A role for BDNF in cocaine reward and relapse (original) (raw)
. Author manuscript; available in PMC: 2009 Feb 15.
Published in final edited form as: Nat Neurosci. 2007 Aug;10(8):935–936. doi: 10.1038/nn0807-935
Abstract
Brain-derived neurotrophic factor (BDNF) is important in regulating synaptic plasticity in the brain areas that process reward information. A new study reports that BDNF in the nucleus accumbens, a brain area critical for the rewarding effects of cocaine, promotes persistent cocaine-seeking behaviors and heightens relapse vulnerability.
Addiction is a life-long struggle. Even after many months of abstinence from drugs, addicts can relapse into drug use when acutely exposed to the drug itself, drug-associated cues or stress. Compulsive drug use and relapse may reflect the ability of addictive drugs to subvert or highjack the brain systems that normally mediate reward learning, causing the neurons in these systems to become persistently more responsive to the addictive drug and relapse-triggering events. BDNF is an important component in the signaling pathways that regulate plasticity in brain regions that process reward-related information. In this issue, Graham et al.1 provide evidence that BDNF in the nucleus accumbens, a brain area critical for the rewarding effects of cocaine, promotes persistent cocaine-seeking behaviors and heightens vulnerability to relapse.
BDNF is important in synaptic plasticity and in the survival and function of midbrain dopamine neurons that project to the striatum, including neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens. BDNF mRNA is expressed in midbrain dopamine neurons2 and can also be detected in the striatum, although its expression is very low under normal conditions3. Acute cocaine exposure modestly increases BDNF protein levels in striatum4, whereas cocaine self-administration and subsequent withdrawal from the drug results in profound long-lasting (and time-dependent) increases in BDNF in the VTA, amygdala and accumbens5.
In rats and mice, BDNF is important in cocaine’s behavioral effects. BDNF injections into the VTA or the accumbens enhance cocaine-induced locomotion6 and cocaine-induced potentiation of responding for cues that are paired with natural rewards7. A single VTA BDNF injection at the end of a cocaine self-administration period enhances responding for cocaine cues for up to 30 d after the cessation of cocaine use, as assessed by extinction tests where rats were exposed to cues previously paired with cocaine injections, but not to cocaine8. In addition, BDNF heterozygote knockout mice are less responsive to cocaine’s rewarding (as assessed in the conditioned place preference procedure) and locomotor activating effects7,9.
However, this previous work has not addressed two important questions. What is the role of BDNF in cocaine reward and relapse, as assessed by established drug self-administration10 and reinstatement11 procedures, and in which brain area(s) does BDNF (which can be transported in both an anterograde and retrograde manner from its local injection site12,13) have its effects on cocaine reward and relapse?
Graham et al.1 addressed these questions with an impressive combination of behavioral, neuroanatomical and molecular techniques. They first trained rats to press a lever to self-administer intravenous cocaine. The authors then examined BDNF protein expression following a 4-h self-administration session. Cocaine self-administration modestly (~20%) and transiently increased BDNF protein levels in the shell, but not in the core region of the accumbens. Graham et al.1 hypothesized that this dynamic cocaine-induced increase in BDNF activity in the accumbens shell promotes both the maintenance of cocaine self-administration and subsequent vulnerability to relapse after cessation of cocaine self-administration.
To assess whether BDNF in the accumbens promotes cocaine self-administration, Graham et al.1 trained rats to self-administer cocaine and then injected BDNF or antibody to BDNF into the accumbens shell immediately after cocaine-self administration training for 5 d. Three to seven days after the last injection, BDNF-exposed rats self-administered more cocaine over a range of cocaine doses (an upward shift in the dose-response curve), suggesting increases in cocaine’s rewarding effects. In contrast, exposure to antibody to BDNF had weak effects in the opposite direction. Injections of BDNF, but not antibody to BDNF, also potently increased the rats’ motivation to work for cocaine when the response requirement for each successive injection exponentially increased under a progressive-ratio reinforcement schedule. To confirm that BDNF had a critical role in the accumbens shell, as opposed to upstream or downstream structures, Graham et al.1 locally injected an adeno-associated viral (AAV) vector to knock down BDNF protein production in the accumbens shell. This manipulation led to a substantial reduction in BDNF levels in the accumbens shell (46%) and caused a downward shift in the dose-response curve, suggesting a decrease in cocaine’s rewarding effects.
