STAT3 activation via interleukin 6 trans‐signalling contributes to ileitis in SAMP1/Yit mice (original) (raw)
Abstract
Background and aim
SAMP1/Yit mice spontaneously develops intestinal inflammation. Previously, we demonstrated that the signal transducer and activator of transcription (STAT)‐3/suppressor of cytokine signalling (SOCS)‐3 pathway is pivotal in human inflammatory bowel disease. In our studies in SAMP1/Yit mice, the aim was to investigate whether STAT3 activation contributes to ileitis and to examine the therapeutic effects of this signal blockade.
Methods
Intestinal expression of phospho‐STAT3 in SAMP1/Yit mice and control AKR/J mice was examined by western blotting and immunohistochemistry. SOCS3 and interleukin 6 (IL‐6) mRNA were determined by northern blotting and reverse transcription‐polymerase chain reaction, respectively. We also examined the effects of intravenously injected hyper‐IL‐6, an IL‐6/soluble IL‐6 receptor fusion protein, and of soluble gp130‐Fc, a specific inhibitor of soluble IL‐6 receptor signalling, on STAT3 phosphorylation and disease severity in SAMP1/Yit mice.
Results
Phospho‐STAT3 was expressed strongly during the disease course in SAMP1/Yit mice but only transiently in AKR/J mice. Phospho‐STAT3 was localised to epithelial and mononuclear cells in the diseased intestine of SAMP1/Yit mice. SOCS3 as well as IL‐6 mRNAs were expressed in affected intestine. Administration of hyper‐IL‐6 caused disease exacerbation and enhancement of STAT3 phosphorylation. In contrast, soluble gp130‐Fc administration ameliorated the disease and suppressed STAT3 phosphorylation.
Conclusion
STAT3 signalling is critical in the development of intestinal inflammation in SAMP1/Yit mice. Blockade of this signalling pathway by soluble gp130‐Fc may have therapeutic effects in inflammatory bowel disease.
Keywords: interleukin 6, gp130, signal transducer and activator of transcription 3, Crohn's disease, ulcerative colitis
The cause of inflammatory bowel disease (IBD), a collective term designating ulcerative colitis and Crohn's disease, remains unclear. However, overwhelming inflammatory and immune responses play major roles in pathogenesis.1,2 A common feature of IBD is a complex interplay of cells and cytokines within the intestine.2,3 Interaction of a cytokine with its specific receptors initiates signals that modify cell functions in both the cytoplasm and nucleus. Signal transducer and activator of transcription (STAT) proteins are a family of regulatory elements participating in this process. After activation by a family of cytoplasmic tyrosine kinases termed Janus kinases (JAK) that are associated with cell surface cytokine receptors, STATs translocate into the nucleus.4,5
We previously found STAT3 to be strongly activated in patients with IBD and in animal models of colitis.6 Very recently, these results were confirmed by a larger scale study of human IBD.7 Serum concentrations of interleukin (IL)‐6, a potent mediator of STAT3 activation, have been shown to be increased in patients with IBD.8,9,10 IL‐6 first binds to a specific receptor, and this complex associates with two molecules of the signal transducing membrane protein gp130, thereby inducing its dimerisation and initiation of signalling.11 The gp130 receptor is expressed by all cells in the body whereas IL‐6 receptor (IL‐6R) is mainly expressed by hepatocytes, neutrophils, monocytes/macrophages, and some lymphocytes. A soluble form of the IL‐6R (sIL‐6R) has been found in various body fluids. Interestingly, sIL‐6R together with IL‐6 stimulates cells which only express gp130, a process which has been named trans‐signalling.12 Moreover, it has recently been found that a soluble form of gp130 (sgp130) serves as a natural inhibitor of trans‐signalling without affecting the activity of membrane bound IL‐6R.12 We have shown that serum concentrations of sIL‐6R are elevated in patients with IBD.13 Recent studies have demonstrated a beneficial effect of antibodies against IL‐6R in patients with Crohn's disease14 as well as in animal models of colitis.15,16 These data strongly indicated that activation of the IL‐6/STAT3 pathway has a key role in the development of IBD.
The SAMP1/Yit mouse is an animal model of spontaneous intestinal inflammation. Unlike most IBD models, intestinal inflammation in SAMP1/Yit mice is unique in its preferential involvement of the small intestine. Animals maintained under specific pathogen free (SPF) conditions develop discontinuous inflammatory lesions of the ileum as early as 10 weeks of age, and show 100% penetrance by 30 weeks of age.17,18 The aims of the present study were to investigate whether STAT3 activation is involved in intestinal inflammation and to determine whether blockade of this signalling has a therapeutic effect in SAMP1/Yit mice.
Materials and methods
Mice and reagents
SAMP1/Yit mice were maintained under SPF conditions at Kurume University. AKR/J mice were purchased from Seiwa Experimental Animals (Fukuoka, Japan). All experiments were conducted with prior approval of the Laboratory Animal Medicine Ethics Committee of Kurume University School of Medicine. Two fusion proteins, hyper‐IL‐619,20 and sgp130‐Fc,19,21,22 were provided by one of the authors (SR‐J).
