinar cells. It was also found in other epithelia with fluid-exchange properties, the choroid plexus epithelium, the ependyma, visceral pleura and peritoneum, bile ducts and intestinal lining epithelium. However, there was little or no expression in vascular endothelial cells, kidney tubules or collecting ducts, lung alveoli, or in sebaceous glands. It is concluded that the channel is present in surface epithelia (e.g. skin) where it has a cell-protective role against osmotic challenge, and in epithelia where there is anion secretion that is facilitated by a K+ current-dependent hyperpolarization. It was also in some epithelial cells where its roles are as yet unknown.
Keywords: cell volume regulation, epithelial transport, potassium channels, secretion
Introduction
Three classes of calcium-activated potassium channels, which are distinguished by their unitary conductances and pharmacological profiles, have been identified. Of these, the intermediate-conductance (IK) channels, coded by the KCNN4 gene, appear to have a limited distribution, being primarily located in non-excitable cells (Jensen et al. 1998; von Hahn et al. 2001; Begenisich et al. 2004; Chen et al. 2004). IK channels were first discovered in cells of haemopoetic lineage, including red blood cells and lymphocytes (Jensen et al. 1999; Hoffman et al. 2003; Maher & Kuchel, 2003). They are referred to by a variety of names, including: KCa3.1; SK4; IK1; KCNN4, Gardos channels; KCa4 and IKCa1 (see IUPHAR database, http://www.iuphar-db.org/iuphar-ic/KCa.html). In red blood cells they have a volume-regulatory role, and in lymphocytes they generate a hyperpolarization that is necessary for mitosis and hence lymphocyte proliferation. They were later detected in secretory epithelia, such as the crypt epithelium in the colon (Warth et al. 1999) and in secretory organs such as the pancreas (Ishii et al. 1997) and salivary glands (Jensen et al. 1998; Nehrke et al. 2003). IK channels in epithelia appear to have two roles, the first in fluid secretion (Joiner et al. 2003; Hayashi et al. 2004) and the second in cell volume regulation, particularly in response to hypotonic stress (Wang et al. 2003; Sand et al. 2004), an analogous role to their function in erythrocytes. It is only in recent years that antibodies to IK channels that are useful for immunohistochemical studies have become available and so in most of these tissues or organs the cellular locations of the channels are not known. Moreover, in many epithelia there is no information as to whether the channels are expressed at all. There is a need to understand the distributions and roles of the channels in tissues where they have not been studied, because the IK channels have been suggested to be targets for a number of therapeutic interventions, including treatment of sickle-cell anaemia (Stocker et al. 2003), immune modulation (Wulff et al. 2003), treatment of obstructive airways disease (Singh et al. 2001), reduction of traumatic brain injury (Mauler et al. 2004), amelioration of proliferative vascular disorders (Neylon, 2002; Grgic et al. 2005), inhibition of the proliferation of cancer cells (Parihar et al. 2003) and treatment of motility disorders in the intestine (Furness et al. 2004a). In order to understand their functions fully, and to anticipate and diminish side-effects of drugs that target IK channels for the treatment of particular disorders, a proper understanding of channel distribution at the cell level is required.
The immunohistochemical studies of IK channel distribution in epithelia that have been published to date reveal immunoreactivity of enterocytes in the duodenum, ileum and colon (Furness et al. 2003; Joiner et al. 2003), where the channels may have roles in active K+ secretion (Joiner et al. 2003), in providing a hyperpolarization to facilitate Cl− secretion (Begenisich et al. 2004) and in regulatory volume control (Wang et al. 2003). IK channel immunoreactivity is present in cells of the oesophageal epithelium and in surface cells of the gastric mucosa, where regulatory volume control is a probable function of the IK channels (Furness et al. 2003).
In order to determine whether patterns of IK channel expression are consistent with their hypothesized roles in cell volume regulation and fluid secretion, this study investigated the distribution of the channels in a range of epithelia that are either exposed to osmotic challenge or are involved in fluid secretion.
Materials and methods
Sprague–Dawley rats of both sexes, 100–300 g in weight, were used in this study. In all, 20 rats were used and all data were confirmed in tissues from at least three animals. All procedures were approved by the University of Melbourne Animal Experimentation Ethics Committee.
Rats were anaesthetized with sodium pentobarbital (40 mg kg−1) given subcutaneously and were then perfused through the heart with isotonic saline followed by fixative (2% formaldehyde plus 0.2% picric acid in 0.1 m sodium phosphate buffer, pH 7.0, for peripheral tissues, 4% paraformaldehyde in 0.1 m sodium phosphate buffer, pH 7.0, for the central nervous system). Tissues were removed and placed in the fixative at ice temperature and fixed overnight at 4 °C. The next day, tissue was cleared of fixative using three 10-min washes in dimethylsulfoxide (DMSO), followed by three 10-min washes in PBS (0.15 m NaCl in 0.01 m sodium phosphate buffer, pH 7.2) and were then placed in PBS-sucrose-azide (PBS containing 0.1% sodium azide as preservative and 30% sucrose as a cryoprotectant) and stored at 4 °C overnight. The following day, small segments of tissue were transferred to a mixture of PBS-sucrose-azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a ratio of 1 : 1 for a further 24 h before being embedded in 100% OCT. Sections 12 µm thick were cut and collected on microscope slides.
