Molecular insights into segmentation along the proximal-distal axis of the nephron (original) (raw)
. Author manuscript; available in PMC: 2008 May 9.
Published in final edited form as: J Am Soc Nephrol. 2007 Jun 13;18(7):2014–2020. doi: 10.1681/ASN.2007040453
Abstract
The structure of a mammalian kidney is parsed into large collections of polarized nephrons, and each segment is home to a diverse community of cells that specialize in renal endocrine and excretory functions. Early developmental lengthening and diversification of nephron segments along a proximal-distal axis initiate all subsequent facets of tubular growth and function. Morphogenic cues and biochemical interactions critical to this process are starting to emerge. The underlying principles of regional cell signaling and transcriptional control organizing early segmentation are the subject of this review.
Of all the events leading to the formation of a functioning kidney, early segmentation of developing nephrons is perhaps most crucial to successful organogenesis. Each nephron comprises a glomerular filter followed by various tubular segments specialized for reabsorption and secretion of solutes and water. All components of the developing nephron derive from different regions along the proximal-distal axis of primitive cell aggregates called the renal vesicle. The sequential timing of lineage-dependent transcription factors and signaling pathways establish the characteristic structure and function of each segment.
Nephron development can be linked and understood by examining several discrete events. These events include the organization of the metanephric blastema, segregation of cell types by differential adhesion, and the regional engagement of specialized transcription factors. The molecular basis of this reciprocal process had been reviewed extensively elsewhere;1-3 we will focus here on aspects that pertain to nephron segmentation, or the process through which regional identities emerge. Proper segmentation of the renal vesicle is critical to all specialization that follows.
Wnt signals from the ureteric bud initiate the organization of the metanephric mesenchyme into units of epithelia. These signals also contribute to early polarization of the nephron along the proximal-distal axis
The ureteric bud, an epithelial outgrowth from the Wolffian duct, instructs the metanephric mesenchyme to undergo mesenchymal-epithelial transition. This mesenchyme in turn induces the ureteric bud to branch. Each branch tip again induces mesenchymal-epithelial transitions and this continues until a species-appropriate number of nephrons form to complete a mammalian kidney.
Since the kidney expands as a sphere from a central starting point, we will consider the center to peripheral axis as the proximal-distal axis in describing the relative position of emerging structures within the metanephric mesenchyme. In this way peripheral structures become distal and closer to the cortex than to the medulla. Developmental biologists recognize three primitive stages forming the nephrogenic body during development: the renal vesicle, the Comma-shaped body, and the S-shaped body. Nascent tubular structures always appear on the proximal side of the ureteric bud tip, and their own proximal-distal axis remains aligned, with the duct tip marking the distal end.
Proximal-distal polarity within nascent nephrogenic bodies is morphologically apparent in the Comma-shaped body. This stereotypical structure forms because the first cells to elongate, change shape, and form a “slit” are located at the proximal end, farthest from the ureteric bud.4 Proliferation and differential adhesion may be the drivers that contort the Comma-shaped body into an S-shaped structure that fuses at its distal end with the ureteric bud, while podocyte precursors emerge at its most proximal end.4 The epithelial precursors for three nephron segments (glomerulus, proximal tubule, and distal tubule) can all be identified within the S-shaped body using several molecular markers, including Pax2, WT-1, Jag1, Cdh6, and E-cadherin.2 The molecular basis for this asymmetry creates a structural polarity.
Is polarization of a nascent nephron along the proximal-distal axis intrinsic to the developing nephron, or is it dependent on signals from the ureteric bud, or both? Isolated metanephric mesenchyme fails to form epithelia, but it can be induced to do so by a signaling source like ectopic ureteric bud or spinal cord. Using this system, Saxen and others speculate that differentiation of tubular structures along the proximal-distal axis requires ureteric bud signals, is sequential, and perhaps interdependent. The most proximal elements, the glomerulus and proximal tubule, appear shortly after induction, whereas all three nephron segments, including the distal tubule, form only when exposure to inducer source is longer than 24 hours.4 In this way, the metanephric mesenchyme resembles other tissues responding to morphogenic gradients. The response to the morphogen is dependent on the distance of the morphogen source from the responding cell (for example, activin 5, 6 or wingless 7, 8), with a low concentration dependent response occurring at a greater distance from the source than a high concentration one, and the higher concentration event inhibiting/replacing the earlier fates thus generating a proximal-distal axis.
