Ciliary Beat Frequency Is Maintained at a Maximal Rate in the Small Airways of Mouse Lung Slices (original) (raw)
Abstract
Ciliary beat frequency (CBF) is a key factor in the defense of the airways, and ATP can stimulate CBF by increasing intracellular calcium concentration ([Ca2+]i). However, the regulatory effects of ATP have been mainly studied in cultured or isolated epithelial cells from the large cartilaginous airways. The aim of this study was to evaluate the regulation of CBF in small airways of lung slices that are representative of in vivo tissue. Mice lungs were inflated with agarose and cut into thin slices with a vibratome. CBF in the small bronchioles was observed with differential interference contrast microscopy and quantified using high-speed digital imaging (at 240 images s−1). We found that the in situ organization of the ciliated cells was well preserved and that their CBF was high. We verified the fidelity of our recording system by analyzing rapid changes in CBF in response to temperature. However, we found that ATP had no effect on CBF, despite the fact that the [Ca2+]i, measured with confocal fluorescence imaging, was increased. Ionomycin and purinergic or β-adrenergic agonists also failed to increase CBF. Similar results were obtained in outgrowths of cells cultured from lung slices. By contrast, ATP increased the slower CBF of outgrowths of ciliated cells cultured from tracheal rings. Therefore, we conclude that CBF in intrapulmonary airways of mice is maintained at a maximum rate and cannot be further increased by agonist stimulation. These conditions would ensure that mucociliary clearance is constantly active to provide continuous airway protection.
Keywords: ATP, Ca2+, high-speed digital microscopy, small airways
Mucociliary clearance is an important defense mechanism that clears foreign particles and chemicals from the airways to maintain healthy lungs. A key parameter determining the rate of mucus clearance is ciliary beat frequency (CBF). The density (cilia per cell and number of ciliated cells) and length of cilia are greater in the trachea than in small airways, and this correlates with an increase in mucus transport and CBF toward the oropharynx (1–5). This suggests that CBF is regulated throughout the airways to provide local control of mucociliary clearance. Unfortunately, this hypothesis is difficult to test in the smaller airways because of the inaccessibility of cilia. Consequently, most studies addressing the regulation of CBF have used cultured epithelial cells from the trachea or the large airways (cartilaginous bronchi) or trachea rings, and have found that CBF increases in response to a variety of stimuli, such as nucleotides (ATP, cAMP, UTP, cGMP) (6–12), mechanical deformation (13), NO (14, 15), and β-agonists (16–18). Frequently, this increase in CBF has been associated with increases in intracellular calcium concentration ([Ca2+]i) (6, 9, 12–15, 19). By contrast, the signaling mechanisms that regulate CBF in the smaller airways remain unknown.
In this article, we describe a unique method to study CBF in the smaller airways. Thin lung slices that retain the in vivo organization of the lung are prepared from mice and provide access to the cilia and epithelial cells at different positions of the bronchial tree. Differential interference contrast (DIC) microscopy and high-speed video recording could then be used to measure CBF. Because CBF is temperature dependent (20), we used this characteristic to validate our methodology for CBF measurements. With this approach, we determined that the CBF within the lung slices is normally maintained at a high rate of 20–25 Hz.
The major result of this study is that CBF in the intrapulmonary airways of the lung slice cannot be further increased by extracellular ATP (1–500 μM), even though ATP increased the [Ca2+]i in the ciliated cells. By contrast, control studies confirmed that the slower CBF of cultured ciliated tracheal cells or tracheal rings could be increased by ATP. These results indicated that the cilia of intrapulmonary airways do not respond to increases in [Ca2+]i, a response confirmed with the Ca2+ ionophore, ionomycin. Similar results were obtained in rat lung slices. Collectively, these results suggest that the CBF of the intrapulmonary airways is not influenced by agents that increase [Ca2+]i. In view of the facts that the basal CBF of cultured cells is always slower than the CBF of lung slices, and that the maximal CBF rates induced in cultured cells approach that of intrapulmonary airway cilia, a simple explanation for the insensitivity of cilia to [Ca2+]i is that their beat frequency is maintained, in situ, at a maximal rate. With this design, mucociliary clearance in the lower airways would be constantly vigilant, and perhaps more reliable, for the immediate removal of microscopic foreign material.
MATERIALS AND METHODS
Solutions and Chemicals
Hanks' balanced salt solution (HBSS) without phenol red, FBS, and Dulbecco's modified Eagle's medium (DMEM) were obtained from GIBCO/Invitrogen Corp. (Carlsbad, CA). HBSS was supplemented with 25 mM HEPES (sHBSS; pH 7.4). Fluo-3AM and Fluo-4AM were obtained from Molecular Probes/Invitrogen Corp.. UTP, forskolin, ionomycin, hexokinase, adenosine deaminase, and pluronic F-127 were obtained from Calbiochem (La Jolla, CA). Sulfobromophthalein, ATP, isoproterenol, apyrase, agarose (type VII-A, low gelling temperature), and DMSO were obtained from Sigma-Aldrich Corp. (St Louis, MO). Fluo-3AM and Fluo-4AM were dissolved in DMSO and diluted in sHBSS to the final working concentrations. Final DMSO concentrations were < 0.1%.
Lung Slice and Tracheal Ring Preparation
Lung slices were prepared by a modification of the method described previously to study airway contraction (21). To reduce movement artifacts resulting from smooth muscle cell (SMC) contraction (22), it was necessary to further stiffen the lung slice by increasing the concentration of agarose used to inflate the lung from 2 to 3%.