Immunohistochemistry
To localize IK channel immunoreactivity, we used a previously characterized antiserum (IK38/6) raised against a 15-amino-acid synthetic peptide corresponding to the N-terminus of the rat IK channel (Furness et al. 2003). Prior to antibody incubation, preparations were exposed to 10% normal horse serum (NHS) plus 1% Triton X100 for 30 min at room temperature. Incubation in anti-IK channel antiserum was at a dilution of 1 : 2000 for 24 h at 4 °C. Following incubation in primary antiserum, preparations were rinsed in PBS (3 × 10 min) and then incubated for 1 h at room temperature with secondary antibody, donkey anti-rabbit IgG coupled to Alexa 488 (Molecular Probes Inc., Eugene, OR, USA).
Preparations were analysed by confocal microscopy on a Bio-Rad MRC1024 confocal scanning laser system installed on a Zeiss Axioplan 2 microscope. The images were of 1024 × 1024 pixels and the thickness of each optical section was nominally 0.5 µm. In some cases, immunoreactive cells were scanned as a series of optical sections with a centre to centre spacing of 0.2 µm. The images were further processed using Confocal Assistant, Corel PhotoPaint and Corel Draw software programs.
Western blots and RT-PCR
Rats were killed by stunning and exsanguination. Samples of bladder mucosa or colon external muscle plus myenteric plexus (EM/MP) were collected in either lysis buffer (CelLytic; Sigma, Sydney, Australia), including a protease inhibitor cocktail (Sigma) plus 5 µm PMSF for protein extraction, or RNAlater (Ambion, Austin, TX, USA) for RNA extraction.
Samples taken for protein extraction were sonicated on ice and frozen at −20 °C until used. Protein extracted from tissue samples was quantified using the Bio-Rad Protein Assay (Bio-Rad, Regent's Park, Australia).
Protein lysate (20 µg total protein) from each sample was resolved by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) through 10% gels under reducing conditions. Samples were run with precision plus protein standard ladders (Bio-Rad) in order to determine molecular mass. Samples were then electrophoretically transferred, 1 h at 100 V, to polyvinylidine fluoride (PVDF) membranes (Hybond-P, Amersham, Melbourne, Australia). Non-specific sites on the membrane were blocked using PBS-T [PBS plus 0.1% (v/v) Tween20] with 5% skim milk. Membranes were washed three times in PBS-T (15/5/5 min) and then exposed to diluted primary antisera for 1 h. Unbound antibodies were removed by three further washes in PBS-T. Blots were exposed to an horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antiserum (Amersham) for 1 h. All incubations and washes were performed at room temperature with constant agitation. Membranes were then washed again in PBS-T and the HRP-labelled proteins detected using the enhanced chemiluminescence detection system (ECL; Amersham) in conjunction with hyperfilm ECL (Amersham).
For reverse transcription-polymerase chain reaction (RT-PCR) detection of IK channel mRNA, tissue was removed into RNAlater and subsequently placed into lysis buffer (buffer RLT; Qiagen, Melbourne, Australia). Total RNA was extracted using the RNeasy Mini Kit (Qiagen). RNA integrity was determined by electrophoresis on an agarose gel. RNA (1 µg) was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen, Melbourne, Australia) and oligo d(T) primers in a 20-µL reaction volume. Two forward primers (F1: 5′-GCTCAACCAAGTCCGMTTCC-3′ and F2: 5′-CAACAAGGCGGAGAAACACG-3′) and two reverse primers (R1: 5′-TGGMCTSCTGRMTGGGTTCTGG-3′ and R2: 5′-GCATCTTGGAGATGTCCAC-3′) were used. Samples of the resultant cDNA (2 µL of a 1 : 5 dilution) were then amplified by PCR using Platinum Taq DNA polymerase (Invitrogen, Melbourne, Australia). Touchdown PCR was performed using annealing temperatures ranging from 68 to 57 °C.
Results
Immunoreactivity for the IK channel was found in cells forming surface epithelia, for example in the skin, in epithelia lining hollow organs and in epithelia within secretory glands.
