Listeria monocytogenes Flagella Are Used for Motility, Not as Adhesins, To Increase Host Cell Invasion (original) (raw)
Abstract
Flagellar structures contribute to the virulence of multiple gastrointestinal pathogens either as the effectors of motility, as adhesins, or as a secretion apparatus for virulence factors. Listeria monocytogenes is a food-borne, gram-positive pathogen that uses flagella to increase the efficiency of epithelial cell invasion (A. Bigot, H. Pagniez, E. Botton, C. Frehel, I. Dubail, C. Jacquet, A. Charbit, and C. Raynaud, Infect. Immun. **73:**5530-5539, 2005; L. Dons, E. Eriksson, Y. Jin, M. E. Rottenberg, K. Kristensson, C. N. Larsen, J. Bresciani, and J. E. Olsen, Infect. Immun. **72:**3237-3244, 2004). In this study, we aimed to elucidate the mechanism by which flagella contribute to L. monocytogenes invasion. To examine the role of flagella as adhesins, invasion and adhesion assays were performed with flagellated motile and nonmotile bacteria and nonflagellated bacteria. We observed that flagellated but nonmotile bacteria do not adhere to or invade human epithelial cells more efficiently than nonflagellated bacteria. These results indicated that flagella do not function as adhesins to enhance the adhesion of L. monocytogenes to targeted host cells. Instead, it appears that motility is important for tissue culture invasion. Furthermore, we tested whether motility contributes to early colonization of the gastrointestinal tract using a competitive index assay in which mice were infected orally with motile and nonmotile bacteria in a 1:1 ratio. Differential bacterial counts demonstrated that motile bacteria outcompete nonmotile bacteria in the colonization of the intestines at early time points postinfection. This difference is also reflected in invasion of the liver 12 h later, suggesting that flagellum-mediated motility enhances L. monocytogenes infectivity soon after bacterial ingestion in vivo.
Listeria monocytogenes is a saprophytic, gram-positive rod, ubiquitously distributed in the environment. It is the etiologic agent of listeriosis, a food-borne disease affecting humans and a variety of vertebrates (26). Listeriosis occurs primarily in immunocompromised individuals, causing septicemia, meningitis, and meningoencephalitis, and in pregnant women, causing spontaneous abortion. Healthy adults may suffer a febrile gastroenteritis from ingesting large numbers of L. monocytogenes cells (5). The bacterium's unique ability to withstand and even thrive under a variety of stress conditions generally considered to be food preservatives makes L. monocytogenes of particular concern to food producers, regulatory agencies, handlers, and consumers (20).
Flagella and flagellum-mediated motility are integral to the virulence of multiple gastrointestinal pathogens (10, 16). There are three main ways the flagella can contribute to bacterial pathogenesis. The flagellum can serve as a secretion apparatus for virulence factors, similar to a type III secretion system. For example, Yersinia enterolitica secretes a virulence-associated phospholipase A, YlpA, through the flagellar apparatus (28). Flagella can also serve as adhesins to tether bacteria to host cells much like fimbrial adhesins. For instance, the flagella of enteropathogenic Escherichia coli contribute to bacterial adhesion to epithelial cells in a manner that is independent of motility (7). More intuitively, flagella can function as a motility and chemotactic device targeting bacteria to specific areas of the gastrointestinal tract, as demonstrated for Helicobacter pylori. Colonization of the stomach by H. pylori is dependent on the spatial orientation of motile bacteria within the pH gradient of the gastric mucus (21). Bacteria do not adhere to the stomach epithelium, instead localizing close to it to avoid being washed away in the lumen. Overall, the roles of flagella in bacterial pathogenesis are diverse, although not necessarily mutually exclusive.
L. monocytogenes produces five to six peritrichous flagella. Motility genes are down regulated at 37°C in vitro, although there is variation from strain to strain (8, 17, 27). Temperature-dependent regulation of motility and chemotaxis genes can be partially attributed to the negative regulator of motility gene expression, MogR (8). A mogR deletion mutant is strongly attenuated in mice infected intravenously, although nonmotile mutants are not (27), suggesting that a mutation in mogR affects more than motility.
Recently, it was reported that flagella contribute to L. monocytogenes adhesion and invasion of human intestinal epithelial Caco-2 cells. Nonmotile mutants lacking either the entire flagellar apparatus (Δ_fliF_ and Δ_fliI_) or only the flagellar filament (Δ_flaA_) and a chemotactic mutant (Δ_cheY/A_) that constantly tumbles were many fold less adherent and invasive than the parent strains (2, 6). These results could suggest that the flagellum is used as an adhesin. Alternatively, motility may contribute to cell adhesion and invasion by increasing the probability of bacterium-host cell contact or perhaps by influencing the spatial orientation of bacterial interaction with host cell membranes. In fact, centrifugation of the Δ_flaA_ and Δ_cheY/A_ mutants onto host cells only partially complemented their adhesion and invasion defect (6). It would be advantageous for bacteria to interact head on with host cell membranes as internalin A, a major cell surface adhesin of L. monocytogenes, accumulates at the bacterial poles (19). In addition, the physical force generated by unidirectional movement may enhance host cell uptake, as was shown for intercellular spread of bacteria that use an actin-based mechanism of motility (15).