Histological evaluation
After mice were killed, the distal small intestine was removed. For histological examination of intestinal inflammation, specimens were fixed in 3% buffered formalin and embedded in paraffin. Multiple 5 μm sections were stained with haematoxylin and eosin using standard techniques. Microscopically examined sections were scored semiquantitatively as described previously.23,24 Briefly, acute and chronic inflammation were assessed separately without knowledge of treatment or strain. Acute inflammation was scored as 0, for 0–1 polymorphonuclear cells per high power field (PMN/hpf) within the mucosa; 1, for 2–10 PMN/hpf; 2, for 11–20 PMN/hpf; 3, for 21–30 PMN/hpf; and 4, for >30 PMN/hpf. Chronic inflammation was scored as 0, for 0–10 mononuclear lymphocytes per hpf (ML/hpf) within the mucosa; 1, for 11–20 ML/hpf; 2, for 21–30 ML/hpf; 3, for 31–40 ML/hpf; and 4, for >40 ML/hpf. Acute and chronic inflammatory scores were added to yield a combined histological score.
Western blot analysis
STAT3 and phospho‐STAT3 were determined in intestinal tissue homogenised in 2–4 ml of lysis buffer (50 mM Tris HCl at pH 8.0; 0.5% NP‐40; 1 mM ethylenediaminetetraacetic acid (EDTA); 150 mM NaCl; 10% glycerol; 1 mM sodium vanadate; 50 mM sodium fluoride; 10 mM sodium pyrophosphate; and 1 mM phenylmethylsulfonyl fluoride) with a protease inhibitor cocktail (Sigma Chemical, St Louis, Missouri, USA).6 Extracts were cleared by centrifugation at 15 000 rpm at 4°C for 15 minutes, and supernatants were diluted with lysis buffer to result in a protein concentration of approximately 2 mg/ml. Extracts were resolved by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. Proteins were detected by immunoblotting using anti‐phospho‐STAT1, 3, 4, 5, and 6 (New England BioLabs, Beverly, Massachusetts, USA), anti‐STAT1, 3, 4, 5, and 6 (Santa Cruz Biotechnology, Santa Cruz, California, USA), anti‐phospho‐p38 mitogen activated protein kinase (p38 MAPK; Cell Signaling Technology, Beverly, Massachusetts, USA), and anti‐p38 MAPK (Cell Signaling Technology).
Northern hybridisation
Total RNA was prepared from intestinal tissue with Trizol (Gibco BRL, Carlsbad, California, USA) according to the manufacturer's instructions.6 After 5 μg of total RNA were separated electrophoretically on 1.0% agarose gels containing 2.4% formaldehyde, the bands were transferred to positively charged nylon membranes. After fixation under calibrated ultraviolet irradiation, the membranes were hybridised with digoxygenin (DIG) labelled cDNA probes and visualised using alkaline phosphatase labelled anti‐DIG antibody according to the manufacturer's instructions (Boehringer, Mannheim, Germany). Probe cDNAs for suppressor of cytokine signalling (SOCS)‐1, SOCS3, and the internal control, glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH), have been described previously.25
Reverse transcription‐polymerase chain reaction (RT‐PCR)
Total RNA was prepared from ileal tissues of SAMP1/Yit mice and AKR/J mice, as described above. RT‐PCR was carried out using a primer specific for IL‐6 or G3PDH, as previously described.17 These primers were purchased from BD Clontech Labs (Palo Alto, California, USA).
Immunohistochemistry
Immunohistochemistry was performed on 5 μm paraformaldehyde fixed cryostat sections using an avidin‐biotin peroxidase procedure (Vector Laboratories, Burlingame, California, USA). Endogenous peroxidase activity was quenched by exposure to 3% H2O2 for 10 minutes. Tissue sections were pretreated with diluted normal goat serum for 20 minutes and incubated with a 1:100 dilution of anti‐phospho‐STAT3 specific antibodies (1:1 mixture of antibodies against tyrosine phosphorylated STAT3 and serine phosphorylated STAT3; New England BioLabs). Sections then were incubated with antirabbit biotinylated IgG for 30 minutes, washed with phosphate buffered saline (PBS), incubated with avidin/biotinylated horseradish peroxidase complex for one hour, and washed with PBS. Reaction product was visualised with Vector VIP (Vector Laboratories), rinsed in tap water for five minutes, counterstained with methyl green, and dipped in saturated lithium carbonate solution for bluing. Normal rabbit IgG diluted to an equivalent protein concentration and applied in place of the primary antibody was used as a negative control. For double immunofluorescence analysis, anti‐phospho‐STAT3 was visualised by fluorescein isothiocyanate labelled streptavidin (BD Pharmingen, San Diego, California, USA) and CD4 by Cy3 labelled G.K.‐1.5. These sections were examined by confocal scanning microscopy (LSM510; Carl Zeiss, Oberkochen, Germany), and findings were analysed with Image Browser software (Carl Zeiss).