Experimentally, ureteric bud or spinal cord inducing mesenchymal-epithelial transitions can be replaced by several Wnt molecules9, 10 or by transient exposure to GSK3ß inhibitors,11 suggesting that Wnt signals likely induce mesenchymal-epithelial transition by increasing the half-life of β-catenin. Indeed, ectopic stabilization of β-catenin in the metanephric mesenchyme triggers conversion to epithelial structures.11 The endogenous ureteric bud signal is most likely Wnt9b. Nephrogenesis arrests in _Wnt9b_-/- embryos after the ureteric bud invades the blastema and the kidney fails to form (Figures 1A and B).12 Thus, the Wnt signal is both sufficient and required for induction of pre-tubular mesenchymal aggregates.12 Since Wnt9b (and its target, Wnt4; see below) can induce a distal marker, Lhx1,13 in the renal vesicle, an early Wnt gradient may provide a polarizing signal. Cells near the source will express Lhx1, a distal marker, while cells further away (exposed to lower morphogen concentrations) will be the first to elongate and later acquire fates for the proximal tubule and glomerular podocytes. This is consistent with short exposure to the morphogen producing the proximal tubule/podocyte precursors. As will be detailed below, Lhx1 is required but not sufficient to secure proper segmentation of the nephron.
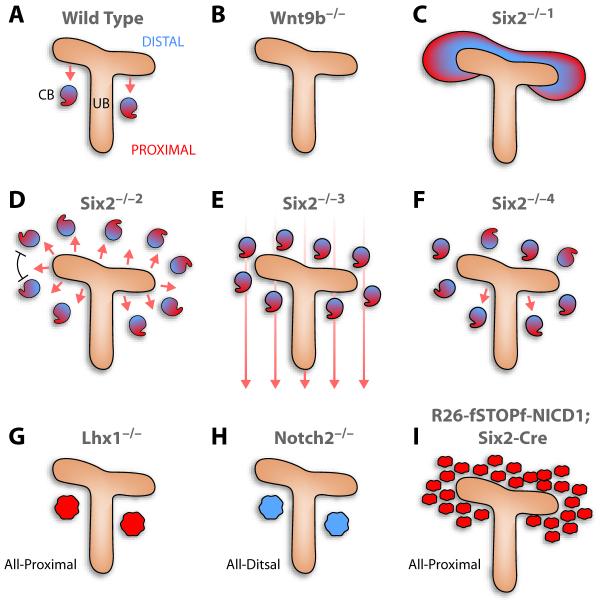
Figure 1. Schematic summary of expected and observed results from genetic manipulation of the metanephros.
A. Wild type renal vesicle acquires a proximal identity first and has a stereotypical position relative to the ureteric bud. B. Without Wnt9b, no renal vesicle develops. C-F. Although a greater sample needs to be analyzed, Six2-/- mice permit discrimination between several mechanistic possibilities. Hypothetically, in the absence of Six2, all metanephric mesenchyme cells could respond to Wnt9b and convert to an epithelial structure (C; Six2-/-1), but in reality, only a subset form ectopic renal vesicle with regular spacing between them (D; Six2-/-2). In addition, the distal ends of the ectopic vesicles are almost always closer to the ureteric bud, suggesting that a signal from the ureteric bud polarizes the vesicles along the proximal/distal axis. E. In theory, if a global patterning cue existed, all ectopic renal vesicles should align to the field (as in Six2-/-3) but this is not the case in the Six2-/- metanephros. F. Another possibility is that only the proximal ureteric bud organizes the renal vesicle along the proximal-distal axis, or the proximal-distal segmentation program is an intrinsic renal vesicle property that does not require external cues. In both these cases randomized proximal-distal acquisition is expected (Six2-/-4). G-I. Polarization defects in Lhx1, Notch2 and NICD1 mutants. Note that over-expression of activated Notch1 differs from loss of Six2 in that it results in multiple, poorly spaced epithelial clusters.
