DTNBP1 Genotype Influences Cognitive Decline in Schizophrenia (original) (raw)
. Author manuscript; available in PMC: 2007 Mar 15.
Abstract
Objective
Intellectual decline is common in schizophrenia and predicts functional outcome. While many patients undergo intellectual decline that typically predates the onset of symptoms, few studies have investigated the underlying mechanism through which this occurs. The current study assessed the relationship between intellectual decline in schizophrenia and genetic variation in dysbindin-1 (DTNBP1).
Methods
We assessed cognitive decline in 183 Caucasian patients with schizophrenia using a proxy measure of premorbid IQ with which current general cognitive ability (g) was compared. We then tested for a relationship between the risk haplotype identified in previous work (CTCTAC) and intellectual decline.
Results
We found that carriers of the CTCTAC haplotype, demonstrated a significantly greater decline in IQ as compared with non-carriers (p=0.05).
Conclusions
These data suggest that DTNBP1 influences the severity of intellectual decline in schizophrenia and may represent one underlying cause for heterogeneity in cognitive course.
Keywords: dysbindin, schizophrenia, cognitive decline, genetics
1.1 INTRODUCTION
Substantial evidence suggests that a large proportion of patients with schizophrenia undergo a decline in intellectual functioning; however, there is considerable inter-individual variation in the degree of decline. Studies that have investigated intellectual functioning prior to the onset of schizophrenia and after illness onset, indicate that approximately 40–50% of patients are “deteriorating”, with an IQ decline of ≥ 10 points from premorbid IQ, while another 50% of patients do not demonstrate significant intellectual decline (Reichenberg et al. 2005; Weickert et al. 2000).
These data also suggest that many patients’ intellectual functioning is impaired prior to the onset of illness (Reichenberg et al. 2005, 2002; Fuller et al. 2002; Weickert et al. 2000). Reichenberg et al. (2005) recently reported that a majority of healthy adolescents who later manifest schizophrenia undergo a significant intellectual decline prior to the onset of psychotic symptoms. Intellectual decline, defined as a significantly lower than expected IQ at age 17, was associated with an increased risk for developing schizophrenia (Reichenberg et al. 2005). Subsequent to the initial episode (Bilder et al. 2006), cognitive performance, although impaired, appears to remain relatively stable in most patients, over short (2-year) and long (5-year and 10-year) term follow-up (Burdick et al. 2006a; Heaton et al. 2001). Given the early presence of decline and its stability over time, it is likely that genetic influences play a role in determining the severity of the decline, yet to date there have been no studies to identify specific genes that may differentiate these heterogeneous cognitive profiles.
Recently, however, a growing body of evidence suggests that the gene coding for dysbindin-1 (DTNBP1), initially identified by Straub and colleagues (2002), might influence intellectual decline in schizophrenia. First, DTNBP1 has been shown to influence risk for schizophrenia in several genetic linkage and association studies (for review see Norton et al. 2006), although not all studies have reported positive results. Second_,_ post-mortem evidence suggests that DTNBP1 is expressed in regions of the brain that are critical to cognitive function and that its expression is reduced in hippocampus and prefrontal cortex in patients with schizophrenia (Weickert et al. 2004). Third, knockdown of endogenous dysbindin in primary cortical neuron culture results in decreased pre-synaptic protein expression and decreased release of glutamate (Numakawa et al. 2004), a key neurotransmitter thought to underlie cognitive dysfunction in schizophrenia. Finally, recent data from our group suggests that a schizophrenia risk haplotype in DTNBP1 (Funke et al. 2004) is associated with decreased general cognitive ability (g) in patients with schizophrenia and healthy volunteers (Burdick et al. 2006b). In this study group, we also have data on premorbid intellectual function and we have now assessed the effects of this 6-locus DTNBP1 risk haplotype (CTCTAC) on premorbid versus post-illness onset intelligence in 183 patients with schizophrenia to test the hypothesis that DTNBP1 influences intellectual decline in schizophrenia.
