5′-AMP-Activated Protein Kinase (AMPK) Is Induced by Low-Oxygen and Glucose Deprivation Conditions Found in Solid-Tumor Microenvironments (original) (raw)
Abstract
Low oxygen gradients (hypoxia and anoxia) are important determinants of pathological conditions under which the tissue blood supply is deficient or defective, such as in solid tumors. We have been investigating the relationship between the activation of hypoxia-inducible factor 1 (HIF-1), the primary transcriptional regulator of the mammalian response to hypoxia, and 5′-AMP-activated protein kinase (AMPK), another regulatory system important for controlling cellular energy metabolism. In the present study, we used mouse embryo fibroblasts nullizygous for HIF-1α or AMPK expression to show that AMPK is rapidly activated in vitro by both physiological and pathophysiological low-oxygen conditions, independently of HIF-1 activity. These findings imply that HIF-1 and AMPK are components of a concerted cellular response to maintain energy homeostasis in low-oxygen or ischemic-tissue microenvironments. Finally, we used transformed derivatives of wild-type and HIF-1α- or AMPKα-null mouse embryo fibroblasts to determine whether AMPK is activated in vivo. We obtained evidence that AMPK is activated in authentic hypoxic tumor microenvironments and that this activity overlaps with regions of hypoxia detected by a chemical probe. We also showed that AMPK is important for the growth of this tumor model.
We have been studying the relationship between the activity of hypoxia-inducible factor 1 (HIF-1), the primary transcriptional regulator of the response of mammalian cells to oxygen deprivation (e.g., see references 21, 43, and 50) and the regulation of c-Jun/AP-1 transcription factors (31, 32). We determined that c-Jun N-terminal phosphorylation is induced by low-oxygen conditions (hypoxia or anoxia; called hypoxia hereafter) in an HIF-1-dependent manner (31) and showed that this HIF-1-dependent c-Jun phosphorylation absolutely requires extracellular glucose utilization (32). Together, these findings suggest that enhanced glucose absorption and/or glycolytic activity mediated by HIF-1 in response to hypoxia activates c-Jun/AP-1, as well as other targets of c-Jun N-terminal kinases. To further investigate this potential mechanism, we focused on determining the contribution of bioenergetics—ATP depletion—to hypoxia-inducible c-Jun phosphorylation in wild-type (WT) and HIF-1-null mouse embryo fibroblasts (MEFs). While exploring cellular mechanisms of ATP regulation, we observed that 5′-AMP-activated protein kinase (AMPK) activity was induced in both cell types, particularly under conditions of hypoxia and glucose deprivation. This observation suggested the hypothesis that AMPK is important for the adaptive responses of energetically stressed cells in the hypoxic and glucose-deprived microenvironments present in solid tumors (e.g., reviewed in references 35 and 59).
AMPK activity is defined by a class of evolutionarily conserved serine/threonine kinases that are sensitive to various environmental stresses, especially those that perturb cellular energy status (reviewed in references 9, 19, and 47). Different members of the AMPK catalytic subunit subfamily have been characterized; the α subunits (collectively, AMPKα1 and -α2) are the most widely expressed in mammalian cells (36). AMPK is a heterotrimeric complex consisting of an α subunit and β and γ regulatory subunits, each of which is encoded by distinct genes (α1 and α2; β1 and β2; γ1, γ2, and γ3) (19). In terms of a role in ATP regulation, decreased cellular ATP levels promote AMPK activation through the allosteric binding of AMP, which in effect enables AMPK to sense increases in the cellular [AMP]/[ATP] ratio. Full activation of AMPK also requires specific phosphorylation within the activation loop of the catalytic domain of the α subunit (at Thr172 in humans and mice) by LKB1, a serine/threonine protein kinase and tumor suppressor (36, 37, 52). LKB1 is thus an AMPK kinase. Recently, mammalian Ca2+/calmodulin-dependent kinase kinases have also been identified as AMPK kinases (reviewed in reference 6). Activated AMPK phosphorylates diverse targets, including many that are directly involved in controlling cellular energy metabolism (22, 34). In cells exposed to an energy-depleting stress, AMPK is believed to function as an energy sensor that inhibits ATP-consuming processes and stimulates ATP-producing processes to optimize total cellular ATP levels for maintaining critical physiological functions (or for survival in response to extreme stress) (19). For example, in cells exposed to hypoxic or ischemic conditions that significantly deplete total ATP, activated AMPK can stimulate ATP generation by increasing both glucose absorption and glycolysis (e.g., see references 2, 19, and 22). AMPK can also generate ATP by phosphorylating and inhibiting the metabolic enzymes acetyl coenzyme A (acetyl-CoA) carboxylases 1 and 2 (ACC1/2), which synthesize malonyl-CoA (19). Malonyl-CoA synthesized by ACC1 is necessary for de novo fatty acid synthesis, whereas that synthesized by ACC2 inhibits fatty acid transport into the mitochondrion, the site of ATP production by the process of fatty acid β oxidation (18). Thus, AMPK-dependent inhibition of ACC1/2 can divert cellular metabolism from consuming ATP during fatty acid biosynthesis to producing ATP by oxidizing fatty acid stores.
In the present study, we found that the combination of hypoxia and glucose deprivation decreased total cellular ATP levels to the same extent in both WT and HIF-1α-null cells. This finding supports our previous conclusion that increased intracellular glucose, rather than decreased ATP levels, is responsible for the stimulation of c-Jun N-terminal kinase activity in WT cells exposed to hypoxia. AMPK activity, conventionally defined by phosphorylation of AMPK target sites on the metabolic enzymes ACC1/2 (19), was strongly activated in both WT and HIF-1α-null cells under the same conditions of hypoxia and glucose deprivation, which is consistent with its function as a sensor of ATP depletion. However, AMPK activity was also rapidly induced in both cell types following exposure to hypoxia in the presence of glucose, even though total cellular ATP was not significantly depleted. By using genetically manipulated MEFs nullizygous for AMPK expression, we directly demonstrated that AMPK activity is sensitive to a wide range of low-oxygen conditions, at least in mesenchymal cells.
To determine whether these hypoxia-inducible responses of AMPK also occur in vivo, we prepared tumor xenografts from transformed derivatives of the same WT and HIF-1α-null cells, and exposed tumor-bearing mice to the hypoxia probe pimonidazole (3, 10, 44, 45). Immunohistochemical analysis of these tumors indicated that AMPK activity was prevalent in hypoxic regions of both tumor types, especially in viable areas near necrosis. By using tumor xenografts prepared from identically transformed WT and AMPKα-null cells, we determined that the absence of AMPK activity greatly inhibited the growth of this experimental tumor type. We propose that activation of AMPK in hypoxic or ischemic microenvironments may be critical for cell survival and thus would represent a novel protective mechanism for metabolically depressed or ATP-deficient cells.
MATERIALS AND METHODS
Materials.
The following antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA): rabbit anti-phospho-acetyl-CoA carboxylase (Ser79) antibody (P-ACC; catalog no. 3661, rat antigen, cross-reactive with mouse P-ACC1/2), rabbit anti-ACC antibody (catalog no. 3662, human antigen, cross-reactive with mouse ACC1/2), rabbit anti-AMPK antibody (catalog no. 2532, human antigen, cross-reactive with mouse AMPKα1 and -α2), and rabbit anti-phospho-AMPK (Thr172) antibody (catalog no. 2531, human antigen, cross-reactive with mouse AMPKα1 and -α2 phosphorylated on Thr172). A monoclonal anti-HIF-1α antibody was obtained from Novus Biologicals, Inc. (Littleton, CO; catalog no. NB100-123, human antigen, cross-reactive with mouse HIF-1α). Affinity-purified sheep anti-AMPKα1 and anti-AMPKα2 antibodies (rat antigen, cross-reactive with mouse AMPKα subunits) were kindly provided by Grahame Hardie (University of Dundee, Dundee, United Kingdom) (60).
Cell culture.
The origin and culture of immortalized WT and HIF-1α-null MEFs have been described previously in detail (31). Mice having the genotype _AMPK_α_1_−/− _AMPK_α_2_−/− (called AMPKα-null mice hereafter) were generated by crossing _AMPK_α_1_−/− and _AMPK_α_2_−/− mice (these genotypes are called AMPK_α_1 null and AMPK_α_2 null hereafter). Details about how mice having the genotype AMPK_α_1 null or AMPK_α_2 null were generated are given in references 26 and 60. Primary AMPKα-null, AMPKα1-null, and AMPKα2-null MEFs, as well as genetically matched WT MEFs, were prepared from 10.5-day postcoitum embryos by conventional methods. Briefly, embryos were harvested, the heads and internal organs were removed, and the carcasses were finely minced with scissors and digested by incubation in 0.05% trypsin-0.5 mM EDTA solution for 30 min at 37°C with gentle agitation. Trypsin was inactivated by adding high-glucose Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) and antibiotics. The cells were further disaggregated by filtering through a 70-μm-pore-size cell strainer. Homogeneous cell suspensions were plated in DMEM containing 10% FBS and maintained at 37°C as monolayer cultures. The genotype of each source of primary MEFs was determined by PCR and immunoblotting analysis as described previously (26, 60). MEFs from the second or third cell culture passage were immortalized by deriving pooled clones that express the simian virus 40 large T antigen, stably expressed from the vector pSV-Ori− (similar to pOT; see references 17, 25, and 31).
