Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells (original) (raw)
Abstract
S-nitrosylation, the selective modification of cysteine residues in proteins to form _S_-nitrosocysteine, is a major emerging mechanism by which nitric oxide acts as a signaling molecule. Even though nitric oxide is intimately involved in the regulation of vascular smooth muscle cell functions, the potential protein targets for nitric oxide modification as well as structural features that underlie the specificity of protein _S_-nitrosocysteine formation in these cells remain unknown. Therefore, we used a proteomic approach using selective peptide capturing and site-specific adduct mapping to identify the targets of S-nitrosylation in human aortic smooth muscle cells upon exposure to _S_-nitrosocysteine and propylamine propylamine NONOate. This strategy identified 20 unique _S_-nitrosocysteine-containing peptides belonging to 18 proteins including cytoskeletal proteins, chaperones, proteins of the translational machinery, vesicular transport, and signaling. Sequence analysis of the _S_-nitrosocysteine-containing peptides revealed the presence of acid/base motifs, as well as hydrophobic motifs surrounding the identified cysteine residues. High-resolution immunogold electron microscopy supported the cellular localization of several of these proteins. Interestingly, seven of the 18 proteins identified are localized within the ER/Golgi complex, suggesting a role for S-nitrosylation in membrane trafficking and ER stress response in vascular smooth muscle.
Keywords: nitric oxide, proteomics, _S_-nitrosothiols
S-nitrosylation, the formal transfer of nitrosonium to a reduced cysteine, is a reversible and selective posttranslational modification that regulates protein activity, localization, and stability, and also functions as a general sensor for cellular redox balance (1–7). The formation of protein _S_-nitrosocysteine requires the removal of a single electron, i.e., the conversion of the nitrogen in nitric oxide from an oxidation state of 2 to 3. Several distinct pathways could satisfy the formation of protein _S_-nitrosocysteine adducts in biological systems, such as autooxidation of nitric oxide forming higher oxides of nitrogen, radical recombination of thiyl radical with nitric oxide, catalysis by metal centers, the direct reaction of nitric oxide with a reduced cysteine followed by electron abstraction, and transnitrosation reactions carried out by _S_-nitrosoglutathione, other small molecular mass _S_-nitrosothiols, and more recently, _S_-nitrosocysteine-containing proteins (8–10).
In vascular smooth muscle cells, nitric oxide derived from endothelium regulates important biological functions beyond relaxation, such as phenotypic changes, proliferation, and commitment to undergo apoptosis (11, 12). Previous studies have shown that the molecular mechanisms underlying the functions of nitric oxide in vascular smooth muscle are mediated by both soluble guanylate cyclase-dependent and independent mechanisms (12–14). It has been suggested that selective S-nitrosylation of protein targets are responsible for the guanylate cyclase-independent regulation of vascular smooth muscle cell biology (13). Despite these critical roles for nitric oxide, the targets of S-nitrosylation in vascular smooth muscle cells are largely unknown. To that end, proteomic approaches are highly informative in providing a global assessment of the modified proteins in cells and tissues.
Proteomic approaches based on the biotin-switch method have been used to identify potential targets of S-nitrosylation in various model systems including murine brain tissue (15) and RAW 264.7 cells (16), Mycobacterium tuberculosis (17), mouse mesangial cells (18), and human aortic endothelial cells (19, 20), yet the structural features that subserve the specificity of S-nitrosylation remain contentious. Recently, a peptide capture approach simultaneously identified 68 unique _S_-nitrosocysteine residues belonging to 56 proteins from _S_-nitrosoglutathione-treated rat cerebellar lysates (21). Analysis of the identified peptides by a machine learning approach did not reveal linear sequence motifs under the experimental conditions used (21). However, subsequent inspection of the identified peptides indicated a prevalence for an acid/base motif, suggesting that exploration of additional _S_-nitrosocysteine proteomes may further clarify the structural motifs that underlie the specificity of S-nitrosylation.
To this end, in intact human aortic smooth muscle cells (HASMC) exposed to _S_-nitrosocysteine (CysNO) or propylamine propylamine NONOate (PAPANO), we identified potential targets of S-nitrosylation and evaluated S-nitrosylation motifs under conditions that preserve the cellular localization of proteins as well as endogenous protein–protein interactions. Using a proteomic approach that selectively identified the modified _S_-nitrosocysteine residues, 18 proteins were identified. The localization of several of these proteins was further supported by high-resolution immunogold electron microscopy. Primary sequence analysis of the _S_-nitrosocysteine-containing peptides revealed the presence of acid/base motifs as well as the occurrence of cysteine residues within hydrophobic pockets.
Results and Discussion
Formation of _S_-Nitrosocysteine Protein Adducts in Human Aortic Smooth Muscle Cells.