Administration of hyper IL‐6 and sgp130‐Fc in SAMP1/Yit mice
Hyper IL‐6 (1 μg intravenously) or vehicle was injected weekly into 20 week old female SAMP1/Yit mice for eight weeks. In a separate experiment, soluble gp130‐Fc (sgp130‐Fc; 2 μg intravenously) or vehicle was injected weekly into 10 week old or 20 week old female SAMP1/Yit mice for eight weeks. These mice then were killed for assessment of the effects of these two agents on STAT3 phosphorylation as well as on two indices of disease severity, ileal tissue weight, and histological scores, as described above.
Statistical analysis
Data are expressed as mean (SEM). Statistical analysis used the Student's t test.
Results
STAT3 expression in SAMP1/Yit mice
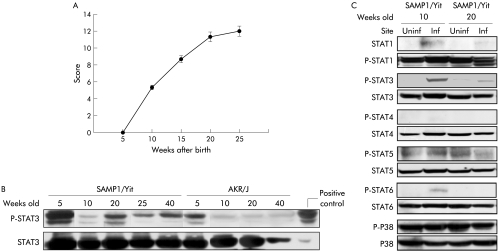
Intestinal inflammation was not present in either SAMP1/Yit mice or control AKR/J mice at five weeks of age. Inflammation was apparent at 10 weeks and worsened with increasing age in the ileum of SAMP1/Yit mice (fig 1A). Figure 1B shows serial changes in ileal STAT3 expression in SAMP1/Yit and AKR/J mice. Phospho‐STAT3 was strongly expressed during the course of ileitis in SAMP1/Yit mice but only transiently in AKR/J mice. In both strains, expression of phospho‐STAT3 was maximal at five weeks of age. Furthermore, STAT3 was phosphorylated only in inflamed areas in SAMP1/Yit mice at 10 and 20 weeks of age (fig 1C). Concomitant determination of other signal transduction components demonstrated that STAT1 and STAT6 but not STAT4, STAT5, or p38 MAPK were more fully phosphorylated in inflamed than in non‐inflamed areas.
Figure 1 Inflammation and regulatory protein expression in mice according to time and sampling site. (A) Blinded histological scores of the ileum of SAMP1/Yit mice at different ages (n = 5 per time point). (B) Temporal pattern of phospho‐signal transducer and activator of transcription (STAT)‐3 expression in the ileum of SAMP1/Yit mice and AKR/J mice, as determined by western blotting. SOCS, suppressor of cytokine signalling; G3PDH, glyceraldehyde‐3‐phosphate dehydrogenase. (C) Comparison of phospho‐STATs and p38 mitogen activated protein kinase expressions between inflamed (Inf) and uninflamed (Uninf) ileal tissues of SAMP1/Yit mice, as determined by western blotting. Data are representative of two independent experiments showing similar results.
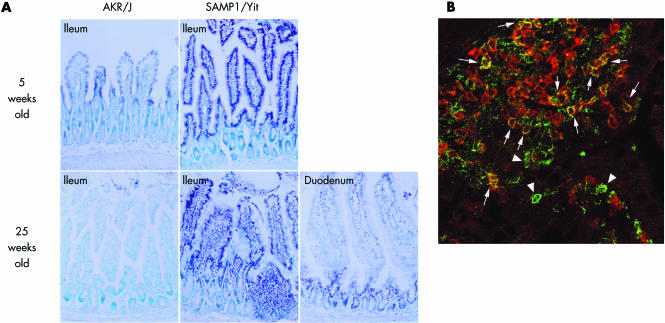
Using immunohistochemical analysis, we identified expression and cellular localisation of phospho‐STAT3 in the ileum. In the ileal mucosa of SAMP1/Yit mice, at five weeks of age, we detected appreciable quantities of phospho‐STAT3 expression almost exclusively in the nuclei of epithelial cells and of mononuclear cells showing either lymphocytic or macrophage morphology; immunostaining was only scattered in AKR/J mice (fig 2A). At 25 weeks of age, we again detected phospho‐STAT3 expression in morphologically identified lymphocytes and macrophages in the inflamed ileal mucosa of SAMP1/Yit mice while expression was very rare in the uninflamed duodenal mucosa of SAMP1/Yit mice and in the ileal mucosa of AKR/J mice (fig 2A). Furthermore, double immunofluorescence analysis demonstrated that among mononuclear cell populations, phospho‐STAT3 localised partly within CD4+ T‐cells (fig 2B) while some CD4− mononuclear cells also showed immunoreactivity for phospho‐STAT3.