Cells were usually plated in 60- or 100-mm-diameter plastic culture dishes (400 to 500 cells/mm2) in DMEM containing 10% FBS (Sigma, St. Louis, MO) and 25 mM HEPES buffer (pH 7.4, DMEMH-10% FBS). To construct in vitro proliferation curves, cells were plated at different densities (250, 500, 1,000, and 2,000 cells/well) in DMEMH-10% FBS in a 48-well cell culture plate (400 μl/well) and incubated overnight at 37°C in 5% CO2-air. The redox-sensitive dye Alamar Blue (BioSource International, Camarillo, CA) was added (40 μl/well), and the cells were incubated for up to 6 h at 37°C before reading fluorescence (590 nm, 530- to 560-nm excitation) from the reduced dye. Fluorescence readings were taken each day for 7 days.
Hypoxia treatments.
Most hypoxia experiments were performed according to a standard protocol in which cells were incubated in a 5% CO2-air atmosphere at 37°C overnight and then placed in aluminum gas exchange chambers maintained at 37°C (31, 32). The chambers containing the cells were then placed in a 37°C circulating water bath, and the original atmosphere was repeatedly exchanged with 5% CO2-95% N2 with a manifold equipped with a vacuum pump and a gas cylinder. Atmospheric oxygen partial pressure (pO2) values of ≤0.01% (relative to air at a pO2 of ∼21%) can be achieved inside the chambers with this system. Following various hypoxic exposures, the chambers were opened in an anaerobic glove box (Bactron X; Sheldon Manufacturing Inc., Cornelius, OR; supplied with 5% CO2-95% N2) to prepare cell lysates without significant reoxygenation. Atmospheric oxygen levels in both the chambers and the glove box were measured and calibrated with a polarographic oxygen electrode (Oxygen Sensors, Inc., Norristown, PA). Hypoxia experiments involving a pO2 of 1% were performed by equilibrating the glove box with an atmosphere of 1% O2-5% CO2-95% N2 held at 37°C with a circulating fan. Cells were incubated inside a humidified space in the glove box, and the medium on the cells was gently and continually agitated with a rotary shaker.
ATP assay.
Cells were plated in 35-mm-diameter plastic culture dishes (5 × 105 cells/dish) in DMEMH-10% FBS and incubated overnight in 5% CO2-air. The following experimental conditions were used for ATP assays (triplicate dishes were harvested at each time point): normoxic cells in normal medium, normoxic cells in glucose-free medium, hypoxic cells in normal medium, hypoxic cells in glucose-free medium, and addition of glucose to normoxic and hypoxic cells in glucose-free medium. Immediately before exposure of cells to hypoxia (pO2 of ≤0.01%) for up to 8 h, the medium was replaced with fresh DMEMH-10% FBS or DMEMH-10% FBS prepared from glucose-free DMEMH. For glucose stress experiments involving glucose reconstitution, cells were incubated on a warm surface maintained at 37°C inside the anaerobic glove box. In these experiments, cells were cultured in normal or glucose-free DMEMH-10% FBS and incubated in air-5% CO2 or exposed to hypoxia for 8 h. Degassed glucose solution (0.2 g/ml in sterile water) was then added to the dishes of normoxic and hypoxic cells in glucose-free medium to a final concentration of 4.5 g/liter (25 mM), and the cells were incubated at 37°C for 30 min or 1 h before harvesting for ATP analysis (glucose solution was added to hypoxic cells in the glove box). All cells were washed twice with phosphate-buffered saline (PBS) and lysed on ice by adding to each dish 0.3 ml of ice-cold lysis reagent from the Roche ATP Bioluminescence Kit HS II (Roche Applied Science, Indianapolis, IN) and scraping with a plastic spatula. Lysates were spun at 9,000 × g for 5 min at 4°C, and the supernatants were kept. The protein concentrations of the supernatants were determined by a bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL), and the concentrations were normalized with the kit lysis buffer. If supernatants were not immediately analyzed for ATP content, they were stored at −20°C until analysis. Total cellular ATP was measured, according to the supplier's instructions, with the luciferase-luciferin assay reagents and standards provided with the kit. This study was performed three times, and the statistical significance of differences in the combined results (averages) for each experimental pair of WT and HIF-1α-null cells was determined with a paired, two-tailed t test.
Immunoblotting analysis.
Immunoblotting protocols have been described in detail in references 31 and 32. Briefly, cells were placed on ice in air or on Super Ice cold packs in the anaerobic glove box, and the medium was removed. For most studies, cells were lysed by adding 200 μl of ice-cold lysis buffer (50 mM Tris-HCl, [pH 7.4], 0.5% NP-40, 250 mM NaCl, 1 mM dithiothreitol, 50 mM NaF, 15 mM Na4P2O7, 25 mM β-glycerophosphate, 1 mM NaVO4, 100 nM okadaic acid, 1× protease inhibitor cocktail III [PIC III; Calbiochem, San Diego, CA]). The lysis buffer was degassed before protein was harvested from hypoxic cells. After spinning at 9,000 × g for 5 min at 4°C, the protein concentrations of the supernatants were determined by a bicinchoninic acid assay. Equal protein samples (typically, 10 to 15 μg) were resolved in 4 to 12% NuPAGE sodium dodecyl sulfate-polyacrylamide gels (Invitrogen) and electroblotted onto Immobilon P membranes (Millipore, Bedford, MA). Blots were blocked in 5% nonfat dried milk in PBS containing 0.1% Tween 20 at 4°C overnight. For protein detection, blots were incubated overnight at 4°C with a primary antibody typically diluted 1:1,000 in PBS-0.1% Tween 20 containing 5% nonfat dried milk and a secondary anti-mouse, anti-rabbit, or anti-sheep immunoglobulin G antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in PBS-0.1% Tween 20 (e.g., 1:5,000). Primary antibody binding was detected and visualized with the ECL Plus Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, NJ) according to the supplier's instructions. Nuclear lysates were used to detect HIF-1α protein by immunoblotting as described in reference 40. In this case, cells were washed twice with deoxygenated, ice-cold PBS and then lysed by adding 800 μl of degassed, ice-cold lysis buffer 1 (10 mM Tris-HCl [pH 8.0], 0.5% NP-40, 150 mM NaCl, 1 mM EDTA, PIC III). After spinning the lysates at 500 × g for 5 min at 4°C, the supernatants were discarded and the pellets were resuspended in 500 μl of ice-cold lysis buffer 2 (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1× PIC III). After spinning at 9,000 × g for 5 min at 4°C, the protein concentrations of the supernatants were determined. Equal protein samples (typically, 5 to 10 μg) were resolved in 4 to 12% NuPAGE sodium dodecyl sulfate-polyacrylamide gels and electroblotted onto Immobilon P membranes (Millipore). The blots were processed with an anti-HIF-1α antibody (diluted 1:500) or other antibodies as described above.
Preparation of mouse fibrosarcoma xenografts.
Tumor xenografts were prepared by implanting female nude mice (BALB/c [nu/_nu_], 18 to 20 g; Taconic, Germantown, NY) subcutaneously (4 × 106 to 5 × 106 cells/mouse; distal dorsal region) with knockout MEFs (HIF-1α-null MEFs [31, 32] or AMPKα-null MEFs) or the corresponding WT MEFs that had been transformed by retroviral infection of an H-ras oncogene (H-ras [_Val12_] expressed from gWz-BLAST; obtained from Martin McMahon, University of San Francisco). These studies were performed in full compliance with current Institutional Animal Care and Use Committee guidelines and requirements. Tumor growth was monitored daily, and tumor volumes were measured twice weekly for up to 4 weeks (volumes, V, were calculated with the formula V = _W_12 × _W_2 × 0.52, where _W_1 and _W_2 are orthogonal tumor diameters and _W_1 ≤ _W_2). Three hours before sacrifice, mice bearing WT or HIF-1α-null tumors were injected intraperitoneally with pimonidazole hydrochloride in sterile PBS (Hypoxyprobe-1; Chemicon International, Temecula, CA; 60 mg/kg body weight). Immediately after sacrifice, tumors were excised, weighed, and divided into two approximately equal fragments. One fragment was fixed overnight in a 10% Zn2+-formalin solution, transferred to 70% ethanol, and embedded in paraffin. The other fragment was embedded in optimum cutting temperature compound and frozen in liquid nitrogen.