Stratified surface epithelia: skin, cornea, oral mucosa, tongue, oesophagus, vagina
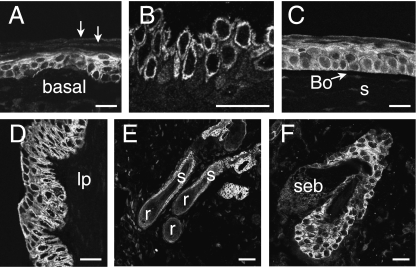
The stratified squamous epithelia of the skin, oral mucosa and tongue all had a similar appearance (Fig. 1A,B). In the skin, a proportion of cells of the basal (germinal layer) and of the adjacent spiny cell layer had immunoreactivity of the cell surface and to a variable extent in the cytoplasm. Cells forming the external surface epithelium of the cornea had cell surface immunoreactivity. In this case, cells throughout the thickness of the epithelium were reactive (Fig. 1C). The underlying Bowman's membrane and stromal cells were not immunoreactive. Cells of the vaginal epithelium, which is non-keratinized, were immunoreactive throughout its thickness (Fig. 1D).
Fig. 1.
Examples of IK channel immunoreactivity in stratified squamous epithelia. (A) Thin skin from the ear. The cells of the basal and spiny cell layers have cell surface immunoreactivity. Cells of the stratum corneum (arrows) are not IK channel immunoreactive. (B) Oral mucosa, showing IK channel immunoreactivity of cells of the basal layers. (C) Cornea. Cells through the full thickness of the cornea have channel immunoreactivity. The underlying Bowman's membrane (Bo) and the corneal stroma (s) are unstained. (D) Vagina. Cells through the full thickness of the epithelium are immunoreactive. Cells in the lamina propria (lp) are not labelled. (E) Hair follicles and dermis. Immunoreactivity can be seen in the dermal invaginations that form the walls of the follicles, but does not occur in the hair root (r) or hair shaft (s). (F) Sebaceous gland (seb) and part of the hair follicle into which it drains. No immunoreactivity occurs in the sebaceous gland, but immunoreactive epithelial cells of the follicle are observed. Scale bars: 20 µm.
Immunoreactive cells of the epidermis continued into the dermal invaginations of the hair follicles, but were absent from the root of the hair and the hair itself (Fig. 1E,F). The immunoreactivity was primarily at the cell surfaces. The cells of the sebaceous glands associated with hair follicles were not immunoreactive (Fig. 1F), and nor was the smooth muscle forming the erector pili.
Epithelia with fluid exchange functions: respiratory epithelium, vascular endothelium, serosal surfaces, kidney, choroid plexus, ependyma
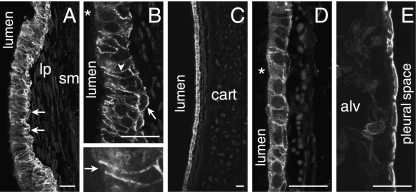
IK channel immunoreactivity occurred at the surfaces of columnar cells of the pseudostratified epithelium of the trachea that are involved in water and electrolyte exchange, but not in the mucus-secreting goblet cells (Fig. 2A,B). IK channel immunoreactivity was not found in the underlying connective tissue, smooth muscle or cartilage. Within the epithelium, immunoreactivity was strongest in the pyramidal basal cells, and basolateral parts of the columnar cells. The apical surfaces, including the cilia, were not immunoreactive. Submucosal glands of the trachea are rare in rodents, and were not encountered in these studies. IK immunoreactivity was also present at the surface membranes of the low columnar or cuboidal epithelial cells lining the bronchi, both in the major bronchi and in the respiratory bronchioles, where there is no cartilage. All bronchial epithelial cells appeared to be immunoreactive (Fig. 2C,D). The pulmonary alveoli did not exhibit IK immunoreactivity, either in the alveolar epithelium or in the capillary endothelium (Fig. 2E). Round cells adjacent to the alveolar epithelium or sometimes within it had IK immunoreactivity. They were about 10 µm in diameter and had the appearance of alveolar macrophages. The simple squamous epithelial cells of the visceral pleura were immunoreactive (Fig. 2E).
Fig. 2.
IK channel immunoreactivity in the respiratory epithelium. (A) The immunoreactivity occurs in the lining of the trachea. It is prominent in the pyramidal basal cells (arrows). The lamina propria (lp) and smooth muscle (sm) are not reactive. (B) Higher magnification view of the tracheal epithelium. IK channel immunoreactivity is seen at the surface membranes of basal cells (arrow) and in the basolateral membranes of the columnar cells (arrowhead). Goblet cells were not immunoreactive (cell adjacent to asterisk). One of the columnar cells is shown at higher magnification (lower micrograph). Note the absence of labelling of the apical membrane (arrow). (C) Immunoreactivity of the cuboidal cells of the bronchial epithelium. The underlying cartilage (cart) is not stained. (D) Bronchial epithelium at higher magnification. Immunoreactivity is observed on the basolateral membranes, and weakly in the cytoplasm (cell adjacent to asterisk). (E) Immunoreactivity of the simple squamous epithelium of the visceral pleural membrane. Note that the alveoli (alv) adjacent to the pleural membrane are not immunoreactive. Scale bars: 20 µm.