An in vivo role for flagella in the pathogenesis of L. monocytogenes has been elusive. The nonflagellated and chemotaxis mutants described above behave similarly to their parent strains when given orally to mice, as determined by bacterial counts from spleens, mesenteric lymph nodes, or intestinal mucosa at 1 and 3 days postinfection or from livers at 3 days postinfection (2, 6, 27). However, one group reported a significant difference at day 3 postinfection, with the Δ_flaA_ mutant being recovered in higher numbers than the wild-type strain from the spleens of infected mice (6). This difference was not observed at day 1 or day 7 postinfection. Overall, these results suggest that flagellum-mediated motility is not important for systemic listeriosis in mice infected per os.
In this study, we aimed to elucidate the mechanism by which flagella contribute to L. monocytogenes invasion of human epithelial cells and the influence of bacterial motility on early colonization of the intestinal tract in mice. Adhesion and invasion assays were performed with flagellated motile and nonmotile bacteria and nonflagellated bacteria. Our results clearly indicate that flagella function as a motility device, but not as adhesins, to enhance L. monocytogenes invasion of epithelial cells. Moreover, we show that nonmotile bacteria do not compete well with motile bacteria for initial colonization of the intestinal tract and early invasion of the liver after oral infection of mice with L. monocytogenes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The L. monocytogenes strains used for this study are listed in Table 1 and were routinely cultured in brain heart infusion broth (BHI) with or without streptomycin (200 μg/ml). E. coli DH5-α carrying pKSV7 (23) was cultured in Luria-Bertani broth (LB) plus ampicillin (100 μg/ml), whereas E. coli SM10 carrying pPL2 (12) was cultured in LB plus chloramphenicol (25 μg/ml). L. monocytogenes strains carrying pKSV7 or pPL2 were cultured in BHI plus chloramphenicol (10 μg/ml). In preparation for tissue culture assays and flagellar staining, L. monocytogenes was cultured overnight in BHI broth with shaking at 250 rpm at 30°C.
TABLE 1.
Characteristics of the L. monocytogenes strains used in this study
| Strain | Genotype | Description of mutation | Source or reference |
|---|---|---|---|
| 10403S | Wild type (serotype 1/2a) | None | 3 |
| DP-L3903 | 10403S erm+ | 10403S Tn_917_ | 1 |
| HEL-304 | Δ_flaA_a | 840-bp in-frame flaA deletion | This study |
| HEL-447 | Δ_flaA flaA_ + | HEL-304 + flaA ORF integrated into tRNAArg site | This study |
| HEL-487 | Δ_flaA_ erm+ | HEL-304 transduced with Tn_917_ from DP-L3903 | This study |
| HEL-742 | _motB_D23A | Substitution of aspartic acid 23 for alanine | This study |
| HEL-758 | motB+ | HEL-742 complemented with wild-type motB allele | This study |
Construction of in-frame deletion mutants.
In-frame deletion mutants were generated by site-directed mutagenesis with overlap extension (SOEing) PCR in the 10403S wild-type background strain (Table 1) (9). Two sets of primers were designed to amplify DNA from regions upstream and downstream of the segment to be deleted and to incorporate restriction sites (Table 2). These two PCR products were used in a SOEing PCR with the outside primers to create a product containing the deletion. This product was digested with BamHI and KpnI, ligated into the shuttle vector pKSV7, and transformed into DH5-α. Purified plasmids were used to sequence cloned fragments to ensure no additional mutations had occurred. Each construct was electroporated into 10403S to replace the wild-type allele with the deletion by allelic exchange (4). Deletions within the appropriate genes were confirmed by analysis of fragment sizes generated by PCR.
TABLE 2.
PCR primers used in this study
| Gene and primer | 5′→3′ Sequence |
|---|---|
| Δ_flaA_ | |
| Marq114 | CGGGATCCGTGAAGAGAAGACGATTTTTATTG |
| Marq115 | TGAATTTGATATGTTATTAGCTGTTAGTATTTACTTTCATTTGTGTTTCC |
| Marq116 | GGAAACACAAATGAAAGTAAATACTAACAGCTAATAACATATCAAATTCA |
| Marq117 | GCGGTACCGAACGGAAAATTTCACTTACTAAC |
| _motB_D23A | |
| Marq278 | CGGGATCCAGTTGTAGATGGTCAATCTTC |
| Marq279 | GCGGTACCCACTTAGTTCCCAGTTGGAA |
| Marq282 | TTCATATAGTGCTTTGTTGACACTT |
| Marq283 | AAGTGTCAACAAAGCACTATATGGA |
| flaA + | |
| Marq169 | CGGGATCCGATATAAAGCCGATATTTCG |
| Marq170 | CGTGGATCCCTTAACATTGGCTCTGTGC |
Construction of the _motB_D23A MotB point mutant.