Figure 2 Findings on immunohistochemistry and double staining using immunofluorescence. (A) Immunohistochemical localisation of phospho‐signal transducer and activator of transcription (STAT)‐3 in ileal mucosa of SAMP1/Yit mice and AKR/J mice at five and 25 weeks of age. (B) Confocal image analysis of phospho‐STAT3 (green) and CD4 (red) in the ileal mucosa of SAMP1/Yit mice at 25 weeks of age. Phospho‐STAT3 was localised to CD4+ T cells (arrows) and also other mononuclear cells (arrowheads) in the lamina propria.
SOCS mRNA expression in SAMP1/Yit mice
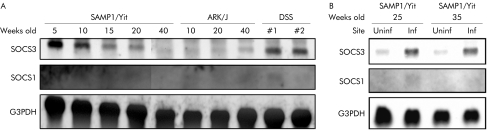
The SOCS family is a negative regulator of the JAK/STAT pathway.26,27,28,29 We investigated how important members of this family affected development of disease in SAMP1/Yit mice. As shown in fig 3A, a developmental increase in the SOCS3 message was observed in the ileum of SAMP1/Yit mice but not in AKR/J mice. Furthermore, this increase was localised to inflamed areas in SAMP1/Yit mice (fig 3B). SOCS1 message was not detectable at any time point in either SAMP1/Yit or AKR/J mice (fig 3A).
Figure 3 Expression of suppressor of cytokine signalling (SOCS)‐1 and SOCS3 mRNA in ileal tissues. Total RNA was extracted from tissues obtained from SAMP1/Yit mice and AKR/J mice. Ileal tissue extracts from mice with dextran sodium sulphate (DSS) induced colitis were used as a positive control for SOCS3 mRNA. Northern hybridisation was performed with cDNA probes for coding regions of murine SOCS1, SOCS3, and for glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH), as an internal control. Data are representative of two independent experiments showing similar results.
IL‐6 mRNA expression in SAMP1/Yit mice
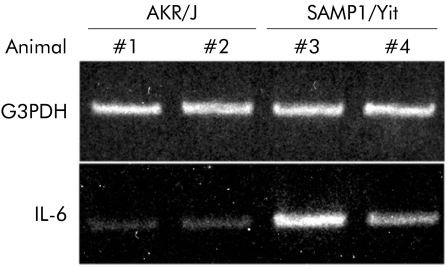
Figure 4 shows representative IL‐6 mRNA expression in ileal tissues from 25 week old SAMP1/Yit mice and AKR/J mice examined by RT‐PCR. IL‐6 mRNA was more abundant in ileal tissues of SAMP1/Yit mice than in those of AKR/J mice.
Figure 4 Expression of interleukin 6 (IL‐6) mRNA in ileal tissues. Total RNA was extracted from ileal tissues from two 25 week old‐SAMP1/Yit mice and two AKR/J mice of similar age. Reverse transcription‐polymerase chain reaction was performed using primers specific for IL‐6 or for glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH) (the control). Data are representative of three independent experiments, each experiment using one to two mice per group.
Effects of STAT3 modulators on SAMP1/Yit mice
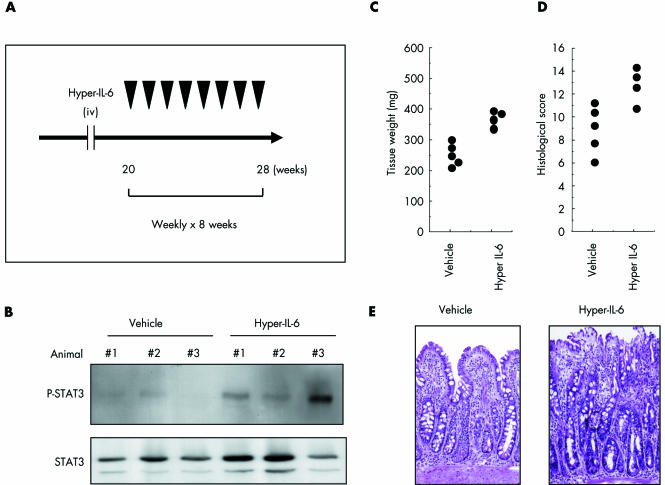
We next focused on the in vivo role of STAT3 in SAMP1/Yit mice using agents capable of regulating STAT3 phosphorylation. Hyper IL‐6 is a fusion protein of IL‐6 and sIL‐6R that enhances STAT3 phosphorylation.19,20 SAMP1/Yit mice were treated weekly with hyper IL‐6 or vehicle from 20 to 28 weeks of age (fig 5A). As shown in fig 5B, STAT3 was more intensely phosphorylated in hyper IL‐6‐treated mice than in vehicle treated mice. Compared with controls, hyper IL‐6 significantly increased tissue weight of the ileum (p<0.05; fig 5C) and increased histological severity of disease when mice were killed at 28 weeks (p<0.05; fig 5D). Figure 5E shows the histological appearance of representative lesions. Destruction of the epithelial layer and inflammatory infiltrates in the lamina propria were prominent in hyper IL‐6 treated mice.