The intracellular protein _S_-nitrosocysteine content was evaluated by reductive chemistries coupled with chemiluminescence detection (22). Naïve HASMC in culture had levels of protein _S_-nitrosocysteine below the lower limits of detection, and Western blot analysis failed to document expression of nitric oxide synthases in these cells (not shown). Therefore, to generate endogenous S-nitrosylated proteins, intact cells were exposed to either PAPANO, a nitric oxide donor with defined release kinetics, or CysNO, an effective transnitrosating agent. Exposure of HASMC to 100 μM CysNO for 20 min generated 3.0 ± 0.3 nmol of protein _S_-nitrosocysteine per mg of protein, whereas exposure to 2 mM PAPANO for 1 h generated 0.40 ± 0.03 nmol of protein _S_-nitrosocysteine per mg of protein (mean ± SD, n = 4). These two conditions were used to explore the _S_-nitrosoproteome of HASMC. The difference in the yield of protein _S_-nitrosocysteine between CysNO and the nitric oxide donor treatment may reflect the higher efficiency of S-nitrosylation by CysNO consistent with previous results (23). Cell culture studies have shown that exogenous CysNO is effectively transported intracellularly via the amino acid transporter system transporter system (23). Consequently, intracellular CysNO may facilitate the formation of protein _S_-nitrosocysteine adducts primarily by replenishment of endogenous _S_-nitrosoglutathione or by direct transnitrosation. In contrast, nitric oxide could be consumed by other cellular targets such as soluble guanylate cyclase and thus a smaller fraction may participate in S-nitrosative chemistries. Therefore, the treatment of HASMC with either CysNO or PAPANO, followed by site-specific proteomic analysis of protein _S_-nitrosocysteine formation, allowed us to evaluate the potential selectivity of S-nitrosylation.
Evaluation of MS/MS Sequence-to-Spectrum Assignments.
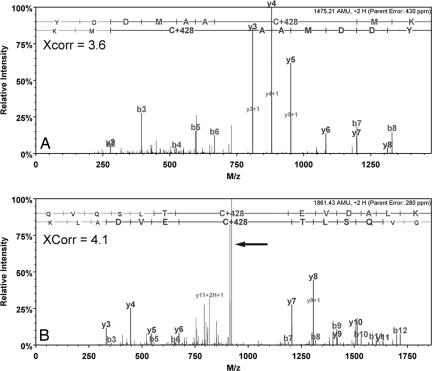
Because of the selectivity of _S_-nitrosocysteine modification and the peptide enrichment strategy used, rigorous selection criteria, as described in Fig. 1, were used to identify peptides from the tandem MS (MS/MS) analysis. Typical MS/MS spectra that either met or failed these criteria are depicted in Fig. 1. Importantly, the mass shift due to the Cys–_N_-[6(biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide (HPDP–biotin) adduct (+428) was present in either the _y_- or _b_-ion series for accepted peptide assignments. Although these selection criteria would minimize false peptide identifications resulting from MS/MS sequence-to-spectrum assignments, they would not prevent false positive peptide assignments arising from biotin–HPDP labeling of cysteine residues that were not completely blocked by methyl methanethiosulfonate. As a control for this nonspecific labeling, ascorbate was omitted to largely prevent reduction of _S_-nitrosocysteine. Although ascorbate-independent biotin-HPDP labeling of _S_-nitrosocysteine is possible, naïve and cysteine-treated HASMC did not contain significant levels of endogenous _S_-nitrosoproteins quantified by reductive chemistries coupled to chemiluminescence detection. Therefore, omission of ascorbate from these conditions served as an appropriate false positive control. MS/MS sequence-to-spectrum assignments from these treatments were evaluated by the same criteria as described in Fig. 1 and were used to eliminate peptide identifications if they were also identified in the NO-treated samples. A total of 18 peptides belonging to 16 proteins were identified as possible false positives (Table 2, which is published as supporting information on the PNAS web site), and therefore, were not considered targets of S-nitrosylation under our experimental conditions.
Fig. 1.
Evaluation of sequest peptide assignments. (A) An MS/MS spectrum (XCorr 3.6) assigned to an _S_-nitrosocysteine-containing peptide from 14-3-3 protein ζ that met all selection criteria and was accepted. (B) An MS/MS spectrum (Xcorr 4.1) assigned to a peptide from vimentin. Although this assignment passed the initial selection criteria, it was ultimately rejected because the top three most intense fragment peaks were not assigned (arrow). The evaluation of sequest peptide assignments was assessed by multiple selection criteria as follows: (i) Only peptide assignments that identified a biotin-HPDP derivitized cysteine (+428) included in the _y_- or _b_-ion series were considered. (ii) Each experimental condition was performed in quadruplicate, with peptide assignments evaluated if they appeared in at least three of the four independent replicates. (iii) Peptide assignments that passed these two selection filters were then evaluated by output scores assigned by sequest and were rejected if they did not meet specific threshold values as described in the Materials and Methods. (iv) If peptide assignments passed this scoring filter, the corresponding MS/MS spectra were manually reviewed. For an assignment to be accepted the MS/MS spectrum must have a continuous _b_- or _y_-ion series of at least five residues and the three most intense fragment peaks assigned to either an _a_-, _b_-, or _y_-ion, to an _a_-, _b_-, or _y_-ion resulting from a neutral loss of water or ammonia, or to a multiply protonated fragment ion. All review of peptide assignments and manual interpretation of MS/MS spectra were facilitated by scaffold, a proteome software package.
