Functional characterization of human PFTK1 as a cyclin-dependent kinase (original) (raw)
Abstract
Cyclin-dependent kinases (CDKs) are crucial regulators of the eukaryotic cell cycle whose activities are controlled by associated cyclins. PFTK1 shares limited homology to CDKs, but its ability to associate with any cyclins and its biological functions remain largely unknown. Here, we report the functional characterization of human PFTK1 as a CDK. PFTK1 specifically interacted with cyclin D3 (CCND3) and formed a ternary complex with the cell cycle inhibitor p21Cip1 in mammalian cells. We demonstrated that the kinase activity of PFTK1 depended on CCND3 and was negatively regulated by p21Cip1. Moreover, we identified the tumor suppressor Rb as a potential downstream substrate for the PFTK1/CCND3 complex. Importantly, knocking down PFTK1 expression by using siRNA caused cell cycle arrest at G1, whereas ectopic expression of PFTK1 promoted cell proliferation. Taken together, our data strongly suggest that PFTK1 acts as a CDK that regulates cell cycle progression and cell proliferation.
Keywords: cyclin D3, p21, retinoblastoma, cell cycle
Cyclin-dependent kinases (CDKs) are crucial regulators of the eukaryotic cell cycle. Cdc2 was identified as the first CDK that was essential for G1/S and G2/M transitions in Schizosaccharomyces pombe (1). Since then, homologs in all species from yeast to mammals have been found. So far, 11 mammalian CDK family members have been described, and that number is increasing rapidly (2). CDKs are a family of Ser/Thr protein kinases that all share a highly conserved motif, known as the PSTAIRE motif, located in subdomain III of the kinase domain. This motif is involved in the binding of a CDK to a cyclin and has been used to classify other newly identified CDK-related kinases, such as PCTAIRE, PITSLRE, PFTAIRE, PITAIRE, KKIALRE, PISSLRE, MAK, and MRK, etc. (3–12). The function of these CDK-related kinases has not been fully understood. Intriguingly, despite their sequence homologies with CDKs, almost all of the members of this group appear to be devoid of cell cycle functions, largely because of their restricted tissue expressions and failure of identifying a regulatory cyclin partner.
The activity of most CDKs requires the formation of holoenzymes. These holoenzymes contain both regulatory (cyclin) and catalytic (CDK) subunits, and, sometimes, additional proteins. Distinct cyclin–CDK complexes drive cells through different phases of the cell cycle (1). Typically, the D-type cyclins (cyclins D1, D2, and D3) act as growth factor sensors, forming active kinases with CDKs in response to extracellular signals. The mitogen-dependent accumulation of cyclin D-CDK (CDK4 and CDK6 in particular) holoenzymes triggers the phosphorylation of Rb, a tumor suppressor, and cancels the growth-repressive functions of hypophosphorylated Rb (1, 13, 14).
In addition to positive regulation by cyclins, CDKs are regulated by several subunits named CKIs (CDK inhibitors) that associate physically with cyclin–CDK complexes to inhibit their activities and promote cell cycle arrest or delay. CKIs include INK4 and Cip/Kip family members (15). Among them, p21Cip1 is mostly documented as a universal inhibitor of CDKs (16).
PFTK1 is a Cdc2-related protein kinase, also named as PFTAIRE1 (17). The gene is highly expressed in the brain, pancreas, kidney, heart, testis, and ovary and minimally expressed in the spleen and thymus. Some interacting proteins of PFTAIRE have been reported in different species. For example, L63, the Drosophila PFTAIRE1, interacts with two novel proteins, PIF-1 and PIF-2 (PFTAIRE interacting factor-1 and -2) (18); two specific sets of endogenous cytosolic proteins of ≈58–60 and 200–205 kDa have been reported to associate with mPFTAIRE1 (19); KIAA0202 and 14-3-3 proteins were identified as hPFTAIRE1-interacting proteins (20, 21). However, none of the interacting partners is cyclin-like, and the functional significance of these PFTAIRE-associated factors has not been fully addressed.
To uncover the biological function of PFTK1, we conducted a complete yeast two-hybrid screening, from which we identified two PFTK1-associating factors as CCND3 and p21Cip1. We showed that these three proteins formed a stable complex in vivo, and p21Cip1 inhibited the CCND3-dependent activation of PFTK1. Further functional characterization of human PFTK1 demonstrated that PFTK1 acts as a CDK likely involved in cell cycle regulation.
Results
Interactions of CCND3 and p21Cip1 with PFTK1 Were Identified by Yeast Two-Hybrid Screening.