Lung slices were prepared from BALB/C mice (7- and 9-wk-old; Charles River Breeding Labs, Needham, MA) killed by intraperitoneal injection of pentobarbital sodium (Nembutal), as approved by the institutional animal care and use committee of the University of Massachusetts Medical School. A solution of 6% agarose was dissolved in distilled water at 60°C, cooled to 37°C, and mixed with 2× concentrated sHBSS to give a 3% agarose–sHBSS solution at 37°C. The trachea was cannulated, and the lungs were inflated with warm agarose–sHBSS (∼ 1.3 ml). Subsequently, 0.1–0.2 ml of air was injected to flush the agarose–sHBSS out of the airways into the distal alveolar space. The lungs were cooled to 4°C to gel the agarose. A lung lobe was mounted in a vibratome (Model EMS-4000; Electron Microscopy Sciences, Hatfield, PA) with the peripheral region uppermost, and lung slices of ∼ 100-μm thick were cut at 4°C in sHBSS. The initial lung slices did not contain airways and were discarded. The subsequent lungs slices contained the distal portions of the small airways, which had a diameter of ∼ 80 μm.
For consistency, the ciliary responses of similar-sized airways were initially compared. This comparison was achieved by collecting the first five lung slices that contained distal airways from different lungs lobes of each mouse. To compare the responses of the airway at different locations, a single lung lobe was sectioned into ∼ 30 sequential lung slices. With these serial slices, an airway was followed from the periphery (distal airway, ∼ 80 μm in diameter) to the central (intermediate airways, 150–210 μm), and proximal zone (∼ 270 μm in diameter) of the lobe.
Lung slices were maintained in DMEM supplemented with 10% FBS, antibiotics, and antimycotics at 37°C in 10% CO2. FBS was added to the media to match the conditions of cultured cells. Experiments were also performed with slices maintained in medium without FBS, but no differences were observed.
Tracheal rings were also prepared from BALB/C mice. The trachea was removed, washed with sHBSS, and manually sliced into rings (∼ 0.5 mm in width) that were maintained in the same medium and conditions as used for lung slices. Lung slices and tracheal rings were used within 3 d.
Cell Culture
Outgrowths of ciliated cells from the epithelium of the airways of lung slices or tracheal rings were obtained by using methods previously described to obtain outgrowth cultures of airway epithelial cells from trachea explants of rabbits (23). In brief, freshly prepared lung slices and tracheal rings were plated on collagen-coated glass coverslips and cultured in DMEM supplemented with 10% FBS, penicillin, and streptomycin at 37°C in 10% CO2 for 5–10 d. For outgrowth of cells, it was important that the lung slices or tracheal rings remained in contact with the collagen. This contact was achieved by using a thin meniscus of culture media. Outgrowths of ciliated cells from the epithelium could be seen attached to the coverslip within the lumen of the airway or tracheal ring after 5 d.
Drug Application
Lung slices or tracheal rings were mounted in a custom-made perfusion chamber and held in place with a small sheet of nylon mesh. A second coverslip, edged with silicone grease, was placed over the tissue. Perfusion of the slice or ring with sHBSS or agonists was performed by a gravity-fed perfusion system (21). The volume of the chamber was ∼ 100 μl, with a perfusion rate of 800 μl/min. When using cultured cells, the coverslip on which the cells were grown formed the bottom of the chamber. The chamber was left open, and had a volume of ∼ 300 μl; drugs were added manually to the chamber by micropipette (9). In experiments with multiple drug exposures, the cells were allowed to recover for at least 20 min between trials; 10 min has been reported to be sufficient time for a full recovery of sensitization of receptors (24).
Measurement of CBF with High-Speed Digital Microscopy
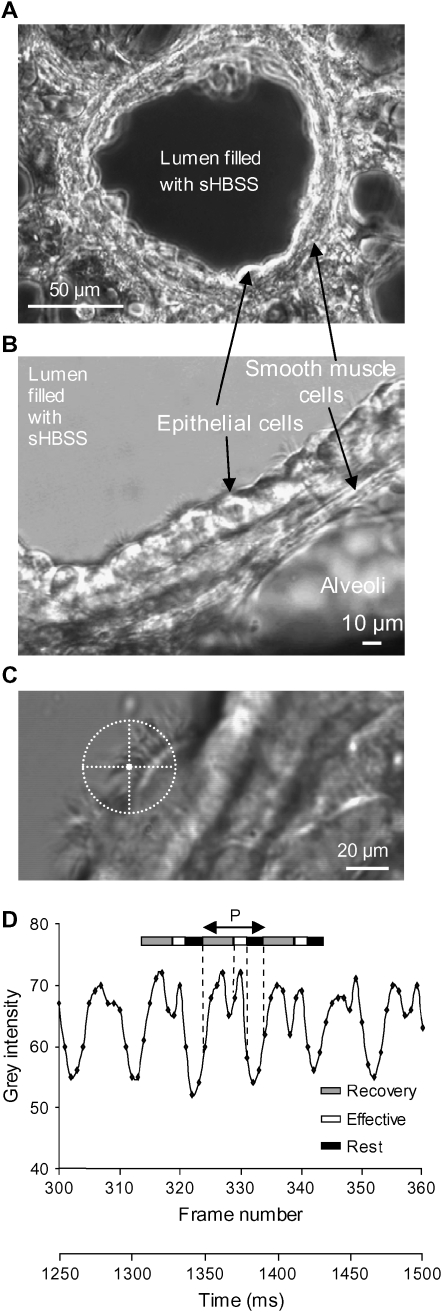
Specimens were observed with DIC microscopy using an inverted microscope (IX71; Olympus, Tokyo, Japan) equipped with a 40× oil immersion objective (numeric aperture: 1.0) or a 60× water immersion objective (numeric aperture: 1.20), and a Nikon closed-circuit television zoom adapter (Nikon, Tokyo, Japan). Images (648 pixels × 200 lines) of ciliary activity were recorded at 240 frames per second (fps) with a high-speed, progressive scan charge-coupled device camera (TM-6710; Pulnix America, Sunnyvale, CA) and image acquisition software (Video Savant; IO Industries, London, ON, Canada), as previously described (9). Changes in the gray intensity of the image that result from the repetitive motion of the cilia (Figure 1D) were analyzed by selecting regions of interest (ROI) of 3 × 3 pixels (0.87 × 0.80 μm) near the base of the cilia (Figure 1C). The CBF (Hz) was calculated from the period (frequency = 1/period) of each cycle of the gray-intensity waveform by beat-by-beat analysis (Figure 1D) (9).