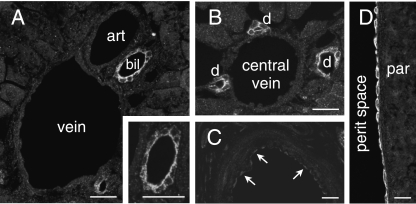
The endothelial cells of muscular arteries and of veins were not immunoreactive, or had very faint staining (Fig. 3A–C). As well as examining vessels in the liver, which are illustrated in Fig. 3, we investigated the aorta, vena cava, pial arteries, muscular arteries in the mesentery and arteries supplying the limbs, as well as encountering arteries in other organs that were investigated. Although no staining occurred in the parenchyma of the liver, the lining cells of the intra-hepatic bile ducts and ductules were immunoreactive (Fig. 3A,B). Faint staining of the vascular endothelium was observed in some muscular arteries, such as the mesenteric arteries (Fig. 3C). Immunoreactivity occurred in the squamous epithelium of the visceral peritoneum, which is shown for the serosa of the spleen in Fig. 3(D). No IK channel immunoreactivity was detected in the parenchyma of the spleen.
Fig. 3.
Blood vessels, intra-hepatic biliary ducts and visceral peritoneum. (A) Branches of the hepatic portal vein (vein), hepatic artery (art) and bile duct (bil) in the parenchyma of the liver. The only IK channel immunoreactivity is in the epithelium of the intra-hepatic biliary duct, which is shown at higher magnification in the inset. The endothelial cells of the vein and artery are negative, as are the hepatocytes. (B) The central vein and bile ductules. The epithelium of the ductules (d) is positively stained, but the endothelium of the central vein is unreactive. (C) Cross-section through a small mesenteric artery. Very faint staining is present in endothelial cells of the intima (arrows). (D) IK channel immunoreactivity of the simple squamous epithelium (visceral peritoneum) on the surface of the spleen. Peritoneal space: perit space. The parenchyma of the spleen itself (par) was negative. Scale bars: 20 µm.
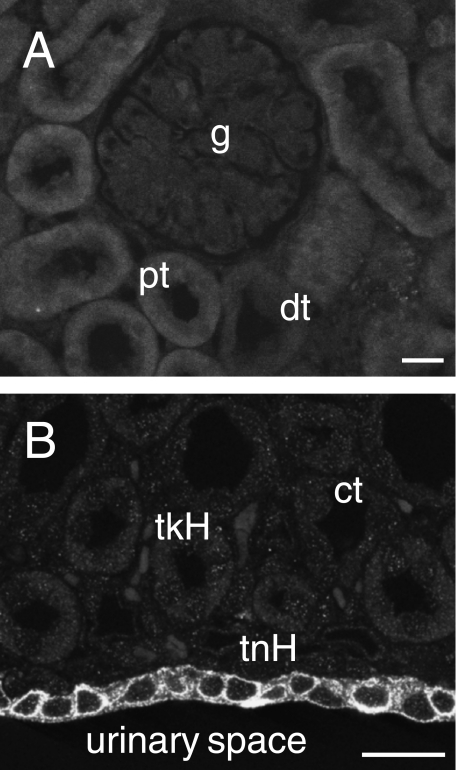
Detectable IK immunoreactivity did not occur in any of the tubular structures of the kidney, the proximal and distal tubules, the loops of Henle or the collecting ducts, or in the glomeruli (Fig. 4A,B). However, it was present in the epithelium of the inner surface of the cortex adjacent to the urinary space of the renal pelvis (Fig. 4B).
Fig. 4.
IK channel immunoreactivity in the kidney. (A) Renal cortex showing the lack of immunoreactivity of a glomerulus (g), the proximal convoluted tubules (pt) and distal convoluted tubules (dt). (B) The renal medulla, with lack of IK staining in thick loops of Henle (tkH), thin loops (tnH) and collecting tubules (ct). The urothelium at the inner surface of the renal medulla, adjacent to the urinary space (urinary space), is immunoreactive. Scale bar: 20 µm.
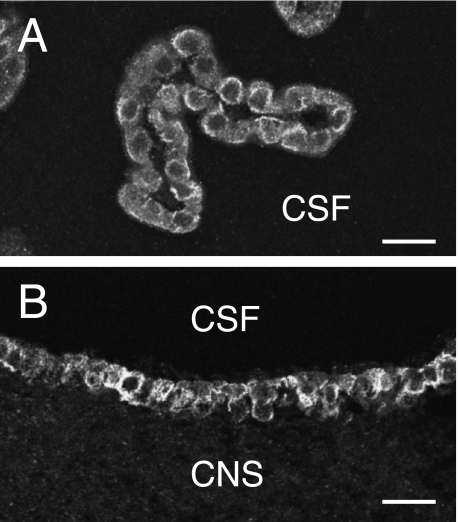
In the choroid plexus (Fig. 5A), there was strong reactivity of the cuboidal epithelial cells of the cerebrospinal fluid surface, but the adjacent vascular endothelium was not immunoreactive. Immunoreactivity was also prominent at the surfaces of ependymal cells lining the cerebral ventricles, but it was absent from the underlying neuropil, glial cells and nerve cell somata (Fig. 5B). Absence of IK channel expression in the central nervous system is consistent with earlier observations (Jensen et al. 1998; Chen et al. 2004).