Wnt9b is expressed in the Wolffian duct, the ureteric bud, and the collecting system.12 Given its broad expression pattern, it was unclear until recently how mesenchymal-epithelial transition is limited only to the proximal aspect of the branch bud tip. Mice deficient in the homeobox gene, Six2, sprout epithelial aggregates both proximal and distal to branches in the ureteric bud (Figure1C).14 Thus, Six2 must either prevent mesenchymal-epithelial transition distally perhaps by antagonizing the effects of Wnt signaling,14 or Wnt9b may reverse an inhibitory influence of Six2 on epithelialization in response to other inducers. Either way, Six2 maintains mesenchyme cells in an undifferentiated state.
The Six2-/- null mice also teach us more about proximal-distal cues. If early renal vesicles normally establish their proximal-distal polarity based on intrinsic cues, they would be expected to display a randomized pattern and the proximal-distal axis would vary relative to the bud branch (Figure 1F). If, however, the renal vesicle reads a global positioning cue, they all would align along the same axis (Figure 1E). Instead, it appears the ureteric bud acts to organize the proximal-distal axis of the renal vesicle, as Six2-/- anlage produce many renal vesicles that align their distal ends towards the ureteric bud and their proximal ends away from it (Figure 1D).14 However, not all do so, indicating perhaps there is some degree of autonomy. This positioning factor may be Wnt9b or additional molecules emanating from the ureteric bud. A second notable feature of _Six2_-/- renal vesicles is their spacing. If Wnt9b is a dominant inducer of mesenchymal-epithelial transitions and Six2 its only adversary, cells lacking Six2 would be expected to convert en mass, eventually forming a continuous epithelial tube surrounding a branching ureteric bud (Figure 1C) or as a cloud of epithelial clusters (see below and Figure 1I). Instead, ectopic renal vesicles are regularly spaced, as if epithelial cells produce an inhibitor preventing adjacent metanephric mesenchyme from becoming epithelial (Figure 1D), which is reminiscent of the mechanism involved in feather patterning.15 No explanation has emerged for this spacing which is not seen when activated Notch1 is expressed in the Six2-expressing cells (below and Figure 1I). 29
Once formed by bud induction, the cellular aggregates that become epithelial also synthesize Wnt4 and no longer require Wnt9b. Wnt4 expression begins in the renal vesicle, becoming restricted to the distal portion of the S-shaped body.16 This observation is consistent with Wnt concentration being highest at the distal end, acting to organize the early proximal-distal axis. Genetic inactivation of Wnt4 in mice results in severely hypoplastic kidneys accompanied by limited branching of the ureteric bud. Although N-myc is still present in the metanephric mesenchyme, and the Wnt targets Pax8 and FGF8 are still detectable in the ureteric bud, the renal vesicle markers N-myc, Pax8, FGF8 and Lhx1 (or Lim1) are either lost shortly after renal vesicle formation or absent entirely.16, 12 This indicates that Wnt9b alone is not sufficient and, as Saxen’s reconstitution experiments show, sustained exposure to Wnt is required to complete segmentation, which is also reminiscent of animal cap induction by activin.5, 6 While Wnt4 or Wnt9b are capable of inducing the formation of renal vesicles and tubulogenesis in isolated metanephric mesenchyme, Wnt9b fails to induce differentiation in _Wnt4_-/- metanephric mesenchyme, whereas Wnt4 can induce _Wnt9b_-/- mesenchyme to form tubular structures.12 These studies support a model in which bud-derived Wnt9b, in a paracrine manner and in opposition to Six2, induces Pax8, FGF8 and Wnt4 gene expression. Wnt4 then acts in an autocrine manner within pre-tubular vesicles to maintain Pax8 and FGF8 and to activate Lhx1 to not only promote epithelialization but to also polarize the renal vesicles.