1.2 EXPERIMENTAL/MATERIALS AND METHODS
The sample consisted of 183 unrelated Caucasian patients with schizophrenia or schizoaffective disorder who were administered a battery of standardized cognitive measures comprised of the Wechsler Adult Intelligence Test-Revised (WAIS-R)-Digit Span; Continuous Performance Test-Identical Pairs Version (CPT-I/P); California Verbal Learning Test (CVLT)-Abridged; Controlled Oral Word Association Test (COWAT), and Trail Making Tests A&B. Subjects were included if they were age 25 to 64 and had an estimated premorbid IQ >70 and represent a subset of the sample reported on in Burdick et al. 2006a.
Genotyping and haplotype procedures are described in detail elsewhere (Funke et al. 2004; Burdick et al. 2006a). Briefly, six single nucleotide polymorphisms [P1583-(rs909706), P1578-(rs1018381), P1763-(rs2619522), P1320-(rs760761), P1765-(rs2619528), and P1325-(rs1011313)] were genotyped and met criteria for Hardy-Weinberg equilibrium. We utilized the SNPHAP program (Department of Medical Genetics, Cambridge Institute for Medical Research, Addenbrooke’s Hospital, Cambridge, U.K) for estimating haplotype frequencies, which uses an estimation method algorithm to calculate maximum likelihood estimates of haplotype frequencies given genotypes which do not specify phase. We included subjects whose haplotypes could be assigned with a confidence of ≥ 95%. We focused our analyses on the single risk haplotype (CTCTAC) for schizophrenia from our previous work (Funke et al. 2004).
When longitudinal data are not available, an alternative approach to measure intellectual decline is to estimate premorbid IQ in patients with schizophrenia by using tests of reading skill. We assessed cognitive decline using the Wide Range Achievement Test-Third Edition-Reading Subtest (WRAT-3) as a proxy for premorbid IQ. WRAT-3 is a test that assesses single word reading skill which, like command of general knowledge and vocabulary, is particularly resistant to the effects of deterioration associated with brain disease and is considered an estimate of pre-morbid IQ in patient populations (Kremen et al. 1996; Goldberg et al. 1995).
As a measure of current IQ, with which WRAT-3 scores were compared, “general cognitive ability”, or (g), was calculated as the first component of an unrotated principal components analysis utilizing all of the cognitive tests administered, except the WRAT-3. All cognitive variable data were transformed to standardized z-scores and missing values were replaced by the mean of the group. No case with more than two missing values was retained in the sample. A single factor model was produced (extracted variables with eigenvalues of > 1.0 using the regression method). This first unrotated factor explained 48.5% of the variance and represented our generalized cognitive ability factor. Each of the individual measures loaded onto the first factor with covariance of > 0.61. A detailed description of the PCA methods used to derive g is provided in Burdick et al. (2006a).
For exploratory purposes, we also characterized our patients, consistent with previous methods (Reichenberg et al. 2005; Weickert et al. 2000), as “deteriorating” if they experienced an IQ decline of ≥ 10 points and as “stable” if they did not meet this threshold.
1.2.1 Statistical Analyses
All scores were transformed to standard scores for uniformity in comparing across measures. Consistent with methodology used in Kremen et al. (2001) we measured putative decline from premorbid IQ by using a regression approach. This method is preferable to using raw score differences because the premorbid-current discrepancy may not be equivalent at all IQ levels. This was done by regressing g on WRAT-3 in our healthy controls (n=126) and using the regression model to generate standardized scores, representing predicted IQ in the schizophrenia group. A residual score (observed minus predicted) reflected whether a subject’s current IQ was above or below their premorbid IQ. Thus, the putative IQ change described below is actually the discrepancy between current IQ, as measured by g, and an expected IQ based on a predicted score (Reichenberg et al. 2005). Hence, data are presented as residual scores as opposed to raw scores.