Figure 1.
Measurement of CBF in small airways of mice. (A) A low-power phase–contrast image showing a small airway obtained with the lung slice preparation. The lumen is devoid of agarose, but filled with sHBSS. (B) A medium-power image of epithelial cells and SMCs of the small airways and agarose-filled alveoli obtained with DIC microscopy. (C) A high-power DIC image showing two ciliated cells. The cross-hair sight indicates the position of the region of interest used to measure CBF. (D) The waveform of the variation in gray intensity extracted from a 250-ms sequence of digital images (60 frames) recorded at 240 fps. Waveform shows the three phases of the beat cycle: recovery phase, effective phase and rest phase. Each beat cycle period (P; bidirectional arrow) was used to calculate CBF (1/period).
Measurement of Intracellular Ca2+ with Confocal Microscopy
Lung slices or cultures were incubated with Fluo-3AM or Fluo-4AM (20 μM), 100 μM sulfobromophthalein (an inhibitor that prevents dye extrusion via anion exchangers), and 0.2% pluronic F-127 for 45 min at 30°C, followed by 45 min in sHBSS containing 100 μM sulfobromophthalein at 30°C. Loaded slices or cultures were mounted in a custom perfusion chamber, and imaging was performed using a video-rate confocal microscope (21, 25, 26). In brief, a 488-nm laser was used as the excitation wavelength, and the emitted fluorescence (> 510 nm) was detected by a photomultiplier tube and a frame-capture board to form an image. Fluorescence images were recorded to a hard disk using Video Savant software at 30 fps. Changes in fluorescence were analyzed by selecting an ROI of ∼ 5 × 5 pixels in a single epithelial cell. Changes in fluorescence (F) were normalized to the initial level of fluorescence (F0). Final fluorescence values were expressed as a ratio (F/F0). The maximum F/F0 ratio obtained was used to compare different experiments.
Statistical Analysis
Data are expressed as means ± SEM. Statistical analysis was assessed by ANOVA. Comparison of paired-data was done using a paired-samples t test. A value of P < 0.05 was considered statistically different.
RESULTS
Measurement of CBF in Small Airways of Mice
To be consistent and allow the comparison of data from different airways in different lung slices, we primarily examined, unless otherwise noted, the responses of the distal small airways within peripheral lung slices. These airways had a diameter of ∼ 80 μm, and retained the morphologic and physiologic characteristics of in vivo airways (Figure 1). The airways were lined with a healthy cuboidal ciliated epithelium. Importantly, the airway lumen was devoid of agarose that might have interfered with CBF, but the periciliary layer was not preserved because the lumen was filled with sHBSS. The airways are surrounded by contractile SMCs and alveoli tissue. To measure CBF, the motion artifacts resulting from SMC contraction were reduced by stiffening the agarose used to stabilize the lung slice. This modification did not appear to affect the lung slice in any other way. The morphology of the lung slice did not substantially change during the period of use. Similarly, no substantial differences were observed in slices cut from different lobes of the lungs.
Lung slices were considerably thicker than cultured ciliated cells and, because the epithelium was viewed in profile, the movement of many overlapping cilia was observed simultaneously (Figure 1). Consequently, we established an imaging system using DIC optics to provide a relatively narrow depth-of-field, so that only a few cilia with good contrast were visible, and reevaluated and confirmed the accuracy of our CBF recording technique. The placement of the analysis points near the base of the cilia provided reliable measurements for CBF (Figures 1C and 1D). With a sampling rate of ∼ 240 fps, the waveform of the variation in gray intensity representing ciliary activity (Figure 1D) allowed the measurement of the duration of each phase of the ciliary beat cycle and any rapid changes in CBF. High-speed recording is essential for accurate CBF measurements at frequencies greater than 15 Hz (9, 16, 20).
CBF in Lung Slices Is Temperature Dependent
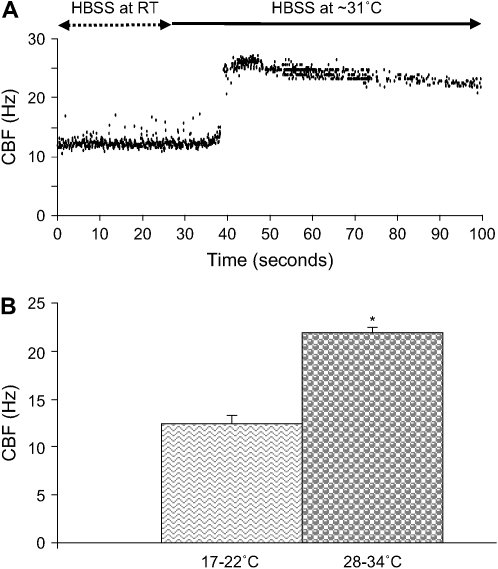
Previous studies indicate that ciliary beating is sensitive to temperature (20). Consequently, we used this characteristic to verify our ability to measure rapid changes in CBF. Lung slices were perfused with sHBSS at room temperature (RT; 20 ± 2°C) until a stable CBF was achieved (CBF = 12.45 ± 0.88 Hz). Temperature was monitored with a thermocouple within the chamber. Subsequently, warm sHBSS (31 ± 3°C) was perfused through the chamber and, after 15–25 s, this treatment increased the CBF to a maximum value of 27.4 ± 1.2 Hz. The CBF subsequently declined and stabilized at 21.88 ± 0.66 Hz after ∼ 120 s (n = 45 cells, 3 mice; P < 0.05; Figure 2). As expected, this process was reversible: CBF decreased to its initial level when the sHBSS was returned to RT. However, CBF decreased slowly, and a minimum of 6 min was necessary to complete the process (data not shown). We also observed in these experiments that the basal CBF was variable. At RT, CBF values from different cells varied from 8.5 to 18.34 Hz, whereas, at 31 ± 3°C, CBF varied from 12.95 to 34.44 Hz. These experiments confirm the fidelity of our system to measure rapid changes in CBF in small airways of mice. It is also important to note that the basal CBF in small airways is very high in comparison to cultured cells of different species (9).