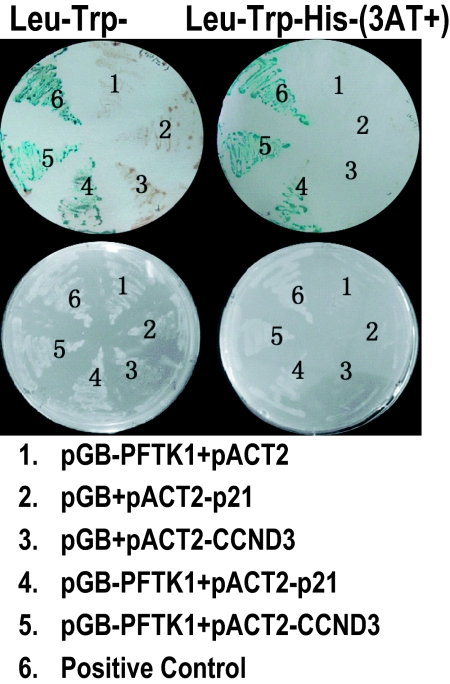
Although PFTK1 orthologs have been identified in many species, including human, mouse, and Drosophila, functionally relevant interacting partners of PFTK1 have yet to be found. To find previously unidentified PFTK1-associated factors, we performed a yeast two-hybrid screen using human PFTK1 as the bait. Eighty-two positive clones were isolated. Interestingly, 42 of them encoded p21Cip1 protein fragments and 6 of them encoded CCND3 protein fragments (Table 1). No other cyclin or cell cycle regulator was identified in the screening, suggesting the specificity of these interactions. Interactions between full-length proteins were confirmed by direct yeast two-hybrid analysis (Fig. 1).
Table 1.
Yeast two-hybrid screening of PFTK1 protein
| Positive clone | No. of positive clones/total no. of positive clones | GenBank accession no. |
|---|---|---|
| p21cip1 | 42/82 | NM_000389 |
| Cyclin D3 | 6/82 | NM_001760 |
Fig. 1.
PFTK1 interacts with p21Cip1 and CCND3 in the yeast two-hybrid assay. pGB or pGB-PFTK1 was cotransformed with pACT2, pACT2-p21Cip1, or pACT2-CCND3 into the yeast strain Y190 along with a positive control pair, and tranformants were streaked on an SD/Leu-Trp plate (Left) and an SD/Leu-Trp-His plate with 3-AT (Right). β-Galactosidase assay was also performed (Upper).
PFTK1 Forms a Ternary Complex with p21Cip1 and CCND3.
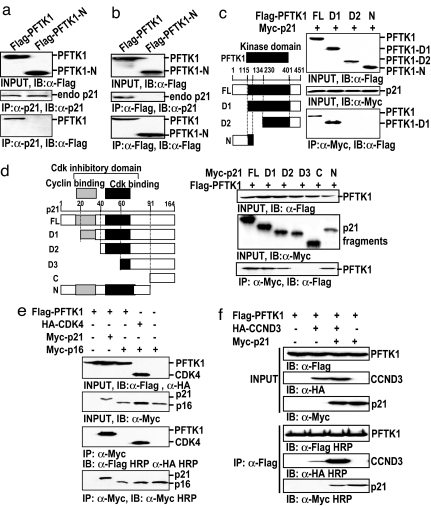
To examine the in vivo interaction and physiological relevance between PFTK1 and p21Cip1 identified in the yeast two-hybrid screening, we first conducted semiendogenous coimmunoprecipitation experiments. Cell lysates prepared from 293T cells transfected with PFTK1 were immunoprecipitated with anti-p21Cip1. As shown in Fig. 2a, PFTK1 interacted with endogenous p21Cip1. In a reciprocal experiment, we also observed that endogenous p21Cip1 coimmunoprecipitated with ectopically expressed PFTK1 (Fig. 2b). Taken together, our data demonstrate a specific interaction between PFTK1 and p21Cip1.
Fig. 2.