Univariate analysis of variance (ANOVA) was used with haplotype group as the between-subjects factor and the residual score reflecting decline as the dependent variable. Haplotype groups were defined as carriers of the CTCTAC risk haplotype (with one or two copies; n=35) versus non-carriers of CTCTAC (no copies; n=148); heterozygote and homozygote carriers were merged due to the low frequency of homozygotes (n=4).
1.3 RESULTS
We found that patients with schizophrenia who carry the CTCTAC risk haplotype demonstrated a significantly greater decline in IQ (residual mean change=13.5±13.6) as compared with patients who do not carry the risk haplotype (residual mean change=8.7±12.4) (F=4.00; df=1, 182; p=0.05; Figure 1). Effect size calculations indicated that DTNBP1 genotype accounted for 2.2% of the variance in intellectual decline.
Figure 1. Intellectual Decline in Patients with Schizophrenia by DTNBP1 Genotype.
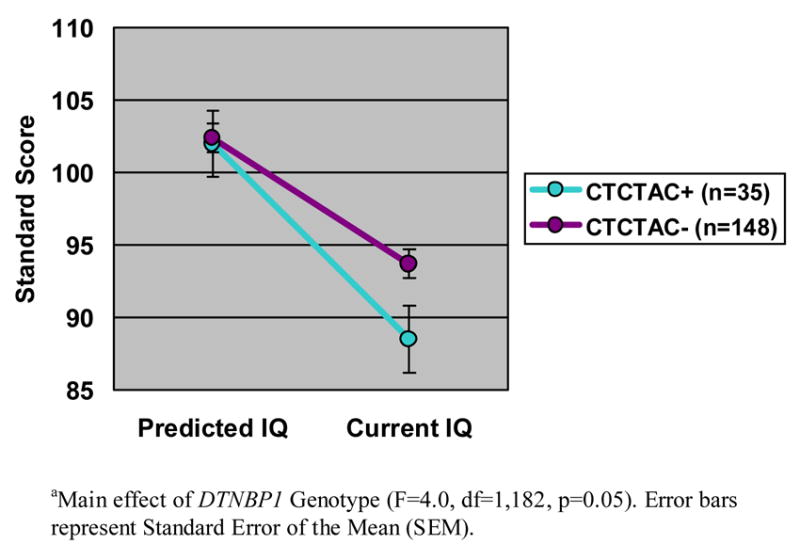
Carriers of the CTCTAC risk haplotype demonstrate a greater intellectual decline than non-carriers. The data represent residual scores (observed minus predicted IQ) and are presented on the y-axis as Standard Scores (with a mean of 100 and a standard deviation of 15). The x-axis illustrates change from predicted IQ to current IQ in carriers versus non-carriers.
Haplotype groups did not differ on demographic or illness characteristics: Carrier vs. non-carrier: (Age=43.7±8.7 years vs. 40.7±8.7 years; Sex 40.0% female vs. 35.8% female; Education=13.0±2.3 years vs. 12.9±3.5 years; Age of onset=18.4±5.7 years vs. 18.4±5.8 years; Global Assessment of Function-GAF score=38.7±12.6 vs. 40.8±13.9; ; Duration of illness 26.5± 9.0 years vs. 22.4± 10.6 years; all p-values > 0.05). Consistent with previous reports (1, 2), 45.4% of patients demonstrated intellectual decline of at least ten points were characterized as “deteriorating”. In the group of deteriorating patients, the risk haplotype had a frequency of 24%, as compared with a frequency of only 15% in the non-deteriorating group, but this difference did not reach statistical significance (Chi2=2.43; p=0.12).