Fig. 5.
Epithelial cells of the choroid plexus and ependyma, showing IK channel immunoreactivity. (A) A region of choroid plexus from the fourth ventricle. The cuboidal cells facing the cerebrospinal fluid (CSF) space are immunoreactive, but no reaction is observed in the underlying vascular endothelium. (B) Immunoreactivity of the ependymal cells lining the third ventricle. Note the lack of immunoreactivity of the adjacent central nervous system (CNS). Scale bars: 20 µm.
Exocrine glands and ducts: salivary glands, lacrimal glands, pancreas, bile ducts, eccrine sweat glands, mammary gland
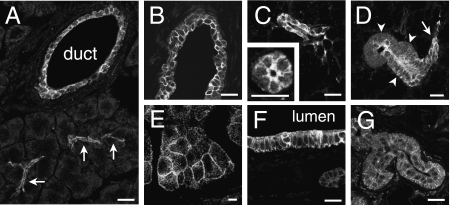
The salivary glands, lacrimal glands and pancreas all presented a similar picture, with immunoreactivity in the intercalated ducts, beginning at the acinus and extending towards the striated portion of the duct (Fig. 6A–D). The striated ducts showed obvious immunoreactivity in the submandibular glands (Fig. 6D), but not in other glands. In addition, strong immunoreactivity was observed in the interlobular and large intralobular ducts (Fig. 6A,B). In both the salivary glands and the pancreas, the surface membranes of the acinar cells were faintly immunoreactive. Immunoreactivity occurred at both the basolateral and the apical membranes of acinar and duct cells (Fig. 6C). The staining of the acinar cells was often punctate, on the surfaces of the cells, suggesting that clumps of channels were distributed along the membranes of acinar cells. IK channel immunoreactivity was less prominent in the cytoplasm.
Fig. 6.
IK channel immunoreactivity in the walls of fluid-secreting glands and ducts. (A) Section through the pancreas. Immunoreactivity is seen in the intercalated ducts (arrows) and in an intra-pancreatic duct (duct). (B) Interlobular duct of the extra-orbital lacrimal gland, showing IK channel immunoreactivity of the duct epithelium. (C) Intercalated ducts shown in longitudinal and in cross-section in the submandibular gland. In the cross-section of the duct, immunoreactivity is clearly seen at the apical surface. (D) Continuity between an intercalated duct (arrow) and a striated duct (arrowheads) in the submandibular gland. The cells forming the walls of the striated duct were immunoreactive. (E) Acinar cells of the parotid gland, showing IK channel immunoreactivity at the basolateral surfaces. (F) The wall of the extra-hepatic bile duct, showing its immunoreactive epithelium. (G) Eccrine sweat gland from the rat paw pad. A low level of IK channel immunoreactivity is seen. Scale bars: 20 µm.
Other ducts that had IK channel immunoreactive cells in their walls were the bile ductules and bile ducts within the liver (Fig. 3A,B), the extra-hepatic bile duct (Fig. 6F) and the lactiferous ducts of the mammary gland. The secretory parts of the eccrine sweat glands of the paw were faintly stained (Fig. 6G). The excretory ducts of sweat glands within the dermis were faintly stained, but not where they passed through the epidermis.
Urinogenital tract
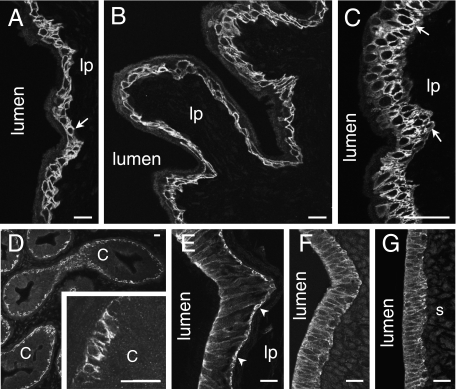
The transitional epithelium that lines the urinary tract had a similar appearance in the bladder, ureters and urethra (Fig. 7A–C). The cells forming the basal 1–2 cell layers had immunoreactivity at their surface membranes, whereas the more superficial cells were negative. The underlying connective tissue and smooth muscle in the walls of these organs were also unstained.
Fig. 7.
