‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore (original) (raw)
Abstract
Territorial animals typically respond less aggressively to neighbours than to strangers. This ‘dear enemy effect’ has been explained by differing familiarity or by different threat levels posed by neighbours and strangers. In most species, both the familiarity and the threat-level hypotheses predict a stronger response to strangers than to neighbours. In contrast, the threat-level hypothesis predicts a stronger response to neighbours than to strangers in species with intense competition between neighbours and with residents outnumbering strangers, as commonly found in social mammals such as the banded mongoose (Mungos mungo). The familiarity hypothesis predicts reduced aggression towards neighbours also in these species. We exposed free-living banded mongoose groups to translocated scent marks of neighbouring groups and strangers. Groups vocalized more and inspected more samples in response to olfactory cues of the neighbours than to the strangers. Our results support the threat-level hypothesis and contradict the familiarity hypothesis. We suggest that increased aggression towards neighbours is more common in social species with intense competition between neighbours, as opposed to reduced aggression towards neighbours typical for most solitary species.
Keywords: olfactory discrimination, neighbour recognition, habituation, territoriality, sociality, Herpestidae
1. Introduction
Relationships between territorial competitors are commonly explained by two hypotheses, both of which are based on the observation that many territorial animals respond less aggressively to neighbours than to strangers (reviewed in Ydenberg et al. 1988; Temeles 1994), a phenomenon termed the ‘dear enemy effect’ (sensu Fisher 1954). First, the familiarity hypothesis argues that, when the relationship between neighbours is settled, reduced aggression towards each other allows conservation of time and energy and reduces the risk of injuries (Wilson 1975), for example, because familiarity reduces the likelihood of role mistakes in territorial contests (Ydenberg et al. 1988). It has also been suggested that residents engage in fights with strangers to gather information about them (Getty 1989). Much evidence has accumulated in support of the familiarity hypothesis (reviewed in Ydenberg et al. 1988; Temeles 1994). In some species, however, the response to neighbours is more intense than the response to strangers (5 out of 55 species reviewed in Temeles 1994) and territory holders may increase aggression towards familiar but untrustworthy neighbours (Godard 1993; Olendorf et al. 2004), suggesting that aggression is not always reduced towards more familiar individuals.
Second, the threat-level hypothesis argues that neighbours and strangers may compete for different resources and, therefore, represent different levels of threat to an established territory holder. The response of residents should, thus, be stronger to the category of conspecifics that represents the bigger threat (Temeles 1994). Strangers often represent ‘floaters’ looking for a territory (Wilson 1975), and may thus be competitors for both territories and mates, whereas neighbours may only compete for mates. In this situation, both the familiarity hypothesis and the threat-level hypothesis predict a more aggressive response to strangers than to neighbours.
Studies contrasting the familiarity and the threat-level hypotheses are scarce, even though neighbour–stranger discrimination (NSD) has been demonstrated in a variety of taxa, including birds, mammals, reptiles and amphibians (reviewed in Ydenberg et al. 1988; Temeles 1994). Solitary northern harriers (Circus cyaneus), for example, defend feeding territories and respond more aggressively to neighbours than to strangers (Temeles 1990). In this species, neighbours may usurp portions of residents' territories, whereas floaters primarily appear to steal food and were never observed to take over territories. The pattern of NSD observed in northern harriers contradicts the familiarity hypothesis.
We suggest that the familiarity and the threat-level hypotheses can be contrasted by studying neighbour recognition in social vertebrates, which have been largely neglected in this regard (Radford 2005). In group-living species, differences in the value of contested resources might not be sufficient to explain the threat levels of neighbours and strangers. An additional parameter is relevant: group size of neighbours and strangers relative to resident groups. Many social animals commonly disperse singly or in small numbers, and pose little threat to larger established groups (Wilson 1975). In contrast, relationships between neighbouring groups of territorial animals are often aggressive. Groups may attempt to expand their territory at the expense of neighbouring groups (Mech & Boitani 2003), and some social mammals engage in fights with neighbouring groups, leading to serious injuries and occasional fatalities (Schaller 1972; Goodall 1986; Mech & Boitani 2003). Thus, neighbours may pose a significant threat to groups defending a territory, whereas strangers are generally outnumbered by established territory holders.