Figure 5 Enhancement of signal transducer and activator of transcription (STAT)‐3 phosphorylation associated with exacerbation of ileitis in SAMP1/Yit mice. (A) Hyper interleukin 6 (IL‐6) (n = 4, 1 μg intravenously) or vehicle (n = 4) was injected weekly from 20 to 28 weeks of age. (B) Expression of phospho‐STAT3 in the ileum by western blotting. (C) Weight of ileal tissue in hyper IL‐6 treated versus untreated mice. (D) Histological scores in hyper IL‐6 treated versus untreated mice. (E) Representative haematoxylin‐eosin staining of ileal sections from SAMP1/Yit mice at 28 weeks of age treated weekly with either hyper IL‐6 or vehicle for eight weeks.
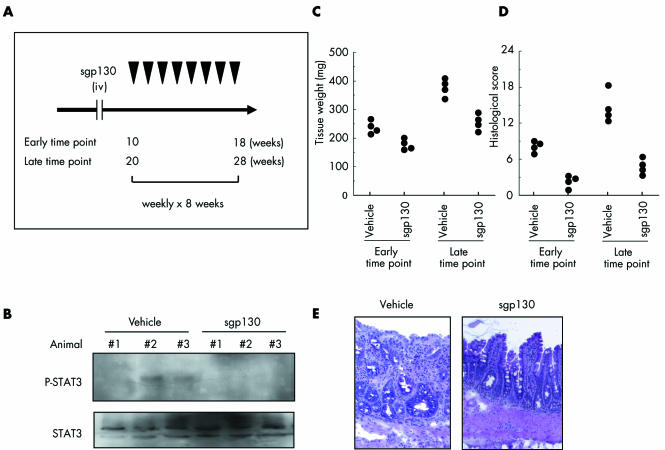
On the other hand, sgp130‐Fc is a dimerised fusion protein of sgp130 that specifically suppresses STAT3 phosphorylation mediated by IL‐6 trans‐signalling.19,21,22 SAMP1/Yit mice were treated weekly with sgp130‐Fc or vehicle from 10 to 18 weeks of age or from 20 to 28 weeks of age (fig 6A). As shown in fig 6B, STAT3 showed less phosphorylation in sgp130‐Fc treated mice than in vehicle treated mice. Furthermore, ileal tissue weight was significantly less in sgp130‐Fc treated mice than in vehicle treated mice at the age of 18 weeks (p<0.01) and 28 weeks (p<0.01) (fig 6C). Histological evaluation showed a significant beneficial effect from sgp130‐Fc at the age of 18 weeks (p<0.0005) and 28 weeks (p<0.05) (fig 6D). As shown in fig 6E, destruction of the epithelial layer and inflammatory infiltrates in the lamina propria were very mild in sgp130‐Fc treated mice.
Figure 6 Suppression of signal transducer and activator of transcription (STAT)‐3 phosphorylation ameliorated ileitis in SAMP1/Yit mice. (A) Either sgp130‐Fc (2 μg intravenously) or vehicle was injected weekly into 10 week old mice (n = 4 for each group, early time point) or 20 week old mice (n = 4 for each group, late time point) for eight weeks. (B) Expression of phospho‐STAT3 in the ileum according to western blotting. Each treatment was performed weekly from 20 to 28 weeks of age. (C) Weight of ileal tissue in sgp130‐Fc treated versus untreated mice. Either sgp130‐Fc or vehicle was injected weekly into 10 week old mice (early time point) or 20 week old mice (late time point) for eight weeks. (D) Histological scores in sgp130‐Fc treated versus untreated mice. Either sgp130‐Fc or vehicle was injected weekly into 10 week old mice (early time point) or 20 week old mice (late time point) for eight weeks. (E) Representative haematoxylin‐eosin staining of ileal sections from SAMP1/Yit mice at 28 weeks of age treated weekly with either sgp130‐Fc or vehicle for eight weeks.
Discussion
At present, very few mouse models encapsulate the anatomical abnormalities of IBD. Colitis induced by chemical agents such as trinitrobenzene sulphonic acid or DSS is very different from the human disease. Genetically manipulated mice such as the IL‐10 and IL‐2 knockout mice are used widely but there is no proof that dysregulation of these targeted genes represent the underlying defects of human IBD. The SAMP1/Yit mouse model is the most appropriate model because mice spontaneously develop ileal inflammation characterised by transmural and segmental inflammation with granulomas, which is mostly related to human Crohn's disease.18 In the present study, we analysed the activation state of STAT3 and the negative regulator SOCS, and examined the effect of STAT3 modulation on development of intestinal inflammation in vivo using SAMP1/Yit mice.