Segregation of cell types into distinct domains is enhanced by differential expression of adhesion molecules within early epithelial structures
The segregation of cells into proximal and distal domains within the renal vesicle may be assisted by differential expression of the cell adhesion molecules E-cadherin (distal) and Cadherin-6 (proximal).17 In _Six2_-/- kidneys, the expression domains of E-cadherin and Cadherin-6 are abnormally expanded, overlapping each other extensively within ectopic renal vesicles. This suggests that polarization of renal vesicles is not entirely an intrinsic property of the nascent epithelium at this stage or that distally, Six2-/- aggregates are exposed to a different signaling environment then those located proximally. Alternatively, _Six2_-/- aggregates suffer from other intrinsic defects; that is, ureteric bud signals may be altered when the metanephric mesenchyme is ablated, or the metanephric mesenchyme itself may contribute to the proper polarization of renal epithelia. Whichever the case, it is apparent the location of the renal vesicle relative to signals emanating from the ureteric bud critically contributes to the establishment of a proper proximal-distal axis within the renal vesicle, in addition to promoting epithelialization of the metanephric mesenchyme.
In wild-type kidneys, the S-shaped body maintains the segregation of a distal E-cadherin+ domain with a medial Cadherin-6+ region containing proximal tubule precursors. The glomerular/podocyte precursors do not express Cadherin-6, indicative of the emergence of yet another domain within the S-shaped body. The expression of E-cadherin in the ureteric bud and the distal portion of the renal tubule likely facilitates fusion between the two.17 Mice deficient for Cadherin-6 are viable and fertile,18 however, epithelialization of the renal vesicle is delayed; that is, neonates have some necrotic glomeruli and adults have reduced nephron number, indicating a role for this molecule in nephrogenesis.
Regional expression of transcription factors within the renal vesicle along the proximal-distal axis is required for the subsequent differentiation of diverse cell fates along the nephron
In addition to Lhx1, a homeobox transcription factor, polarized expression patterns of genes encoding other transcription factors are also evident within the renal vesicle. This suggests that renal vesicle cells are not equivalent and these additional factors are likely to mediate the differentiation of distal versus the proximal elements within the renal vesicle. Lhx1 induces the expression of Brn-1, a POU domain-containing transcription factor, in the distal domain of the renal vesicle and subsequently in the distal portion of the S-shaped body.19, 13 Genetic inactivation of Brn-1 in mice results in postnatal lethality within the first 48 hours after birth due to renal insufficiency. Brn1-/- kidneys develop a normal number of mature glomeruli and proximal tubules, but display a dramatic reduction in numbers of mature loops of Henle, macula densa, and distal convoluted tubules. Nephron development in these mice is arrested at a primitive stage before the loops of Henle elongate. Brn-1 is thus a transcription factor that specifies the distal domain within the renal vesicle and subsequently contributes to differentiation of distal derivatives.
Conditional inactivation of Lhx1 in the metanephric mesenchyme results in the formation of renal vesicles that express Wnt4, Pax8 and FGF8 but lack the distal-specifying factor Brn-1. They also lack delta-like-1 (Dll1) expression, a distally restricted Notch ligand. The failure of Lhx1-/- renal vesicles to regionalize along the proximal-distal axis arrests nephron development at this stage and does not allow for the polarized elongation of the vesicles into Comma-shaped bodies (Figure 1G). In chimera experiments, Lhx1-/- embryonic stem cells initially contribute to the entire renal vesicle but later are excluded from the distal region. In S-shaped bodies, where at least three distinct molecular domains are evident, Lhx1-/- cells could only contribute to the precursors of Bowman’s capsule and podocytes, consistent with the importance of Lhx1 and Brn-1 in distal fate acquisition. Lhx1 thus acts downstream of the organizer signal, is required for distal domain specification within the renal vesicle, and may function latter to specify the proximal domain of the S-shaped bodies along with Notch, as we detail below.
A number of studies also implicate Notch signaling in segmentation of the nephron. The asymmetric expression of the Notch ligand, Dll1, in the distal domain of the renal vesicle, its persistence in the medial portion of the S-shaped bodies, and the enrichment of Notch signaling components and targets in the proximal domain of the S-shaped bodies,20-24 raise the possibility that Notch signaling is involved in nephron segmentation. Mammals express 4 different Notch genes, Notch 1-4, all of which are type-I transmembrane proteins with an extracellular ligand binding domain and an intracellular domain that regulates transcription. Notch1 and Notch2 are expressed in renal epithelial cells.