We experimentally tested the threat-level and the familiarity hypotheses in the banded mongoose (Mungos mungo), a small (less than 2 kg), territorial, cooperatively breeding carnivore. Banded mongoose groups are stable units formed either when a single-sex splinter group is joined by an opposite-sex splinter group, or when a single-sex splinter group takes over a small group, chasing away their same-sexed rivals (Cant et al. 2001). Such splinter groups disperse from their original groups via eviction by co-residents, displacement by immigrants or voluntary emigration. Home ranges may overlap considerably and borders are demarcated by faeces, urine and secretions of the anal glands (Rood 1975; C. A. Mu¨ller 2005, personal observation), which are inspected intensively when encountered by neighbours. Group sizes in banded mongooses vary over a large scale (range 5–44 individuals, mean 20 individuals; Cant 2000), and groups may expand their home ranges at the expense of smaller neighbouring groups (Rood 1975; and see electronic supplementary material). Competition between groups is intense, resulting in inter-group encounters with sometimes fatal consequences to members of the inferior group (Rood 1975; Cant et al. 2002; Gilchrist & Otali 2002). Strangers, in contrast, represent single individuals or splinters that disperse up to 20 km (Cant et al. 2001) and probably cross several established territories in the process. These splinters are commonly small (interquartile range=2–6.5, _N_=28; Banded Mongoose Project 2005, unpublished data), are outnumbered by resident groups and, thus, pose little threat to them. This is also the case when considering that single-sex splinters may compete only with their same-sexed rivals in resident groups when they attempt to take over. In the six documented group takeovers between 1998 and 2005, only small groups with no more than two residents of one sex were affected (Banded Mongoose Project 2005, unpublished data). This indicates that already groups of moderate size are at low risk of takeovers.
The familiarity and the threat-level hypotheses make contrasting predictions in banded mongooses. The familiarity hypothesis predicts that resident groups respond more intensely to strangers than to neighbours. The threat-level hypothesis predicts that residents react more strongly to neighbours than to strangers. Both hypotheses also predict that residents further discriminate between different neighbouring groups, an ability that has been demonstrated in a subset of the species that show NSD (Cheney & Seyfarth 1982; Davis 1987; Stoddard 1996). The familiarity hypothesis predicts neighbour–neighbour discrimination if reduced aggression towards neighbours is based on reciprocation (Godard 1993). The threat-level hypothesis predicts more intense responses to larger than to smaller neighbouring groups. The ability to discriminate both between neighbours and strangers and between different neighbours has rarely been tested in group-living species.
We tested these predictions using scent-mark translocation experiments. In addition, we used repeated exposures to scent marks of strangers to test if banded mongoose groups habituate to olfactory stimuli of unfamiliar groups. Since we presented secondary cues, we could not measure aggressivity of the response directly. Instead, we used worry-calling propensity, counter-marking propensity and inspection as measures of response intensity. Worry calls are harmonic calls with a fundamental frequency between 0.4 and 0.7 kHz and most of the energy concentrated between 0.4 and 2.0 kHz (for spectrogram see electronic supplementary material). They occur singly or in sequences of several calls and they are given when mongooses encounter secondary cues of other mongooses or of predators and commonly result in recruitment of other group members (C. A. Mu¨ller 2005, personal observation; see electronic supplementary material, video). They have not been observed in any non-threatening context. We assumed that they reflected how unsettling the stimuli were to the inspecting animals, as in sciurids, for example, calling propensity is correlated with level of danger (Swaisgood et al. 1999) and with faecal glucocorticoid levels (Blumstein et al. 2006). Inspection behaviour was assumed to be influenced by the familiarity of the stimulus, but it may also reflect gathering of additional information about the counterparts such as reproductive state of females, age and health (Sliwa & Richardson 1998; Swaisgood et al. 2002; White et al. 2003).
2. Material and methods
This study was conducted on a wild population of individually marked banded mongooses in Queen Elizabeth National Park, Uganda (0°12′ S, 29°54′ E) between April 2004 and August 2005. The study population remained largely constant in size throughout this period and consisted of 210–240 individuals in nine groups, seven of which were habituated to close observation and included in the experiments described below. The size of these seven groups ranged from 8 to 44 individuals. Animals were classified in age classes as adults (greater than 12 months), subadults (6–12 months) and infants (less than six months). Date of birth was known for all individuals except for nine adult immigrants. All animals were trapped on a regular basis to refresh individual marks (colour-coded plastic collars or small shaves on the rump), detect pregnancies, take morphometric measures and estimate ectoparasite load (see Cant 2000 for details). For trapping as well as for scent-mark presentations, small amounts of bait were used (a mix of rice and gravy).
Life-history data were collected during daily visits to the groups. For all visits, we recorded location (Magellan GPS Companion and Garmin GPS 12) and occurrence of births and deaths to monitor changes in the size of groups and their home ranges. Additionally, we recorded all events of encounters between neighbouring groups (two groups which occupy adjacent territories) and between resident groups and floaters (animals not defending a territory but travelling singly or in small numbers over large distances).