Figure 2.
Effect of temperature changes on CBF in ciliated cells of small airways. (A) A representative experiment of a lung slice perfused with sHBSS maintained at RT (∼ 20°C) and with a basal CBF of 12.17 Hz. After 25 s of recording, “warm” sHBSS (∼ 31°C) was perfused which increased the CBF to a maximum value of 26.5 Hz before declining and stabilizing after ∼ 120 s at 23.1 Hz. (B) Summary of the increase in CBF from experiments performed as described in (A). CBF is significantly higher at 28–34°C (21.88 ± 0.66 Hz) than at RT (12.45 ± 0.88 Hz; *P < 0.05; n = 45 cells, 3 mice). (Values are mean ± SEM).
ATP Does Not Affect CBF
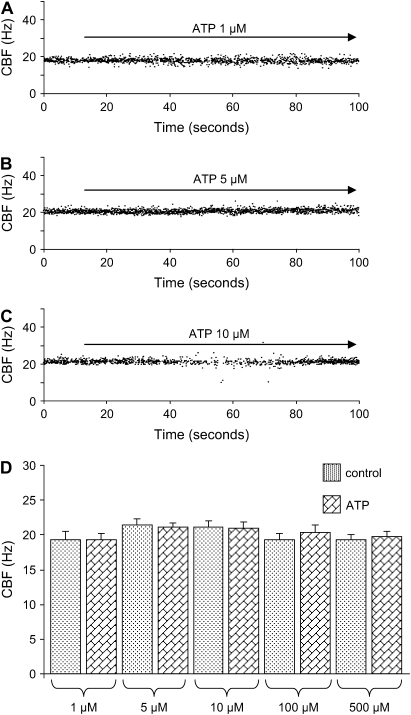
Because many studies of cultured or trachea ciliated cells show that extracellular ATP enhances CBF (6, 9), we examined the effect of ATP on CBF in lung slices. Figures 3A–3C show examples of experiments performed at ∼ 30°C with 1, 5, and 10 μM ATP. Surprisingly, the CBF remained unaltered at any concentration of ATP tested (Figure 3D). By contrast, the SMCs of airways displayed some contraction, an indication that lung slices were sensitive to ATP. Repetitive stimulation with ATP (same or different concentrations) also did not change the CBF. Similar results were obtained at RT, but with a lower CBF. This lack of a response to ATP was not related to the age of the lung slices, because similar results were obtained with freshly isolated slices and slices maintained in medium for up to 3 d. It is also important to note that no differences in the basal CBF were observed between freshly prepared lung slices and slices maintained in medium for several days. We observed that CBF in rat lung slices was also not sensitive to ATP, a result suggesting that this response is not specific to mice (data not shown).
Figure 3.
Effect of ATP on CBF in the small airways of mice. (A_–_C) Representative measurements of CBF during 100 s in 3 cells from different lung slices. Application of ATP (A) 1 μM, (B) 5 μM, and (C) 10 μM did not change CBF (temperature 29.6, 31.2, and 30.6°C, respectively). (D) Summary of CBF measurements in response to ATP 1 μM (19.35 ± 0.77 Hz; n = 44 cells, 3 mice), 5 μM (21.04 ± 0.67 Hz; n = 72 cells, 3 mice), 10 μM (21.02 ± 0.82 Hz; n = 112 cells, 4 mice), 100 μM (20.29 ± 1.08 Hz; n = 48 cells, 3 mice), and 500 μM (19.76 ± 0.71 Hz; n = 48 cells, 3 mice) with respect to the control CBF (1 μM ATP 19.30 ± 1.25 Hz; ATP 5 μM, 21.48 ± 0.84 Hz; ATP 10 μM, 21.05 ± 0.97 Hz; ATP 100 μM, 19.33 ± 0.87 Hz) obtained from the first 10 s of recording in the absence of ATP. The temperature during the experiments was between 29.3 and 34°C. (Values are mean ± SEM.)
With serial slices, we also found no significant difference in the CBF when comparing the distal (∼ 80 μm in diameter: 22.73 ± 0.74 Hz, 10 cells, 3 mice), intermediate (∼ 150–210 μm in diameter: 22.46 ± 0.65 Hz, 10 cells, 3 mice), and proximal airways (∼ 270 μm in diameter: 21.26 ± 0.89 Hz, 10 cells, 3 mice), and that ATP (10 μM) did not change the CBF (distal airways: 23.03 ± 0.73 Hz; intermediate airways: 21.73 ± 0.93 Hz; proximal airways: 21.42 ± 1.16 Hz). Similar results were obtained with 100 μM ATP. These results indicate that the insensitivity of CBF to ATP was not dependent on the airway size or the location within the intrapulmonary tract.