Notch receptors participate in essential, short-range, signal transduction pathways used during development to help cells select one of several future options by altering the transcriptional landscape in cells experiencing Notch activation. Signaling is initiated when ligand-binding induces ectodomain shedding of Notch, which is followed by intramembrane cleavage, mediated by the enzyme γ-secretase, to release the Notch intracellular domain (NICD). NICD translocates to the nucleus to affect transcription through one protein, the transcription factor, RBP-J.25 Under basal conditions, RBP-J functions as a transcriptional repressor, but upon complex formation with NICD it is converted transiently to an activator through the recruitment of additional cofactors and the transcriptional machinery.26 The discovery that cleaved, intracellular domains of Notch1 (NICD1) are present in renal vesicles and the S-shaped bodies indicates that Notch1 is activated early in nephrogenesis and prompted investigators to pharmacologically block Notch signaling with γ-secretase inhibitors (GSI).27 A GSI-sensitive window was discovered early in nephrogenesis, opening after initial mesenchymal-epithelial transition and closing as the S-shape body forms. When exposed to the drug27 or deprived of γ-secretase components genetically,28 metanephric mesenchyme produces nephrons lacking glomeruli, proximal tubules, and loops of Henle, but contain distal tubules that correctly fused to the collecting duct.29 Interestingly, inactivation of Lhx1 results in loss of Dll1, and Dll1 hypomorphic animals have a severe reduction in nephron numbers accompanied by loss of proximal segments.29 This finding is consistent with the assumption that Dll1 is the first Notch ligand to act in the renal vesicle. Following loss of Lhx1, at least three other Notch signaling components, jagged-1 (Jag1), hairy and enhancer of split 5 (Hes5), and musashi homolog2 (Msi2), fail to appear.30 These results indicate that Notch signaling plays a role in nephron segmentation, alone or in conjunction with other γ-secretase substrates.
Further dissection of the Notch pathway reveals that removal of the receptor Notch2 is sufficient to mimic the full effect of GSI or γ-secretase inactivation, confirming that Notch signaling is indeed critical for nephron segmentation and permitting detailed analysis of its role in this process. Importantly, renal vesicles deficient in Notch2 attain the normal, distally restricted expression of Lhx1, demonstrating that the initial proximal-distal axis is correctly specified upstream of Notch2 and independent of it. Similar results are seen when RBP-J is removed, but not when Notch1 is lost. This observation explains why Alagille syndrome occurs in humans lacking one copy of Notch2;31 Notch1 simply cannot do the job. Given that Notch1 can activate RBP-J effectively,32 understanding how to reinvigorate Notch1 in human kidneys may provide a key to the treatment of Alagille syndrome, which should now be thought of as a proximal-distal polarization defect occurring during kidney development.
As Lhx1 and its targets are properly expressed in the Notch2 deficient renal vesicles, why does the renal vesicle fail to segment? It appears that cells expressing Jag1 and accumulating NICD1 (both are excellent markers for the earliest proximal progenitors) are specified in the Notch2-/- renal vesicle, but these cells fail to proliferate and fail to down-regulate Pax2. Pax2 is normally expressed in the entire renal vesicle, but is later suppressed in the mid-section of the S-shaped body where Jag1 expression predominates. It is thus likely that Notch2 signals not only promote proliferation, but are also involved in separating distal (dominated by Pax2 and Lhx1) and proximal (dominated by WT-1) regions while establishing a distinct third entity—the future proximal tubule. In the absence of Notch2 activity, the initial separation of the renal vesicle into Wt1 and Pax2 expressing domains occurs, but is halted and then reversed.29
Consistent with a role for Notch signaling in promoting proximal nephron cell fates, ectopic over expression of activated Notch1 within the metanephric mesenchyme results in the premature commitment of these cells to epithelial structures that express proximal tubular and podocyte markers, but not distal nephron markers (unpublished observations). Thus, Notch activity provides proximal identity to the renal vesicle, whereas its loss causes the renal vesicle to be entirely distal (Figure 1H).29
As Notch signaling can be used to induce or suppress a structural option as many times as needed during organogenesis, additional roles for Notch signaling during nephron patterning are likely (we use this language to dissuade the reader from thinking that Notch only maintains stem or progenitor cells or promotes proliferation). Notch can impact any biological process in a context-dependent manner. For example, Notch signaling is required to suppress multi-ciliated cells that are interspersed among transporting cells within the zebrafish pronephros.33 Blocking the Notch signaling pathway results in conversion of transporting epithelial cells into multi-ciliated cells. Similarly, different cell types are intermingled in the mature mammalian nephron, particularly in the collecting system, where principal cells transporting water are interspersed with intercalated cells. Notch signaling may impact the distribution of these cells as well.