(a) Scent-mark translocation experiments
In separate trials, each group was presented with excreta collected from four different donor groups: two neighbouring groups, a non-neighbouring group (‘strangers’) and the group itself (‘own group’). In a control condition prior to each experiment, the subject groups were exposed to fresh samples of herbivore faeces (warthog (Phacochoerus aethiopicus) or waterbuck (Kobus ellipsiprymnus)) and samples of water (1 ml with a spoonful of soil) to control for variable scent-marking and worry-calling propensity. The scent marks of each group were presented in two different locations in separate trials: the centre and the border of the experimental group's territory. Home ranges were divided into border and centre areas based on sightings recorded by GPS over the preceding 12 months. To test for discrimination between different neighbours, we presented scent marks of the neighbouring groups at the shared border as well as at the border with a different group (opposite border). To test for NSD, only the experiments at the shared border and in the centre were used. Experiments on the same group were spaced at least 14 days apart to minimize carry-over effects.
For each trial, six or seven samples of fresh scat and urine were collected from the donor group within 1 h. The set of scent marks consisted of scat and urine samples from 5 to 7 individuals (4–7 adults and 0–3 subadults and infants) and included samples of adult males and adult females and of both excretion types. Only samples with known identity of the excreting animal were used. If insufficient samples were collected ad libitum, we trapped several individuals and collected excreta from the traps. This procedure represented only minimal stress, since all individuals in the study population have been trapped on a regular basis (2–4 times a year) and they are used to it (Cant 2000). All animals were released within 15 min of trapping. This is well below the delay time between peak of hormones in the blood and in the faeces for mammals (Palme et al. 2005). However, we cannot exclude the possibility that faecal samples collected by trapping were more or less likely to include secretions from the anal glands (Asa et al. 1985). Less than 20% of all the samples were collected by trapping, and collection did not differ systematically between donor categories.
The collected samples were stored on ice and presented to the experimental group on the same day (on average 2 h after collection). Since banded mongooses often use open patches for territorial marking (C. A. Mu¨ller 2005, personal observation), the samples were arranged in a circle on open ground (spaced apart 30–50 cm). This enabled accurate observation of the mongooses' response from 5–10 m distance. We scattered 20–50 g of bait in a circle at 2–4 m distance to the samples to make sure that the mongooses would find the presented stimuli. The experiments were recorded for later analysis using a digital video camera (Panasonic NV-GX7) and a Sennheiser ME 66/K6 directional microphone. Recording was stopped when no individual had approached any of the presented samples for 60 s.
The following response variables were evaluated: (i) number of individuals emitting worry calls; (ii) number of individuals counter-marking; and (iii) number and duration of inspection bouts (nose within 1 cm of a sample). Data on different types of counter-marks were pooled (urinating, defecating and anal marking). The duration of inspection bouts (one individual inspecting one sample) was determined frame-by-frame in Windows Movie Maker (1 frame=0.08 s). Only responses of adults were included in the analyses presented here, since younger individuals may not have learned to recognize neighbours yet.
To investigate how strangers become neighbours, we simulated the settling of a new group by repeatedly presenting scent marks of an unfamiliar (non-neighbouring) group to experimental groups. Six groups were exposed to scent marks of an unfamiliar group four times in a row (separated by 3–5 days). The experimental protocol was the same as described above. For the second, third and last experiments in these series, at least two samples were from individuals that had contributed to the set of samples earlier in the series. This allowed the experimental group to recognize the presented samples as from the same group, even if scent marks of banded mongooses do not contain group-specific information (Brown & MacDonald 1985). The series of repeated exposures to samples of an unfamiliar group were performed after the set of experiments investigating neighbour recognition had been completed.
(b) Statistical analyses
The number of worry calls and counter-marks observed during the control condition (prior to each trial) was deducted from the experimental condition. To avoid pseudoreplication, responses to the two neighbouring groups were averaged for the different locations. If the comparison of responses to stimuli of the three donor categories (‘own’, ‘neighbour’ and ‘stranger’) was significant, we conducted a planned post hoc comparison of responses to stimuli of neighbouring groups and strangers.
Group-level responses to scent-mark translocation experiments (number of individuals giving worry calls, number of individuals counter-marking and number of inspections) were normalized by square-root transformation and analysed in linear mixed models (LMM) using the restricted maximum likelihood method and type I sums of squares. Since group size changed markedly throughout the study period for some groups, group size of the experimental group (number of adults) was included as a covariate in the initial model, but dropped if the _p_-values for the main effect and all interactions were larger than 0.1. Group identity was included as a random factor but dropped if redundant (variance component less than 10−5). In the latter case, a linear model (LM) was calculated.
On the individual level, we analysed the duration of single inspection bouts (log-transformed) in a LMM, additionally controlling for sex of the inspecting individual, sex and age of the animal that had contributed the sample, sample type and inspection order (first, second, … sample a particular individual inspected). Identity of the inspecting individual (nested within group) was included as an additional random factor. For the latter analysis, we used only bouts with known identity of the inspecting animal and with bout length determined to the nearest 2 frames (0.16 s), in total 3133 bouts of 142 individuals in seven groups and ten trials per group.