Immunohistochemical evaluation of histological sections.
Histological sections (4 μm thick) were prepared from the paraffin fragments of the mouse fibrosarcomas by standard methods. Sections were deparaffinized by successive treatments with xylene and ethanol solutions, and then endogenous peroxidase activity was quenched by treatment with 3% hydrogen peroxide for 10 min. Reconstitution or retrieval of the P-ACC1/2 antigen was achieved by immersing sections in 10% citrate buffer (pH 6.0) for 4 min and then exposing them to microwave radiation (e.g., 750 W, two exposures, 5 min per exposure). After the sections were blocked with 10% donkey serum for 15 min, they were incubated with the primary anti-P-ACC1/2 antibody (1:50 dilution) overnight at 4°C. As a negative control, some sections were incubated with the same solution lacking the primary antibody. All sections were thoroughly washed with PBS and incubated with a biotinylated donkey anti-rabbit secondary antibody (1:2,000 dilution) for about 20 min at 37°C. A streptavidin-peroxidase complex (ABC kit; Vector Laboratories, Burlingame, CA) was then added, and the mixture was incubated for the same length of time. Immunostaining was completed by adding the peroxidase substrate diaminobenzidine and incubating the mixture for 3 min, and then the sections were washed and counterstained with hematoxylin and eosin. Hematoxylin-and-eosin staining was done to enable the routine morphological evaluation of the tumors and to estimate the extent of necrosis in a section. Immunostaining for pimonidazole bound to hypoxic regions of the original tumors was performed by following the protocol for paraffin sections provided with the Hypoxyprobe-1 kit.
Immunostained sections from the mouse fibrosarcomas were evaluated for the location and other parameters of the P-ACC1/2 and pimonidazole antigens by using an imaging system consisting of a charge-coupled device camera coupled to a fluorescence microscope (Axioskop 2 plus; Zeiss) and an associated software package for the semiquantitative analysis of captured images (KS300; Imaging Associates, Bicester, United Kingdom). The semiquantitative analysis of such images is described in detail in references 3 and 45. Briefly, to estimate the percentage of a tumor immunostained for pimonidazole or containing necrotic regions, images of whole sections were inspected at low magnification and medium-high magnifications (×25, ×50, and ×100) and scored as percentages of the area involved. To estimate the intensity of immunostaining for the P-ACC1/2 antigen in tumor cells, images were first inspected at low magnifications (×25 and ×50) to evaluate the percentages of positively staining cells and then they were inspected at medium and medium-high magnifications (×100 and ×200) to assign intensity values on the semiquantitative 0-to-3 scale described in Table 1. Immunostaining intensity scores for P-ACC1/2 were calculated by using the following formula: weighted signal intensity = percentage of immunostained cells × average intensity score (Table 1). Pimonidazole immunostaining was not scored for intensity to avoid potential problems associated with the microregional analysis of hypoxia, such as pimonidazole pharmacokinetics, tissue redox states, and the time to xenograft excision (45). Finally, comparisons of microregional pimonidazole and P-ACC1/2 immunostaining were made for individual microscopic fields of contiguous sections at medium and medium-high magnifications (×100 and ×200) according to the following criteria: overlap, no overlap, mixture of overlap and no overlap, and absence of signal (45).
TABLE 1.
Semiquantitative microscopic analysis of immunostaining and necrotic regions for sections of WT and HIF-1α-null mouse fibrosarcoma xenografts
| Parameter | Pimonidazole staininga | Necrosis | P-ACC1/2 stainingb | |||
|---|---|---|---|---|---|---|
| WT | HIF-1α null | WT | HIF-1α null | WT | HIF-1α null | |
| Avg % of tumors | 45c | 25c | 45c | 5c | 150d | 91.2d |
| No. of tumors | 4 | 4 | 4 | 4 | 4 | 4 |
| SD | 0.58 | 0.58 | 1.3 | 0.58 | 25.8 | 16.5 |
| P valuee | ≤0.0027 | ≤0.0013 | ≤0.0086 |
RESULTS
Hypoxia combined with glucose deprivation depletes total cellular ATP levels to the same extent in WT and HIF-1α-null cells.
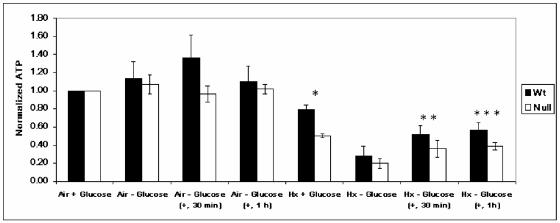
To further investigate the dependence of hypoxia-inducible c-Jun phosphorylation on glucose utilization, we measured total cellular ATP levels in WT and HIF-1α-null cells under identical normoxic and hypoxic conditions in the presence and absence of glucose. Figure 1 shows that ATP levels did not change significantly in normoxic WT and HIF-1α-null cells exposed to glucose-free medium for 8 h (Air − Glucose). In contrast, ATP levels in WT and HIF-1α-null cells deprived of glucose for 8 h under hypoxia (Hx − Glucose) declined to ≤30% of the control values for both normoxic cell types cultured in complete medium (Air + Glucose). There was no significant difference in the relative amount of total ATP between WT and HIF-1α-null cells exposed to hypoxia and glucose deprivation (significant differences between each pair of cell types for each condition are designated by asterisks in Fig. 1). Therefore, although ATP was depleted as expected in both WT and HIF-1α-null cells in response to the combined stress of hypoxia and glucose deprivation, ultimately ATP was conserved similarly in both cell types. Figure 1 also shows that when glucose-free medium on hypoxic WT and HIF-1α-null cells (Hx − Glucose) was reconstituted to the glucose concentration in complete medium (+, 30 min; +, 1 h), the relative levels of ATP increased under hypoxia in both cell types with similar kinetics (during 1 h after the addition of glucose). Both WT and HIF-1α-null cells remained viable in response to the energy depletion conditions used for these studies, as determined by a proliferation assay (51; data not shown).
FIG. 1.
Total cellular ATP levels in WT and HIF-1α-null cells exposed to prolonged hypoxia in the presence and absence of glucose. ATP levels were measured in whole-cell lysates of WT and HIF-1α-null MEFs exposed to normoxia (Air + Glucose) or hypoxia (Hx) in complete (+ Glucose) or glucose-free (− Glucose) medium for 8 h. In glucose reconstitution experiments, degassed glucose solution was added to a final concentration of 4.5 g/liter at either 30 min (+, 30 min) or 1 h (+, 1 h) before cell lysis. Data were normalized to measurements of total ATP in normoxic cells in complete medium (Air + Glucose). Error bars, standard deviations from three independent experiments. Statistical comparisons were made between WT and HIF-1α-null cells for each condition shown (only the differences indicated by one or more asterisks are statistically significant). *, P ≤ 0.02, Hx + Glucose; **, P ≤ 0.04, Hx − Glucose (+, 30 min); ***, P ≤ 0.02, Hx − Glucose (+, 1 h). The addition of glucose to both WT and HIF-1α-null cells exposed to hypoxia and glucose deprivation increased total cellular ATP levels by 1 h after the addition [WT cells, P ≤ 0.005; HIF-1α-null cells, P ≤ 0.005; Hx − Glucose compared with Hx − Glucose (+, 1 h)].
These experiments provide evidence that the loss of HIF-1-dependent c-Jun phosphorylation in WT cells subjected to the stress of hypoxia and glucose deprivation (32) is unlikely to be the consequence of a depleted ATP reserve, since the relative levels of ATP were conserved to the same extent in both WT and HIF-1α-null cells exposed to the same stress. This particular finding also implies the existence of a biochemical mechanism (or mechanisms) at least partially independent of HIF-1 activity for regulating ATP levels in cells exposed to severe energy stress.
AMPK is strongly activated in WT and HIF-1α-null cells exposed to hypoxia combined with glucose deprivation.