Apyrase, Hexokinase, and Adenosine Deaminase Do Not Affect CBF
Endogenous ATP or adenosine has been detected in airway surface liquid (27–29), and could explain why the basal CBF in small airways is already high and not increased by exogenous ATP. Consequently, apyrase, hexokinase (with 5 mM glucose), and adenosine deaminase (5 U/ml for each) were used separately or together to metabolize and inhibit any effects of endogenous ATP or adenosine. All enzymes failed to slow down the CBF in lung slices. These results indicate that basal CBF does not result from endogenous stimulus. This is consistent with the fact that the lung slices were subject to constant perfusion with sHBSS, which would prevent the accumulation of locally secreted factors to regulate CBF.
ATP Increases Intracellular Calcium in Airway Epithelial Cells
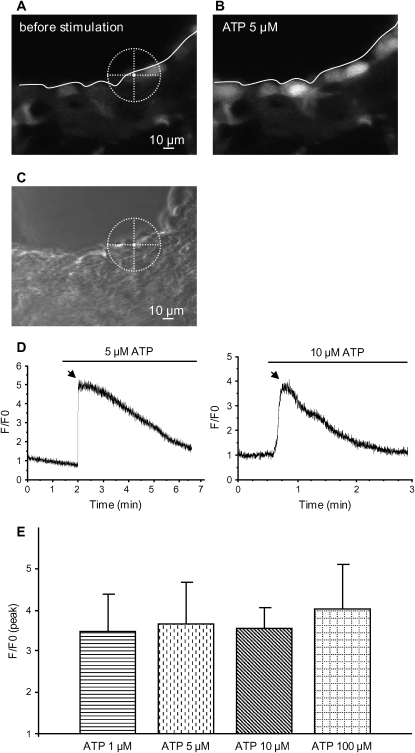
In previously published reports, the main mechanism by which ATP increased CBF was by an increase in [Ca2+]i in epithelial cells (6, 9, 14). Because we observed that CBF in lung slices did not change upon stimulation with ATP, we investigated whether ATP induced changes in [Ca2+]i in small airways. Using confocal microscopy, we found a strong increase in fluorescence (Fluo-3AM or Fluo-4AM) of ciliated epithelial cells in lung slices in response to ATP (1–100 μM; Figure 4). This increase was homogenous within the cells, and no differences between the apical and basal regions of the cell were observed. There were no significant differences between the increases in fluorescence induced by the concentrations of ATP tested (Figure 4E). This suggests that 1 μM ATP induced a maximal increase in fluorescence. However, individual cells displayed variation in the basal and stimulated fluorescence.
Figure 4.
Effect of ATP on Ca2+ signaling in epithelial cells of lung slices. (A and B) Fluorescence images obtained with confocal microscopy and Fluo-3AM. The basal fluorescence was low before addition of ATP (A) and increased with addition of 5 μM ATP (B), indicating an increase in [Ca2+]i. The cross-hair sight indicates the position of the ROI used in this figure. (C) A phase–contrast image showing the localization of ROI on the slice. (D) In the ciliated cell, 5 or 10 μM ATP induced a large and transient increase of fluorescence. Slices were exposed to only one concentration of ATP. The arrow indicates the peak used to compare the maximum levels of fluorescence reached between different experiments. (E) Summary of the increase of the fluorescence ratio (F/F0, peak) after addition of 1, 5, 10, or 100 μM ATP. No significant differences were found between the different concentrations tested. Each concentration was tested at least three times with three different mice.
Ionomycin Increase of [Ca2+]i but Not CBF
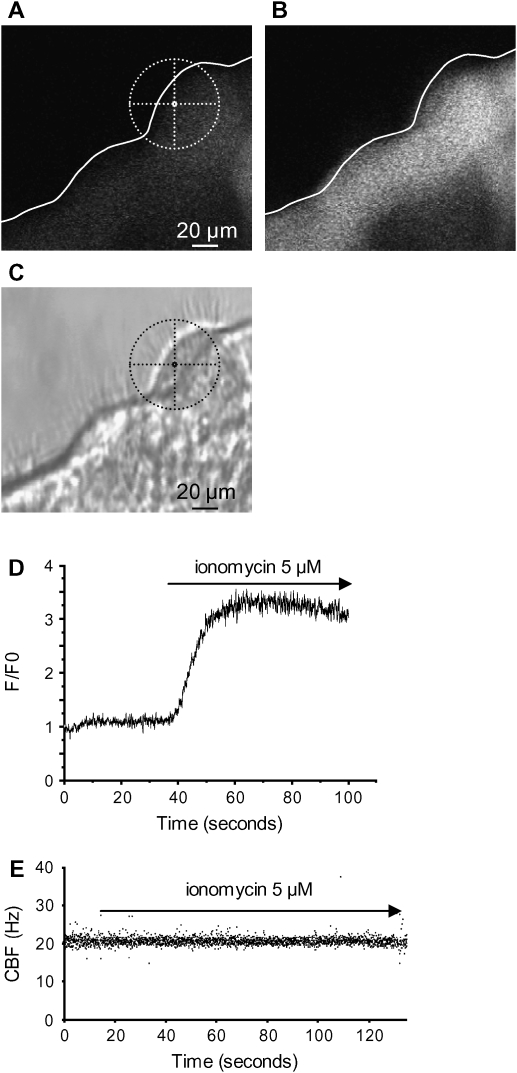
To determine if the lack of a CBF response, despite the increase of [Ca2+]i, was specific to ATP, we investigated the effect of a nonspecific increase in [Ca2+]i induced by the Ca2+ ionophore, ionomycin (8, 15). As expected, ionomycin induced large increases in fluorescence in the ciliated cells (Figures 5A–5C). This increase was homogenous throughout the cell, and was sustained for at least 1 min or more (Figure 5C). However, ionomycin had no effect on CBF: CBF was 19.17 ± 0.66 Hz before and 19.95 ± 0.79 Hz or 19.41 ± 0.63 Hz after treatment with 5 or 10 μM ionomycin (112 cells, 4 mice; Figure 5D). Similar to the effect of ATP, the SMCs displayed some contraction in response to ionomycin.