For the series of exposures to scent marks of an unfamiliar group, three response variables were analysed on group level: number of worry calls emitted; number of counter-marks (both square-root transformed); and total duration of interest measured as the amount of time for which at least one individual was inspecting the presented excreta. Since group sizes changed by no more than one individual throughout these series, we analysed these data using repeated measures ANOVA. Data analysis was carried out in R v. 2.2.1 (R Development Core Team 2005).
3. Results
(a) Life history
During the course of this study, 233 animals were born and 211 animals died or disappeared. Twelve of 51 animals, for which the cause of mortality was known, were killed by neighbouring groups (eight infants and four adults). The size of some groups changed considerably between years due to death and recruitment of offspring. Of the seven groups studied, two increased in size (group size in March 2004, 11 and 18, respectively; in March 2005, 19 and 29, respectively), one decreased (34 to 23 individuals) and four remained largely constant. The two groups increasing in size expanded their home ranges considerably at the expense of neighbouring groups (see electronic supplementary material).
Seventy-three aggressive interactions between neighbouring groups were observed during the course of the study (0.02 interactions per observation hour). At least 22 of these interactions included serious aggression (body contact). Floaters were seen near the studied groups on 13 occasions, but no serious aggression towards them was observed.
(b) Neighbour–stranger discrimination
For the subset of experiments for which we had recorded the means of collection, samples collected ad libitum were neither inspected longer than samples collected by trapping (LMM with group and individual as random factors and controlling for significant effects of sample type, sample age and inspection order; _F_1,672=0.70, _p_=0.40) nor did groups inspect them more often (LMM with group as random factor and controlling for significant effects of sample type, location of the experiment and donor category; _F_1,57=0.001, _p_=0.98; ‘trapped’ samples: _N_=8, ‘ad libitum’ samples: _N_=62).
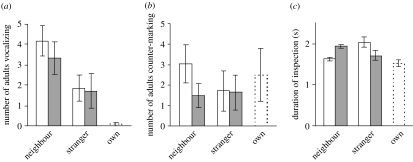
Presenting excreta of neighbours or strangers elicited worry calls in 80% of all experiments. Worry calls never occurred during the control condition, when herbivore faeces and water were presented. Only in one out of twelve experiments was a worry call given in response to samples of the own group. The number of individuals giving worry calls differed among the three donor categories (LMM, _F_2,27=26.3, p<0.001; figure 1a) and was twice as high for the neighbour treatments as for the stranger treatments (_F_1,18=9.27, _p_=0.007). The response did not differ between locations of the presentation (centre versus border; _F_1,18=1.09, _p_=0.31). In response to two out of the four neighbour treatments, one group emitted acoustically different calls typically given during agonistic group interactions (‘war cries’) in addition to worry calls (for spectrograms see electronic supplementary material).
Figure 1.
Responses of seven banded mongoose groups to excreta of neighbouring groups and strangers. Mean±s.e. are shown. Open bars, at the border of the experimental group's home range. Filled bars, in the centre of the experimental group's home range. Responses to scent marks of the group itself are shown on the far right in each panel. (a) Number of adults emitting worry calls. (b) Number of adults counter-marking. (c) Duration of single inspection bouts.
In 75% of all experiments, the presented scent marks evoked counter-marking. In 10% of the experiments, scent marking was also observed during the control condition. Taking this into account, the number of adults counter-marking neither differed among treatments (LMM, _F_2,27=0.59, _p_=0.56; figure 1b) nor between locations (_F_1,27=0.97, _p_=0.33), nor was there an interaction between the two factors (_F_2,27=0.44, _p_=0.65).
The number of inspection bouts differed among the three donor categories (LMM, _F_2,27=9.39, p<0.001). The number of bouts was higher during the neighbour treatments than the stranger treatments (_F_1,17=9.04, _p_=0.008) and higher at the border of the home ranges than in the centre (_F_1,17=6.16, _p_=0.024). The duration of single inspection bouts also differed among treatments (LMM after controlling for significant effects of sex of the inspecting individual, sex and age of the animal that had contributed the sample, sample type and inspection order; _F_2,2437=9.31, p<0.001; figure 1c). When comparing inspection bouts between ‘neighbour’ and ‘stranger’ treatments, we found no treatment effect but a significant interaction between treatment and location of the experiment (_F_1,2108=30.4, p<0.001; figure 1c). Inspection bouts to samples of neighbours were longer in the home range centre than at the border. In contrast, inspection bouts to samples of strangers were longer at the border than in the centre of the home range.