Identification of Proteins and Sites of S-Nitrosylation by LC-MS/MS.
Employing selective peptide capture followed by LC-MS/MS analysis, 18 _S_-nitrosocysteine-containing peptides belonging to 16 proteins were identified in HASMC exposed to CysNO (Table 1). The identification of S-nitrosylated proteins with diverse molecular weights and cellular roles such as cytoskeletal proteins, chaperones, proteins of the translational machinery, calcium-binding proteins, and an ion channel protein supported the robustness of this technique. From the 16 proteins identified as potential targets of S-nitrosylation, 14-3-3 protein θ, 14-3-3 protein ζ, annexin A2, elongation factor 2, and elongation factor 1 A-1 had been identified by the biotin switch method in various other systems (17–19, 24). In addition, Cys-137 of RAB3B has been proposed as susceptible to S-nitrosylation based on a conserved NKCD motif (25). Because these experiments identified _S_-nitrosocysteine at residue 184, further work will be necessary to examine the site-specificity of S-nitrosylation in RAB3B. Additionally, four _S_-nitrosocysteine-containing peptides belonging to four proteins were identified after exposure to a nitric oxide donor (Table 1). Two of the proteins, 14-3-3 ζ and GRP75, were also identified as S-nitrosylated at the same residue after CysNO treatment, whereas microtubule-associated protein 4 and myoneurin were exclusive to PAPANO-treated HASMC.
Table 1.
HASMC _S_-nitrosoproteome
| Biological function, protein name | Uniprot accession no. | Sequence† | Residue‡ | Z§ | XCorr¶ |
|---|---|---|---|---|---|
| Cell growth and maintenance | |||||
| Myosin heavy chain 9 | P35579 | KQELEEIC*HDLEAR | 916 | 3 | 4.3 |
| LQLQEQLQAETELC*AEAEELR | 895 | 3 | 5.8 | ||
| VEDMAELTC*LNEASVLHNLK | 90 | 3 | 3.7 | ||
| Vinculin | Q5SWX2 | VENAC*TK | 85 | 2 | 2.8 |
| Microtubule-associated protein 4‖ | P27816 | C*SLPAEEDSVLEK | 635 | 3 | 3.9 |
| Signal transduction | |||||
| 14-3-3 protein ζ | P63104 | YDDMAAC*MK | 25 | 2 | 3.6 |
| 14-3-3 protein ζ‖ | P63104 | YDDMAAC*MK | 25 | 2 | 3.7 |
| 14-3-3 protein θ | P27348 | YDDMATC*MK | 25 | 2 | 3.5 |
| Annexin A2 | Q567R4 | GLGTDEDSLIEIIC*SR | 133 | 3 | 4.2 |
| Annexin A11 | P50995 | GVGTDEAC*LIEILASR | 294 | 3 | 3.5 |
| VAV-like protein | Q6TPQ2 | C*RSLSQGMELSC#PGSR | 33 | 3 | 3.0 |
| Protein metabolism | |||||
| Elongation factor 2 | P13639 | DLEEDHAC*IPIK | 567 | 3 | 2.9 |
| Elongation factor 1 A-1 | P68104 | DGNASGTTLLEALDC*ILPPTR | 234 | 3 | 4.1 |
| Eukaryotic initiation factor 5AII | Q9GZV4 | YEDIC*PSTHNMDVPNIK | 73 | 3 | 4.0 |
| T-complex protein 1, ζ subunit | P40227 | NAIDDGC*VVPGAGAVEVAMAEALIK | 405 | 3 | 5.0 |
| Cyclophilin B | P23284 | DVIIADC*GK | 194 | 2 | 3.1 |
| GRP75 | P38646 | VC*QGER | 487 | 2 | 2.4 |
| GRP75‖ | P38646 | VC*QGER | 487 | 2 | 2.3 |
| Transport | |||||
| COP-A | Q8IXZ9 | AWEVDTC*R | 245 | 2 | 2.5 |
| Ras-associated protein 3B | P20337 | LVDAIC*DK | 184 | 2 | 3.1 |
| Chloride intracellular channel 4 | Q9Y696 | DEFTNTC*PSDK | 234 | 2 | 3.5 |
| Nucleic acid metabolism | |||||
| Myoneurin‖ | Q8WX93 | VSSC*EQR | 740 | 2 | 2.7 |
The ability of this method to identify S-nitrosylated proteins from as little as 0.4 nmol of _S_-nitrosocysteine per mg of protein is an improvement in sensitivity and, hence, proteome coverage over the traditional biotin-switch approach. For example, exposure of RAW 264.7 cells to 250 μΜ CysNO generated ≈5.5 nmol of _S_-nitrosocysteine per mg of protein from which the standard biotin-switch assay identified three S-nitrosylated proteins (24). This increase in sensitivity was likely due to the enrichment of _S_-nitrosocysteine-containing peptides and subsequent MS/MS analysis using electrospray ionization and linear ion trap detection. Critically, the increase in sensitivity did not sacrifice selectivity, as nearly 90% (43 of 49) of the unique peptides that passed the selection criteria contained a Cys–HPDP–biotin adduct. The capture of six nonspecific peptides lacking a biotinylated adduct was likely due to the harsher elution conditions required to denature avidin and release the biotinylated peptides. Overall, the selectivity and increased sensitivity of this method, as well as the ability to identify both the modified proteins and the sites of S-nitrosylation in a single experiment represent a significant advantage for elucidating the _S_-nitrosoproteome in complex biological mixtures.