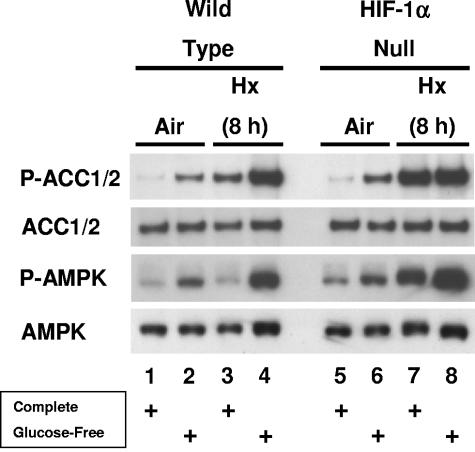
Many researchers have reported that AMPK activity—generally attributed to α subunits rather than to other members of the AMPK class—is induced by various hypoxic or ischemic conditions in diverse cell types and tissues (e.g., see references 5, 12, 13, 20, 28, 33, 38, 39, 41, 58, and 61). Therefore, we reasoned that AMPK could be activated in WT and HIF-1α-null cells stressed by hypoxia and glucose deprivation as part of a mechanism to conserve ATP levels. To investigate this possibility, we exposed WT and HIF-1α-null cells to the same normoxic and hypoxic conditions as those used for the measurement of total cellular ATP (Fig. 1). As before, control cells were incubated in complete or glucose-free medium under normoxia or hypoxia. To detect AMPK activity, we performed immunoblotting studies of whole-cell lysates with an antibody that recognizes a consensus phosphorylation site on the metabolic enzyme ACC-1 (phospho-ACC1 Ser79, mouse), which is an established AMPK target (19). This antibody may also recognize the equivalent phosphorylation site on ACC2 (Ser212, mouse), which is also an AMPK target (19); here we designate both phosphorylated forms of mouse ACC P-ACC1/2. To corroborate the immunoblotting results obtained with the P-ACC1/2 antibody, we probed replicate blots with an antibody that recognizes a phosphorylation site required for the activation of mammalian AMPK (phospho-AMPK-Thr172, P-AMPK) (36, 47).
Figure 2 shows specific bands for P-ACC1/2 and P-AMPK levels, as well as specific bands for the corresponding total ACC1/2 and AMPK protein levels. The results in Fig. 2 demonstrate that phosphorylation of ACC1/2 on the AMPK target site was induced in both WT and HIF-1α-null cells in normoxic glucose-free medium (lanes 2 and 6 compared with lanes 1 and 5) and in hypoxic complete medium (lanes 3 and 7 compared with lanes 1 and 5). ACC1/2 phosphorylation in the WT cells was more responsive to the combination of hypoxia and glucose deprivation than to hypoxia alone, whereas it was similarly induced in the HIF-1α-null cells under both conditions (lanes 4 and 8 compared with lanes 3 and 7). The relatively high induction of ACC1/2 phosphorylation in hypoxic HIF-1α-null cells both in the presence and in the absence of glucose could be explained by their diminished ability compared with WT cells to utilize glucose for glycolytic ATP production (32), effectively creating a combined stress of hypoxia and glucose deprivation in complete medium. Thus, the HIF-1α-null cells showed maximal ACC1/2 phosphorylation following 8 h of hypoxia in complete medium, whereas the hypoxic WT cells showed relatively less ACC1/2 phosphorylation as they adapted to this energy stress through increased glucose utilization. Overall, these findings demonstrate that phosphorylation of ACC1/2 on the AMPK target site was induced by either glucose deprivation or hypoxia, independently of HIF-1 activity, and that hypoxia combined with glucose deprivation was a more potent signal than hypoxia alone for the induction of this phosphorylation.
FIG. 2.
Activation of AMPK in WT and HIF-1α-null cells exposed to prolonged hypoxia in the presence and absence of glucose. Immunoblot assays of total protein from WT and HIF-1α-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, P-AMPK, or total AMPK. For details, see Materials and Methods. These results are representative of at least three independent experiments.
Figure 2 also shows that AMPK Thr172 phosphorylation was increased in both WT and HIF-1α-null cells in normoxic, glucose-free medium (lanes 2 and 6 compared with lanes 1 and 5) and in hypoxic, glucose-free medium (lanes 4 and 8 compared with lanes 3 and 7). These findings indicate that glucose deprivation under normoxia or following 8 h of hypoxia caused activation of AMPK (indicated by the induction of Thr172 phosphorylation) in both cell types, which is consistent with the concurrent induction of ACC1/2 phosphorylation shown in Fig. 2. Unlike ACC1/2 phosphorylation, however, AMPK phosphorylation was increased in the HIF-1α-null cells, but not in the WT cells, in hypoxic complete medium (lanes 3 and 7 compared with lanes 1 and 5). This finding could also be explained by the ability of the WT cells to adapt to the energy stress of prolonged hypoxia through increased glucose utilization: AMPK activity was attenuated in hypoxic WT cells in response to increased glycolytic ATP production, whereas it remained high in hypoxic HIF-1α-null cells. The transient induction of AMPK activity in response to hypoxia is considered in detail below.
Hypoxia-inducible phosphorylation of ACC1/2 in the presence and absence of glucose requires AMPK.
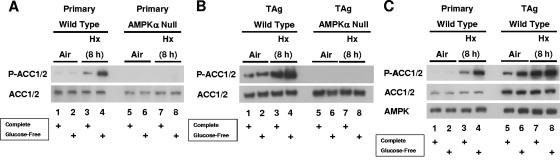
To determine whether AMPK is required for the hypoxia-inducible phosphorylation of ACC1/2 described above or whether a different AMPK activity can also contribute to this phosphorylation, we exposed MEFs nullizygous for both α subunits (AMPKα-null cells) to hypoxia in the presence and absence of glucose. Figure 3, which shows the effect of hypoxia on ACC1/2 phosphorylation in both primary (Fig. 3A) and simian virus 40 large T antigen-immortalized (Fig. 3B) WT and AMPKα-null cells, demonstrates that AMPKα activity is essential for this phosphorylation in response to both hypoxia (lane 7 compared with lane 3, Fig. 3A and B) and hypoxia combined with glucose deprivation (lane 8 compared with lane 4, Fig. 3A and B). In agreement with the results shown in Fig. 2, hypoxia combined with glucose deprivation was a more potent signal than hypoxia alone for the induction of ACC1/2 phosphorylation in the WT cells. The induction of ACC1/2 phosphorylation by glucose deprivation under normoxic conditions shown in Fig. 2 was evident in the immortalized cells (Fig. 3C; lane 6 compared with lane 5), which expressed higher basal levels of ACC1/2 protein than the primary cells from which they were derived (Fig. 3C, ACC1/2, lanes 1 to 4 compared with lanes 5 to 8). All subsequent studies involving AMPKα-null cells were performed with these immortalized MEFs and the corresponding immortalized WT MEFs. In summary, by using genetically modified MEFs nullizygous for expression of both α subunits, we determined that AMPK activity is solely responsible for the phosphorylation of ACC1/2 induced by prolonged hypoxia in the presence and absence of glucose, at least in mesenchymal cells. Therefore, we used changes in ACC1/2 phosphorylation as a direct readout for hypoxia-inducible AMPK activity in MEFs cultured in complete or glucose-free medium.
FIG. 3.
Requirement for AMPK to phosphorylate ACC1/2 in cells exposed to prolonged hypoxia in the presence and absence of glucose. (A) Immunoblot assays of total protein from primary WT and AMPK-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (B) Immunoblot assays of total protein from immortalized WT and AMPK-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (C) Immunoblot assays of total protein from primary and immortalized WT and AMPK-null MEFs exposed to these conditions. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, and total AMPK. Immortalized MEFs were used for all other studies involving genetic knockouts of the AMPKα subunits. TAg, simian virus 40 large T antigen.
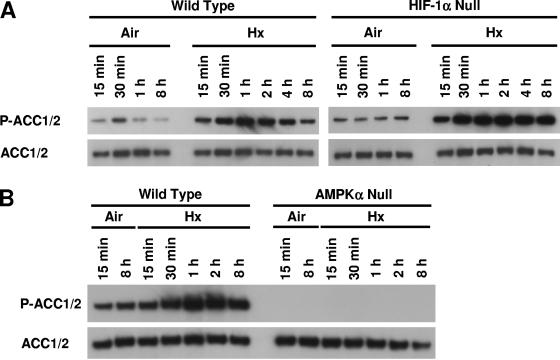
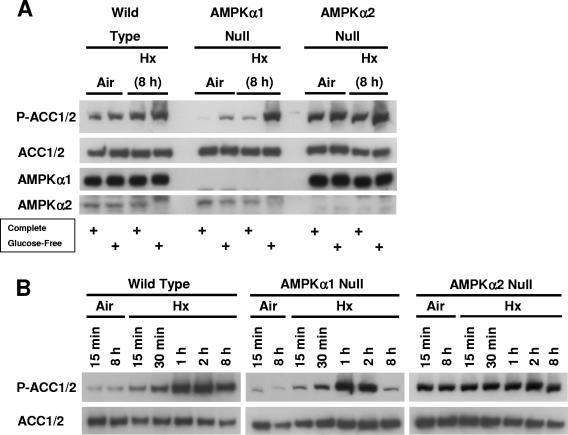
To further investigate the relationship between hypoxia-inducible AMPK activation and ACC1/2 phosphorylation, we used the same phosphorylation site-specific ACC1/2 antibody to perform immunoblotting experiments with lysates of WT and HIF-1α-null cells cultured in complete medium under normoxia or exposed to hypoxia for various times (Fig. 4A). These experiments revealed that ACC1/2 phosphorylation was rapidly induced by hypoxia in both WT and HIF-1α-null cells: increased P-ACC1/2 signals were detected in lysates of both cell types harvested as early as 15 min after exposure to hypoxia. Consistent with the idea that HIF-1α-null cells enter a state of glucose deprivation following exposure to hypoxia, hypoxia-inducible ACC1/2 phosphorylation persisted in these cells for up to 8 h of stress, whereas this phosphorylation was attenuated in the WT cells. Figure 4B, which shows results from an identical hypoxia study involving the WT and AMPKα-null cells used for the studies presented in Fig. 3, demonstrates that AMPK activity is responsible for the rapid hypoxia-inducible ACC1/2 phosphorylation found in both WT and HIF-1α-null cells in complete medium. We emphasize that the WT cells used for Fig. 4B also showed an attenuated pattern of ACC1/2 phosphorylation following 4 to 8 h of hypoxia similar to that found for the different WT cells used for Fig. 4A.