(c) Neighbour–neighbour discrimination
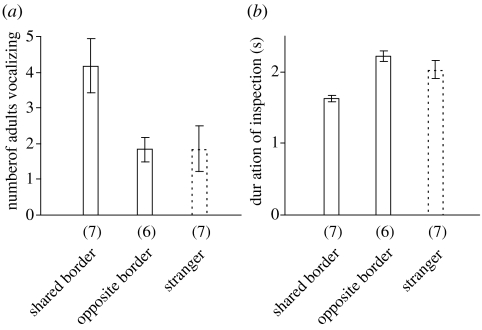
When presented with samples of a neighbouring group at the opposite border, fewer individuals gave worry calls than when samples of the same group were presented at the shared border (LM, _F_1,11=5.11, _p_=0.045; figure 2a). The number of individuals counter-marking did not differ between shared and opposite border (LMM, _F_1,5=0.14, _p_=0.73), nor did the number of inspections (LMM, _F_1,4=0.16, _p_=0.71). However, single inspection bouts were longer at the opposite border than at the shared border (LMM, _F_1,1710=34.2, p<0.001; figure 2b).
Figure 2.
Responses of banded mongoose groups to excreta of neighbouring groups at the shared and opposite borders of the experimental group's home range. Mean±s.e. are shown. Reactions to excreta of strangers at the border of the home range are given for reference. Numbers in brackets give sample sizes. (a) Number of individuals emitting worry calls. (b) Duration of single inspection bouts.
When categorizing each neighbouring group used in the experiments as either larger (_N_=13) or smaller (_N_=12) than the resident group, we found no effect of relative group size on the number of individuals emitting worry calls (LM correcting for location of the experiment, _F_1,22=1.45, _p_=0.24), on the number of individuals counter marking (LMM, _F_1,16=0.08, _p_=0.78) or on the number of inspection bouts (LMM, _F_1,16=0.31, _p_=0.58). However, single inspection bouts were longer when samples of a smaller rather than a larger neighbouring group where inspected (LMM, _F_1,1710=6.26, _p_=0.012). This effect was restricted to urine samples and did not occur for faeces (sample type×donor size interaction, _F_1,1710=13.8, p<0.001).
(d) Repeated exposure to scent marks of strangers
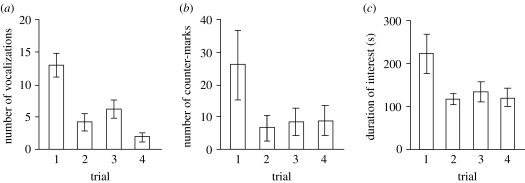
The intensity of the response to repeated presentation of scent marks from strangers declined over time (figure 3). During the later trials, fewer worry calls were emitted (repeated measures ANOVA, _F_3,15=9.84, _p_=0.0008) and the duration of interest was reduced (_F_3,15=4.79, _p_=0.016). The number of counter-marks tended to be lower during the later trials (_F_3,15=2.46, _p_=0.10).
Figure 3.
Reactions of six banded mongoose groups to repeated exposure to excreta of strangers. Mean±s.e. are shown. (a) Number of worry calls. (b) Number of counter-marks. (c) Duration of interest measured as amount of time for which at least one individual was inspecting the presented excreta.
4. Discussion
We tested two hypotheses that attempt to explain relationships between territorial neighbours, and in particular, the wide occurrence of NSD throughout the animal kingdom. The familiarity hypothesis and the threat-level hypothesis (Temeles 1994) make contrasting predictions when neighbours represent a higher threat to residents than strangers. This situation might be common in social species, in which large stable groups defend territories, such as in the banded mongoose.
Adult banded mongooses discriminated between neighbours and strangers. More animals emitted worry calls and individuals performed more inspection bouts in response to scent marks of neighbouring groups than to scent marks of strangers. We interpret worry calls, which recruited other group members to the site, as a correlate of response intensity. Inspection of the scent marks from neighbours may be increased because individuals gather information about dispersal opportunities as well as age, health and reproductive status of members of the neighbouring groups. The same information about strangers may also be relevant, but less so, since they likely represent transient animals that may not be encountered again. The number of animals counter-marking did not differ between ‘neighbour’, ‘stranger’ and ‘own group’ treatments. This suggests that counter-marking is not exclusively used for territory defence, but may serve other purposes within the group such as group cohesion or mate guarding (Jordan et al. in press).
As predicted by the threat-level hypothesis, neighbours elicited a stronger response than strangers. In banded mongooses, neighbours pose a considerable threat as potential usurpers of territories, opponents in lethal fights and competitors for mates (Cant et al. 2002). Strangers, in contrast, commonly represent small, single-sex dispersing splinters that are typically outnumbered by their same-sexed rivals in established groups (Cant et al. 2001; Banded Mongoose Project 2005, unpublished data) and, thus pose little threat. The stronger response to scent marks of neighbours than to samples of strangers cannot be explained by habituation. Neighbouring groups meet regularly (Cant et al. 2002; Gilchrist & Otali 2002) and encounters with scent marks of neighbouring groups at the territory border occur on a daily to weekly basis (C. A. Mu¨ller 2005, personal observation). The familiarity hypothesis, thus, predicts a reduced response to the stimuli of neighbouring groups, which is the opposite of what we found. Even so, repeated exposure to scent marks of the same unfamiliar group, simulating a new group settling nearby, led to weaker responses over time. Thus, even though mongooses habituate to olfactory stimuli from foreign groups, the response to scent marks of neighbours is increased. This suggests that, only after physical encounters have taken place, are neighbouring groups treated as a larger threat than strangers. These encounters may be seen as invasion attempts and, thus, as indication that the neighbouring group cannot be trusted (Godard 1993; Olendorf et al. 2004). Since all groups regularly engaged in fights with all of their neighbours, ‘trustworthy’ neighbours, which could be expected to be treated like dear enemies, did not occur in our study population.