Evaluation of S-Nitrosylation in HASMC by Immunogold Electron Microscopy.
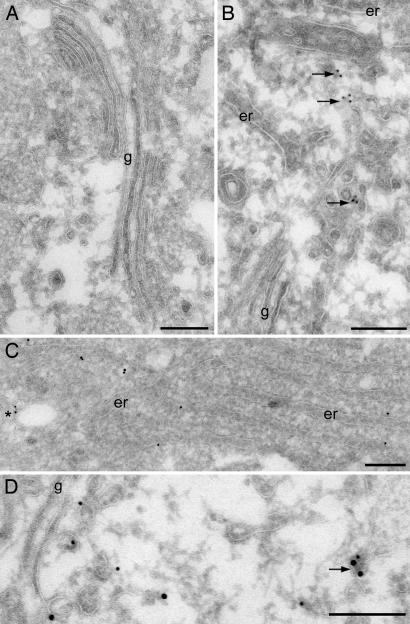
The cellular distribution of protein _S_-nitrosocysteine was explored by high-resolution electron microscopy and immunogold labeling using monoclonal and polyclonal anti-_S_-nitrosocysteine antibodies. After treatment of HASMC with 100 μM CysNO, significant immunoreactivity for protein _S_-nitrosocysteine was observed in distinct cellular compartments such as the endoplasmic reticulum membrane and vesicular membrane structures near the Golgi complex (Fig. 2B and C), consistent with the proposed subcellular localizations of several of the identified proteins in Table 1. Treatment with _para_-hydroxymercuricbenzoate, which displaces _S_-nitrosocysteine, significantly abolished _S_-nitrosocysteine immunoreactivity (Fig. 2A). Of particular interest was the immunogold labeling located in close vicinity to the Golgi complex, which was largely associated with membranes of the endoplasmic reticulum and on vesicular membrane profiles near the Golgi (Fig. 2 B and C). Based on the proteomic data and the specific location of these vesicles at lateral rims and _cis_-Golgi facing ER exit sites, these membranes could represent COP-I-coated vesicles. Immunogold double labeling against _S_-nitrosocysteine and COP-I was performed, revealing low but distinct labeling on ER membranes (Fig. 2D) as well as occasional localization on vesicular membranes of the Golgi complex. (Fig. 2D, arrow). Recent studies have suggested that, besides the COP-I vesicle coat, proteins of the 14-3-3 family also recognize arginine-based ER localization signals on multimeric membrane proteins (26). Because this proteomic study identified COP-A, 14-3-3 ζ, RAB3B, cyclophilin B, and chloride intracellular channel protein, which have proposed roles in ER/Golgi transport and ER protein folding, this finding suggested a regulatory role for S-nitrosylation in these cellular processes. Interestingly, recent studies have revealed a role for S-nitrosylation in the regulation of vesicular trafficking in endothelial and epithelial cells (4, 7), platelets (5), and neurons (6). In addition, nitric oxide has been identified as a proximal mediator of ER stress responses, although the role of S-nitrosylation was not evaluated (27).
Fig. 2.
High-resolution immunoelectron microscopy. HASMC exposed to 100 μM CysNO for 20 min were fixed and processed for EM. Immunoreactivity for _S_-nitrosocysteine-containing proteins was visualized by 10-nm protein A gold particles. COP-1 immunoreactivity was visualized by 15-nm protein A gold particles. (A) Sections were treated with _para_-hydroxymercuricbenzoate to displace the _S_-nitrosocysteine adducts and then stained with monoclonal anti-_S_-nitrosocysteine antibody (26). (B) _S_-nitrosocysteine immunoreactivity (monoclonal antibody) was associated with endoplasmic reticulum (er) and small vesicular structures (arrows) in the vicinity of the Golgi complex (g). (C) A similar pattern of staining obtained with a polyclonal anti-_S_-nitrosocysteine antibody (asterisk indicates labeling of small vesicle). (D) Double labeling for _S_-nitrosocysteine (10-nm gold) and COP-1 (15-nm gold) showed localization on vesicular membrane profiles (arrow). (Scale bar, 200 nm.)