PFTK1 forms a ternary complex with p21Cip1 and CCND3. (a) The 293T cells were transfected with indicated constructs of PFTK1. Lysates were immunoprecipitated with anti-p21Cip1 and blotted with anti-p21Cip1 and anti-Flag. (b) Anti-Flag was used to immunoprecipitate the same lysates used in a. The immunoprecipitants were blotted with anti-p21Cip1 and anti-Flag. (c Left) Schematic representation of Flag-tagged PFTK1 truncations. (c Right) The 293T cells were transfected with indicated constructs. Lysates were immunoprecipitated with anti-Myc and blotted with anti-Flag. Lysates were also blotted with anti-Myc and anti-Flag. (d Left) Schematic representation of Myc-tagged p21Cip1 truncations. (d Right) PFTK1 interacts with full-length p21Cip1, D1, D2, and N fragments of p21Cip1. (e) The 293T cells were transfected with indicated constructs. Lysates were immunoprecipitated with anti-Myc and blotted with anti-Myc, anti-Flag, and anti-HA. (f) The 293T cells were transfected with indicated constructs. Lysates were immunoprecipitated with anti-Flag. Both lysates and immunoprecipitants were blotted with anti-Flag-HRP, anti-HA-HRP, and anti-Myc-HRP separately.
To further map the p21Cip1-interacting region of PFTK1, we constructed additional PFTK1 fragments (Fig. 2c Left), including D1 (115–451 aa), D2 (230–451 aa), and N (1–134 aa), and transfected each cDNA construct into 293T cells together with p21Cip1. As shown in Fig. 2c, p21Cip1 was coimmunoprecipitated with full-length PFTK1 and its D1 derivative but not D2 and N, demonstrating that the kinase domain of PFTK1 was the p21Cip1-interacting region, in which amino acids 115 to 230 are necessary for binding. Similarly, we constructed p21Cip1 truncation fragments (Fig. 2d Left), including D1 (20–164 aa), D2 (40–164 aa), D3 (60–164 aa), C (91–164 aa), and N (1–91 aa). As shown in Fig. 2d, PFTK1 interacted with D1, D2, and N fragments of p21Cip1, indicating that the region between amino acids 40 and 60 within p21Cip1 is necessary for its association with PFTK1. It is well documented that the CDK-binding region is composed of amino acids 42–82 on p21Cip1 (22). Our observation of PFTK1 binding to p21Cip1 through the CDK-binding domain of p21Cip1 provided the first evidence that PFTK1 might be a CDK. On the other hand, another CDK inhibitor p16INK4a failed to interact with PFTK1 although it associated with its known binding partner CDK4 readily, showing the specificity of the PFTK1–p21Cip1 interaction (Fig. 2e).
The association between CCND3 and PFTK1 was relatively weak (Fig. 2f). However, the association between PFTK1 and CCND3 was markedly enhanced in the presence of p21Cip1. The result is reminiscent of the previous finding that p21Cip1 helps the formation of CDK–cyclin complexes during cell cycle regulation (23, 24). The p21Cip1 association with PFTK1 was independent of the presence of CCND3 (Fig. 2f), again consistent with the observation that CKIs can bind to both monomeric CDKs and cyclin-bound CDKs and also use multiple mechanisms to inhibit CDKs and halt the progression of the cell cycle.
Endogenous Interaction Between PFTK1 and CCND3.
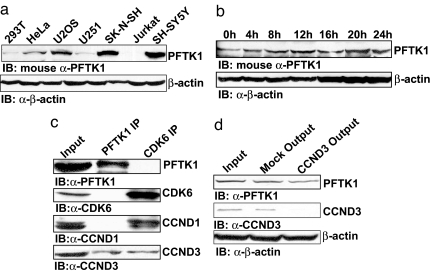
To examine the endogenous association between PFTK1 and CCND3, we generated a PFTK1 monoclonal antibody. As shown in Fig. 3a, the PFTK1 antibody detected a specific protein band with the predicted molecular mass of PFTK1 in different cell lines. The expression level of PFTK1 stayed relatively constant during the cell cycle (Fig. 3b), similar to the pattern seen with other CDKs. We then used the anti-PFTK1 antibody to immunoprecipitate PFTK1 in the neuroblastoma cell line SH-SY5Y. We could readily detect CCND3 in the PFTK1 immunoprecipitates, indicating that these proteins indeed interact in vivo (Fig. 3c). In addition, this association appears specific, because we could not detect any endogenous cyclin D1 (CCND1) in the PFTK1 precipitates. In contrast, CDK6 was coimmunoprecipitated with both CCND1 and CCND3 (Fig. 3c). Moreover, we used an anti-CCND3 antibody to immunodeplete CCND3 from cells lysates and found that the PFTK1 level was decreased (Fig. 3d), suggesting that at least part of the cellular PFTK1 is associated with CCND3. These data convincingly demonstrate that the association between PFTK1 and CCND3 is not only specific but also physiologically relevant.
Fig. 3.