The motB gene was mutated by SOEing PCR, substituting the aspartic acid at position 23 for an alanine. Amplification of the upstream segment was performed from 10403S genomic DNA with the primer pair Marq278 and Marq283 (Table 2). Marq278 incorporates a BamHI restriction site, and Marq283 provides the necessary codon change as well as a silent mutation to create a novel HincII restriction site for screening. Amplification of the downstream segment was performed from 10403S genomic DNA with the primer pair Marq282 and Marq279. Marq282 also includes the necessary codon change and the HincII site. Marq279 incorporates a KpnI restriction site. These two amplification products were then used in a SOEing PCR with Marq278 and Marq279. This product was digested with BamHI and KpnI and ligated into the shuttle vector pKSV7 and transformed into DH5-α. Purified plasmids were used to sequence the cloned fragment to ensure no additional mutations had occurred. The construct was electroporated into 10403S to replace the wild-type motB allele with _motB_D23A by allelic exchange (4). Mutants were initially screened for loss of motility on motility agar plates. The motB gene from nonmotile mutants was amplified by PCR and digested with HincII to confirm the allelic exchange.
Complementation of the Δ_flaA_ mutant.
The flaA deletion strain was complemented using the site-specific shuttle integration vector pPL2 (12). The flaA open reading frame (ORF) and promoter region were PCR amplified using forward primer Marq169 and reverse primer Marq170. This PCR product was digested with BamHI and KpnI, ligated into pPL2, and transformed into E. coli SM10, creating HEL-449. This plasmid construct was transferred into L. monocytogenes strain HEL-304 by conjugation. In short, log-phase cultures of HEL-304 and HEL-449 were washed, concentrated ≈10-fold, mixed in a ratio of 1:2 in a final volume of 40 μl LB, and spotted onto BHI agar plates with oxacillin (8 μg/ml) (25). After a 5-h incubation period at 30°C, plates were washed with 1 ml of LB and 100 μl was plated onto BHI agar with streptomycin (200 μg/ml) and chloramphenicol (20 μg/ml). Colonies were screened for gain of motility on motility agar plates. Motile colonies were screened by PCR using primers NC16 and PL95 (12) to amplify a 499-bp product, confirming integration of the vector.
Complementation of the _motB_D23A mutant.
The _motB_D23A mutant strain was complemented by inserting a wild-type copy of the motB gene back into the chromosome by allelic exchange. The wild-type copy of the gene was amplified using forward primer Marq278 and reverse primer Marq279. The PCR product was digested with BamHI and KpnI, ligated into the allelic exchange vector pKSV7, and transformed into DH5-α. Purified plasmid was used to sequence the cloned fragment to ensure no additional mutations had occurred. The construct was electroporated into HEL-742 for allelic exchange. Successful allelic exchange was confirmed by screening for wild-type motility on motility agar and chloramphenicol sensitivity.
Flagellar staining.
Bacteria were stained with crystal violet (11) and viewed by bright-field microscopy with a ×60 oil immersion lens. Digital photographs were taken with an Olympus DP70 digital camera.
Western blotting.
Bacterial cell surface proteins were extracted by boiling the equivalent of 10 ml of washed bacteria in 200 μl of 2× sample buffer (125 mM Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate [SDS], 20% glycerol, 10% β-mercaptoethanol) for 5 min. Extracted proteins were resolved on 12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride using a semidry electroblotting apparatus. The protein blot was reacted with rabbit immune serum to L. monocytogenes flagella (H-AB from Denka Seiken Co., Ltd.) at a 1/100 dilution followed by a goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (24 ng/ml) (Jackson ImmunoResearch Laboratories, Inc.). Enzymatic reactivity was detected with nitroblue tetrazolium (0.33 mg/ml) and 5-bromo-4-chloro-3-indolyl phosphate (0.17 mg/ml).
Motility agar.
An LB-0.4% agar plate was stab inoculated from isolated colonies and incubated at room temperature for 36 h. Motility was assessed by the radius of the growth ring.
Adhesion and invasion assays.
Caco-2 cells were maintained in Eagle's minimal essential medium plus 20% fetal bovine serum, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. For some invasion assays, 35-mm tissue culture dishes were seeded with 3 × 105 Caco-2 cells in antibiotic-free medium, but for most assays, 24-well tissue culture plates were seeded with 5 × 104 Caco-2 cells per well in antibiotic-free medium. Cells were seeded on a 12-mm-diameter glass coverslip treated with type I rat tail collagen (0.1 mg/ml). Cells were incubated for 24 h at 37°C with 5% CO2, allowing a semiconfluent monolayer to form. Cells were infected with a multiplicity of infection of approximately 500, and when indicated, one plate was centrifuged at 40 × g for 5 min at 22°C while the other plate remained stationary. Infected cells were then incubated at 37°C with 5% CO2 and washed with phosphate-buffered saline (PBS) at 30 min postinfection. For adhesion assays, the coverslips were harvested after the PBS washes. For invasion assays, prewarmed medium supplemented with gentamicin (150 μg/ml) was added after the washes, and coverslips were harvested at 75 min postinfection after one additional PBS wash. Bacterial counts were determined by transferring each coverslip into a 15-ml conical tube containing 5 ml of sterile water and plating the lysate on LB agar (18). For each sample, three to five coverslips were used for bacterial counts and one coverslip was stained with DiffQuick. Bacterial counts were also determined for the initial inoculum by plating serial dilutions of the inoculum on LB agar.