Sequence Analysis of _S_-Nitrosocysteine-Containing Peptides.
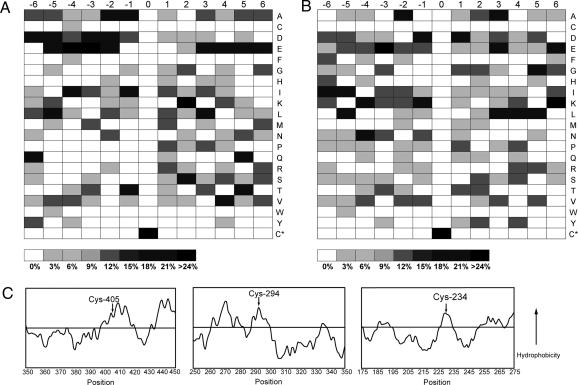
The site-specific mapping of _S_-nitrosocysteine residues allowed direct comparison of primary peptide sequences for motifs that may govern S-nitrosylation specificity. It has been proposed that there is a predisposition toward flanking basic (Lys, Arg, His) and acidic (Asp, Glu) residues, and if positioned within 6 Å of the modified cysteine, these residues could regulate S-nitrosylation and denitrosation by altering thiol nucleophilicity (1). Sequence alignment of the 18 S-nitrosylated peptides identified from CysNO-treated smooth muscle cells revealed that the highest occurrence of acidic (D, E) residues was ≈50% and 40%, at positions −3 and −4, respectively, relative to the modified cysteine. The highest occurrence of basic (K, R, H) residues was ≈30% at position 2 (Fig. 3A). Interestingly, there were no basic residues in position −3 and −4, and acidic residues at position 2 only occurred at a 10% frequency. Given the relatively small number of peptides being compared, the differences observed may result by chance; therefore, the same analysis was performed for the 18 false-positive peptide identifications (Fig. 3B). These peptides were excluded because they were not thought to contain _S_-nitrosocysteine, and therefore, they served as an appropriate peptide population for comparison. Sequence alignment of these 18 sequences revealed that at positions −3 and −4 acidic residues occurred at lower frequency, 34% and 17%, compared to 50% and 40% for the _S_-nitrosocysteine-containing peptides, respectively. Similarly, the frequency of basic residues at position 2 dropped to 6% compared to the _S_-nitrosocysteine-containing peptides (30%). Given the strong trend for flanking acidic/basic residues revealed by alignment of _S_-nitrosocysteine-containing peptides, this finding provides some of the best direct evidence supporting the acid/base motif. Another factor that may govern S-nitrosylation specificity is the occurrence of local hydrophobicity surrounding the cysteine residue (28). Construction of Kyte–Doolittle hydropathy plots revealed that the _S_-nitrosocysteine residues identified in T-complex protein 1, ζ subunit, annexin A11, and elongation factor 1 A-1 were located in discrete motifs of increased hydrophobicity (Fig. 3C).
Fig. 3.
S-nitrosylation specificity motifs. (A) Sequence alignments of 18 _S_-nitrosocysteine-containing peptides identified from CysNO-treated HASMC comparing the occurrence of amino acids at positions flanking the modified cysteine. (B) Sequence alignments of 18 false positive peptides comparing the occurrence of amino acids at positions flanking the cysteine residue. (C) Kyte–Doolittle hydropathy plots from regions flanking the identified _S_-nitrosocysteine residue (arrow). The identified _S_-nitrosocysteine residues from T-complex protein 1, ζ subunit (Left), annexin A11 (Center), and elongation factor 1 A-1 (Right) were located within hydrophobic pockets. Hydropathy plots were constructed by using a window of 13 aa.
Although primary sequence analyses are useful for determining structural features that underlie the specificity of posttranslational modifications, they do not reveal motifs that result from three-dimensional protein structure. Therefore, proteins identified in Table 1 and for which the crystal structures (>85% homology to the identified proteins) have been determined were evaluated for acid/base motifs. Four of the 20 proteins, 14-3-3 ζ, 14-3-3 θ, RAB3B, and chloride intracellular channel 4 met these criteria. Evaluation of the molecular models revealed that for each protein, an acid/base motif opposing the identified cysteine was present within a molecular radius ranging from 2.4 to 7.1 Å (Fig. 4, which is published as supporting information on the PNAS web site). Because the proteomic studies identified 14-3-3 ζ and GRP75 as targets of S-nitrosylation in both CysNO and PAPANO-treated HASMC, these agents may share similar molecular specificities with respect to protein _S_-nitrosocysteine formation. On the other hand, myoneurin and microtubule-associated protein 4, which were identified only from PAPANO-treated HASMC, did not contain acid/base or hydrophobic motifs by primary sequence analysis. Also, the crystal structures for these proteins have not been determined. Therefore, the presence of common motifs for some but not all proteins identified from CysNO and PAPANO treatments suggests that protein _S_-nitrosocysteine formation derived from the nitric oxide radical donor include both secondary reactions of nitric oxide to generate transnitrosating species as well as other potential chemistries (8, 9).