Endogenous interaction between PFTK1 and CCND3. (a) Expression of endogenous PFTK1 in different cell lines. Lysates of different cell lines were blotted with monoclonal anti-PFTK1 and anti-β-actin. (b) U2OS cells were arrested in G1/G0 phase by treatment with 0.5 mM l-mimosine for 24 h. Cells were then released and harvested at indicated time points. Immunoblotting for endogenous PFTK1 was performed along with an anti-β-actin blot. (c) Lysates from 1×108 SH-SY5Y cells were immunoprecipitated with anti-PFTK1 or anti-CDK6, and the immunoprecipitants were blotted with anti-PFTK1, anti-CDK6, anti-CCND1, or anti-CCND3. (d) Lysates from SH-SY5Y cells were immunoprecipitated by using anti-CCND3 or mIgG as the control. The resulting supernatant was blotted with anti-PFTK1, anti-CCND3, or anti-β-actin.
PFTK1 Is an Active Kinase, and Its Activity Is Enhanced by CCND3 and Inhibited by p21Cip1.
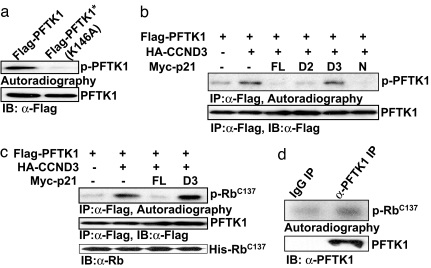
Previously, human PFTK1 has not been examined for its kinase activity and potential substrates. Stable cell lines expressing mouse PFTK1 were used by Besset et al. to examine its kinase activity toward different substrates. Although several substrates, including histone H1, casein, p56 Rb protein, neurofilaments, and myelin basic protein have been examined, none of them was found to be phosphorylated by mPFTK1 (3). The discovery of CCND3 and p21Cip1 as PFTK1-associated factors prompted us to further investigate the in vitro kinase activity of PFTK1. The 293T cells were transfected with PFTK1 or PFTK1 (K146A), in which the invariant lysine in subdomain II critical for ATP binding was mutated (25). Immunoprecipitated PFTK1 was subjected to an in vitro kinase assay, followed by SDS/PAGE and visualized by autoradiography. An ≈50-kDa 32P-labeled band corresponding to PFTK1 was observed only when the wild-type protein was immunoprecipitated (Fig. 4a). Taken together, these observations are consistent with PFTK1 being a bona fide protein kinase with intrinsic kinase activity.
Fig. 4.
PFTK1 is an active kinase that phosphorylates Rb. (a) The 293T cells transfected with indicated constructs were lysed, and lysates were immunoprecipitated with anti-Flag. Half of the precipitates were blotted with anti-Flag, and the other half were used for kinase assay. (b) The kinase activity of PFTK1 is enhanced by CCND3 and inhibited by p21Cip1. The 293T cells transfected with indicated constructs were harvested and analyzed as described above. The p21Cip1 derivatives used were the same as described in Fig. 2d Left. (c) Phosphorylation of His-RbC137 by PFTK1. The 293T cells transfected with indicated constructs were harvested and analyzed as described above. Two micrograms of His-RbC137 protein was added to each reaction as the substrate. (d) Activity of endogenous PFTK1 in U2OS cells. U2OS cells (1×108) were harvested and immunoprecipitated with mouse anti-PFTK1 (mouse IgG was used as the control). Half of the precipitates were used for blotting with rabbit anti-PFTK1 (Lower), and the other half were used for kinase assay containing 2 μg of RbC137 protein as the substrate (Upper).
Furthermore, we investigated whether the kinase activity of PFTK1 is regulated by CCND3 and p21Cip1. PFTK1 and CCND3 were cotransfected with p21Cip1 or its fragments in 293T cells. Our data revealed that the autophosphorylation activity of PFTK1 could be enhanced and inhibited by CCND3 and p21Cip1, respectively (Fig. 4b). The inhibition by p21Cip1 was specific, because a truncated version of p21Cip1 (p21Cip1-D3), which failed to interact with PFTK1, did not show any inhibitory effect on PFTK1 activity. This result suggests that the association of p21Cip1 with PFTK1 is required to exert the inhibitory effect of p21Cip1. Taken together, we conclude that PFTK1 is an active kinase whose activity is enhanced by CCND3 and inhibited by p21Cip1.
PFTK1 Phosphorylates the Tumor Suppressor Rb.