FIG. 4.
Time courses of ACC1/2 phosphorylation in WT, HIF-1α-null, and AMPKα-null cells exposed to hypoxia in complete medium. (A) Immunoblot assays of total protein from WT and HIF-1α-null MEFs harvested following exposure to normoxia or hypoxia for the indicated times. (B) Immunoblot assays of total protein from WT and AMPKα-null MEFs harvested following exposure to normoxia or hypoxia for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2 and total ACC1/2.
To determine whether both AMPKα subunits (α1 and α2) contribute to hypoxia-inducible ACC1/2 phosphorylation, we separately exposed immortalized AMPKα1-null and AMPKα2-null cells to the same conditions as those used for the studies shown in Fig. 2 and 4. Figure 5 shows that ACC1/2 phosphorylation was more responsive to induction in AMPKα1-null cells (containing AMPKα2) than in AMPKα2-null cells (containing AMPKα1) by all stress conditions: glucose deprivation under normoxia and the combination of hypoxia and glucose deprivation (Fig. 5A) and hypoxia in complete medium (Fig. 5B). In fact, the basal level of ACC1/2 phosphorylation was constitutively high in the AMPKα2-null cells under these conditions, with little if any evidence of inducibility. Presumably, the corresponding patterns of stress-inducible ACC1/2 phosphorylation in WT cells represent some integration of the activities of both AMPKα1 and -α2 (Fig. 5). The different response of stress-inducible ACC1/2 phosphorylation in MEFs containing either an AMPKα1 or an AMPKα2 subunit suggests that the AMPKα2 subunit is more important in this cell type for the activation of AMPK by hypoxia or glucose deprivation under normoxia. In support of the possibility that AMPKα2 activation is particularly sensitive to hypoxia, it was recently reported that both the expression and activity AMPKα2 were increased by this stress in various hypoxic human glioma cell lines, whereas the expression and activity of AMPKα1 were constitutive under the same conditions (42). It is conceivable that the relatively high basal ACC1/2 phosphorylation found in the AMPKα2-null cells is a compensatory response involving activation of AMPK containing the remaining α1 subunit.
FIG. 5.
ACC1/2 phosphorylation in AMPKα1-null and AMPKα2-null cells exposed to hypoxia in the presence and absence of glucose. (A) Immunoblot assays of total protein from WT, AMPKα1-null, and AMPKα2-null MEFs lysed following exposure to normoxia or hypoxia in complete or glucose-free medium for 8 h. (B) Immunoblot assays of total protein from AMPKα1-null and AMPKα2-null MEFs lysed following exposure to normoxia or hypoxia for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2 and total ACC1/2. These results are representative of two independent experiments.
In summary, these findings obtained from experiments involving MEFs nullizygous for α subunit expression indicate that AMPK responds to a range of low-oxygen conditions in the presence and absence of glucose. Our overall finding that hypoxia-inducible ACC1/2 phosphorylation was not detectable in AMPKα-null cells indicates either that other AMPK-like enzymes are much less responsive to hypoxia than AMPK or that they are not highly expressed in MEFs.
Physiological hypoxia simultaneously induces AMPK activity and accumulation of HIF-1α protein.
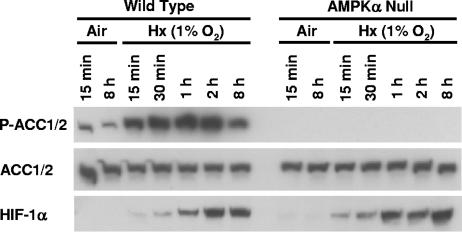
To determine whether AMPK is activated by physiological hypoxia (e.g., 1% O2), as well as pathophysiological hypoxia (e.g., ≤ 0.01% O2), we investigated the induction of ACC1/2 phosphorylation in WT and AMPKα-null cells cultured in complete medium under normoxia or exposure to 1% O2 for various lengths of time. Figure 6 shows that ACC1/2 phosphorylation was rapidly induced in response to this low-oxygen condition with essentially the same kinetics as its induction by pathophysiological hypoxia (Fig. 4). Moreover, HIF-1α protein accumulated in both hypoxic cell types in parallel with hypoxia-inducible ACC1/2 phosphorylation. This experiment demonstrates that AMPK activity is sensitive to a physiologically relevant hypoxic stress and follows induction kinetics that parallel the induction of HIF-1α protein expression. This rapid coinduction of AMPK activity and HIF-1α protein expression indicates that the AMPK and HIF-1 systems operate in parallel in hypoxic MEFs and suggests a rapid concerted response to this stress.
FIG. 6.
ACC1/2 phosphorylation in WT and AMPKα-null cells exposed to physiological hypoxia. Immunoblot assays of total protein from WT and AMPKα-null MEFs harvested following exposure to normoxia or hypoxia (pO2 = 1%) in complete medium for the indicated times. Replicate blots were probed for the relative levels of P-ACC1/2, total ACC1/2, and HIF-1α.
Phosphorylation of ACC1/2 at the AMPK target site overlaps with hypoxic microregions within xenografts prepared from H-Ras-transformed WT and HIF-1α-null MEFs.
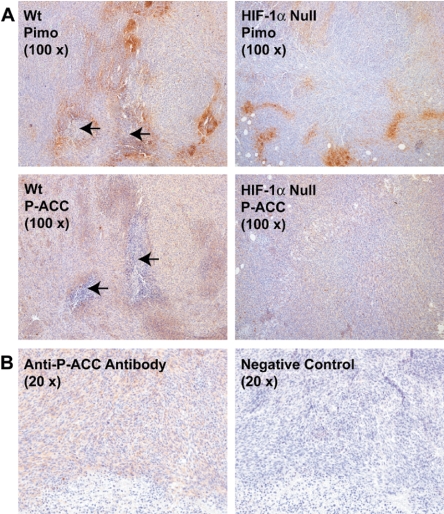
Our in vitro findings for the activation of AMPK (defined as specific ACC1/2 phosphorylation) by hypoxia and hypoxia combined with glucose deprivation suggested that oxygen and glucose gradients in solid tumors would be correlated or overlap with AMPK activity, independently of the induction of HIF-1 activity. To investigate this possibility, we prepared tumor xenografts by implanting nude mice with WT and HIF-1α-null MEFs that had been transformed by retroviral infection of an H-ras oncogene. These cells exhibited the same in vitro patterns of inducible ACC1/2 phosphorylation as those shown in Fig. 2 and 4 for the immortalized parental cells (data not shown). We injected the 2-nitroimidazole pimonidazole hydrochloride 3 h before sacrificing tumor-bearing mice to enable the detection of hypoxic microregions within the fibrosarcomas by immunohistochemistry (3, 10, 44, 45). Figure 7 shows representative examples of adjacent histological sections from WT and HIF-1α-null fibrosarcomas that had been immunostained for pimonidazole binding or ACC1/2 phosphorylation (P-ACC). As expected from similar studies with H-Ras-transformed MEFs (e.g., see references 14 and 48), we found that the WT tumors were significantly larger than the HIF-1α-null tumors (WT, 187 ± 47 mm3; HIF-1α null, 85 ± 15 mm3 [averages ± standard deviations, n = 4]), and contained both more hypoxic regions and areas of necrosis (Table 1). We observed that the WT tumors had more intense P-ACC1/2 staining than the HIF-1α-null tumors (average score, 150 for WT tumors; average score, 91 for HIF-1α-null tumors; Table 1), but in general, P-ACC1/2 and pimonidazole immunostaining (indicating hypoxic microregions) overlapped in both tumor types. However, we observed that P-ACC1/2 immunostaining was particularly intense in regions adjacent to necrosis that were also hypoxic (Fig. 7). These observations are consistent with the induction of AMPK activity by oxygen gradients (low-oxygen conditions) in microenvironments within these experimental tumors, possibly coupled with glucose deprivation. It is striking that P-ACC1/2 immunostaining—and thus evidently AMPK activity—was most intense near areas of necrosis, which probably developed in response to severe oxygen and glucose deprivation.