Epithelia of urinogenital organs. The lumen and lamina propria (lp) are indicated. (A) Transitional epithelium of the ureter. Immunoreactivity occurs at the surface membranes of the basal epithelial cells (arrow). Slight protein autofluorescence reveals the luminal surface of the epithelium. (B) Epithelium lining the bladder. Note that there is no immunoreactivity of the underlying connective tissue of the lamina propria (lp) or muscle. (C) IK channel immunoreactivity in the urethra. Cells of the basal layer and adjacent layers are immunoreactive. (D) Immunoreactivity in the epidydimis. The only prominent immunoreactivity is in the basal cells of the epithelium. Part of an epidydimal duct is shown at higher magnification in the inset. The tall columnar cells (c) of the epidydimal lining were unstained. (E) Vas deferens. The epithelial cells show immunoreactivity of their surface membranes. Basal cells (arrowheads) are more strongly immunoreactive than columnar cells. The underlying lamina propria (lp) and smooth muscle were not immunoreactive. (F) Fallopian tube. The IK channel immunoreactivity is seen in the basolateral membranes, but the cilia are not reactive. (G) Uterus. The columnar epithelial cells exhibit IK channel immunoreactivity, but the underlying stroma (s) is not immunoreactive. Scale bars: 20 µm.
Cells in the epithelium lining the epididymis and vas deferens were immunoreactive (Fig. 7D,E). Only the basal cells, not the ciliated, columnar epithelial cells, were stained in the epididymis, whereas in the vas deferens the basal cells were strongly stained and there was also staining, although less intense, of the columnar epithelium (Fig. 7E). In all cases, the cell membrane was well stained and there was little cytoplasmic staining. The smooth muscle of the vas deferens was unstained.
IK channel immunoreactivity occurred in the lining epithelium of the female genital tract, including the vaginal epithelium (Fig. 1D) and the pseudostratified epithelial cells lining the fallopian tubes (Fig. 7F). In the fallopian tubules, immunoreactivity occurred on the basolateral membranes, but not on the cilia. The columnar epithelial cells of the uterine lining also showed basolateral staining (Fig. 7G). Cells in the underlying endometrial stroma were not IK channel immunoreactive.
Intestine
We have previously described and illustrated IK immunoreactivity in the intestinal epithelium (Furness et al. 2003). In brief, in the small intestine, enterocytes of the villi were immunoreactive and, in the duodenum, enterocytes of the crypts and villi were both immunoreactive. In the colon, enterocytes of the surface and most of the depth of the colonic glands were immunoreactive.
Western blot analysis and RT-PCR
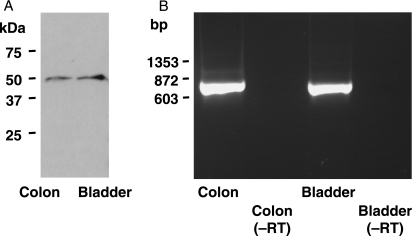
Channel protein from an epithelial source, the transitional epithelium of the ureter, was compared with the channel protein from enteric neurons. Analysis of Western blots probed with the anti-IK channel antibody revealed immunoreactive protein of ∼50 kDa, the molecular weight for the IK channel monomer (Fig. 8). PCR products obtained using primers directed against the IK channel mRNA extracted from the urinary epithelium also revealed products of the correct size. The primer pair F1–R1 (see Methods) yielded the predicted product size of 724 bp (Fig. 8). Similarly, the primer pair F2–R1 yielded the predicted product size of 403 bp and the primer pair F1–R2 yielded the predicted product size of 571 bp.
Fig. 8.
Evidence for IK channel protein and gene expression in epithelia. Samples were taken from the urothelium and, for comparison, from the external muscle/myenteric plexus of the intestine, where IK channels occur in neurons. (A) Western blot analysis of protein extracted from the epithelium of the urinary bladder shows a single band at approximately 50 kDa, the predicted size of the channel protein monomer. (B) RT-PCR product from an extract of urinary bladder endothelium and from the colon, showing bands of the predicted size.
Discussion
The present work demonstrates the presence of IK channel immunoreactivity in epithelia in a range of organs. In many cases, the tissues correspond with those where IK channels have been demonstrated by RT-PCR or functional studies, but have not been previously localized to particular cell types. This applies to regions of the gastrointestinal tract, pancreas, salivary gland, skin, trachea and urinary bladder (Ishii et al. 1997; Jensen et al. 1998; Warth et al. 1999; Furness et al. 2003; Nehrke et al. 2003; Chen et al. 2004).
One of the first roles for IK channels to be defined was that of volume regulation in red blood cells, where they were originally known as Gardos channels (Maher & Kuchel, 2003). When exposed to hypotonic challenge, these cells extrude K+ through IK channels, which is accompanied by loss of cell water. The outward flux of water reduces osmotic swelling of the cells. The present work suggests that the presence of IK channels is common to epithelia that are exposed to fluids of low ionic strength. This applies to the skin, cornea and oral cavity (present work), the oesophagus and the surface cells of the gastric lining (Furness et al. 2003). Osmotic challenge also occurs in other organs where substantial changes in osmolarity can occur. For example, the osmolarity of urine changes over a large range, depending, for example, on changes in dietary intake and on environment. It is thus interesting that the urothelium of bladder, urethra and ureters, and the epithelium of the inner surface of the kidney, which has the urine on one side and interstitial fluid on the other, all have strong immunoreactivity for the IK channel. Tracheal and bronchial epithelial cells, which separate the fluid on their apical surfaces (whose osmotic strength is modified by the humidity of the air) and the interstitial fluid on their basolateral surfaces, also exhibit IK immunoreactivity. In addition, the respiratory epithelium has an excretory role (see below). In stratified epithelia, IK channels are expressed most strongly in the basally located cells (e.g. in skin, oral mucosa, oesophagus and urinary tract). At these sites, the more superficial cells may provide the first protection against hypotonic insult through other mechanisms.