In summary, the proteomic approach used permitted not only the evaluation of the _S_-nitrosoproteome in HASMC, but facilitated the elucidation of two S-nitrosylation motifs that govern the selectivity of modification. By systematically evaluating potential peptide sequence-to-spectrum assignments and by eliminating false positive _S_-nitrosocysteine-containing peptide identifications, 20 unique _S_-nitrosocysteine-containing peptides belonging to 18 proteins were identified. The identification of cytoskeletal, signal transduction, and ER-associated proteins implicates S-nitrosylation in the regulation of smooth muscle cell proliferation, apoptosis, and ER protein folding. The detection of proteins that participate in the ER/Golgi transport system is consistent with previous reports implicating S-nitrosylation in the regulation of vesicular trafficking in other cell types (4–7). Significantly, through regulation of vascular smooth muscle ER/Golgi function, S-nitrosylation may influence vascular wall stress responses.
Materials and Methods
Chemicals and Reagents.
Unless otherwise indicated, chemicals were purchased from Sigma. Kaighn’s modification of Ham’s F12 medium with 2 mM l-glutamine (F12K), Earle’s Balanced Salt Solution (EBSS), and SDS/PAGE 4–12% Bis-Tris gradient gels were purchased from Invitrogen. Micro Bio-spin P6 columns were obtained from Bio-Rad. PAPANO was purchased from Cayman Chemicals. Biotin-HPDP and streptavidin–agarose were purchased from Pierce. Ultrafree-MC filters, PVDF Immobilon-FL, and ZipTipC18 P10 were from Millipore. Trypsin Gold (mass spectrometry grade) was purchased from Promega. Mouse monoclonal and rat polyclonal anti-nitrosocysteine antibodies were obtained from A.G. Scientific.
CysNO was prepared by mixing equimolar amounts of l-cysteine and NaNO_2_ under acidic conditions (0.25 M HCl) in the presence of 0.1 mM DTPA. CysNO stock solutions (500 mM) were prepared fresh. The final concentration of CysNO was determined from absorbance at 334 nm by using the extinction coefficient 900 M_−1_·cm_−1_. Immediately before exposure to cell cultures, an intermediate dilution of the stock solution was prepared in HEN buffer (250 mM Hepes, pH 7.7/1 mM EDTA/0.1 mM neocuproine). PAPANO was prepared as a concentrated stock in 0.01 M NaOH, and the final concentration was determined by absorbance at 250 nm using the extinction coefficient 8050 M_−1_·cm_−1_. All stock solutions were stored on ice in the dark.
Cell Culture and Treatment with NO Agents.
HASMCs were obtained at passage 15 or 16 from American Type Culture Collection (Manassas, VA) and cultured in F12K supplemented with 10 mM Hepes, 10 mM TES, 10% FBS, ITS (0.01 mg/ml insulin/0.01 mg/ml transferrin/10 ng/ml sodium selenite), 0.03 mg/ml ECGS, and 0.05 mg/ml ascorbic acid. Cells were maintained in a 5% CO_2_ incubator at 37°C in T175 flasks. Experiments were performed between passages 16 and 21. When intact cells were ready for analysis (≈85% confluency) they were washed twice with EBSS and incubated in the dark for 20 min with 100 μM l-cysteine or 100 μM CysNO at 37°C. For PAPANO treatments, cells were exposed to 2 mM PAPANO for 1 h at 37°C in FBS/ITS/Asc-free medium (basal media) or in basal media alone as a control.
Quantitation of Protein _S_-Nitrosocysteine.
Intracellular S-nitroso-protein content was determined from HASMC cellular lysates by chemiluminescence using a Sievers 280 nitric oxide analyzer. HASMC were treated with NO agents as described above, and cell extracts were obtained as described below. Lysates were then passed over two successive Micro Biospin P6 columns to remove low molecular weight _S_-nitrosothiols, and protein concentration was determined. Lysates were incubated with 0.1% SNA/10% glacial acetic acid for at least 15 min to remove nitrite contamination. ≈0.12 mg of cellular lysates were routinely injected into the reaction vessel containing 5 ml of 60 mM potassium iodide (KI) and 10 mM iodine (I2) in glacial acetic acid at 37°C. Under these conditions, the lower limit of detection was 0.03 pmol of SNO per mg of total protein. Equivalent results for _S_-nitrosoprotein content were also obtained by using Cu(I)/Ascorbate reduction method. As a negative control for detection of S_-nitrosocysteine, lysates were incubated with 3.5 mM HgCl_2 for 20 min at 4°C.
Cell Extract Preparation and Biotin Switch Assay.