Western analysis showed strong activation of STAT3 in association with disease development in SAMP1/Yit mice. This alteration is not limited to the SAMP1/Yit model as it has been demonstrated in other experimental models, as well as in human IBD.6,7 Thus activation of STAT3 is likely to represent a general mechanism of response of the intestine to a variety of inflammatory stimuli. We further assessed topographic distribution of phospho‐STAT3, demonstrating an increased number of immunoreactive cell nuclei, especially in mononuclear and epithelial cell populations. A similar distribution has been reported in human IBD.6,7 Together with strong activation of STAT3 shown by western blotting, these findings suggest an important role for STAT3 in the development of ileal inflammation.
We observed phospho‐STAT3 predominantly in T cells in diseased areas of the intestine. This result is consistent with recent findings demonstrating constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease.7,30 Recent reports have shown that T cells were rescued from apoptosis through induction of STAT3 dependent genes Bcl‐2 and Bcl‐xL while blockade of sIL‐6R signalling in experimental colitis prevented disease onset by suppressing expression of these antiapoptotic regulators.15 Increased numbers of T cells positive for phospho‐STAT3 therefore may be associated with development and perpetuation of disease in SAMP1/Yit mice. The observation that disease can be transferred to severe combined immunodeficiency (SCID) mice by introducing CD4+ T cells from SAMP1/Yit mice23 is strongly supportive of this conclusion.
We observed that some lamina propria mononuclear cells apart from T cells, especially apparent macrophages, express phospho‐STAT3. Interestingly, Takeda et al reported that macrophage‐STAT3 knockout mice developed chronic enterocolitis as they aged.31 However, we have previously demonstrated that STAT3 in whole tissue extracts was strongly activated even in these knockout mice,6 suggesting that STAT3 in cell types other than macrophages may take part in intestinal inflammation. In fact, disease cannot be transferred to SCID mice by introducing macrophages from SAMP1/Yit mice rather than T cells (one of the authors, SM, personal communication). Together with our data showing strong activation of STAT3 in several colitis models with different aetiologies,6 STAT3 would seem to be involved in progression rather than initiation of intestinal inflammation.
We also identified intestinal epithelial cells as expressing phospho‐STAT3. The actions of STAT3 in cells of epithelial lineage remain unclear. However, previous reports have demonstrated that intestinal epithelial cells produce and release cytokines such as IL‐6 or IL‐15 and several chemokines in response to various stimuli32,33,34; these cytokines may affect epithelial cells themselves as well as immune cells within the intestinal mucosa, such as lymphocytes and macrophages. Such interaction may be important in the process of inflammation and development of disease in SAMP1/Yit mice.
The SOCS gene family consists of SOCS1 to SOCS7, all of which contain a central SH2 domain and a C terminal region, termed the “SOCS box”. SOCS genes are involved in negative regulation of the JAK/STAT pathway induced by cytokine signalling.26,27,28,29 We previously observed that both SOCS3 and STAT3 were strongly expressed in human IBD. We also observed that in mice with DSS induced colitis, SOCS3 was induced as colitis developed while a high degree of SOCS3 expression suppressed STAT3 activation.6 In the present study, both STAT3 and SOCS3 were highly expressed in SAMP1/Yit mice, suggesting STAT3 activation is associated with SOCS3 induction. Amounts of SOCS3 in the inflamed intestine may be insufficient to shut off STAT3 activation, thereby inducing chronicity of inflammation. Modifications to SOCS3 and rapid degradation by proteasomes could be other reasons for incomplete suppression.
We previously reported that DSS induced colitis was mild in mice lacking STAT3 activation but severe in mice genetically manipulated to hyperactivate STAT3.6 This suggested that an agent capable of modulating STAT3 activity could control intestinal inflammation. We therefore first demonstrated that hyper‐IL‐6, an IL‐6/sIL‐6R fusion protein capable of enhancing STAT3 activity,19,20 exacerbated intestinal inflammation in SAMP1/Yit mice. Strong evidence indicates that IL‐6 can interact with cells not expressing IL‐6R by a process known as trans‐signalling where IL‐6 forms a complex with sIL‐6R that can transduce a signal to target cells.19 Therefore, hyper‐IL‐6 seems likely to enhance STAT3 activation via trans‐signalling, increasing disease severity in SAMP1/Yit mice.