Figure 5.
Effect of ionomycin on the [Ca2+]i and CBF of ciliated cells in lung slices. (A) A fluorescence and (C) phase–contrast image showing the basal fluorescence of Fluo-3AM in the epithelial cells, and the position of a selected ROI in a ciliated cell. (B) The increase in fluorescence in the epithelial cells after addition of 5 μM ionomycin. The cross-hair sights indicates the position of the ROI used in this figure. (D) Ionomycin (5 μM) increased the fluorescence ratio (F/F0) in the selected ROI. (E) Representative data showing no change in CBF in a lung slice after addition of 5 μM ionomycin (n = 112 cells, 4 mice).
UTP, Forskolin, and Isoproterenol Did Not Increase CBF
The lack of a response of CBF to ATP in the small airways suggests that the cilia were already at maximal CBF and could increased no further. To test this hypothesis, mouse lung slices were exposed to other purinergic agonists and β-adrenergic agonists. UTP, forskolin, and isoproterenol (10–100 μM) all failed to increase CBF.
Extracellular ATP Increases CBF in Tracheal Rings
As described previously here, many other studies have shown that ATP increases CBF in tracheal cells. This raises the possibility that the regulation of CBF in the very large airways (cartilaginous bronchi or trachea) is different. We found that 5 μM (n = 64 cells, 5 mice), 10 μM (n = 48 cells, 4 mice), and 20 μM (n = 51 cells, 4 mice) ATP increased CBF in mouse tracheal rings to a similar extent (increase of ∼ 45%; control: 20.19 ± 0.84 Hz; n = 102 cells, 5 mice; Figure 6). Lower concentrations of ATP (1 μM) did not enhance CBF (n = 63 cells, 4 mice). Repetitive stimulation with ATP (same or different concentrations) induced the same increase in CBF.
Figure 6.
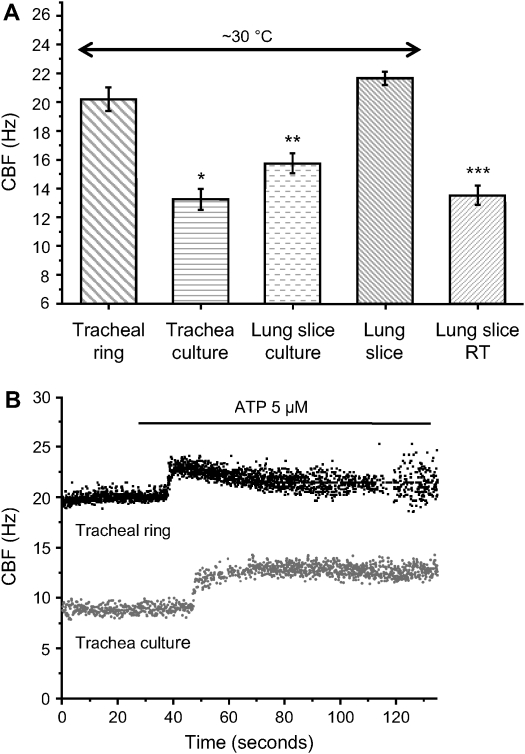
A comparison of the basal CBF and the effects of ATP on CBF in noncultured and cultured lung slices or tracheal rings. (A) The basal CBF of tracheal rings (20.19 ± 0.84 Hz; n = 102 cells, 5 mice) and lung slices (21.65 ± 0.47 Hz; n = 112 cells, 5 mice) are similar, whereas both are significantly higher than the basal CBF of cultured outgrowths from tracheal rings (*13.23 ± 0.72 Hz; n = 56 cells, 6 mice; P < 0.05) or lung slices (**15.73 ± 0.70 Hz; n = 112 cells, 7 mice; P < 0.05). CBF measured at RT in lung slices is significantly lower (***13.52 ± 0.70 Hz; n = 188 cells, 5 mice; P < 0.05) than CBF measured at ∼ 30°C. (B) ATP (5 μM) induced a similar increase in CBF in both tracheal rings (45 ± 3%; n = 64 cells, 5 mice) and tracheal cultures (51 ± 5%; n = 22 cells, 6 mice). However, CBF decreased gradually in tracheal rings to reach the prestimulatory basal rate after ∼ 2 min, while CBF was sustained in tracheal cultures. (Values are mean ± SEM.)
ATP Increases CBF in Cultures of Trachea Cells but Not in Cultures of Lung Slices
A possible explanation for the differences in basal CBF and the responses in CBF and [Ca2+]i to ATP of airway epithelial cells is that the culturing process has altered the cell properties. To investigate this idea, tracheal rings and lung slices were plated onto collagen-coated coverslips and cultured for 5–10 d. After ∼ 5 d, outgrowths of ciliated cells from the tracheal rings and lung slices were observed. These cultured cells were maintained for a further 5 d, and no differences in CBF were observed with the age of the outgrowths of ciliated cells.
In view of the idea that small airway cilia may be beating at a maximal frequency, it is important to note that the basal CBF of cultured cells in outgrowths from tracheal rings (13.23 ± 0.72 Hz; n = 56 cells, 6 mice) and lung slices (15.73 ± 0.70 Hz; n = 112 cells, 7 mice) was significantly lower in comparison with the CBF of uncultured tracheal rings (20.19 ± 0.84 Hz; n = 102 cells, 5 mice) and lung slices (21.65 ± 0.47 Hz; n = 112 cells, 5 mice; P < 0.05; Figure 6A).