To assess the role of BDNF in relapse vulnerability, the authors again injected BDNF or antibody to BDNF into the accumbens shell during cocaine self-administration training. Then, three to seven weeks later, they assessed the strength of responding upon termination of cocaine delivery (extinction) and subsequent reinstatement of responding (relapse) induced by exposure to cocaine-priming injections, cues that had been associated with cocaine during training, or a footshock stressor. These reinstating stimuli model the human condition, in which relapse to drugs following a period of abstinence is more likely to occur after acute re-exposure to drug, drug-associated cues or stress11. Exposure to BDNF caused slower extinction of the cocaine self-administration habit when the drug was no longer available and stronger reinstatement (relapse) after extinction had been achieved. Again, antibody to BDNF injections had modest effects in the opposite direction on some, but not all, of these relapse-related measures.
Overall, the present results provide the most dramatic demonstration to date of the ability of exogenous BDNF injections to promote cocaine-taking behavior over a range of experimental conditions and over an extended period of time. Particularly notable is the finding that several days of BDNF injections into the accumbens shell during cocaine self-administration increased the rats’ vulnerability to relapse, even after several weeks of abstinence and subsequent extinction of the drug-reinforced responding. Remarkably, the effect of BDNF on relapse to cocaine seeking was evident across several different reinstating stimuli (cocaine priming, cocaine cues and stress). The authors also reached two major conclusions concerning the mechanisms underlying the behavioral effects of exogenous BDNF. First, cocaine-induced dynamic induction and release of BDNF during the initial phases of cocaine use promotes the development of the self-administration habit and increases propensity for subsequent relapse. Second, the critical site for these effects of BDNF is the accumbens shell.
Some intriguing questions regarding the temporal relationship between BDNF injections and self-administration remain unanswered by these results. It would be of interest to know whether BDNF that was injected many hours after self-administration or even after the cessation of self-administration would be equally effective in increasing the rats’ vulnerability to relapse. Such data would provide insight into the mechanistic relationship between the modest increase in accumbens BDNF expression after the cocaine self-administration sessions and the potent and long-lasting effects of BDNF injections on cocaine-taking behavior. For example, one study8 suggests that the precise temporal relationship between BDNF brain injections and repeated sessions of cocaine intake may not be critical for the behavioral effects of these injections. Future work examining this temporal relationship will be of interest, particularly if one of the goals of this work would be to eventually develop treatment strategies that temporarily target brain BDNF.
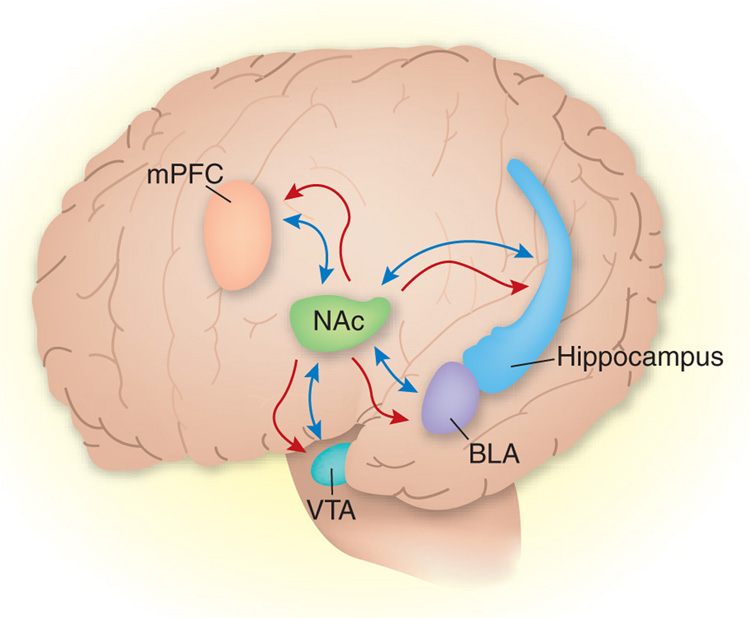
In addition, although the results of Graham et al.1 with BDNF and antibody to BDNF injections and the AAV vector suggest that the accumbens shell is the site of action for BDNF, both BDNF and the viral vector, over time, can undergo transport and therefore exert their biological effects in other brain areas12–14 (Fig. 1). In the current study, BDNF and the AAV vector may have been transported in a retrograde manner to accumbens projection areas such as hippocampus, basolateral amygdala or prefrontal cortex, where BDNF mRNA expression is substantially higher than in the accumbens2,3. Thus, BDNF in these areas may be important for cocaine’s behavioral effects. Retrograde or anterograde transport is unlikely to be involved in the effects of the antibody to BDNF that was used; however, it is possible that this agent diffused into the ventricles and then to other brain areas. This is a common issue in neuroanatomical studies that target the accumbens shell, because cannulae often pass through the lateral ventricles to reach this area, leading to drug diffusion into the ventricles and subsequently to other brain areas15. These issues not withstanding, the striking findings of Graham et al.1 should promote additional research on the brain sites mediating the effects of brain injections of BDNF on cocaine-taking behaviors.