Rb has been shown to be a substrate for all cyclin D-associated CDKs. We went on to test the possibility of Rb being a substrate of PFTK1–CCND3 complex in vitro. In agreement with our previous finding, the phosphorylation of RbC137 (amino acids 792–928 of Rb) by PFTK1 was marginal and greatly enhanced in the presence of CCND3 (Fig. 4c), again supporting the notion that PFTK1 is a cyclin-dependent kinase. Moreover, the CCND3-dependent PFTK1 phosphorylation of RbC137 was significantly inhibited by p21Cip1 but not by its truncated fragment p21Cip1-D3 (Fig. 4c). These data demonstrate that PFTK1 can phosphorylate Rb, at least in vitro, and this phosphorylation is enhanced by CCND3 and inhibited by p21Cip1. Moreover, endogenous PFTK1 protein immunoprecipitated with an anti-PFTK1 antibody was also capable of phosphorylating Rb (Fig. 4d), further suggesting that Rb is a bona fide substrate of PFTK1.
Expression of PFTK1 Promotes the S-Phase Entry.
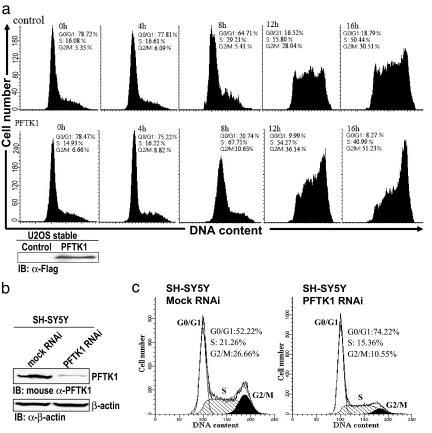
The facts that PFTK1 could be a CDK and that it phosphorylates Rb prompted us to investigate the role of PFTK1 in cell cycle regulation. We established a cell line stably overexpressing PFTK1 by retroviral infection of U2OS cells. A control cell line using empty vector was also established. We next evaluated their growth characteristics by delineating the growth curve of these two stable cell lines synchronized at G1 with 0.5 mM l-mimosine. After incubation with l-mimosine for 24 h, cells were released from drug treatment and harvested every 4 h for cell cycle analysis. As shown in Fig. 5a, cells with stably expressed PFTK1 entered S-phase faster than control cells by FACS analysis after removal of l-mimosine, indicating that PFTK1 promotes cell cycle progression. Overall, cells with PFTK1 stably expressed displayed a reduced doubling time compared with the control cell line. However, there is no growth difference between the two cell lines released from G2/M arrest induced by 50 ng/ml nocodazole (data not shown), suggesting that PFTK1 may specifically promote G1 to S transition. This result is consistent with the observation that PFTK1 could phosphorylate Rb to exert its function.
Fig. 5.
PFTK1 regulates G1/S transition of cell cycle. (a) Stably expressed PFTK1 promotes cell cycle progression. A stable PFTK1-expressing cell line (U2OS) and a control cell line were synchronized by 0.5 mM l-mimosine for 24 h. The drug-containing medium was removed, and fresh medium was added (marked as 0 h) to allow cells to exit from G0/G1 arrest. Cells were collected for FACS analysis every 4 h. Lysates were blotted with anti-Flag to confirm the expression of PFTK1. (b) siRNA-mediated knockdown of PFTK1 in SH-SY5Y cells. Cells were harvested 48 h after transfection. The knockdown effect of siRNA against PFTK1 was examined by using an anti-PFTK1 monoclonal antibody. The blot was also probed with anti-β-actin antibody. (c) siRNA against PFTK1 leads to cell cycle arrest at G1. Representative histograms for cell cycle distribution in SH-SY5Y cells transfected with control siRNA (Left) and PFTK1-specific siRNA (Right) are shown.
siRNA Knockdown of PFTK1 Results in G1 Arrest.
To further verify the above observation, we designed and tested siRNA against PFTK1, which showed efficient knockdown effect in SH-SY5Y cells (Fig. 5b). We found that siRNA against PFTK1 induced a profound G1 arrest compared with the control siRNA in SH-SY5Y cells (Fig. 5c). The percentage of G1 cells increased from 52.22% to 74.22%. Taken together, these data strongly suggest that PFTK1 may represent a new member of CDK family involved in cell cycle regulation.