Sodium azide assay.
Assays performed in the presence of sodium azide (NaN3) were conducted as described for the adhesion assay above, with the following changes. Overnight cultures of bacteria were washed and suspended in antibiotic-free tissue culture medium. NaN3 was added to a concentration of 20 mM, and the suspension was incubated at room temperature for 5 min. Tissue culture medium on host cells was replaced with 37°C antibiotic-free tissue culture medium with or without 20 mM NaN3 immediately before infection.
Mouse infections.
Competitive index (CI) assays were performed as described with some modifications (1, 14). Bacteria were grown in BHI with streptomycin at 250 rpm at 30°C to an optical density at 600 nm of approximately 0.4. Bacteria were centrifuged at 3,200 × g at 20°C for15 min, washed once with PBS, pH 7.1, and suspended to a calculated concentration of 1.6 × 1010 to 7.0 × 1010 CFU/ml. The two strains being used in the infection were mixed in a 1:1 ratio, and the numbers of CFU were determined by plating serial dilutions onto LB with streptomycin and LB with streptomycin and erythromycin in parallel. Alternatively, the inoculum was plated onto LB plus streptomycin and colonies were individually picked and inoculated onto LB plus streptomycin and erythromycin. Five- to 8-week-old pathogen-free female BALB/c mice (Taconic) were fasted for 2 h before and after gavage. Gavage was performed using a 20-gauge by 1.5 in. animal feeding needle (Popper and Sons, Inc.) with a dose of 4 × 109 to 1.75 × 1010 bacteria in a volume of 250 μl. Mice were sacrificed at 4 to 6, 18, or 42 h after gavage. Livers were harvested and homogenized in 0.05% NP-40, streptomycin (200 μg/ml), and erythromycin (0.1 μg/ml) to induce the erm gene. The entire intestines were removed and cut open longitudinally, and the contents were flushed out using PBS. Intestines were then placed in homogenization buffer supplemented with 0.2 mM EDTA and incubated for up to 1 h at room temperature, and homogenates were plated as described above. Animal protocols were approved by the Cornell University Institutional Animal Care and Use Committee and in accordance with all legal requirements.
Statistical analysis.
Percent invasion or adhesion was calculated using invasion or adhesion efficiencies (number of internalized or adherent bacteria divided by total number of bacteria used in infection) normalized to the wild-type strain set to 100% for each experimental repetition. Invasion and adhesion assays were analyzed using a one-way analysis of variance with Bonferroni's multiple comparison test to determine if invasion levels of individual strains were different from the wild type set at 100% or from those of other strains. The mouse competitive index assay was analyzed with a one-sample Student's t test (one tailed) using log-transformed competitive indexes to determine if the indexes were significantly less than 1.
RESULTS
Construction and characterization of motility mutants.
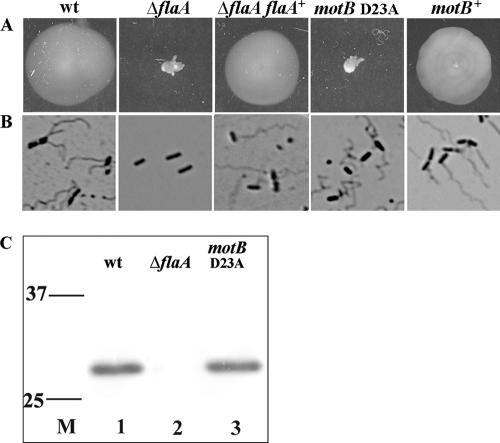
To assess the role of flagella and flagellum-mediated motility in L. monocytogenes infection of host cells, an in-frame deletion was made in flaA, the gene encoding flagellin, the monomer that makes up the long helical flagellar filament. This strain is denoted as the Δ_flaA_ mutant. Motility phenotypes were confirmed by inoculation into motility agar and observation of wet mounts. As predicted, the Δ_flaA_ mutant was nonmotile by wet mount and produced a compact colony on motility agar plates (Fig. 1A). The wild-type strain (10403S) was motile and produced a colony with a large growth radius (Fig. 1A). Bacteria were stained with crystal violet to visualize the flagella. Flagella were not visible at the surface of the Δ_flaA_ mutant, but were detectable at the surface of the wild-type strain (Fig. 1B). SDS-extractable bacterial surface proteins were examined by Western immunoblotting using an antibody to L. monocytogenes flagella. As expected, flagellin was detected from the wild-type strain but not from Δ_flaA_ (Fig. 1C). Additionally, we constructed a complemented Δ_flaA_ strain (Δ_flaA flaA_+) by inserting the flaA open reading frame into the tRNAArg site on the chromosome. This complemented strain was flagellated (Fig. 1B) and motile (Fig. 1A).
FIG. 1.
Characteristics of the wild-type and motility mutant strains. (A) Detection of motility. Strains of L. monocytogenes were stabbed into soft LB agar and incubated at room temperature for 36 h. A large area of bacterial growth is indicative of bacterial motility. (B) Detection of bacterium-associated flagella. Bacteria grown in BHI at 30°C with shaking were stained with crystal violet to visualize flagella by bright-field microscopy. (C) Immunodetection of flagellin from L. monocytogenes. Bacterial surface-extracted proteins were resolved by SDS-polyacrylamide gel electrophoresis, and flagellin was detected by Western immunoblotting. Lane M, prestained markers with molecular mass indicated in kDa on the left. wt, wild type.