Figure 1.
Potential mechanisms underlying the effects of accumbens injections of BDNF or adeno-associated viral vector, which encodes CRE-recombinase to knock down local BDNF protein production, on cocaine reward and relapse. Exogenous BDNF can act locally in the nucleus accumbens (NAc) or act after retrograde transport to accumbens projection areas such as the hippocampus, basolateral amygdala (BLA), prefrontal cortex (mPFC) and VTA. Similarly, the viral vector can either act at the accumbens injection site to decrease local BDNF production or it can act distally after retrograde transport to the hippocampus, BLA, mPFC and VTA. These projection regions are also involved in cocaine’s behavioral effects, and BDNF mRNA expression in these areas is substantially higher than it is in the accumbens. Blue arrows, direction of transport of exogenous BDNF; red arrows, direction of transport of viral encapsulated BDNF.
The present results may have important implications for the treatment of addiction. A unique finding in this report is that injections of antibody to BDNF after the establishment of the cocaine self-administration habit decreased subsequent relapse to cocaine-seeking in tests conducted weeks later. This observation suggests that short-term treatment approaches that transiently target brain BDNF may have enduring effects on the subject’s motivation to seek cocaine over extended time periods. Such a treatment approach would have obvious benefits; it could be readily applied to addicts who are actively using drugs, especially if the temporal relationship between drug-taking and treatment is more permissive than that proposed by Graham et al.1. Furthermore, short-term treatment approaches would be particularly desirable when targeting brain BDNF, as prolonged interference with the functioning of this growth factor will likely lead to undesirable dopamine-deficiency Parkinsonian-like side-effects. The results from Graham et al.1 should encourage addiction researchers to examine the effects of short-term pharmacological and molecular interventions, aimed at reversing long-term drug-induced brain neuroadaptations, on subsequent drug relapse.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
References
- 1.Graham DL, et al. Nat. Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 2.Seroogy KB, et al. J. Comp. Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- 3.Meredith GE, Callen S, Scheuer DA. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, et al. J. Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 5.Grimm JW, et al. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce RC, Bari AA. Rev. Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- 7.Horger BA, et al. J. Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. J. Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall FS, Drgonova J, Goeb M, Uhl GR. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- 10.Schuster CR, Thompson T. Annu. Rev. Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 11.Epstein DH, Preston KL, Stewart J, Shaham Y. Psychopharmacology (Berl.) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altar CA, DiStefano PS. Trends Neurosci. 1998;21:433–437. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- 13.DiStefano PS, et al. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 14.Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Mol. Ther. doi: 10.1038/sj.mt.6300227. published online 12 June 2007. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AK, Epstein AN. Brain Res. 1975;896:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]