Only few studies to date have investigated responses to neighbours and strangers in social vertebrates. Recently, a stronger response to stimuli of neighbours than of strangers has been shown in another social mammal with intense competition between neighbouring groups, the chimpanzee (Pan troglodytes verus; Herbinger 2004). In contrast, green woodhoopoe (Phoeniculus purpureus) groups respond less intensely to neighbours than to strangers (Radford 2005). However, in green woodhoopoes, group sizes are considerably smaller (2–9, mean=3, _N_=31, not including dependent young; Radford & Du Plessis 2004) than in the chimpanzees studied by Herbinger (10–52, mean=28, _N_=3; Herbinger 2004) or in banded mongooses (5–60, mean=24, _N_=9, present study). Thus, a numerical disparity between neighbouring groups and strangers is probably reduced or absent in woodhoopoes. Furthermore, when woodhoopoe groups are defeated in territorial disputes with neighbouring groups, they lose little, since victorious neighbours only briefly intrude into the defeated group's territory and no permanent changes in the territory boundaries are observed. However, woodhoopoe groups may lose their territory to strangers (Radford 2005). The weaker response to neighbours than to strangers observed in woodhoopoes is thus in accordance with both the familiarity and the threat-level hypotheses.
The duration of inspection bouts in banded mongooses was influenced by the source of the samples as well as by their spatial occurrence. Excreta of neighbours were inspected longer when encountered in the centre of the focal group's home range than when encountered at the border. In contrast, samples of strangers were inspected longer when encountered at the border than in the centre. Samples from strangers encountered at the border may represent a new group settling nearby or a recent takeover in a neighbouring group. Thus, it may pay to gather additional information about these potential new neighbours. Conversely, samples from strangers encountered in the centre of a group's home range are probably from transients, which are less likely to be encountered again. The pattern found for neighbours may be explained by increased inspection when excreta are encountered out of the usual (spatial) context, which may represent an attempt of a neighbouring group to expand its territory. The duration of inspection bouts during the ‘neighbour’ treatments increased from shared border to centre to opposite border of the focal group's home range (figures 1_c_ and 2_b_).
The threat-level hypothesis not only predicts a stronger response to neighbours than to strangers in banded mongooses, but also a stronger response to larger compared to smaller neighbours. However, we found that the response to larger neighbouring groups was not stronger than to smaller ones. This indicates that banded mongooses distinguish between different threat levels only in a crude way (even smaller neighbouring groups are typically still considerably larger than dispersal splinters representing strangers). Alternatively, mongoose groups may be unable to monitor the size of their neighbours. We believe this is unlikely since fights between groups are decided by group size (Cant et al. 2002) and, thus, groups remembering the outcome of recent fights also know if the respective neighbouring group is larger or smaller than themselves. Furthermore, we found that mongooses inspected urine samples of smaller neighbouring groups longer than urine of larger ones. This may reflect that smaller neighbours more likely offer an opportunity to disperse and take over. It also indicates that mongooses are able to distinguish larger from smaller neighbouring groups.
Although adult banded mongooses did not discriminate between neighbouring groups according to relative group size, they nevertheless discriminated between different neighbours. Excreta were inspected longer and elicited fewer worry calls when presented at the opposite border than when presented at the shared border. The response to neighbours at the opposite border was not different from the response to strangers (figure 2). These results suggest that stimuli of neighbours, when encountered at the ‘wrong’ border, are considered to represent dispersing animals and are therefore treated like stimuli of strangers, even though neighbours are probably still recognized when encountered in a novel location (as in frogs, Bee & Gerhardt 2002). Presence of NSD at the shared border and absence at the opposite border has also been found for species exhibiting a ‘dear enemy effect’ (e.g. Stoddard et al. 1991; Radford 2005). Therefore, stimuli of familiar conspecifics encountered in a novel location do not automatically lead to a stronger response, but may lead to a weaker response (in this case fewer worry calls). The latter finding cannot be explained by dishabituation.