To directly determine if the flagellar filament is used as an adhesin, we needed to construct a flagellated but nonmotile mutant. This was initially attempted by creating an in-frame deletion in motB, a gene encoding one of the flagellar motor proteins, creating the Δ_motB_ mutant. The Δ_motB_ mutant was nonmotile by wet mount and motility agar. However, although flagella were detectable at the surface of the Δ_motB_ mutant, 55% or less of the bacteria were flagellated and flagellated bacteria appeared to carry fewer flagella than wild-type bacteria when examined by crystal violet staining. Additionally, by Western immunoblotting, the Δ_motB_ mutant appeared to have considerably less flagellin than the wild-type strain. We rationalized that MotB is structurally important for assembly of the flagellar apparatus and therefore created a different mutant preserving the MotB structure. This mutant, the _motB_D23A strain, contains an alanine in position 23 of the MotB open reading frame instead of an aspartic acid. The corresponding aspartic acid in E. coli has been shown to be essential for torque generation (29). This mutant was nonmotile by wet mount and motility agar (Fig. 1A), and fully flagellated by crystal violet staining (Fig. 1B) and Western immunoblotting (Fig. 1C). The _motB_D23A mutant is therefore a fully flagellated but nonmotile strain and an ideal tool for addressing the question of flagellar filaments as adhesins. The _motB_D23A mutant was complemented by inserting the wild-type gene into the chromosome by allelic exchange. The motB+ complemented strain was flagellated and motile by wet mount and motility agar (Fig. 1A and B).
Functional flagella are required for optimal invasion of human intestinal epithelial cells by L. monocytogenes.
The human intestinal epithelial cell line Caco-2 was used to evaluate the invasion phenotypes of L. monocytogenes motility mutants. First, as previously shown by other groups, we observed that the Δ_flaA_ mutant is significantly less invasive than the wild-type strain, but has no defect in intracellular growth and cell-to-cell spread (6, 27) (Table 3) (data not shown). Similarly, we confirmed that centrifugation of nonmotile nonflagellated bacteria onto host cells only partially complements the invasion defect, indicating that flagella contribute more to invasion than a mere increase in the probability of bacterium-host cell contact (6) (Table 3). The complemented Δ_flaA flaA_+ strain did not invade Caco-2 cells to the same level as the wild-type strain, but this difference was not significant (Table 3). This incomplete complementation may be caused by the exclusion of the recently described MogR binding promoter region III (22) and the integration of the flaA promoter and ORF sequences at a new location on the chromosome.
TABLE 3.
L. monocytogenes adhesion to and invasion of Caco-2 cells
| Strain type | Treatment with: | % Adhesiona (n) | % Invasiona (n) | |
|---|---|---|---|---|
| Centrifugation | NaN3 | |||
| Δ_flaA_ | − | − | 38.25 ± 9.01* (3) | 0.71 ± 0.31** (4) |
| Δ_flaA_ | + | − | 88.31 ± 38.26 (3) | 16.73 ± 8.99** (4) |
| Δ_flaA flaA_+ | − | − | ND | 58.76 ± 30.14 (5) |
| _motB_D23A | − | − | 3.86 ± 0.94** (3) | 0.32 ± 0.22** (4) |
| _motB_D23A | + | − | 18.18 ± 5.39** (**) (3) | 3.68 ± 1.85** (4) |
| motB+ | − | − | 95.41 ± 26.39 (3) | 153.10 ± 51.38 (4) |
| Wild type | − | + | 7.94 ± 0.40** (3) | ND |
| _motB_D23A | − | + | 9.15 ± 7.76** (3) | ND |
Next, to determine whether the flagellar filament per se contributes to the ability of L. monocytogenes to invade epithelial cells independent of motility, we tested the flagellated but nonmotile motB_D23A mutant. Results indicate that the flagellated but nonmotile motB_D23A strain was as deficient as the nonflagellated nonmotile Δ_flaA strain, suggesting that nonmotile flagella do not contribute to invasion of epithelial cells by L. monocytogenes (Table 3). Similarly to what we observed for the Δ_flaA strain, centrifugation of the _motB_D23A strain onto host cells only partially complemented the invasion defect. The motB+ complemented strain behaved like the wild-type strain.
The flagellar filament does not improve adhesion of L. monocytogenes to cultured human intestinal epithelial cells.
Adhesion assays were performed with the Δ_flaA_ and motB_D23A mutants to determine whether the flagellar filament contributes independently of motility to the ability of L. monocytogenes to adhere to epithelial cells. Semiconfluent monolayers of Caco-2 cells were infected for 30 min and washed before determination of counts of cell-associated bacteria. Results indicate that flagellated nonmotile and nonflagellated bacteria were significantly deficient in their ability to adhere to Caco-2 cells (Table 3). The adhesion rate of the Δ_flaA strain was not significantly different from that of the wild-type strain after centrifugation, whereas that of the motB_D23A strain was. Moreover, the levels of adhesion of the Δ_flaA and _motB_D23A strains were significantly different from each other after centrifugation, indicating that nonmotile flagella interfere with adhesion of L. monocytogenes to epithelial cells. The adhesion rate of the motB+ complemented strain was equivalent to that of the wild-type strain.