Other regions in which we have located IK channel immunoreactivity include organs that secrete considerable fluid volume, notably lacrimal glands, salivary glands and pancreas. However, there are some differences in the channel distributions in these glands. In all serous glands, channel immunoreactivity was weak in the secretory acini. Nevertheless, functional studies reveal an important role for IK channels in providing the electrochemical driving force for Cl− efflux from the acinar cells (Melvin et al. 2005). These studies also indicate that the K+ channels involved in this driving current occur at low densities (Melvin et al. 2005), which is what we have observed. Consistent with low protein expression in the pancreas is a low level of mRNA as compared with other tissues (Jensen et al. 1998; Chen et al. 2004). Immunoreactivity was prominent in the intercalated ducts of fluid-secreting glands. The intercalated ducts and small ducts of the pancreas are acted upon by the hormone secretin, which stimulates the production of a Cl−-rich and 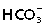 -rich secretion (Owyang & Williams, 2003). It is possible that current flowing through IK channels brings the inside of the cell to a more negative potential, which provides an increased driving force for the secretion of these anions (Begenisich et al. 2004). Chloride channels (the cystic fibrosis transmembrane conductance regulator) are also present in the respiratory epithelium, where IK channel opening could also contribute to the electrochemical potential that drives Cl− secretion (Cuthbert et al. 1999).
-rich secretion (Owyang & Williams, 2003). It is possible that current flowing through IK channels brings the inside of the cell to a more negative potential, which provides an increased driving force for the secretion of these anions (Begenisich et al. 2004). Chloride channels (the cystic fibrosis transmembrane conductance regulator) are also present in the respiratory epithelium, where IK channel opening could also contribute to the electrochemical potential that drives Cl− secretion (Cuthbert et al. 1999).
It is notable that IK immunoreactivity was not detected in the renal tubules, despite the fact that they are clearly involved in K+ exchange. There is in fact a great diversity of K+ channels in the kidney: about 30 channels have so far been identified in this organ, but this does not include the IK channel (Chen et al. 2004; Hebert et al. 2005). Although staining for IK channels was very faint in the vascular endothelium, there is both immunohistochemical and functional evidence for their presence in these cells (Furness et al. 2003; Marrelli et al. 2003). Weak staining of vascular endothelium has also been reported by Chen et al. (2004). Smooth muscle cells, which were encountered in many organs, including the airways, arteries, bladder and genital organs, were not immunoreactive. This is consistent with the channel being present in smooth muscle in the proliferative state, but not in cells in the fully differentiated, contractile state (Neylon et al. 1999). IK channel immunoreactivity is also absent from arteriolar and intestinal smooth muscle in human tissue samples (Chen et al. 2004; Furness et al. 2004b).
In colonic, small intestinal and respiratory epithelia, where IK channel opening provides a driving force for chloride secretion, physiological evidence indicates that functional IK channels are on the basolateral surfaces (Rufo et al. 1997; Cuthbert et al. 1999; Cuthbert & MacVinish, 2003). In the present work, immunohistochemistry has confirmed that IK channel immunoreactivity is confined to the basolateral surfaces of cells of the respiratory epithelium. However, we have found an additional apical surface localization on enterocytes in the small intestine and colon, which confirms previous observations (Furness et al. 2003; Joiner et al. 2003). Whether these apical channels have functional roles may depend on whether Ca2+ concentrations become elevated in proximity to these channels.
IK channels were expressed in the basal cells of the epithelium lining the epididymis and vas deferens. The epididymis, and possibly the vas deferens, are involved in the re-absorption of fluid that is secreted from the testis into the spermatic fluid (Hinton & Palladino, 1995; Leung et al. 2001). This process involves activity of the Na+/H+ exchanger, the Na+/K+ pump, K+ channels, and other exchangers and channels that influence water and ion movement (Leung et al. 2001). Physiological studies have not yet uncovered a role for IK channels, although the present observations suggest that there may be such a role.
In summary, IK channels are expressed in epithelia that are exposed to osmotic stress (skin, oral mucosa, oespohagus, urinary tract and respiratory tract) and in epithelia where there is a need for K+ current to drive anion secretion (small intestine, colon, pancreas, salivary glands, lacrimal glands, respiratory epithelium). They are also expressed in other epithelia where their functions have yet to be determined.