Although Notch impacts proximal-distal nephron specification via RBP-J in mouse kidney,29 the transcriptional targets of the Notch-RBP-J complex are unclear. Some insight into this issue is emerging from studies in lower vertebrates. The pronephros, mesonephros, and metanephros all share the nephron as their functional unit, though they vary greatly in their organization and number of nephrons. The pronephros, the functional embryonic kidney in amphibians and fish, consists of three defined segments: the glomus, where blood is filtered, the proximal tubule, where selective resorption of filtrate occurs, and the distal nephric duct, which ushers waste out of the organism.
In the Xenopus pronephros, Notch-mediated proximal-distal specification relies on activation of Xenopus Hey1 (xHRT1),34 a direct target of the Notch signaling pathway.35 XHRT1 expression in the glomus is dependent on the Xenopus Wilms’ tumor suppressor 1 (xWT1), whereas the proximal tubule expression of xHRT1 may depend on Notch. xHRT1 over-expression mimics Notch activation and inhibits the formation of the distal tubule and duct, perhaps suppressing Evi1 (ecotropic viral integration site 1) in the distal tubule and duct of the Xenopus and zebrafish pronephros.36 The genes encoding Hey1 and Evi1 are expressed in the mouse kidney 37 and may act down-stream of Notch, but cannot account for the Notch phenotype, as deleting Hey genes produce cardiovascular defects but do not distalize the nephron.38-40
Conclusions
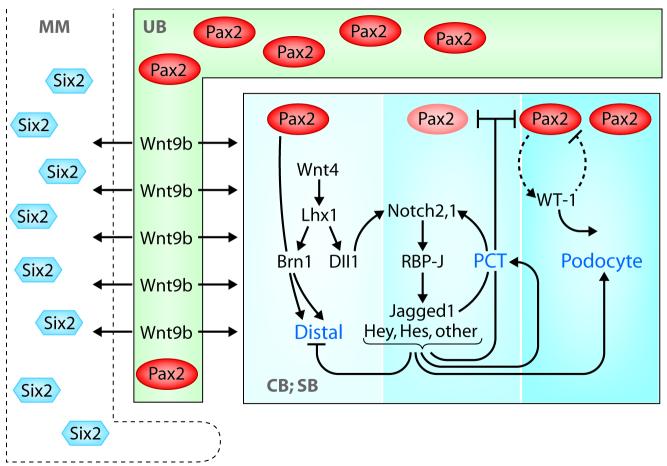
The molecular basis for the proximal-distal nephron segmentation is just beginning to be elucidated. As summarized in Figure 2, the early renal vesicle first divides into broad proximal and distal domains and then into the glomerular, proximal, and distal tubular segments. These are morphologically evident in the S-shaped body. This specification occurs as a sequential process involving multiple genes and signaling pathways. Pax2 and Pax8 may facilitate the formation of active chromatin through their interactions with PTIP (Pax transactivation-domain interacting protein41). Wnt9b and Six2 (most likely with additional co-modulators) position the renal vesicle to the proximal aspect of the ureteric bud; Wnt4/FGF8 may activate Lhx1 in the distal domain of the renal vesicle, where Lhx1 activates Brn1 and Dll1. Dll1 leads to an initial Notch2 activation, which amplifies its own signal by activating the ligand Jag1, producing a proliferative burst in the midsection of the renal vesicle and contributing to S-shaped body formation.