Alternatively, we used an energy poison to determine whether the flagellar filament contributes independently of motility to the ability of L. monocytogenes to adhere to epithelial cells. Conditions for this experiment were optimized by incubating bacteria for 5 min in various concentrations of the ATPase inhibitor NaN3 and checking for motility by wet mount. At 20 mM NaN3, very few bacteria remained motile. This concentration of NaN3 was nonlethal to L. monocytogenes within the time frame of the assay as determined by bacterial counts before and after a 30-min treatment. The adhesion assay consisted of comparing motile wild-type bacteria to NaN3-treated nonmotile wild-type bacteria. As a control for the effect of NaN3 on bacterial adhesion, we tested the adhesion levels of flagellated but nonmotile _motB_D23A bacteria with NaN3 present. The addition of NaN3 brought wild-type adhesion levels down to the levels observed with the _motB_D23A mutant (Table 3). Adhesion levels of NaN3-treated wild-type and _motB_D23A strains as well as those of untreated and treated _motB_D23A mutants to host cells were not significantly different from each other. Together, these data show that the presence of nonmotile flagella is not beneficial for L. monocytogenes adhesion to host cells, indicating that the flagellar filament does not function as an adhesin.
Motile bacteria outcompete nonmotile bacteria in initial colonization of the mouse intestines and liver.
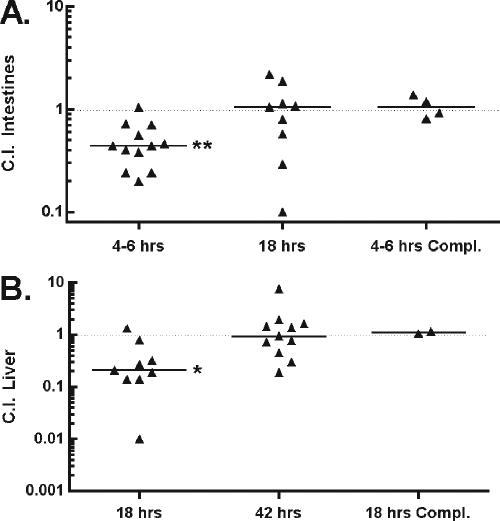
The previous assays clearly demonstrate that flagellum-mediated motility contributes significantly to the ability of L. monocytogenes to invade epithelial cells. To assess the importance of flagellum-mediated motility for early colonization of the intestines, a CI assay was performed by orally infecting 5- to 8-week-old female BALB/c mice with a 1:1 mixture of strain DP-L3903, an erythromycin-resistant derivative of 10403S that is fully virulent, and the isogenic Δ_flaA_ mutant strain. At 4 to 6 h postinfection, the Δ_flaA_ mutant showed a 2.2-fold decrease in colonization of the mouse intestines (CI of 0.46; P < 0.001) (Fig. 2A). However, by 18 h postinfection, this attenuation was no longer detectable. At 18 h postinfection, very few mice had detectable numbers of bacteria in their liver, but the colonization deficiency observed in the intestines was mimicked in the liver of infected mice, with a 4.1-fold decrease in colonization (CI of 0.24; P = 0.011) (Fig. 2B). This deficiency was no longer present by 42 h postinfection (CI of 0.930) (Fig. 2B). The Δ_flaA flaA_+ complemented strain was not deficient in colonization of the intestines or liver compared to the wild-type strain (Fig. 2A and B).
FIG. 2.
Motile bacteria outcompete nonmotile bacteria in initial colonization of the murine intestines and liver. Mice were infected orally with a 1:1 ratio of 10403S erm+ and Δ_flaA_, 10403S and Δ_flaA erm_+, or 10403S erm+ and Δ_flaA flaA_+ L. monocytogenes strains as described in detail in Materials and Methods. CIs were determined for colonization of the intestines (A) and liver (B) at indicated time points postinfection. Each data point represents the CI of one mouse. Data points were collected from a minimum of two independent experiments, except for the complemented (Compl.) Δ_flaA_ mutant strain, for which a single experiment was performed. A CI value of less than 1 indicates that the mutant strain was outcompeted by the wild-type strain. No differences were observed when mice were infected with 10403S erm+ and Δ_flaA_ versus 10403S and Δ_flaA erm_+ strains, indicating that the erythromycin resistance gene did not influence CI results. Horizontal bars indicate the median. Statistical differences from 1.0 were determined by Student's t test. *, P < 0.05; **, P ≤ 0.005.
To ensure that the erythromycin resistance cassette did not cause the phenotypes by providing an advantage to DP-L3903, the experiment was also conducted using 10403S and HEL-487, a Δ_flaA_ strain transduced with the erythromycin cassette from DP-L3903. We observed that the CIs were not influenced by the erythromycin cassette. Overall, these results indicate that flagellum-mediated motility influences L. monocytogenes infectivity in mice at an early time point after oral infection.