Acknowledgments
These studies were funded by the National Health and Medical Research Council (Australia). We thank Michelle Thacker, Vicky Staikopoulos and Anderson Hind for assistance with experiments and Daniel Poole for comments on the manuscript. Y.S. was on leave from Gifu University.
References
- Begenisich T, Nakamoto T, Ovitt C, et al. Physiological roles of the intermediate conductance, Ca2+-activated K channel, Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- Chen MX, Gorman SA, Benson B, et al. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn-Schmiedeberg's Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Hickman ME, Thorn P, MacVinish LJ. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. Am J Physiol. 1999;277:C111–C120. doi: 10.1152/ajpcell.1999.277.1.C111. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, MacVinish LJ. Mechanisms of anion secretion in Calu-3 human airway epithelial cells by 7,8-benzoquinoline. Br J Pharmacol. 2003;140:81–90. doi: 10.1038/sj.bjp.0705403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Robbins HL, Selmer I-S, et al. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res. 2003;314:179–189. doi: 10.1007/s00441-003-0808-z. [DOI] [PubMed] [Google Scholar]
- Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004a;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kearney K, Robbins HL, et al. Intermediate conductance potassium (IK) channels occur in human enteric neurons. Autonom Neurosci. 2004b;112:93–97. doi: 10.1016/j.autneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Grgic I, Eichler I, Heinau P, et al. Selective blockage of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- von Hahn T, Thiele I, Zingaro L, et al. Characterisation of the rat SK4/IK1 K+ channel. Cell Physiol Biochem. 2001;11:219–230. doi: 10.1159/000051936. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kunii C, Takahata T, Ishikawa T. ATP-dependent regulation of SK4/IK1-like currents in rat submandibular acinar cells: possible role of cAMP-dependent protein kinase. Am J Physiol. 2004;286:C635–C646. doi: 10.1152/ajpcell.00283.2003. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton BT, Palladino MA. Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc Res Techn. 1995;30:67–81. doi: 10.1002/jemt.1070300106. [DOI] [PubMed] [Google Scholar]
- Hoffman JF, Joiner W, Nehrke K, Potapova O, Foye K, Wickrema A. The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (gardos channel) in human red blood cells. Proc Natl Acad Sci USA. 2003;100:7366–7371. doi: 10.1073/pnas.1232342100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS, Strøbaek D, Christophersen P, et al. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol. 1998;275:C848–C856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- Jensen BS, Ødum N, Jørgensen NK, Christophersen P, Olesen S-P. Inhibition of T cell proliferation by selective block of Ca2+-activated K+ channels. Proc Natl Acad Sci USA. 1999;96:10917–10921. doi: 10.1073/pnas.96.19.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Basavappa S, Vidyasagar S, et al. Active K+ secretion occurs through multiple types of KCa channels and is regulated by IKCa channels in rat proximal colon. Am J Physiol. 2003;285:G185–G196. doi: 10.1152/ajpgi.00337.2002. [DOI] [PubMed] [Google Scholar]
- Leung GPH, Tse CM, Chew SBC, Wong PYD. Expression of multiple Na+/H+ exchanger isoforms in cultured epithelial cells from rat efferent duct and cauda epididymidis. Biol Reprod. 2001;64:482–490. doi: 10.1095/biolreprod64.2.482. [DOI] [PubMed] [Google Scholar]
- Maher AD, Kuchel PW. The Gárdos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35:1182–1197. doi: 10.1016/s1357-2725(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- Mauler F, Hinz V, Horváth E, et al. Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma. Eur J Neurosci. 2004;20:1761–1768. doi: 10.1111/j.1460-9568.2004.03615.x. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol. 2003;284:C535–C546. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle. Relationship between KCa channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- Neylon CB. Potassium channels and vascular proliferation. Vascular Pharmacol. 2002;38:35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- Owyang C, Williams JA. Pancreatic secretion. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia: Lippincott, Williams & Wilkins; 2003. pp. 340–366. [Google Scholar]
- Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh C-C. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003;471:157–164. doi: 10.1016/s0014-2999(03)01825-9. [DOI] [PubMed] [Google Scholar]
- Rufo PA, Merlin D, Riegler M, et al. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. J Clin Invest. 1997;100:3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand P, Anger A, Rydqvist B. Hypotonic stress activates an intermediate conductance K+ channel in human colonic crypt cells. Acta Physiol Scand. 2004;182:361–368. doi: 10.1111/j.1365-201X.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- Wang J, Morishima S, Okada Y. IK channels are involved in the regulatory volume decrease in human epithelial cells. Am J Physiol. 2003;284:C77–C84. doi: 10.1152/ajpcell.00132.2002. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, et al. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Arch Eur J Physiol. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Wulff H, Gutman GA, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Current Opinion Drug Discovery Development. 2003;6:640–647. [PubMed] [Google Scholar]