The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate (original) (raw)
Abstract
Male excess mortality is widespread among mammals and frequently interpreted as a cost of sexually selected traits that enhance male reproductive success. Sex differences in the propensity to engage in risky behaviours are often invoked to explain the sex gap in survival. Here, we aim to isolate and quantify the survival consequences of two potentially risky male behavioural strategies in a small sexually monomorphic primate, the grey mouse lemur Microcebus murinus: (i) most females hibernate during a large part of the austral winter, whereas most males remain active and (ii) during the brief annual mating season males roam widely in search of receptive females. Using a 10-year capture–mark–recapture dataset from a population of M. murinus in Kirindy Forest, western Madagascar, we statistically modelled sex-specific seasonal survival probabilities. Surprisingly, we did not find any evidence for direct survival benefits of hibernation—winter survival did not differ between males and females. By contrast, during the breeding season males survived less well than females (sex gap: 16%). Consistent with the ‘risky male behaviour’ hypothesis, the period for lowered male survival was restricted to the short mating season. Thus, sex differences in survival in a promiscuous mammal can be substantial even in the absence of sexual dimorphism.
Keywords: sex differences in survival, seasonal survival, risky male behaviour, female hibernation, male roaming, Microcebus murinus
1. Introduction
In most mammalian species, including humans, female average lifespan exceeds that of males (e.g. Promislow 1992; Moore & Wilson 2002; Owens 2002; Toigo & Gaillard 2003; Austad 2006; Clutton-Brock & Isvaran 2007). Apart from effects of deleterious recessive alleles in the heterogametic sex (XY), most hypotheses trying to explain male-biased mortality invoke arguments from sexual selection theory. Since male mammals usually have higher potential reproductive rates than females, male fitness is primarily limited by the number of available females, resulting in strong intrasexual competition among males over access to receptive females (Clutton-Brock & Parker 1992; Kvarnemo & Ahnesjo 1996; Reynolds 1996). Accordingly, male excess mortality is viewed as a cost of sexual selection paid for traits that enhance reproductive success (Moore & Wilson 2002).
Proximate hypotheses to explain sex differences in survival include the following: (i) the costs of growing large, and the resulting, sexual size dimorphism characteristic for many mammals lead to lower survival in the larger sex (Promislow 1992; Ricklefs & Scheuerlein 2001; de Magalhaes et al. 2007); (ii) lower immunocompetence in males, mediated by androgens such as testosterone, renders them more susceptible to parasitic and infectious diseases (Zuk & McKean 1996; Moore & Wilson 2002; Klein 2004; Hau 2007) and (iii) the higher propensity of males to engage in potentially risky behaviours, such as dispersal, physical combat and roaming decreases male survival (Greenwood 1980; Johnson & Gaines 1990; Magnhagen 1991; Alberts & Altmann 1995; Rödel et al. 2004). These hypotheses are not mutually exclusive, and presumably, several processes jointly shape the sex gap in survival in interaction with a given environment. Indeed, sexual size dimorphism, sex-biased parasite load and risky male behaviours seem to be tightly linked across mammals (Moore & Wilson 2002; Brei & Fish 2003). This strong covariation renders it difficult to separate support for any single hypothesis, and to quantify the contribution of the proximate factors invoked to explain sex differences in survival.
Owing to several idiosyncrasies, the primates of Madagascar (Lemuriformes) provide an excellent model to investigate several of the hypotheses regarding male excess mortality, particularly to evaluate the importance of risky male behaviours. First, lemurs are sexually monomorphic (Kappeler 1990), and thus, in case they do exhibit male-biased mortality, we can exclude sex differences in growth and maintenance of a larger body size as the proximate cause (see also Leigh & Terranova 1998). Second, lemurs are highly seasonal breeders with hormone profiles that parallel reproductive activity and are synchronized by photoperiod (Kraus et al. 1999; Perret & Aujard 2001; Ostner et al. 2002). In several seasonally breeding species, males are most susceptible to infections during the breeding season when androgen levels are high (Klein 2004). Consequently, sex differentials in survival due to differences in immunocompetence should be larger during the time of elevated testosterone levels. Finally, lemur males engage in many of the same supposedly risky behaviours as anthropoid primates and other mammals (Setchell & Kappeler 2003).