DISCUSSION
Flagella and flagellum-mediated motility are integral to the virulence of several gastrointestinal bacterial pathogens (10). For L. monocytogenes, no link has been made between flagella and virulence, although the flagella are important for efficient invasion of tissue culture cells (2, 6). In this study, we investigated the mechanism by which flagella influence the ability of L. monocytogenes to invade host cells and the role of flagella in colonizing mice early in infection. Our results clearly indicate that L. monocytogenes flagella do not function as adhesins to enhance bacterial attachment to and invasion of epithelial cells, but rather function as motility devices contributing more to invasion than a mere increase in probability of bacterium-host cell interaction. Moreover, we show that motile bacteria outcompete nonmotile bacteria for initial colonization of the intestinal tract and liver by L. monocytogenes.
Flagella can function as adhesins, independent of motility, to enhance bacterial invasion of host cells, as shown for enteropathogenic E. coli (7). For L. monocytogenes, the presence of flagella increases bacterial adherence to host cells (2, 6), but it is not known whether the flagellum itself serves as an adhesin. The present study addressed this question directly by comparing the behavior of flagellated motile (wild type), flagellated nonmotile (motB_D23A), and nonflagellated (Δ_flaA) bacteria, as well as that of flagellated motile (wild type) and nonmotile (wild type treated with NaN3) bacteria. In both instances, the outcome indicated that flagella do not serve as adhesins for attachment of L. monocytogenes to host cells. Moreover, the fact that flagellated nonmotile bacteria (motB_D23A) attach to host cells less efficiently than nonflagellated bacteria (Δ_flaA) also indicated that nonmotile flagellar filaments interfere with L. monocytogenes adhesion to host cells. This could be due to the nonmotile filaments preventing direct bacterial cell surface interactions with host cell receptors. Similarly, wild-type bacteria attached less efficiently to host cells when motility was inhibited with an energy poison. Therefore, L. monocytogenes peritrichous flagella function as motility devices and not as adhesins to enhance host cell invasion.
Analysis of the efficacy of adhesion relative to that of invasion reveals that flagellum-mediated motility influences L. monocytogenes invasion of epithelial cells to a much larger extent than adhesion. When absolute adhesion and invasion numbers are taken into consideration, the invasion level of the wild-type strain was approximately threefold lower than its adhesion level; however, the invasion levels of Δ_flaA_ and motB_D23A mutants were more than 100-fold lower than adhesion levels. When bacteria were centrifuged onto host cells, the invasion level of the wild-type strain was approximately 2-fold lower than its adhesion level, whereas invasion levels of Δ_flaA and _motB_D23A mutants were more than 10-fold lower than adhesion levels. These observations suggest that flagellum-mediated motility contributes more to invasion of epithelial cells than a mere increase in the probability of bacterium-host cell contact. Perhaps flagellum-mediated motility is required for L. monocytogenes to maintain contact with host cells until high-affinity ligand-receptor binding has been established. It is conceivable that the nonmotile mutants are unable to sustain contact with host cells long enough to ensure this type of interaction. It is also possible that the physical force generated by flagellum-mediated motility against the host cell membrane contributes to invasion in a manner similar to intercellular spread of bacteria that use an actin-based mechanism of motility (15). Consistent with this hypothesis, physical force generated by centrifugation of nonmotile bacteria onto host cells improved invasion, although not to wild-type levels. Moreover, flagellum-mediated motility may be required for proper spatial orientation of bacteria upon attachment to host cells. Nonmotile L. monocytogenes mutants are less defective at invading professional phagocytic cells than epithelial cells (6; and data not shown). Professional phagocytic cells are equipped with cell-surface receptors that recognize common gram-positive surface molecules (24). The only limitation for L. monocytogenes invasion of professional phagocytes may be the probability of bacterium-host cell interaction, since ligands are distributed evenly on the bacterial surface and ligand-receptor interactions lead to rapid phagocytosis. On the contrary, invasion of epithelial cells by L. monocytogenes requires the presence of specialized invasins at the bacterial surface, which may have a polar distribution, as recently shown for InlA (19). Efficient engagement of InlA with its receptor, E-cadherin, would require that bacteria interact head on with host cells. The requirement for spatial orientation could also explain why centrifugation of nonmotile L. monocytogenes onto host cells does not rescue the invasion deficiency to the wild-type level (Table 3) (6). Overall, it appears that the contribution of flagellum-mediated motility to host cell invasion possibly encompasses mechanisms related to the probability of host cell contact, physical force, and spatial orientation.