1.4 DISCUSSION
General intellectual decline is an important feature of schizophrenia and has been demonstrated to predict both functional outcome and variance in other more specific cognitive measures (Weickert et al. 2000). Here we show a significant effect of a DTNBP1 risk haplotype (CTCTAC) on IQ decline in patients with schizophrenia. CTCTAC carriers demonstrated a significantly greater decline in IQ (13.5 units) as compared with non-carriers (8.7 units), as measured via proxy measurements of premorbid IQ and current IQ. Among those patients who were characterized as deteriorating, the risk haplotype had a frequency of 24%, as compared with a frequency of only 15% in the non-deteriorating group. These data are consistent with a study by Williams et al. (2004) in which a 3-locus haplotype in DTNBP1 was associated with educational attainment in patients with schizophrenia and in healthy controls. These data also suggest that different mechanisms may play a role in cognitive function in schizophrenia patients prior to the onset of illness versus healthy controls, as our previous study found an effect of DTNBP1 on general cognitive ability in healthy controls (Burdick et al. 2006a).
The mechanism underlying the effect of DTNBP1 genotype on intellectual decline is unknown, although its broad distribution in brain, along with reported reductions of DTNBP1 expression in regions critical to cognitive function (Weickert et al.2004), suggests that intellectual decline may be related to decreased DTNBP1 expression. This is supported by preliminary data from a knockdown model demonstrating that reduced DTNBP1 expression results in dysfunction within the glutamatergic system (Numakawa et al. 2004), a system believed to be related to cognitive function and disrupted in schizophrenia.
This study has several limitations including a relatively small sample of CTCTAC carriers due to the low frequency of the risk haplotype. This likely impacted the statistical power, resulting in significant, but modest p-value. In addition, we utilized a proxy measure for estimating premorbid IQ, as opposed to measuring decline via a longitudinal design. While longitudinal studies that measure IQ prior to disease onset are preferable, they are difficult to conduct given the low prevalence of the disease and the difficulties inherent in long-term follow-up of individuals prior to the onset of illness. Finally, although our data suggest that DTNBP1 genotype has an effect on intellectual decline, to date, a number of overlapping but not identical DTNBP1 risk haplotypes have been identified but no functional variant has been found within the gene.
In conclusion, we report an association between DTNBP1 and intellectual decline in patients with schizophrenia. These data provide evidence for an underlying genetic vulnerability that may influence the developmental trajectory of schizophrenia, at least with regard to cognitive symptoms.
Acknowledgments
This work was supported by the Stanley Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JM. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28(2):270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, Kucherlapati R, Malhotra AK. Genetic Variation in DTNBP1 Influences General Cognitive Ability. Hum Mol Genet. 2006a;15(10):1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. JNMD. 2006b;194(4):255–260. doi: 10.1097/01.nmd.0000207360.70337.7e. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159(7):1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Funke B, Finn CT, Plocik AM, Lake S, DeRosse P, Kane JM, Kucherlapati R, Malhotra AK. Association of the DTNBP1 locus with schizophrenia in a U.S. population. Am J Hum Genet. 2004;75:891–898. doi: 10.1086/425279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E, Weinberger DR. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schiz Res. 1995;17(1):77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT. The “3 Rs” and neuropsychological function in schizophrenia: An empirical test of the matching fallacy. Neuropsychology. 1996;10:22–31. doi: 10.1016/0165-1781(94)02652-1. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. Intelligence quotient and neuropsychological profiles in patients with schizophrenia and in normal volunteers. Biol Psychiatry. 2001;50(6):453–462. doi: 10.1016/s0006-3223(01)01099-x. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13(21):2699–708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- Norton N, Williams HJ, Owen MJ. An update on the genetics of schizophrenia. Curr Opin Psychiatry. 2006;19(2):158–64. doi: 10.1097/01.yco.0000214341.52249.59. Review. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry. 2002;159(12):2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, Knobler HY, Lubin G, Nahon D, Harvey PD, Davidson M. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62(12):1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O’Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–48. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Williams NM, Preece A, Morris DW, Spurlock G, Bray NJ, Stephens M, Norton N, Williams H, Clement M, Dwyer S, Curran C, Wilkinson J, Moskvina V, Waddington JL, Gill M, Corvin AP, Zammit S, Kirov G, Owen MJ, O’Donovan MC. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1. Arch Gen Psychiatry. 2004;61:336–344. doi: 10.1001/archpsyc.61.4.336. [DOI] [PubMed] [Google Scholar]