Discussion
PFTK1 belongs to the CDK-like kinase family, which include mammalian PFTAIRE, PCTAIRE, PITSLRE, etc. Only one of the family members, p58 (PITSLRE), was reported to associate with a particular cyclin; coincidentally, that cyclin was CCND3 (6). No other member of this family has been identified to have a cyclin or a cyclin-like protein as a partner. Moreover, although many associated factors of PFTK homologs have been identified in different species, none of them is cyclin-like, and their functional relevance remains to be demonstrated. Interestingly, the possibility of cyclin-based activation of Drosophila PFTK1, called L63, was raised by Stowers et al. (26). They showed that L63 (G243A) failed to rescue the lethal phenotype in L63-deleted mutant animals when glycine 243, a residue conserved for cyclin binding, was mutated. Here, we report that CCND3 is a specific regulatory subunit for PFTK1, and further confirm the interaction between PFTK1 and CCND3 both in vitro and in vivo.
D-type cyclins, in association with CDKs, promote cell division by inactivating negative regulators of S-phase gene expression. The catalytic activities of cyclin D-dependent kinases have been well documented only in relationship to their phosphorylation and inactivation of the transcriptional corepressor activity of the Rb family members. Our observation that PFTK1–CCND3 complex could phosphorylate Rb coincided with this notion. Moreover, the fact that PFTK1–CCND3–p21 could form a stable ternary complex makes PFTK1 fit well in the cyclin D-associated CDK category.
To date, almost all of the members of Cdc2-like protein kinases, such as PFTAIRE, PCTAIRE, PITSLRE, PITAIRE, KKIALRE, PISSLRE, etc. have restricted tissue expression and appear to be devoid of cell cycle function. However, human PFTK1 is widely expressed in different tissues in contrast to the restricted expression of mPFTK1 in testis and the nervous system, which prompted us to explore its role in cell cycle regulation. Our results demonstrate that PFTK1 impels the transition from G1 to S phase, not only in established stable cell lines but also in unmodified SH-SY5Y cells as shown by our RNAi assay (Fig. 5). However, from this study, we cannot rule out the possibility that PFTK1 may also function in cell differentiation in specific tissues.
The recent generation of targeted gene-disruption mouse models for individual cyclin Ds and CDK4/6, and different combination of them, has revealed that a majority of cell types can arise in the absence of cyclin D-CDK complexes. However, it is important to note that knockout embryos of these strains show reduced body size, highlighting the significance of these cyclin D–CDK complexes in controlling cell growth and/or cell proliferation (27). Moreover, the most drastic consequence of the absence of cyclin D–CDK complexes arises in tissues with high proliferative demand such as the fetal hematopoietic compartment. For example, CCND3-null T cell progenitors are severely impaired in their ability to undergo pre-TCR expansion (28). Likewise, PFTK1–CCND3 active complexes may also couple various kinds of homeostatic signals (growth factors, nutrients, oxygen, etc.) to cell cycle progression. In fact, Hashida et al. (29) reported that PFTK1 mRNA levels in the euthyroid TRH−/− cerebellum supplemented with thyroid hormone were significantly decreased compared with those in the wild type. Recently, Tang et al. (30) reported PFTK1 as one of the four negative regulators of insulin-responsive glucose transport. These findings also suggest a regulatory role of PFTK1 in response to particular extracellular signals in specific tissues.
In this study, we demonstrate that endogenous CCND3, but not CCND1, interacts with endogenous PFTK1 in SH-SY5Y cells (Fig. 3c). CCND3 is the least studied member of the D-type cyclins. It shows the broadest expression pattern of all three D-type cyclins, and its abundance in quiescent tissues suggests that it has dual roles in proliferation and differentiation (31). Because CCND3 is expressed ubiquitously, it is not surprising that PFTK1, another broadly expressed protein, acts as its binding partner. In addition, CCND3's selective binding to another CDK-related kinase has been reported (6). PFTK1's specific pairing with CCND3 may suggest a link between PFTK1 and cancer cell growth. Like all D-type cyclins, CCND3 is overexpressed in many human cancers. Studies by Sicinska et al. found that mice lacking CCND3 showed greatly reduced sensitivity to malignancies triggered by specific oncogenic pathways in immature T lymphocytes. This requirement for CCND3 also operates in human malignancies, because knockdown of CCND3 inhibited proliferation of T cell acute lymphoblastic leukemia (T-ALL) cells (28). It would be intriguing to investigate whether the association of PFTK1 with CCND3 is related to specific oncogenic pathways involving CCND3.
Our identification and characterization of CCND3 and p21Cip1 as specific regulatory proteins of PFTK1 added a previously unidentified member to the CDK family. We propose that cell cycle regulation by PFTK1–CCND3 complex would presumably be tightly linked to the growth and stimulation of the cell. Further investigation of how PFTK1 responds to particular extracellular signals will help us understand the fine-tuning of cell growth and proliferation in specific tissues.