The fact that no association has been made between flagellum-mediated motility and in vivo virulence is surprising considering the major invasion defect observed in tissue culture epithelial cells (2, 6, 27). We rationalized that if flagellum-mediated motility contributes to infection in vivo, it would be, as for other food-borne pathogens, at the level of intestinal colonization early after oral infection. The ability of the nonmotile mutant (Δ_flaA_) to colonize the intestines of mice was tested in a competitive assay with the wild-type strain. The competitive index assay is more statistically powerful than comparing bacterial counts between mice because it eliminates mouse-to-mouse variation. Additionally, competitive index experiments can elucidate differences in bacterial fitness due to the added element of competition. In that context, the nonmotile L. monocytogenes strain was deficient (2.2-fold) in colonization of the mouse intestines at 4 to 6 h postinfection. Moreover, this deficiency was reflected in colonization of the liver (4.1-fold) 12 h later, consistent with the liver being directly connected to the intestines through the portal circulation system. These observations were not made in previous studies presumably because colonization of the intestines and liver was not evaluated at these early time points postinfection (2, 6). However, in agreement with these previous studies, differences in colonization of the intestines and liver by motile and nonmotile bacteria were no longer detected at later time points. It has been hypothesized that, in mice, L. monocytogenes invasion occurs through Peyer's patches rather than through the intestinal epithelium as the major L. monocytogenes invasin, InlA, does not bind the mouse E-cadherin (13). Considering that flagellum-mediated motility is far less important for infection of phagocytic cells than epithelial cells (2; data not shown), its importance for L. monocytogenes virulence is likely to be underestimated in a mouse model. Perhaps, in a susceptible human, the contribution of flagellum-mediated motility on the ability of L. monocytogenes to colonize the intestinal tract would have a greater impact on the outcome of infection.
Acknowledgments
This work was supported by a USDA National Needs predoctoral fellowship to H.S.O.
We thank Alan Bitar and Min Cao for optimizing the conjugation protocol.
Footnotes
▿
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Auerbuch, V., L. L. Lenz, and D. A. Portnoy. 2001. Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69**:**5953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigot, A., H. Pagniez, E. Botton, C. Fréhel, I. Dubail, C. Jacquet, A. Charbit, and C. Raynaud. 2005. Role of FliF and FliI of Listeria monocytogenes in flagellar assembly and pathogenicity. Infect. Immun. 73**:**5530-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139**:**2005-2009. [PubMed] [Google Scholar]
- 4.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8**:**143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffin. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336**:**100-105. [DOI] [PubMed] [Google Scholar]
- 6.Dons, L., E. Eriksson, Y. Jin, M. E. Rottenberg, K. Kristensson, C. N. Larsen, J. Bresciani, and J. E. Olsen. 2004. Role of flagellin and the two-component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect. Immun. 72**:**3237-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44**:**361-379. [DOI] [PubMed] [Google Scholar]
- 8.Grundling, A., L. S. Burrack, H. G. Bouwer, and D. E. Higgins. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. USA 101**:**12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77**:**51-59. [DOI] [PubMed] [Google Scholar]
- 10.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291**:**605-614. [DOI] [PubMed] [Google Scholar]
- 11.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49**:**581-590. [DOI] [PubMed] [Google Scholar]
- 12.Lauer, P., M. Y. N. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184**:**4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292**:**1722-1725. [DOI] [PubMed] [Google Scholar]
- 14.Lenz, L. L., S. Mohammadi, A. Geissler, and D. A. Portnoy. 2003. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc. Natl. Acad. Sci. USA 100**:**12432-12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monack, D. M., and J. A. Theriot. 2001. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 3**:**633-647. [DOI] [PubMed] [Google Scholar]
- 16.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24**:**1109-1117. [DOI] [PubMed] [Google Scholar]
- 17.Peel, M., W. Donachie, and A. Shaw. 1988. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and Western blotting. J. Gen. Microbiol. 134**:**2171-2178. [DOI] [PubMed] [Google Scholar]
- 18.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167**:**1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafelski, S. M., and J. A. Theriot. 2006. Mechanism of polarization of Listeria monocytogenes surface protein ActA. Mol. Microbiol. 59**:**1262-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts, A. J., and M. Wiedmann. 2003. Pathogen, host and environmental factors contributing to the pathogenesis of listeriosis. Cell. Mol. Life Sci. 60**:**904-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber, S., M. Konradt, C. Groll, P. Scheid, G. Hanauer, H. O. Werling, C. Josenhans, and S. Suerbaum. 2004. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl. Acad. Sci. USA 101**:**5024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen, A., and D. E. Higgins. 2006. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2**:**e30. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74**:**705-711. [DOI] [PubMed] [Google Scholar]
- 24.Stuart, L. M., and R. A. Ezekowitz. 2005. Phagocytosis: elegant complexity. Immunity 22**:**539-550. [DOI] [PubMed] [Google Scholar]
- 25.Trieu-Cuot, P., E. Derlot, and P. Courvalin. 1993. Enhanced conjugative transfer of plasmid DNA from Escherichia coli to Staphylococcus aureus and Listeria monocytogenes. FEMS Microbiol. Lett. 109**:**19-23. [DOI] [PubMed] [Google Scholar]
- 26.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14**:**584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Way, S. S., L. J. Thompson, J. E. Lopes, A. M. Hajjar, T. R. Kollmann, N. E. Freitag, and C. B. Wilson. 2004. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 6**:**235-242. [DOI] [PubMed] [Google Scholar]
- 28.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96**:**6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou, J., L. L. Sharp, H. L. Tang, S. A. Lloyd, S. Billings, T. F. Braun, and D. F. Blair. 1998. Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB. J. Bacteriol. 180**:**2729-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]