FIG. 7.
Phosphorylation of ACC1/2 in hypoxic regions in experimental tumors prepared from H-Ras-transformed MEFs. (A) Representative images of immunostaining for pimonidazole (Pimo) binding and ACC1/2 phosphorylation (P-ACC) on 4-μm-thick contiguous sections of paraffin-embedded tissue blocks from WT and HIF-1α-null MEF xenografts. Arrows indicate necrotic regions in the sections. (B) Images of immunostaining for ACC1/2 phosphorylation on 4-μm-thick contiguous sections of a paraffin-embedded block from a WT MEF xenograft. The image on the right, which was obtained without using the primary anti-P-ACC1/2 antibody, is a negative control for the immunological detection of ACC1/2 phosphorylation.
AMPK expression is important for promoting the growth of xenografts prepared from H-Ras-transformed MEFs.
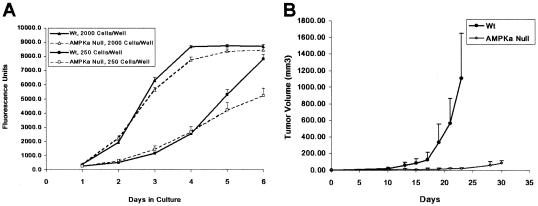
The distribution of P-ACC1/2 immunostaining found on sections of tumor xenografts prepared from H-Ras-transformed WT and HIF-1α-null MEFs (Fig. 7) is consistent with the broad activation of AMPK within gradients of oxygen and glucose present within the microenvironments of these tumor types (e.g., see references 8, 14, and 49). To determine whether the prevalence of AMPK activity in these tumors is biologically significant, we prepared xenografts from H-Ras-transformed WT and AMPKα-null cells and measured the relative growth rates of the two experimental groups. Figure 8 shows the effect of the absence of AMPK expression on the proliferation of these H-Ras-transformed MEFs in vitro (Fig. 8A) and on the growth of tumor xenografts prepared from the same cells (Fig. 8B). There were no significant differences between the in vitro proliferation rates of the transformed WT and AMPKα-null cells (Fig. 8A) until the medium had been depleted as the cells approached confluence (e.g., day 5 of culture for the lower plating value). This outcome is expected for AMPK activation in vitro, which is sensitive to glucose stress under normoxia (Fig. 2 and 3). Although much smaller than the WT tumors at the end of the study (Fig. 8B), the AMPKα-null tumors grew in all eight animals in the experimental group. The striking effect of the AMPKα null mutation on the growth of these tumor xenografts suggests that full AMPK activity is necessary for the H-Ras-transformed MEFs to adapt to the energy demands of solid-tumor growth.
FIG. 8.
Effect of loss of AMPK activity on the proliferation of H-Ras-transformed MEFs. (A) In vitro proliferation of WT (closed symbols) and AMPKα-null (open symbols) MEFs determined by an Alamar Blue assay. Cells/well refer to 96-well plates (triangles, 2,000 cells/well; squares, 250 cells/well). (B) Growth of tumor xenografts prepared from the WT and AMPKα-null MEFs used for the in vitro proliferation assay. There were eight mice in each of the two groups corresponding to the WT (closed symbols) and AMPKα-null (AMPKa, open symbols) tumor genotypes (for details, see Materials and Methods). Error bars are standard deviations.
DISCUSSION
An important inference from our present studies is that AMPK, which is considered to monitor the level of total cellular ATP (19), acts in concert with HIF-1 as part of the adaptive response of mammalian cells to hypoxia or ischemia. This inference is based on two major findings. First, we found that AMPK was strongly activated in both WT and HIF-1α-null cells exposed to hypoxia combined with glucose deprivation (Fig. 2), a severe energy stress that depleted total cellular ATP levels to the same extent in both cell types (Fig. 1). Presumably, HIF-1 activity must be supplemented to conserve total cellular ATP under these conditions. Second, we found that AMPK was rapidly activated in both WT and HIF-1α-null cells exposed to hypoxia in complete medium (Fig. 4). The induction of AMPK activity in WT MEFs occurred with kinetics similar to those of the accumulation of HIF-1α protein in these cells under different hypoxic conditions (pO2 = 1% or ≤0.01%; Fig. 4 and 6). Evidently, AMPK contributes to the general cellular response to hypoxia involving HIF-1, even when ATP levels are not significantly depleted by energy stress. AMPK has been reported to mobilize glucose transporters such as GLUT1 and to directly activate the key glycolytic enzyme 6-phosphofructo-1/2-kinase (2, 19). Therefore, one mechanism by which AMPK and HIF-1 could adapt cells to hypoxia is to generate ATP by enhancing glucose absorption and glycolytic activity. The concept that AMPK is part of a concerted cellular response to hypoxia or ischemia is consistent with a systems (physiological) model in which the adaptation to energy stress involves a complex network of signaling pathways in which HIF-1 is a highly connected node (4, 30).
In severely hypoxic cells, AMPK could be more important than HIF-1 for sustaining ATP levels because HIF-1 activity may be diminished (24). Considering that microenvironments having heterogeneous oxygen and glucose levels commonly exist within solid tumors (35, 59), we prepared experimental tumors from H-Ras-transformed derivatives of the WT and HIF-1α-null cells used for our in vitro studies to investigate hypoxia-inducible AMPK activation in a tissue. Some pioneering studies of AMPK or AMPK-like activity in tumor cells and experimental tumors have been reported. For example, AMPK has been shown to promote the survival of glucose-deprived human pancreatic carcinoma cell lines in vitro and to support the growth of xenografts prepared from one of these cell lines (PANC-1) in nude mice (27). In this study, transiently or stably transfected human α subunit antisense constructs were used to suppress the expression of endogenous α subunits. Some results of the study indicated that the prosurvival function of AMPK in energetically stressed tumor cells may require cooperation with an AMPK-like activity. In this connection, stable overexpression of ARK5, a member of the AMPK class (36), has been shown to promote the in vitro survival of HepG2 human hepatocarcinoma cells subjected to glucose deprivation or exposed to the proapoptotic cytokines tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and tumor necrosis factor alpha (55). Constitutive overexpression of the sucrose-nonfermenting protein kinase/AMP-activated protein kinase-related protein kinase (SNARK), another member of the AMPK class, in HepG2 cells also showed a protective effect in response to glucose deprivation in vitro (54). In vivo, constitutive overexpression of ARK5 in PANC-1 cells was found to enhance the growth and metastatic potential of transfected compared with control tumor xenografts in nude mice (29, 57). In other work, it was proposed that ARK5 is phosphorylated by AKT in glucose-deprived HepG2 and PANC-1 cells (53, 57), suggesting that AKT activation is central to inducing tolerance to this energy stress (11). ARK5 has been shown to be transcriptionally activated in hypoxic and glucose-deprived HepG2 cells through a transforming growth factor β signaling process (56). We were unable to detect an induction of either ARK5 activity or protein expression in MEFs exposed to the hypoxic conditions used for our present in vitro studies (unpublished results). Instead, by using MEFs nullizygous for α subunit expression, we found that AMPK is rapidly induced by hypoxia in this cell type both in the presence and in the absence of glucose and in response to glucose deprivation under normoxia (Fig. 3 to 6).
As described in Results, we used immunostaining of pimonidazole binding to tumor tissue to detect hypoxic microregions in histological sections of fibrosarcoma xenografts prepared from transformed WT and HIF-1α-null cells (Table 1 and Fig. 7). Hypoxic microregions appeared as focal patches of pimonidazole immunostaining in both types of tumors; such patterns have been reported by others for the binding of chemical hypoxia probes to experimental rodent fibrosarcomas (8, 14, 49). In general, this study demonstrated that microregions containing tumor cells with enhanced ACC1/2 phosphorylation (P-ACC1/2 immunostaining) overlapped with hypoxic microregions, particularly around areas of necrosis (Fig. 7). Such areas can develop in solid tumors along gradients of oxygen, glucose, and other substances that emanate from functional tumor microvasculature (35, 59). Given our conclusion that the induction of ACC1/2 phosphorylation in hypoxic MEFs cultured in the presence and absence of glucose requires AMPK activity, we infer that oxygen and glucose gradients also induced AMPK activity in the tumors prepared from these cells. We emphasize that because the HIF-1α-null tumors were significantly smaller than the WT tumors—the presence of HIF-1α confers a growth advantage on such fibrosarcomas (48)—they contained less hypoxia and much less necrosis than the WT tumors and consequently less obvious overlap between P-ACC1/2 and pimonidazole immunostaining (Fig. 7). It is possible that the more aggressively growing WT tumors contained more severe oxygen gradients than the slower-growing HIF-1α-null tumors, leading to relatively more hypoxic and necrotic areas on sections from WT compared with HIF-1α-null tumor tissue. Both WT and HIF-1α-null tumors also exhibited P-ACC1/2 immunostaining that was distributed beyond viable regions surrounding necrosis and hypoxic microregions detected by pimonidazole immunostaining, indicating that AMPK activity was induced in these tissue microenvironments.