Materials and Methods
Yeast Two-Hybrid Analysis.
The full-length human PFTK1 (GenBank, NM_012395) cDNA was cloned into modified pGBKT7 vector and was used as the bait to screen three pACT2-human cDNA libraries (HeLa, human T/B lymphocyte, and human fetal brain). Positive interactions were verified by β-galactosidase assay.
Mammalian Expression Constructs.
Human PFTK1 and its derivative cDNAs were cloned into a mammalian expression vector pEF with an N-terminal Flag tag. Human p16INK4a, p21Cip1 cDNA, and its truncations were cloned into a pCDEF3 vector with an N-terminal Myc tag. Human CCND3 and CDK4 were cloned into a pCDEF3 vector with an N-terminal hemagglutinin (HA) tag. For making the plasmid used for retroviral infection of U2OS cells, Flag-tagged PFTK1 was constructed into a pMXN vector. pCDEF3, pEF, pMXN, pCI-VSV, and pCI-PG8 vectors were gifts from Arthur Weiss (University of California, San Francisco, CA).
Antibodies.
Anti-HA, anti-Flag, and anti-Myc monoclonal antibodies were generated in-house; anti-β-actin antibody was obtained from Sigma (St. Louis, MO); monoclonal anti-PFTK1 antibody was generated by using His-PFTK1 expressed in Escherichia coli BL21 (DE3); polyclonal anti-PFTK1 antibody was generated by using a PFTK1 peptide (LPHFKPERFTLYSSKNLRQC); anti-p21Cip1 and anti-CCND3 antibodies were obtained from BD Biosciences PharMingen (San Diego, CA); polyclonal rabbit antibodies against CCND1 and CDK6 were gifts from Yue Xiong (University of North Carolina, Chapel Hill, NC).
Cell Culture and Stable Transfectants.
Cell lines were obtained from ATCC (American Type Culture Collection, Manassas, VA). The 293T and U2OS cells were maintained in DMEM (PAA, Pasching, Austria) and RPMI medium 1640 (PAA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA) respectively. SH-SY5Y cells were maintained in MEM/F-12 (50/50 mixture; JRH, Lenexa, KS) supplemented with 10% FBS. Cell line stably expressing PFTK1 was generated by retroviral infection of U2OS cells. Briefly, a retroviral vector containing human PFTK1 cDNA with an N-terminal Flag-tag, pCI-PG8, and pCI-VSV were cotransfected into 293T cells. Viral supernatants were collected and used to infect U2OS cells. Drug-resistant cells were then selected, cloned, and screened for PFTK1 expression.
Transfections, Immunoprecipitations, and Immunoblotting.
Transfection, immunoprecipitation and Western blotting were carried out as described (32).
In Vitro Kinase Assay.
Kinase assay was performed as described (32). Recombinant C-terminal retinoblastoma protein (His-RbC137) was expressed and purified from a BL21 (DE3) E. coli strain.
siRNA Analysis.
RNA interference was performed in SH-SY5Y cells. The siRNA sequence used to target PFTK1 was from position 376 to 395 relative to the first nucleotide of the start codon of human PFTK1 (5′-GGAUCUUAUGCUACAGUAUtt-3′). The sequence of control siRNA was 5′-UUCUCCGAACGUGUCACGUtt-3′. siRNAs were chemically synthesized by Shanghai GenePharma (Shanghai, China).
Cell Cycle Analysis.
U2OS and SH-SY5Y cells at a density of 2.5×105 per ml were cultured in the presence of 0.5 mM l-mimosine for 24 h to induce G1 cell cycle arrest. For siRNA analysis, cells were harvested 48 h after transfection with siRNA. Cells were then labeled with propidium iodide (PI) and analyzed by using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Acknowledgments
We thank Arthur Weiss for pCDEF3 vector and Lewis L. Lanier (University of California, San Francisco, CA) for pMX vector. This work was supported by National Natural Science Foundation of China (NSFC) Grant 30430630, National Outstanding Youth Fund of NSFC Grant 30225022, Chinese National 863 program Grant 2002BA711A01-08, and Key Program of Basic Research, Shanghai Grant STC 05JC14087.