Next we demonstrated that sgp130‐Fc, which is capable of suppressing STAT3 activity, could ameliorate intestinal inflammation of SAMP1/Yit mice. The dimer sgp130‐Fc can suppress the action of the IL‐6/sIL‐6R complex without affecting the activity of membrane bound IL‐6R.19,21,22 According to recent reports, activated T cells from Crohn's disease patients are resistance to apoptosis; these T cells produced large amounts of IL‐6 but lacked membrane bound IL‐6R, indicating that sIL‐6R rather than membrane bound IL‐6R was responsible for T cell stimulation.15,19 Accordingly, treatment with sgp130‐Fc may represent a rational therapeutic approach to Crohn's disease. In fact, it has already been demonstrated that blockade of STAT3 activity using antibodies against IL‐6R is effective in patients with Crohn's disease14 as well as in animal models of colitis.15,16 Furthermore, as STAT3 is highly activated in intestinal inflammation irrespective of cause, sgp130‐Fc could be beneficial in IBD as well as in other chronic intestinal inflammatory disorders. In addition, Becker et al have shown that in the azoxymethan/DSS colon cancer model, in which the inflammatory condition in the colon develops into colorectal cancer, development of tumours could be strongly reduced by blockade of IL‐6 signalling with neutralising antibodies and with the sgp130‐Fc.35
There are several lines of evidence showing that cytokines are involved in the pathogenesis of IBD. Several cytokines act through activation of the STAT family. Experiments in mouse models have highlighted STAT3 as well as other STAT family members, such as STAT1 and STAT4, as potential culprits in the pathogenesis of IBD. STAT1 is important in the development and perpetuation of a helper 1 (Th1) immune response and is the signature transcription factor activated by interferon γ.36 Interferon γ blockade is currently being tested as a therapeutic intervention in Crohn's disease.37 STAT4 is activated specifically in Th1 cells following activation by IL‐12.37 IL‐12 has been suggested to be important in Crohn's disease,38 and blockade of this cytokine is currently being evaluated.39 Furthermore, other signal transducing pathways, including nuclear factor κB40 and p38 MAPK,41 also have been shown to contribute to the development of IBD while blockade of these pathways might benefit IBD patients.42 A better understanding of how multiple transcription pathways involved in inflammatory and immune reactions are regulated is hoped, to guide development of new treatments for patients with IBD.
In summary, our study demonstrated activation of STAT3 in SAMP1/Yit mice, as well as downregulation of STAT3 activation in response to injected sgp130‐Fc with attenuation of inflammation. These results identify STAT3 as both a principal regulator of the inflammatory response in IBD and a potentially important therapeutic target.
Abbreviations
DIG - digoxygenin
DSS - dextran sodium sulphate
EDTA - ethylenediaminetetraacetic acid
G3PDH - glyceraldehyde‐3‐phosphate dehydrogenase
hpf - high power field
IBD - inflammatory bowel disease
IL‐6 - interleukin 6
IL‐6R - interleukin 6 receptor
sIL‐6R soluble interleukin 6 receptor -
JAK - Janus kinase
MAPK - mitogen activated protein kinase
ML - mononuclear lymphocytes
PBS - phosphate buffered saline
PMN - polymorphonuclear cells
RT‐PCR - reverse transcription‐polymerase chain reaction
SCID - severe combined immunodeficiency
sgp130 - soluble form of gp130
SOCS - suppressor of cytokine signalling
SPF - specific pathogen free
STAT - signal transducer and activator of transcription
Footnotes
The present work was supported in part by a Grant‐in‐Aid from the Japanese Ministry of Education, Culture, and Science (14770262) and Ministry of Health and Welfare.
Conflict of interest: None declared.
References
- 1.Katz J A, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol 199915291–297. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky D K, Fiocchi C. Cytokine, chemokines, growth factors, eicosanoids, and other bioactive molecules in inflammatory bowel disease. In: Kirsner JB, ed. Inflammatory bowel disease, 5th edn. Philadelphia: W B Saunders, 2000191–207.
- 3.Sartor R B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 1994106533–539. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E., Jr STATs and gene regulation. Science 19972771630–1635. [DOI] [PubMed] [Google Scholar]
- 5.Fu X Y, Schindler C, Improta T.et al The proteins of ISGF‐3, the interferon alpha‐induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A 1992897840–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki A, Hanada T, Mitsuyama K.et al CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med 2001193483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso A, Dentelli P, Carlino A.et al Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis 20051191–98. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuyama K, Sasaki E, Toyonaga_et al_ Colonic mucosal interleikin‐6 in inflammatory bowel disease. Digestion 199150104–111. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuyama K, Sata M, Tanikawa K. Significance of interleukin‐6 in patients with inflammatory bowel disease. Gastroenterol Jpn 19912620–28. [DOI] [PubMed] [Google Scholar]
- 10.Gross V, Andus T, Caesar I.et al Evidence for continuous stimulation of interleukin‐6 production in Crohn's disease. Gastroenterology 1992102514–519. [DOI] [PubMed] [Google Scholar]
- 11.Taga T, Kishimoto T. Gp130 and the interleukin‐6 family of cytokines. Annu Rev Immunol . 1997;15797–819. [DOI] [PubMed]