Similar to freshly prepared tracheal rings, the outgrowths of tracheal ciliated cells displayed a sustained increase in CBF in response to ATP (51 ± 5%, 5 μM ATP; Figure 6B). By contrast, but in keeping with the response of the uncultured lung slice, no change in CBF was observed in response to ATP in outgrowths of cultured cells from lung slices, even after 10 d of culture. However, ATP induced similar increases in [Ca2+]i in both cultured cells of tracheal rings and lung slices.
DISCUSSION
The observation that CBF can be increased in cultured or isolated ciliated cells from the large airways by a variety of stimuli has led to the hypothesis that mucociliary clearance is locally regulated throughout the airways. A major obstacle to the exploration of this hypothesis has been the incompatibility of the cilia of the smaller airways to microscopic analysis. To overcome this problem, we refined the preparation of mouse lung slices to gain access to the ciliated epithelium of the small airways. The lung slice preparation has several advantages, the most important of which is that it retains much of the in vivo organization of the lung (21). The activity of the ciliated cells can be studied without exposure to proteolytic enzymes, the disruption of cell contacts, or the influence of cell culture. In addition, it is relatively easy to prepare and maintain serial lung slices that allow the study of different parts of the airway.
To measure ciliary activity, we adapted our previously proven technique of high-speed digital recording (9) by upgrading to DIC microscopy. However, to ensure that we accurately measured CBF in lung slices, we verified the fidelity of our technique by quantifying changes in CBF in response to temperature. With these calibration experiments, we confirmed the reliably of our method to measure rapid changes in CBF from one beat cycle to the next for extended periods (9). We emphasize this confidence in the quantification of CBF to underscore the fact that, in many experimental conditions, no changes in CBF occurred.
CBF in isolated and cultured tracheal ciliated cells has been frequently found to be increased through increases in [Ca2+]i induced by ATP (6, 9, 11). By contrast, we consistently found, irrespective of concentration, that ATP had no effect on CBF in the intrapulmonary airways of mice. A prior stimulation of the ciliated cells within the airways by ATP or adenosine secreted into the airway surface liquid (27–29) might explain why CBF cannot be further increased in lung slices. However, apyrase, hexokinase, or adenosine deaminase, enzymes that metabolize ATP and adenosine, failed to slow CBF. In addition, in contrast to the air-filled lumen of the in vivo airway, the lumen of the airway of the lung slice is filled with fluid. This experimental condition, together with the constant perfusion of the preparation, would quickly dilute and remove any endogenous factors. Consequently, these results suggest that the CBF was inherently high and independent of secreted ATP or related compounds.
However, a maximal increase in [Ca2+]i was induced by low concentrations of ATP. Although unexpected, the implication of these responses is that the cilia of small airways are insensitive to increases of [Ca2+]i. Support for this hypothesis is provided by the finding that, although ionomycin increased [Ca2+]i, it did not increase CBF. Similarly, ciliated cells in outgrowths cultured from the small airways of slices also did not show changes in CBF in response to ATP. The failure of increased [Ca2+]i to increase CBF also emphasizes that other endogenous factors, such as zinc, that act in conjunction with ATP to increase Ca2+ influx (30, 31), are unlikely to be responsible for the inherent high CBF. This insensitivity of CBF to regulation by ATP and [Ca2+]i was not specific to mice, because we found similar results with rat lung slices. Another study, by Hayashi and colleagues (32), performed with thicker rat lung slices (5–6 mm × 5–6 mm blocks) and lower CBF (9.2 ± 0.2 Hz), showed that ATP also failed to increase CBF in rat airways, whereas a significant increase could be observed in tracheal rings exposed to ATP. In contrast to our results, they found that ATP could not increase intracellular Ca2+ in rat lung slices.
This insensitivity of CBF to ATP and [Ca2+]i appears to be a property of the ciliated cells lining the intrapulmonary airways of mice, because we found, in serial slices cut across the lung lobe, that CBF was also not stimulated in the distal or proximal airways. However, ATP increased CBF in both mouse tracheal rings and cultured ciliated cells from trachea rings. The important implication of these results is that the sensitivity of CBF to Ca2+ is related to the location of the cell in the respiratory tract, and that this characteristic is retained during culture. The possibility that this insensitivity to Ca2+ is related to the preparation of the lung slice is inconsistent with the numerous markers, including the morphological appearance, the Ca2+ response to ATP, and the ability of airways to contract and display dynamic Ca2+ signaling (21, 22), that indicate that the lung slice is representative of the in situ condition. From these results, it would appear that the ciliated cells acquire sensitivity to Ca2+ in the very large airways (cartilaginous bronchi or trachea).
A second important observation that implies an autonomous CBF is that the CBF in small airways was always high in comparison to the basal CBF of cultured cells in both this study and studies using different species (13 ± 0.3 Hz in rabbit tracheal epithelial cells [9]). In this study, we show that CBF of both the small airway and trachea cells is slowed down by being placed in culture. The reason for this slowed CBF is unknown, but it provides (at least in tracheal cells) the potential for subsequent increases in CBF (to approach the maximal rate of CBF) in response to agonists. Therefore, we propose that the insensitivity of cilia to ATP in lung slices is a result of CBF already operating at a maximum (at the optimal temperature). Our findings that other purinergic or β-adrenergic agonists also failed to increase CBF in small airways is consistent with this hypothesis. By contrast, increases in airway mucoclearance in response to purinergic agonists or UTP in patients with primary ciliary dyskinesia have been reported (33). However, in view of the inherent ciliary dysfunction, it is likely that these increases in mucus transport result from changes of other components of the mucociliary interface rather than being the result of a direct effect on the cilia. A common hypothesis for increased mucociliary clearance in cystic fibrosis, where ciliary activity is believed not to be fundamentally different, is the rehydration of mucus and/or increases in the depth of airway surface liquid that would allow normal ciliary activity to resume. Changes in the tonicity, or other modifications of the microenvironment of ciliated cells, could be a potential way to regulate CBF, but in view of our data, this effect would have to be mediated via a pathway independent of Ca2+. Further studies will be required to investigate this potential regulation of CBF.