Abbreviations
CDK
cyclin-dependent kinase
CCND3
cyclin D3
CIP
CDK-interacting protein
RB
retinoblastoma.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sherr CJ, Roberts JM. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 2.Malumbres M, Barbacid M. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Besset V, Rhee K, Wolgemuth DJ. Mol Reprod Dev. 1998;50:18–29. doi: 10.1002/(SICI)1098-2795(199805)50:1<18::AID-MRD3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla R, Draetta G. Oncogene. 1994;9:3037–3041. [PubMed] [Google Scholar]
- 5.Charrasse S, Carena I, Hagmann J, Woods-Cook K, Ferrari S. Cell Growth Differ. 1999;10:611–620. [PubMed] [Google Scholar]
- 6.Zhang S, Cai M, Zhang S, Xu S, Chen S, Chen X, Chen C, Gu J. J Biol Chem. 2002;277:35314–35322. doi: 10.1074/jbc.M202179200. [DOI] [PubMed] [Google Scholar]
- 7.Marques F, Moreau JL, Peaucellier G, Lozano JC, Schatt P, Picard A, Callebaut I, Perret E, Geneviere AM. Biochem Biophys Res Commun. 2000;279:832–837. doi: 10.1006/bbrc.2000.4042. [DOI] [PubMed] [Google Scholar]
- 8.Taglienti CA, Wysk M, Davis RJ. Oncogene. 1996;13:2563–2574. [PubMed] [Google Scholar]
- 9.Abe S, Yagi T, Ishiyama S, Hiroe M, Marumo F, Ikawa Y. Oncogene. 1995;11:2187–2195. [PubMed] [Google Scholar]
- 10.Matsushime H, Jinno A, Takagi N, Shibuya M. Mol Cell Biol. 1990;10:2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazzaro MA, Julien JP. Genomics. 1997;42:536–537. doi: 10.1006/geno.1997.4760. [DOI] [PubMed] [Google Scholar]
- 12.Sauer K, Weigmann K, Sigrist S, Lehner CF. Mol Biol Cell. 1996;7:1759–1769. doi: 10.1091/mbc.7.11.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagano M, Jackson PK. Cell. 2004;118:535–538. doi: 10.1016/j.cell.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Sherr CJ. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 15.Vidal A, Koff A. Gene. 2000;247:1–15. doi: 10.1016/s0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Chen JY. Gene. 2001;267:165–172. doi: 10.1016/s0378-1119(01)00391-2. [DOI] [PubMed] [Google Scholar]
- 18.Rascle A, Stowers RS, Garza D, Lepesant JA, Hogness DS. Mech Dev. 2003;120:617–628. doi: 10.1016/s0925-4773(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 19.Lazzaro MA, Albert PR, Julien JP. J Neurochem. 1997;69:348–364. doi: 10.1046/j.1471-4159.1997.69010348.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Jiang M, Yang T, Ni J, Chen J. Cell Res. 2006;16:539–547. doi: 10.1038/sj.cr.7310071. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Gao YK, Chen JY. Acta Biochim Biophys Sinica. 2002;34:520–525. [PubMed] [Google Scholar]
- 22.Mutoh M, Lung FD, Long YQ, Roller PP, Sikorski RS, O'Connor PM. Cancer Res. 1999;59:3480–3488. [PubMed] [Google Scholar]
- 23.Weiss RH, Joo A, Randour C. J Biol Chem. 2000;275:10285–10290. doi: 10.1074/jbc.275.14.10285. [DOI] [PubMed] [Google Scholar]
- 24.Dash BC, El-Deiry WS. Mol Cell Biol. 2005;25:3364–3387. doi: 10.1128/MCB.25.8.3364-3387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanks SK, Lindberg RA. Methods Enzymol. 1991;200:525–532. doi: 10.1016/0076-6879(91)00168-v. [DOI] [PubMed] [Google Scholar]
- 26.Stowers RS, Garza D, Rascle A, Hogness DS. Dev Biol. 2000;221:23–40. doi: 10.1006/dbio.2000.9685. [DOI] [PubMed] [Google Scholar]
- 27.Santamaria D, Ortega S. Front Biosci. 2006;11:1164–1188. doi: 10.2741/1871. [DOI] [PubMed] [Google Scholar]
- 28.Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, et al. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 29.Hashida T, Yamada M, Hashimoto K, Shibusawa N, Monden T, Satoh T, Mori M. Endocrinology. 2002;143:2808–2811. doi: 10.1210/endo.143.7.8963. [DOI] [PubMed] [Google Scholar]
- 30.Tang X, Guilherme A, Chakladar A, Powelka AM, Konda S, Virbasius JV, Nicoloro SM, Straubhaar J, Czech MP. Proc Natl Acad Sci USA. 2006;103:2087–2092. doi: 10.1073/pnas.0507660103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartkova J, Lukas J, Strauss M, Bartek J. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]