Our in vivo studies with tumor xenografts prepared from H-Ras-transformed derivatives of WT and AMPKα-null MEFs demonstrate that the absence of AMPK activity greatly inhibits the growth of this particular tumor model (Fig. 8), supporting the hypothesis that AMPK is important for the adaptation of tumor cells to the hypoxic and glucose-deprived microenvironments common in solid tumors (35, 59). However, it has also been reported that AMPK activation can inhibit the growth of some experimental tumors (e.g., see references 15, 16, and 46). Further work is necessary to determine the effect of inhibition of AMPK activity on the growth of both model and spontaneous tumors and to understand the mechanistic basis for the suppression of experimental mouse fibrosarcoma growth.
Although other microenvironmental factors cannot be discounted, it is possible that tumor microregions transiently deficient in glucose rather than oxygen could induce ACC1/2 phosphorylation, which is sensitive to glucose deprivation in oxygenated medium (Fig. 2 and 3). It is also worth stating that while studies of pimonidazole binding have demonstrated significant microregional overlap between regions of hypoxia and the location of some hypoxia-inducible proteins, such as carbonic anhydrase IX, GLUT1, involucrin, and erythropoietin (1, 3, 10), there are significant exceptions. For example, no positive correlation was found for the colocalization of pimonidazole binding with that of HIF-1α protein expression in sections of human head and neck tumors (23).
In current physiological models of AMPK function, the holoenzyme is activated in cells that have undergone significant ATP depletion to transmit both positive and negative signals to the metabolic apparatus through key control points, ultimately helping to restore energy homeostasis or promote survival (9, 19, 47). Here we have used genetic models (MEFs defective for AMPKα subunit or HIF-1α expression) to explore the relationship between the AMPK and HIF-1 systems in hypoxic cells cultured in the presence and absence of glucose. In summary, we have demonstrated that AMPK activity (detected by phosphorylation at the AMPK target site of ACC1/2) is induced not only by severe energy stress (prolonged hypoxia combined with glucose deprivation) but also soon after the onset of either physiological or pathophysiological hypoxia, when total cellular ATP levels are not significantly depleted. In the context of tumor biology, it will be fundamentally important to rigorously investigate the oxygen sensitivity of this response by measuring the kinetics of AMPK activation at various defined oxygen levels in both normal and related transformed cells. More generally, it will also be important to understand the signaling processes that operate upstream of rapid hypoxia-inducible activation of AMPK, which occurs on a time scale similar to that of the hypoxia-inducible accumulation of HIF-1α protein. It has been proposed that inhibition of oxidative phosphorylation by hypoxia activates AMPK through an increased [AMP]/[ATP] ratio, at least in certain oxygen-sensitive cell types (12). However, we have not been able to detect significant changes in this ratio in MEFs exposed to hypoxia in complete medium for up to 8 h (unpublished results). We also obtained evidence from studies of experimental tumor xenografts prepared from transformed derivatives of WT and HIF-1α-null cells that AMPK is activated by hypoxia combined with glucose deprivation, as well as by hypoxia within glucose gradients. It is possible that the activation of AMPK in hypoxic tumor microenvironments is a mechanism by which tumor cells can enter a protective state of quiescence. Such viable cells might support tumor growth with adequate reoxygenation, achieved either naturally or in response to therapeutic intervention. Indeed, literature reports suggest that AMPK-dependent regulation of cellular energy metabolism and aspects of malignant progression could be a general phenomenon in human cancer (e.g., see references 7, 27, 33, 41, and 52). We hypothesize that AMPK and HIF-1 act in a concerted manner during the general adaptive response of mammalian cells to hypoxia. Investigating this hypothesis will further increase our knowledge of how signal transduction and cellular energy metabolism are integrated at a network level.
Acknowledgments
This work was supported by Public Health Service grant CA73807 from the National Cancer Institute and grants QLG1-CT-2001-01488 and LSHM-CT-2004-005272 from the European Commission.
We thank Grahame Hardie for the gift of antibodies capable of specifically recognizing mouse AMPKα subunits.
REFERENCES
- 1.Airley, R. E., J. Loncaster, J. A. Raleigh, A. L. Harris, S. E. Davidson, R. D. Hunter, C. M. West, and I. J. Stratford. 2003. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104**:**85-91. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, A., S. Moncada, and J. P. Bolanos. 2004. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6**:**45-51. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy, M. O., K. Amin, S. C. Chou, Z. A. Haroon, M. Varia, and J. A. Raleigh. 2005. Erythropoietin and erythropoietin receptor expression in head and neck cancer: relationship to tumor hypoxia. Clin. Cancer Res. 11**:**20-27. [PubMed] [Google Scholar]
- 4.Barabasi, A. L., and Z. N. Oltvai. 2004. Network biology: understanding the cell's functional organization. Nat. Rev. Genet. 5**:**101-113. [DOI] [PubMed] [Google Scholar]
- 5.Beauloye, C., A. S. Marsin, L. Bertrand, U. Krause, D. G. Hardie, J. L. Vanoverschelde, and L. Hue. 2001. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 505**:**348-352. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum, M. J. 2005. Activating AMP-activated protein kinase without AMP. Mol. Cell 19**:**289-290. [DOI] [PubMed] [Google Scholar]
- 7.Brugarolas, J., and W. G. Kaelin, Jr. 2004. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 6**:**7-10. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas-Navia, L. I., D. Yu, R. D. Braun, D. M. Brizel, T. W. Secomb, and M. W. Dewhirst. 2004. Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res. 64**:**6010-6017. [DOI] [PubMed] [Google Scholar]
- 9.Carling, D. 2004. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem. Sci. 29**:**18-24. [DOI] [PubMed] [Google Scholar]
- 10.Chou, S. C., Y. Azuma, M. A. Varia, and J. A. Raleigh. 2004. Evidence that involucrin, a marker for differentiation, is oxygen regulated in human squamous cell carcinomas. Br. J. Cancer 90**:**728-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esumi, H., K. Izuishi, K. Kato, K. Hashimoto, Y. Kurashima, A. Kishimoto, T. Ogura, and T. Ozawa. 2002. Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent manner. J. Biol. Chem. 277**:**32791-32798. [DOI] [PubMed] [Google Scholar]
- 12.Evans, A. M., K. J. W. Mustard, C. N. Wyatt, C. Peers, M. Dipp, P. Kumar, N. P. Kinnear, and D. G. Hardie. 2005. Does AMP-activate protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J. Biol. Chem. 280**:**41504-41511. [DOI] [PubMed] [Google Scholar]
- 13.Frederich, M., L. Zhang, and J. A. Balschi. 2005. Hypoxia and AMP independently regulate AMP-activated protein kinase activity in the heart. Am. J. Physiol. Heart Circ. Physiol. 288**:**2412-2421. [DOI] [PubMed] [Google Scholar]
- 14.Grunstein, J., W. G. Roberts, O. Mathieu-Costello, D. Hanahan, and R. S. Johnson. 1999. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 59**:**1592-1598. [PubMed] [Google Scholar]
- 15.Han, S., F. R. Khuri, and J. Roman. 2006. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 66**:**315-323. [DOI] [PubMed] [Google Scholar]
- 16.Han, S., and J. Roman. 2006. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol. Cancer Ther. 5**:**430-437. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan, D., D. Lane, L. Lipsich, M. Wigler, and M. Botchan. 1980. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell 21**:**127-139. [DOI] [PubMed] [Google Scholar]
- 18.Hardie, D. G., and D. A. Pan. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30**:**1064-1070. [DOI] [PubMed] [Google Scholar]
- 19.Hardie, D. G., J. W. Scott, D. A. Pan, and E. R. Hudson. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546**:**113-120. [DOI] [PubMed] [Google Scholar]
- 20.Horman, S., C. Beauloye, D. Vertommen, J. L. Vanoverschelde, L. Hue, and M. H. Rider. 2003. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J. Biol. Chem. 278**:**41970-41976. [DOI] [PubMed] [Google Scholar]
- 21.Huang, L. E., and H. F. Bunn. 2003. Hypoxia-inducible factor and its biomedical relevance. J. Biol. Chem. 278**:**19575-19578. [DOI] [PubMed] [Google Scholar]