Unless otherwise indicated, all steps were performed in the dark. After the treatment medium was removed, the cells were quickly trypsinized at 37°C, inactivated with F12K containing 0.1% FBS, and centrifuged at 130 × g for 6 min at 4°C. Cell pellets were washed three times with ice-cold PBS containing 1 mM EDTA and 0.1 mM neocuproine. The biotin-switch assay was performed with between 0.5 and 1 mg of cellular protein as described (15) with minor modification. Cell pellets were resuspended in lysis buffer (HEN buffer containing 1% Triton X-100).
Resuspended pellets were then centrifuged at 12,000 × g, 4°C for 10 min. The biotin switch assay was performed with between 0.5 and 1 mg of protein. The lysates were adjusted to 0.5 mg/ml containing 2.5% SDS and 200 mM methyl methanethiosulfonate and incubated at 50°C for 20 min, vortexing every 4 min to block free thiols. After blocking, cell extracts were precipitated with two volumes of −20°C acetone, incubated at −20°C for 20 min, centrifuged at 12,000 × g, 4°C for 10 min, washed four times with acetone, and resuspended in 0.2 ml of HENS buffer (25 mM Hepes, pH 7.7/0.1 mM EDTA/0.01 mM neocuproine/1% SDS). To the blocked proteins, 0.4 mM biotin-HPDP and 5 mM ascorbate were added and incubated at 25°C for 1 h while rotating. To control for nonspecific HPDP labeling of unmodified cysteines, ascorbate was omitted. After incubation, proteins were precipitated with acetone as described above. Samples in which protein digestion was performed were resuspended in 0.45 ml of 0.1 M ammonium bicarbonate and 0.5% SDS. Protein concentration was checked by the BCA assay (Pierce).
Protein Digestion and Affinity Peptide Capture.
Biotinylated proteins were incubated with trypsin (1:30 enzyme/protein ratio) at 37°C for 18–24 h in the dark. Samples were then passed through Ultrafree-MC 10-kDa cutoff filters that had been previously rinsed with methanol and washed with H_2_O. The filtrate containing the peptides was recovered and incubated with ≈50 μl of dry, washed streptavidin–agarose beads per mg of initial protein for 30 min with gentle mixing. Samples were centrifuged at 5,000 × g for 5 min, and the supernatants were discarded. The beads were washed five times with 10 volumes of 1 M ammonium bicarbonate, followed by five washes with 10 volumes of deionized water. Between washes, samples were centrifuged at 1,000 × g for 1 min. Elution buffer containing 70% formic acid (FA) was incubated with the beads for 30 min with gentle mixing. The captured peptides were recovered by centrifuging beads at 5,000 × g for 4 min and collecting the supernatant. To ensure complete removal of streptavidin–agarose, the samples were centrifuged again. The captured peptides were evaporated to ≈5 μl in vacuo, resuspended in 20 μl of 0.1% FA, and desalted by using Zip-Tips.
Analysis by LC-MS/MS.
Desalted samples were analyzed on a Thermo LTQ linear trap instrument equipped with a Thermo micro electrospray source, and a Thermo Surveyor pump and autosampler (Thermo Electron Corporation, San Jose, CA). LC-MS/MS analyses were done by reverse phase chromatography on an 11-cm fused silica capillary column (100 μm i.d.) packed with Monitor C-18 (5 μm) (Column Engineering, Ontario, CA) with the flow set at 700 nl/min. The mobile phase consisted of 0.1% formic acid in either HPLC grade water (A) or acetonitrile (B). Peptides were eluted initially with 99% A, then 95% A for 3–5 min, then a linear gradient to 72% A by 33 min, then to 20% A at 40 min and held to 45 min, then to 99% A at 52 min and held until 60 min. MS/MS spectra were acquired by using a full scan, which was followed by four data-dependent scans on the four most intense precursor ions. Precursors that were detected twice within 15 s were put on a dynamic exclusion list for a period of 60 s. MS/MS spectra were matched to human NCBI RefSeq database sequences with sequest (Bioworks Browser 3.1 SR1) (Thermo Electron, San Jose, CA). Cysteine modification by methyl methanethiosulfonate (+46 atomic mass units) and by biotin-HPDP (+428 atomic mass units) was specified as variable modifications. For further details, see Supporting Text and Figs. 5–23, which are published as supporting information on the PNAS web site.
Immunoelectron Microscopy.
HASMC cells were treated with CysNO as described above and immediately fixed in 2% paraformaldehyde (PFA) and 0.2% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for 2 h at room temperature. Fixed cells were stored at 4°C in 1% PFA until cyrosectioning. Fifty-nanometer-thick cryosections were cut at −120°C by using an Ultracut S ultramicrotome (Leica). The sections were collected on carbon-coated formvar grids using a mixture of 1.8% methylcellulose and 2.3 M sucrose (29) and incubated with primary nitrosocysteine antibodies (30) and 10 nm of protein A gold (31). After labeling, the sections were fixed with 1% glutaraldehyde, counterstained with uranyl acetate, and embedded in methylcellulose–uranyl acetate. The specificity of the labeling was verified in control experiments where sections were treated with 3.5 mM _p-_hydroxymercuricbenzoate for 30 min (three 10-min treatments). Immunogold double labeling was performed by using 10 and 15 nm of protein A gold. After labeling, the sections were fixed with 1% glutaraldehyde, counterstained with uranyl acetate, and embedded in methyl cellulose–uranyl acetate. The sections were viewed in a JEOL 1200CX electron microscope.