Under conditions of maximal CBF, it would be predicted that inhaled particles, irrespective of size, would be quickly removed. This autonomous activity in the intrapulmonary airways of mice would seem to have an advantage over a local regulatory system that would have to be able to detect very small particles, such as bacteria, if a hazardous accumulation of material is to be avoided. Because of the convergence of the airway surface area, mucociliary loads increase toward the oropharynx. It is possible that, in the larger airways, local regulation of CBF, perhaps via mechanical stimulation (34), becomes more important and necessary to cope with the increased load. Although we found that rat intrapulmonary airways also displayed an autonomous CBF, the correlation of local regulation of CBF within the larger airways emphasizes caution when extrapolating these studies to larger animals, including humans.
In summary, we demonstrate that a variety of agonists or increases in [Ca2+]i have no effect on CBF in small airways. We conclude that CBF is differentially regulated in the upper airways, and that culturing epithelial cells can modify CBF regulation. Finally, we propose that maximal CBF is maintained in vivo in small airways to sustain optimal mucociliary clearance.
This work was supported by National Institutes of Health grant HL70882 (M.J.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0417OC on February 16, 2006
Conflict of Interest Statement: Neither of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Joki S, Toskala E, Saano V, Nuutinen J. Correlation between ciliary beat frequency and the structure of ciliated epithelia in pathologic human nasal mucosa. Laryngoscope 1998;108:426–430. [DOI] [PubMed] [Google Scholar]
- 2.Lucas A, Douglas L. Principles underlying ciliary activity in the respiratory tract. Acta Otolaryngol 1934;20:518. [Google Scholar]
- 3.Toskala E, Nuutinen J, Rautiainen M, Torkkeli T. The correlation of mucociliary transport and scanning electron microscopy of nasal mucosa. Acta Otolaryngol 1995;115:61–65. [DOI] [PubMed] [Google Scholar]
- 4.Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. Temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L454–L459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res 1980;208:65–84. [DOI] [PubMed] [Google Scholar]
- 6.Korngreen A, Priel Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J Physiol 1996;497:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyatt TA, Forget MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol 2005;288:L546–L551. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Sanderson MJ. The role of cGMP in the regulation of rabbit airway ciliary beat frequency. J Physiol 2003;551:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol 2003;546:733–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 2002;538:633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol 2001;280:C1485–C1497. [DOI] [PubMed] [Google Scholar]
- 12.Ma W, Korngreen A, Uzlaner N, Priel Z, Silberberg SD. Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature 1999;400:894–897. [DOI] [PubMed] [Google Scholar]
- 13.Lansley AB, Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+. Biophys J 1999;77:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertsberg I, Hellman V, Fainshtein M, Weil S, Silberberg SD, Danilenko M, Priel Z. Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway. J Gen Physiol 2004;124:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol 1999;516:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Han D, Sanderson MJ. Effect of isoproterenol on the regulation of rabbit airway ciliary beat frequency measured with high-speed digital and fluorescence microscopy. Ann Otol Rhinol Laryngol 2005;114:399–403. [DOI] [PubMed] [Google Scholar]
- 17.Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J Aerosol Med 2004;17:107–115. [DOI] [PubMed] [Google Scholar]
- 18.Frohock JI, Wijkstrom-Frei C, Salathe M. Effects of albuterol enantiomers on ciliary beat frequency in ovine tracheal epithelial cells. J Appl Physiol 2002;92:2396–2402. [DOI] [PubMed] [Google Scholar]
- 19.Salathe M, Bookman RJ. Coupling of [Ca2+]i and ciliary beating in cultured tracheal epithelial cells. J Cell Sci 1995;108:431–440. [DOI] [PubMed] [Google Scholar]
- 20.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003;211:103–111. [DOI] [PubMed] [Google Scholar]
- 21.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 2005;125:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol 2002;283:L1271–L1279. [DOI] [PubMed] [Google Scholar]
- 23.Dirksen ER, Felix JA, Sanderson MJ. Preparation of explant and organ cultures and single cells from airway epithelium. Methods Cell Biol 1995;47:65–74. [DOI] [PubMed] [Google Scholar]
- 24.Morales B, Barrera N, Uribe P, Mora C, Villalon M. Functional cross talk after activation of P2 and P1 receptors in oviductal ciliated cells. Am J Physiol Cell Physiol 2000;279:C658–C669. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson MJ, Parker I. Video-rate confocal microscopy. Methods Enzymol 2003;360:447–481. [DOI] [PubMed] [Google Scholar]
- 26.Sanderson MJ. Acquisition of multiple real-time images for laser scanning microscopy. Microscopy and Analysis 2004;18:17–23. [Google Scholar]
- 27.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 2004;279:36855–36864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 2003;64:785–795. [DOI] [PubMed] [Google Scholar]
- 30.Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets: a review. Pharmacol Ther 2005;105:127–149. [DOI] [PubMed] [Google Scholar]
- 31.Zsembery A, Fortenberry JA, Liang L, Bebok Z, Tucker TA, Boyce AT, Braunstein GM, Welty E, Bell PD, Sorscher EJ, et al. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem 2004;279:10720–10729. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Kawakami M, Sasaki S, Katsumata T, Mori H, Yoshida H, Nakahari T. ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium. Exp Physiol 2005;90:535–544. [DOI] [PubMed] [Google Scholar]
- 33.Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, Boucher RC, Knowles MR. Effect of aerosolized uridine-5′-triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. Am J Respir Crit Care Med 1999;160:144–149. [DOI] [PubMed] [Google Scholar]
- 34.Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 2005;168:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]