Grey mouse lemurs Microcebus murinus are small (approx. 60 g), nocturnal and arboreal primates (Cheirogaleidae) that can be classified as solitary foragers (Kappeler & van Schaik 2002). They show a unique seasonally fluctuating sexual dimorphism in body mass with males being heavier during austral spring when mating begins, and females being heavier in late autumn, prior to hibernation. Overall, they are sexually monomorphic in body mass as well as with regard to other body measurements (Schmid & Kappeler 1998). Females and males follow strikingly different lifestyles. Females are philopatric whereas most juvenile males disperse before their first breeding season (Wimmer et al. 2002; Radespiel et al. 2003; Eberle & Kappeler 2004; Fredsted et al. 2005). Moreover, whereas most males sleep alone, most females form stable sleeping groups (Radespiel 2000; Eberle & Kappeler 2006). In the Kirindy Forest population in western Madagascar, the majority of males remain active with only short daily bouts of torpor throughout the dry winter, but most adult females stay inactive for several months (Schmid 1999; Rasoazanabary 2006). During the short annual mating period, males roam extensively in search of receptive females (Radespiel 2000; Eberle & Kappeler 2004), and also guard and aggressively defend access to them (Eberle & Kappeler 2004).
In this study, we test the hypothesis that risky male behaviours carry survival costs in a wild population of grey mouse lemurs, which lead to sex differences in survival despite the lack of male-biased sexual dimorphism. To this end, we link seasonal differences in sex-specific survival to two sex-specific behavioural strategies of adult M. murinus: female hibernation and male roaming. It has been suggested that remaining inactive during the dry winter protects females during periods of low food availability and high predation risk (Rasoloarison et al. 1995; Schmid & Kappeler 1998; Schmid 1999). Similarly, roaming during mate search probably renders males more conspicuous to predators. Accordingly, we predict that males survive less well than females during both the non-breeding season, i.e. the dry austral winter, and the breeding season, i.e. the wet austral summer. Lowered male survival during the breeding season would be less straightforward to interpret, however, because it would be consistent with the ‘risky male behaviour hypothesis’ as well as the ‘adverse effects of androgens hypothesis’. In order to tease apart these two hypotheses, we determine the temporal course of sex differentials in survival over the breeding season. If male excess mortality was primarily a direct effect of risky male behaviour, it should be restricted to the period when the behaviour concerned, here roaming, actually occurs, i.e. to the brief mating season within the early austral summer. On the other hand, because male summer testosterone levels are elevated approximately two months before and beyond the actual mating period (Perret & Aujard 2001), we would expect male survival to be constantly lower than that of females over the entire breeding season (elevated testosterone levels) if males mainly suffer higher mortality due to androgen-mediated decreased immunocompetence.
2. Material and methods
(a) Study area and study population
We have been studying a population of grey mouse lemurs (M. murinus) in Kirindy Forest, a dry deciduous forest located approximately 60 km northeast of Morondava in western Madagascar (Sorg et al. 2003) since 1994. The region is characterized by pronounced seasonality with a cool dry season from April/May to September (austral winter), a hot dry transitory period in October/November and a hot wet season from December to March (austral summer; Sorg & Rohner 1996). Reproduction is highly seasonal and starts shortly after female emergence from hibernation in late September. The mating period is limited to four weeks from mid-October to mid-November (Eberle & Kappeler 2004). Mouse lemurs mate promiscuously and most litters are of mixed paternity (Eberle & Kappeler 2004). After gestation of two months, one to three young are born and weaned approximately two months later (Eberle & Kappeler 2006). Females are philopatric and dispersal of juvenile males takes place from April to August; there is no evidence for secondary dispersal in this population (Eberle & Kappeler 2004).