- 22.Hue, L., C. Beauloye, L. Bertrand, S. Horman, U. Krause, A. S. Marsin, D. Meisse, D. Vertommen, and M. H. Rider. 2003. New targets of AMP-activated protein kinase. Biochem. Soc. Trans. 31**:**213-215. [DOI] [PubMed] [Google Scholar]
- 23.Janssen, H. L., K. M. Haustermans, D. Sprong, G. Blommestijn, I. Hofland, F. J. Hoebers, E. Blijweert, J. A. Raleigh, G. L. Semenza, M. A. Varia, A. J. Balm, M. L. van Velthuysen, P. Delaere, R. Sciot, and A. C. Begg. 2002. HIF-1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 54**:**1537-1549. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B. H., G. L. Semenza, C. Bauer, and H. H. Marti. 1996. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271**:**C1172-C1180. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, R., B. Spiegelman, D. Hanahan, and R. Wisdom. 1996. Cellular transformation and malignancy induced by ras require c-jun. Mol. Cell. Biol. 16**:**4504-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen, S. B., B. Viollet, F. Andreelli, C. Frosig, J. B. Birk, P. Schjerling, S. Vaulont, E. A. Richter, and J. F. Wojtaszewski. 2004. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 279**:**1070-1079. [DOI] [PubMed] [Google Scholar]
- 27.Kato, K., T. Ogura, A. Kishimoto, Y. Minegishi, N. Nakajima, M. Miyazaki, and H. Esumi. 2002. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21**:**6082-6090. [DOI] [PubMed] [Google Scholar]
- 28.Kim, K. H., M. J. Song, J. Chung, H. Park, and J. B. Kim. 2005. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem. Biophys. Res. Commun. 333**:**1178-1184. [DOI] [PubMed] [Google Scholar]
- 29.Kusakai, G., A. Suzuki, T. Ogura, M. Kaminishi, and H. Esumi. 2004. Strong association of ARK5 with tumor invasion and metastasis. J. Exp. Clin. Cancer Res. 23**:**263-268. [PubMed] [Google Scholar]
- 30.Laderoute, K. 2005. The interaction between HIF-1 and AP-1 transcription factors in response to low oxygen. Semin. Cell Dev. Biol. 16**:**502-513. [DOI] [PubMed] [Google Scholar]
- 31.Laderoute, K. R., J. M. Calaoagan, C. Gustafson-Brown, A. M. Knapp, G. C. Li, H. L. Mendonca, H. E. Ryan, Z. Wang, and R. S. Johnson. 2002. The response of c-Jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1α dependent. Mol. Cell. Biol. 22**:**2515-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laderoute, K. R., J. M. Calaoagan, M. Knapp, and R. S. Johnson. 2004. Glucose utilization is essential for hypoxia-inducible factor 1α-dependent phosphorylation of c-Jun. Mol. Cell. Biol. 24**:**4128-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, M., J. T. Hwang, H. J. Lee, S. N. Jung, I. Kang, S. G. Chi, S. S. Kim, and J. Ha. 2003. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J. Biol. Chem. 278**:**39653-39661. [DOI] [PubMed] [Google Scholar]
- 34.Leff, T. 2003. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem. Soc. Trans. 31**:**224-227. [DOI] [PubMed] [Google Scholar]
- 35.Leo, C., A. J. Giaccia, and N. C. Denko. 2004. The hypoxic tumor microenvironment and gene expression. Semin. Radiat. Oncol. 14**:**207-214. [DOI] [PubMed] [Google Scholar]
- 36.Lizcano, J. M., O. Goransson, R. Toth, M. Deak, N. A. Morrice, J. Boudeau, S. A. Hawley, L. Udd, T. P. Makela, D. G. Hardie, and D. R. Alessi. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23**:**833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marignani, P. A. 2005. LKB1, the multitasking tumour suppressor kinase. J. Clin. Pathol. 58**:**15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsin, A., C. Bouzin, L. Bertrand, and L. Hue. 2002. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 277**:**30778-30783. [DOI] [PubMed] [Google Scholar]
- 39.Marsin, A. S., L. Bertrand, M. H. Rider, J. Deprez, C. Beauloye, M. F. Vincent, G. Van den Berghe, D. Carling, and L. Hue. 2000. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10**:**1247-1255. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, B. J., B. Sato, T. P. Dalton, and K. R. Laderoute. 2005. The metal responsive transcription factor 1 (MTF-1) contributes to HIF-1 activation during hypoxic stress. Biochem. Biophys. Res. Commun. 337**:**860-867. [DOI] [PubMed] [Google Scholar]
- 41.Nagata, D., M. Mogi, and K. Walsh. 2003. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278**:**31000-31006. [DOI] [PubMed] [Google Scholar]
- 42.Neurath, K. M., M. P. Keough, T. Mikkelsen, and K. P. Claffey. 2006. AMP-dependent protein kinase α2 isoform promotes hypoxia-induced VEGF expression in human glioblastoma. Glia 53**:**733-743. [DOI] [PubMed] [Google Scholar]
- 43.Poellinger, L., and R. S. Johnson. 2004. HIF-1 and hypoxic response: the plot thickens. Curr. Opin. Genet. Dev. 14**:**81-85. [DOI] [PubMed] [Google Scholar]
- 44.Raleigh, J. A., D. P. Calkins-Adams, L. H. Rinker, C. A. Ballenger, M. C. Weissler, W. C. Fowler, Jr., D. B. Novotny, and M. A. Varia. 1998. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 58**:**3765-3768. [PubMed] [Google Scholar]
- 45.Raleigh, J. A., S. C. Chou, D. P. Calkins-Adams, C. A. Ballenger, D. B. Novotny, and M. A. Varia. 2000. A clinical study of hypoxia and metallothionein protein expression in squamous cell carcinomas. Clin. Cancer Res. 6**:**855-862. [PubMed] [Google Scholar]
- 46.Rattan, R., S. Giri, A. K. Singh, and I. Singh. 2005. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 280**:**39582-39593. [DOI] [PubMed] [Google Scholar]
- 47.Rutter, G. A., G. Da Silva Xavier, and I. Leclerc. 2003. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 375**:**1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan, H. E., M. Poloni, W. McNulty, D. Elson, M. Gassmann, J. M. Arbeit, and R. S. Johnson. 2000. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 60**:**4010-4015. [PubMed] [Google Scholar]
- 49.Schroeder, T., H. Yuan, B. L. Viglianti, C. Peltz, S. Asopa, Z. Vujaskovic, and M. W. Dewhirst. 2005. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res. 65**:**5163-5171. [DOI] [PubMed] [Google Scholar]
- 50.Semenza, G. L. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3**:**721-732. [DOI] [PubMed] [Google Scholar]
- 51.Shan, S., A. C. Lockhart, W. Y. Saito, A. M. Knapp, K. R. Laderoute, and M. W. Dewhirst. 2001. The novel tubulin-binding drug BTO-956 inhibits R3230ac mammary carcinoma growth and angiogenesis in Fischer 344 rats. Clin. Cancer Res. 7**:**2590-2596. [PubMed] [Google Scholar]
- 52.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 101**:**3329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, A., G. Kusakai, A. Kishimoto, J. Lu, T. Ogura, M. F. Lavin, and H. Esumi. 2003. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J. Biol. Chem. 278**:**48-53. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki, A., G. Kusakai, A. Kishimoto, Y. Minegichi, T. Ogura, and H. Esumi. 2003. Induction of cell-cell detachment during glucose starvation through F-actin conversion by SNARK, the fourth member of the AMP-activated protein kinase catalytic subunit family. Biochem. Biophys. Res. Commun. 311**:**156-161. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki, A., G. Kusakai, A. Kishimoto, Y. Shimojo, S. Miyamoto, T. Ogura, A. Ochiai, and H. Esumi. 2004. Regulation of caspase-6 and FLIP by the AMPK family member ARK5. Oncogene 23**:**7067-7075. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, A., G. I. Kusakai, Y. Shimojo, J. Chen, T. Ogura, M. Kobayashi, and H. Esumi. 2005. Involvement of TGF-β1 signaling in hypoxia-induced tolerance to glucose starvation. J. Biol. Chem. 280**:**31557-31563. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, A., J. Lu, G. Kusakai, A. Kishimoto, T. Ogura, and H. Esumi. 2004. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol. Cell. Biol. 24**:**3526-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terai, K., Y. Hiramoto, M. Masaki, S. Sugiyama, T. Kuroda, M. Hori, I. Kawase, and H. Hirota. 2005. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol. Cell. Biol. 25**:**9554-9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaupel, P. 2004. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 14**:**198-206. [DOI] [PubMed] [Google Scholar]
- 60.Viollet, B., F. Andreelli, S. B. Jorgensen, C. Perrin, A. Geloen, D. Flamez, J. Mu, C. Lenzner, O. Baud, M. Bennoun, E. Gomas, G. Nicolas, J. F. Wojtaszewski, A. Kahn, D. Carling, F. C. Schuit, M. J. Birnbaum, E. A. Richter, R. Burcelin, and S. Vaulont. 2003. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 111**:**91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun, H., M. Lee, S. S. Kim, and J. Ha. 2005. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J. Biol. Chem. 280**:**9963-9972. [DOI] [PubMed] [Google Scholar]