Figure 2. A model for the molecular circuitry involved in segmenting the nephron.
Pax2, Lhx1, and Brn1 are involved in establishing the distal segment; Notch2 is required to fix the proximal identities and to establish proximal precursors. MM—metanephric mesenchyme; UB— ureteric bud; and CB and SB—Comma and S-shaped bodies. See text for details.
Notch activation during a critical window of development, preceding S-shape body formation, is required for fixation of a proximal identity. This event is subdivided further into podocyte precursors that lose Notch activity, perhaps due to an increase in the Notch antagonist COUP-TFII,42 whereas Notch continues to promote the establishment of proximal precursors. Although not much is known about specific factors required for podocytes determination within the early S-shape body, the three-nephron segment identities are stable by the time the S-shaped body is visible and no longer require Notch or Wnt to generate a morphologically recognizable nephron. Further development of S-shaped bodies requires proximal specification by WT-1.43 The proximal-distal nephron polarity mutants described in this review (Lhx1, Brn1 and Notch2) are valuable starting points leading to further identification of down-stream components in the polarity program.
Acknowledgments
The authors wish the thank Drs. Jeff Miner, Scott Boyle, Andy McMahon and Jordan Kreidberg for commenting on the manuscript. This work was supported by a grant from the National Institute of Health DK066408. R.K., and Washington University may receive income based on a license of Notch-related technology by the University to Merck. Merck did not support this work.
References
- 1.Boyle S, de Caestecker M. Role of transcriptional networks in coordinating early events during kidney development. Am J Physiol Renal Physiol. 2006;291:F1–8. doi: 10.1152/ajprenal.00447.2005. [DOI] [PubMed] [Google Scholar]
- 2.Dressler GR. The Cellular Basis of Kidney Development. Annu Rev Cell Dev Biol. 2006 doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev. 2004;14:550–7. doi: 10.1016/j.gde.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Saxen L. Organogenesis of the kidney. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- 5.Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487–92. doi: 10.1038/371487a0. see comments. [DOI] [PubMed] [Google Scholar]
- 6.Gurdon JB, Dyson S, St Johnston D. Cells’ perception of position in a concentration gradient. Cell. 1998;95:159–62. doi: 10.1016/s0092-8674(00)81747-x. [DOI] [PubMed] [Google Scholar]
- 7.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–44. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–9. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 10.Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 11.Kuure S, Popsueva A, Jakobson M, Sainio K. Glycogen Synthase Kinase-3 Inactivation and Stabilization of ß-Catenin Induce Nephron Differentiation in Isolated Mouse and Rat Kidney Mesenchymes. J Am Soc Nephrol. 2007 doi: 10.1681/ASN.2006111206. H., S. Published on line as doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- 12.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–23. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 14.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler RD, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung HS, Franciswest PH, Widelitz RB, Jiang TX, Tingberreth S, Tickle C, Wolpert L, Chuong CM. Local Inhibitory Action of Bmps and Their Relationships With Activators in Feather Formation - Implications For Periodic Patterning. Developmental Biology. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 16.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–83. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 17.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:803–12. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- 18.Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR. Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol. 2000;223:38–53. doi: 10.1006/dbio.2000.9738. [DOI] [PubMed] [Google Scholar]
- 19.Nakai S, Sugitani Y, Sato H, Ito S, Miura Y, Ogawa M, Nishi M, Jishage K, Minowa O, Noda T. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development. 2003;130:4751–9. doi: 10.1242/dev.00666. [DOI] [PubMed] [Google Scholar]