Our findings support the hypothesis that NSD in banded mongooses is based on varying threat levels represented by neighbours and strangers. For this species, we can reject the hypothesis that neighbours and strangers get treated differently because residents are more familiar with neighbours than with strangers. However, banded mongooses may respond to different threat levels in a crude way without discriminating further between larger and smaller neighbouring groups. We suggest that ‘nasty neighbours’ instead of ‘dear enemies’ are commonly found in social species with intense competition between neighbours and with large numerical differences between groups of neighbours and strangers. We believe that studies of taxa with differences in their social system, as well as studies of species in different contexts (e.g. breeding versus non-breeding, Leiser 2003; more or less attractive/aggressive neighbours, Olendorf et al. 2004; Hyman & Hughes 2006) will help to elucidate the causes of the taxonomically widespread phenomenon of neighbour recognition and promote understanding of the relationships between territorial competitors.
Acknowledgments
We are grateful to Uganda Wildlife Authority for permission to work in Queen Elizabeth National Park and Chief Wardens J. Bosco and T. Okello for their support in the park. S. Kyabulima and F. Mwanguhya were a great help in the field. We thank Mike Cant for logistical support and the opportunity to work on the Banded Mongoose Project. Particular thanks to Matthew Bell who was an invaluable and stimulating co-worker and contributed to life-history and GPS data. Mike Cant, Jason Gilchrist and Sarah Hodge kindly provided access to their life-history database. The comments of Hansjoerg Kunc, Rob Olendorf, Peter Pearman, Andy Radford and an anonymous reviewer greatly improved the manuscript. The presented work was funded by the Swiss National Science Foundation (Förderprofessur no. 631-066129 to M.B.M.). The study was carried out under licence from Uganda National Council for Science and Technology and Uganda Wildlife Authority.
Supplementary Material
Supplementary figures
Home ranges, changes in home ranges, sightings of a floater, and spectrograms
Supplementary video
Video clip showing the recruitment effect of worry calls
References
- Asa C.S, Peterson E.K, Seal U.S, Mech L.D. Deposition of anal-sac secretions by captive wolves (Canis lupus) J. Mammal. 1985;66:89–93. doi:10.2307/1380960 [Google Scholar]
- Bee M.A, Gerhardt H.C. Individual voice recognition in a territorial frog (Rana catesbeiana) Proc. R. Soc. B. 2002;269:1443–1448. doi: 10.1098/rspb.2002.2041. doi:10.1098/rspb.2002.2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein D.T, Patton M.L, Saltzmann W. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol. Lett. 2006;2:29–32. doi: 10.1098/rsbl.2005.0405. doi:10.1098/rsbl.2005.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.E, MacDonald D.W. Oxford University Press; New York, NY: 1985. Social odours in mammals. [Google Scholar]
- Cant M.A. Social control of reproduction in banded mongooses. Anim. Behav. 2000;59:147–158. doi: 10.1006/anbe.1999.1279. doi:10.1006/anbe.1999.1279 [DOI] [PubMed] [Google Scholar]
- Cant M.A, Otali E, Mwanguhya F. Eviction and dispersal in co-operatively breeding banded mongooses (Mungos mungo) J. Zool. 2001;254:155–162. doi:10.1017/S0952836901000668 [Google Scholar]
- Cant M.A, Otali E, Mwanguhya F. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology. 2002;108:541–555. doi:10.1046/j.1439-0310.2002.00795.x [Google Scholar]
- Cheney D.L, Seyfarth R.M. Recognition of individuals within and between groups of free-ranging vervet monkeys. Am. Zool. 1982;22:519–529. [Google Scholar]
- Davis M.S. Acoustically mediated neighbor recognition in the North-American bullfrog, Rana catesbeiana. Behav. Ecol. Sociobiol. 1987;21:185–190. doi:10.1007/BF00303209 [Google Scholar]
- Fisher J.B. Evolution and bird sociality. In: Huxley J, Hardy A.C, Ford E.B, editors. Evolution as a process. Allen & Unwin; London, UK: 1954. pp. 71–83. [Google Scholar]
- Getty T. Are dear enemies in a war of attrition? Anim. Behav. 1989;37:337–339. doi:10.1016/0003-3472(89)90125-5 [Google Scholar]
- Gilchrist J.S, Otali E. The effects of refuse-feeding on home-range use, group size, and intergroup encounters in the banded mongoose. Can. J. Zool. 2002;80:1795–1802. doi:10.1139/Z02-113 [Google Scholar]
- Godard R. Tit-for-tat among neighboring hooded warblers. Behav. Ecol. Sociobiol. 1993;33:45–50. doi:10.1007/BF00164345 [Google Scholar]
- Goodall J. The Belknap Press of Harvard University Press; Cambridge, MA: 1986. The chimpanzees of Gombe: patterns of behavior. [Google Scholar]
- Herbinger, I. 2004 Inter-group aggression in wild West African chimpanzees (Pan troglodytes verus): mechanisms and functions. Ph.D. thesis, University of Leipzig.