Supplementary Material
Supporting Information
Acknowledgments
We are grateful to Sheryl Stamer for her assistance with mass spectrometry and MS/MS data analysis. We thank the Protein Core at the Stokes Research Institute for their assistance with sequest analysis and the Proteome Software development team for their technical assistance and expertise with scaffold.
Abbreviations
HASMC
human aortic smooth muscle cell
CysNO
_S_-nitrosocysteine
PAPANO
propylamine propylamine NONOate
HPDP–biotin
_N_-[6(Biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide
MS/MS
tandem MS.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. Nat. Rev. Mol. Cell. Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Hara M. R., Agrawal N., Kim S. F., Cascio M. B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J. H., Tankou S. K., Hester L. D., et al. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo M. A., Piston D. W. J. Cell Biol. 2003;161:243–248. doi: 10.1083/jcb.200301063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K., Morrell C. N., Cambien B., Yang S. X., Yamakuchi M., Bao C., Hara M. R., Quick R. A., Cao W., O’Rourke B., et al. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrell C. N., Matsushita K., Chiles K., Scharpf R. B., Yamakuchi M., Mason R. J. A., Bergmeier W., Mankowski J. L., Baldwin W. M., Faraday N., Lowenstein C. J. Proc. Natl. Acad. Sci. USA. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y., Man H. Y., Sekine-Aizawa Y., Han Y. F., Juluri K., Luo H. B., Cheah J., Lowenstein C., Huganir R. L., Snyder S. H. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang G. F., Moniri N. H., Ozawa K., Stamler J. S., Daaka Y. Proc. Natl. Acad. Sci. USA. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gow A. J., Farkouh C. R., Munson D. A., Posencheg M. A., Ischiropoulos H. Am. J. Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell D. A., Marletta M. A. Nat. Chem. Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 10.Pawloski J. R., Hess D. T., Stamler J. S. Proc. Natl. Acad. Sci. USA. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett M. R., Evan G. I., Schwartz S. M. J. Clin. Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincoln T. M., Wu X., Sellak H., Dey N., Choi C. S. Front. Biosci. 2006;11:356–367. doi: 10.2741/1803. [DOI] [PubMed] [Google Scholar]
- 13.Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. J. Pharmacol. Exp. Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 15.Jaffrey S. R., Erdjument-Bromage H., Ferris C. D., Tempst P., Snyder S. H. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 16.Gao C. J., Guo H. T., Wei J. P., Mi Z. Y., Wall P. Y., Kuo P. C. Nitric Oxide. 2005;12:121–126. doi: 10.1016/j.niox.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Rhee K. Y., Erdjument-Bromage H., Tempst P., Nathan C. F. Proc. Natl. Acad. Sci. USA. 2005;102:467–472. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuncewicz T., Sheta E. A., Goldknopf I. L., Kone B. C. Mol. Cell. Proteomics. 2003;2:156–163. doi: 10.1074/mcp.M300003-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Ruiz A., Lamas S. Arch. Biochem. Biophys. 2004;423:192–199. doi: 10.1016/j.abb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Loscalzo J. Proc. Natl. Acad. Sci. USA. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao G., Derakhshan B., Shi L., Campagne F., Gross S. S. Proc. Natl. Acad. Sci. USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang K., Ragsdale N. V., Carey R. M., MacDonald T., Gaston B. Biochem. Biophys. Res. Commun. 1998;252:535–540. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. H., Hogg N. Proc. Natl. Acad. Sci. USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y. H., Keszler A., Broniowska K. A., Hogg N. Free Radical Biol. Med. 2005;38:874–881. doi: 10.1016/j.freeradbiomed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Lander H. M., Hajjar D. P., Hempstead B. L., Mirza U. A., Chait B. T., Campbell S., Quilliam L. A. J. Biol. Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 26.Yuan H., Michelsen K., Schwappach B. Curr. Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 27.Xu W., Liu L., Charles I. G., Moncada S. Nat. Cell Biol. 2004;6:1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- 28.Hess D. T., Matsumoto A., Nudelman R., Stamler J. S. Nat. Cell Biol. 2001;3:E46–E49. doi: 10.1038/35055152. [DOI] [PubMed] [Google Scholar]
- 29.Liou W., Geuze H. J., Slot J. W. Histochem. Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 30.Gow A. J., Chen Q. P., Hess D. T., Day B. J., Ischiropoulos H., Stamler J. S. J. Biol. Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 31.Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. J. Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information