- 20.Leimeister C, Bach A, Woolf AS, Gessler M. Screen for genes regulated during early kidney morphogenesis. Developmental Genetics. 1999;24:273–83. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<273::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Leimeister C, Schumacher N, Gessler M. Expression of Notch pathway genes in the embryonic mouse metanephros suggests a role in proximal tubule development. Gene Expression Patterns. 2003;3:595–598. doi: 10.1016/s1567-133x(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 22.Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns. 2004;4:707–11. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–52. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- 24.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 25.Mumm JS, Kopan R. Notch Signaling: From the Outside In. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 26.Lubman OY, Korolev SV, Kopan R. Anchoring notch genetics and biochemistry; structural analysis of the ankyrin domain sheds light on existing data. Mol Cell. 2004;13:619–26. doi: 10.1016/s1097-2765(04)00120-0. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, Miner J, Lin M, Tansey MG, Roth KA, Kopan R. g-Secretase Activity is Dispensable for the Mesenchyme-to-Epithelium Transition but Required for Proximal Tubule Formation in Developing Mouse Kidney. Development. 2003;130:5031–5041. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development. 2003;130:5019–5029. doi: 10.1242/dev.00682. [DOI] [PubMed] [Google Scholar]
- 29.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007 doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YT, Kobayashi A, Kwan KM, Johnson RL, Behringer RR. Gene expression profiles in developing nephrons using Lim1 metanephric mesenchyme-specific conditional mutant mice. BMC Nephrol. 2006;7:1. doi: 10.1186/1471-2369-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–73. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong C, Cheng H, Chang LW, Ohtsuka T, Kageyama R, Stormo DG, Kopan R. Target selectivity of vertebrate Notch proteins: collaboration between discrete domains and CSL binding site architecture determine activation probability. J Biol Chem. 2006;281:5106–5119. doi: 10.1074/jbc.M506108200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–22. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 34.Taelman V, Van Campenhout C, Solter M, Pieler T, Bellefroid EJ. The Notch-effector HRT1 gene plays a role in glomerular development and patterning of the Xenopus pronephros anlagen. Development. 2006;133:2961–71. doi: 10.1242/dev.02458. [DOI] [PubMed] [Google Scholar]
- 35.Rones MS, Woda J, Mercola M, McLaughlin KA. Isolation and characterization of Xenopus Hey-1: a downstream mediator of Notch signaling. Dev Dyn. 2002;225:554–60. doi: 10.1002/dvdy.10192. [DOI] [PubMed] [Google Scholar]
- 36.Van Campenhout C, Nichane M, Antoniou A, Pendeville H, Bronchain OJ, Marine JC, Mazabraud A, Voz ML, Bellefroid EJ. Evi1 is specifically expressed in the distal tubule and duct of the Xenopus pronephros and plays a role in its formation. Dev Biol. 2006;294:203–19. doi: 10.1016/j.ydbio.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 37.Morishita K, Parganas E, Parham DM, Matsugi T, Ihle JN. The Evi-1 zinc finger myeloid transforming gene is normally expressed in the kidney and in developing oocytes. Oncogene. 1990;5:1419–23. [PubMed] [Google Scholar]
- 38.Fischer A, Klamt B, Schumacher N, Glaeser C, Hansmann I, Fenge H, Gessler M. Phenotypic variability in Hey2 -/- mice and absence of HEY2 mutations in patients with congenital heart defects or Alagille syndrome. Mamm Genome. 2004;15:711–6. doi: 10.1007/s00335-004-2389-x. [DOI] [PubMed] [Google Scholar]
- 39.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–11. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, Knobeloch KP, Gessler M. Combined Loss of Hey1 and HeyL Causes Congenital Heart Defects Because of Impaired Epithelial to Mesenchymal Transition. Circ Res. 2007 doi: 10.1161/01.RES.0000260913.95642.3b. [DOI] [PubMed] [Google Scholar]
- 41.Lechner MS, Levitan I, Dressler GR. PTIP, a novel BRCT domain-containing protein interacts with Pax2 and is associated with active chromatin. Nucleic Acids Res. 2000;28:2741–51. doi: 10.1093/nar/28.14.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh JM, Yu CT, Tang K, Tanaka T, Kodama T, Tsai MJ, Tsai SY. The expression profiles of nuclear receptors in the developing and adult kidney. Mol Endocrinol. 2006;20:3412–20. doi: 10.1210/me.2006-0312. [DOI] [PubMed] [Google Scholar]
- 43.Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–57. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]