- 12.Jones S A, Rose‐John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL‐6R/IL‐6 complex. Biochim Biophys Acta 20021592251–263. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuyama K, Toyonaga A, Sasaki_et al_ Soluble interleukin‐6 receptors in inflammatory bowel disease: relation to circulating interleukin‐6. Gut 19953645–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito H, Takazoe M, Fukuda Y.et al A pilot randomized trial of a human anti‐interleukin‐6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology . 2004;126989–996. [DOI] [PubMed]
- 15.Atreya R, Mudter J, Finotto S.et al Blockade of interleukin 6 trans signaling suppresses T‐cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 20006583–588. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yoshizaki K, Kishimoto T.et al IL‐6 is required for the development of Th1 cell‐mediated murine colitis. J Immunol 20001644878–4882. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Okabe Y, Setoyama H.et al Inflammatory bowel disease‐like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 19984371–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strober W, Nakamura K, Kitani A. The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease. J Clin Invest . 2001;107667–670. [DOI] [PMC free article] [PubMed]
- 19.Rose‐John S. Interleukin‐6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim Pol . 2003;50603–611. [PubMed]
- 20.Fischer M, Goldschmitt J, Peschel C.et al A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol . 1997;15142–145. [DOI] [PubMed]
- 21.Nowell M A, Richards P J, Horiuchi S.et al Soluble IL‐6 receptor governs IL‐6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J Immunol . 2003;1713202–3209. [DOI] [PubMed]
- 22.Jostock T, Mullberg J, Ozbek S.et al Soluble gp130 is the natural inhibitor of soluble interleukin‐6 receptor transsignaling responses. Eur J Biochem . 2001;268160–167. [DOI] [PubMed]
- 23.Kosiewicz M M, Nast C C, Krishnan A.et al Th1‐type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest . 2001;107695–702. [DOI] [PMC free article] [PubMed]
- 24.Takedatsu H, Mitsuyama K, Matsumoto S.et al Interleukin‐5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur J Immunol . 2004;341561–1569. [DOI] [PubMed]
- 25.Suzuki R, Sakamoto H, Yasukawa H.et al CIS3 and JAB have different regulatory roles in interleukin‐6 mediated differentiation and STAT3 activation in M1 leukemia cells. Oncogene 1998172271–2278. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura A, Ohkubo T, Kiguchi T.et al A novel cytokine‐inducible gene CIS encodes an SH2‐containing protein that binds to tyrosine‐phosphorylated interleukin 3 and erythropoietin receptors. EMBO J 1995142816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starr R, Willson T A, Viney E M.et al A family of cytokine‐inducible inhibitors of signalling. Nature 1997387917–921. [DOI] [PubMed] [Google Scholar]
- 28.Naka T, Narazaki M, Hirata M.et al Structure and function of a new STAT‐induced STAT inhibitor. Nature 1997387924–929. [DOI] [PubMed] [Google Scholar]
- 29.Endo T A, Masuhara M, Yokouchi M.et al A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997387921–924. [DOI] [PubMed] [Google Scholar]
- 30.Lovato P, Brender C, Agnholt J.et al Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem . 2003;27816777–16781. [DOI] [PubMed]
- 31.Takeda K, Clausen B E, Kaisho T.et al Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 19991039–49. [DOI] [PubMed] [Google Scholar]
- 32.McGee D W, Beagley K W, Aicher W K.et al Transforming growth factor‐beta and IL‐1 beta act in synergy to enhance IL‐6 secretion by the intestinal epithelial cell line, IEC‐6. J Immunol 1993151970–978. [PubMed] [Google Scholar]
- 33.Reinecker H C, MacDermott R P, Mirau S.et al Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 19961111706–1713. [DOI] [PubMed] [Google Scholar]
- 34.Yang S K, Eckmann L, Panja A.et al Differential and regulated expression of C‐X‐C, C‐C, and C‐chemokines by human colon epithelial cells. Gastroenterology 19971131214–1223. [DOI] [PubMed] [Google Scholar]
- 35.Becker C, Fantini M C, Schramm C.et al TGF‐beta suppresses tumor progression in colon cancer by inhibition of IL‐6 trans‐signaling. Immunity 200421491–501. [DOI] [PubMed] [Google Scholar]
- 36.Ramana C V, Gil M P, Schreiber R D.et al Stat1‐dependent and ‐independent pathways in IFN‐gamma‐dependent signaling. Trends Immunol 20022396–101. [DOI] [PubMed] [Google Scholar]
- 37.Papachristou G I, Plevy S. Novel biologics in inflammatory bowel disease. Gastroenterol Clin North Am 200433251–269. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka K, Inoue N, Sato T.et al T‐bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn's disease. Gut 2004531303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt C, Marth T, Wittig B M.et al Interleukin‐12 antagonists as new therapeutic agents in inflammatory bowel disease. Pathobiology 200270177–183. [DOI] [PubMed] [Google Scholar]
- 40.Neurath M F, Pettersson S, Meyer zum Buschenfelde K H.et al Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF‐kappa B abrogates established experimental colitis in mice. Nat Med . 1996;2998–1004. [DOI] [PubMed]
- 41.Hommes D, van den Blink B, Plasse T.et al Inhibition of stress‐activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology 20021227–14. [DOI] [PubMed] [Google Scholar]
- 42.Mitsuyama K, Suzuki A, Tomiyasu N.et al Transcription factor‐targeted therapies in inflammatory bowel disease. Digestion . 2001;6368–72. [DOI] [PubMed]