- Hyman J, Hughes M. Territory owners discriminate between aggressive and nonaggressive neighbours. Anim. Behav. 2006;72:209–215. doi:10.1016/j.anbehav.2006.01.007 [Google Scholar]
- Jordan, N. R., Cherry, M. I. & Manser, M. B. In press. The spatial and temporal distribution of meerkat latrines reflects intruder diversity and suggests a role in mate-defence. Anim. Behav
- Leiser J.K. When are neighbours ‘dear enemies’ and when are they not? The responses of territorial male variegated pupfish, Cyprinodon variegatus, to neighbours, strangers and heterospecifics. Anim. Behav. 2003;65:453–462. doi:10.1006/anbe.2003.2087 [Google Scholar]
- Mech L.D, Boitani L. University of Chicago Press; Chicago, IL: 2003. Wolves: behavior, ecology, and conservation. [Google Scholar]
- Olendorf R, Getty T, Scribner K, Robinson S.K. Male red-winged blackbirds distrust unreliable and sexually attractive neighbours. Proc. R. Soc. B. 2004;271:1033–1038. doi: 10.1098/rspb.2004.2687. doi:10.1098/rspb.2004.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme R, Rettenbacher S, Touma C, El-Bahr S.M, Mostl E. Stress hormones in mammals and birds—comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann. N. Y. Acad. Sci. 2005;1040:162–171. doi: 10.1196/annals.1327.021. doi:10.1196/annals.1327.021 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2005 R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org
- Radford A.N. Group-specific vocal signatures and neighbour–stranger discrimination in the cooperatively breeding green woodhoopoe. Anim. Behav. 2005;70:1227–1234. doi:10.1016/j.anbehav.2005.04.002 [Google Scholar]
- Radford A.N, Du Plessis M.A. Green woodhoopoe Phoeniculus purpureus territories remain stable despite group-size fluctuations. J. Avian Biol. 2004;35:262–268. doi:10.1111/j.0908-8857.2004.03235.x [Google Scholar]
- Rood J.P. Population dynamics and food habits of the banded mongoose. East Afr. Wildl. J. 1975;13:89–111. [Google Scholar]
- Schaller G.B. University of Chicago Press; Chicago, IL: 1972. The Serengeti lion: a study of predator–prey relationships. [Google Scholar]
- Sliwa A, Richardson P.R.K. Responses of aardwolves, Proteles cristatus, Sparrman 1783, to translocated scent marks. Anim. Behav. 1998;56:137–146. doi: 10.1006/anbe.1998.0757. doi:10.1006/anbe.1998.0757 [DOI] [PubMed] [Google Scholar]
- Stoddard P.K. Vocal recognition of neighbours by territorial passerines. In: Kroodsma D.E, Miller E.H, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 356–374. [Google Scholar]
- Stoddard P.K, Beecher M.D, Horning C.L, Campbell S.E. Recognition of individual neighbors by song in the song sparrow, a species with song repertoires. Behav. Ecol. Sociobiol. 1991;29:211–215. doi:10.1007/BF00166403 [Google Scholar]
- Swaisgood R.R, Owings D.H, Rowe M.P. Conflict and assessment in a predator–prey system: ground squirrels versus rattlesnakes. Anim. Behav. 1999;57:1033–1044. doi: 10.1006/anbe.1998.1069. doi:10.1006/anbe.1998.1069 [DOI] [PubMed] [Google Scholar]
- Swaisgood R.R, Lindburg D.G, Zhang H. Discrimination of oestrous status in giant pandas (Ailuropoda melanoleuca) via chemical cues in urine. J. Zool. 2002;257:381–386. doi:10.1017/S0952836902000985 [Google Scholar]
- Temeles E.J. Northern harriers on feeding territories respond more aggressively to neighbors than to floaters. Behav. Ecol. Sociobiol. 1990;26:57–63. doi:10.1007/BF00174025 [Google Scholar]
- Temeles E.J. The role of neighbors in territorial systems—when are they dear enemies? Anim. Behav. 1994;47:339–350. doi:10.1006/anbe.1994.1047 [Google Scholar]
- White A.M, Swaisgood R.R, Zhang H. Chemical communication in the giant panda (Ailuropoda melanoleuca): the role of age in the signaller and assessor. J. Zool. 2003;259:171–178. doi:10.1017/S0952836902003187 [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1975. Sociobiology: the new synthesis. [Google Scholar]
- Ydenberg R.C, Giraldeau L.A, Falls J.B. Neighbors, strangers, and the asymmetric war of attrition. Anim. Behav. 1988;36:343–347. doi:10.1016/S0003-3472(88)80004-6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Home ranges, changes in home ranges, sightings of a floater, and spectrograms
Supplementary video
Video clip showing the recruitment effect of worry calls