(b) Capture–mark–recapture
Captures have been conducted in a 9 ha study area on a monthly basis. The study area is equipped with a rectangular system of foot trails at 25 m intervals to facilitate trapping in this dense continuous forest. To trap mouse lemurs, we baited Sherman live traps with small pieces of banana and set them near trail intersections in the late afternoon on three consecutive nights per month. A series of three such nights of trapping will be referred to as a trapping session. Captured animals were collected in the early morning, individually marked with subdermally injected transponders (or re-identified in case of recaptures), subjected to standard morphometric measurements and released at the site of capture the following late afternoon. From 1999 onwards, all adult animals inhabiting the study area have been individually marked (approx. 75 at a time; for details see Eberle & Kappeler 2004). Our records of animals present in this area are therefore as complete as possible for a small nocturnal mammal. In total, 435 individuals (171 females, 264 males) have been caught and marked in the study area up to April 2005. Both this long-term study and surveys on a larger geographical scale revealed that this mouse lemur study population is spatially structured in that it is separated from other sub-populations to the south and west, whereas mouse lemurs live to the north and east immediately adjacent to this study population (Wimmer et al. 2002; Fredsted et al. 2004, 2005).
In order to estimate seasonal survival probabilities for the years 1995–2005, we used data from the trapping session conducted at the onset of the dry winter (9 April to 27 May) and those after female emergence from hibernation, i.e. at the start of the mating period in the first half of October (1–17 October), which we define here as the onset of summer. In some years, some of the monthly trapping sessions had to be skipped due to logistic reasons, and in 2 years (1998 and 2004), trapping sessions were earlier (August) or later (November) than normal. Including these sessions would have potentially confounded the effects of female hibernation and male roaming. We therefore created a ‘dummy’ trapping session (10 October) with zero recapture probabilities for these years. In total, this dataset included 796 captures (only one capture per trapping session considered) of 330 mouse lemurs, 134 females and 196 males; 96 of the females and 140 of the males, respectively, were caught within their first year of life.
During three annual mating seasons between 1999 and 2001, we trapped once per week in the 9 ha study area between mid-October and mid-November to determine exact dates for the start and the end of the mating period. These data in combination with those from regular trapping allowed us to estimate sex-specific survival probabilities before, during and after the mating period for 1999 and 2000. In 2001, very few animals were captured in the trapping session conducted after the mating period to estimate short-term survival. The datasets for these two mating seasons include 486 captures of 78 individuals (36 females, 42 males) in 1999 and 424 captures of 64 individuals (31 females, 33 males) in 2000.
(c) Modelling outline
In order to statistically model survival (ϕ) and recapture probabilities (p), we used the Cormack–Jolly–Seber (CJS) approach for open populations (Cormack 1964; Jolly 1965; Seber 1965) implemented in the program Mark (White & Burnham 1999). For model selection and inference, we followed the analysis strategies outlined by Burnham & Anderson (2002). This entailed constructing only biologically plausible models a priori, tailored to reflect our hypotheses outlined in §1. Model selection was based on Akaike's information criterion (AIC) or one of its appropriate variants (AIC_c_, for small samples; QAIC in the presence of overdispersion). We interpreted model selection results in a weight of evidence context based on AIC differences (Δ_i_) and normalized Akaike weights (w i), as described by Burnham & Anderson (2002). Briefly, Δ_i_ is the difference between the AIC_c_ of the top model versus the model considered, and thus reflects the likelihood of a given model relative to the best-supported model that has the lowest AIC. Akaike weights derive from this measure and are normalized so that the weights of all models in the set sum to 1.
The Akaike weights of the top model of each of our three datasets (seasonal survival, breeding season survival 1999 and 2000; tables 1 and 2) were below 0.9, suggesting considerable model selection uncertainty. Therefore, we used multi-model inference techniques to judge the relative importance of model variables (or structural elements), and to obtain model-averaged parameter estimates and standard errors unconditional on a given model (Buckland et al. 1997; Burnham & Anderson 2002). For seasonal survival, we averaged estimates only over models in a confidence subset of models (table 1). This set included all models for which the relative likelihood was above 0.05, which corresponds to a Δ_i_=6. By doing so, we excluded the negligibly supported models containing a year effect for winter survival, and thus only one model-averaged maximum likelihood estimate (MLE) for each age/sex group had to be derived (figure 1a). The relative importance of predictor variables was assessed by summing Akaike weights for all models in which the predictor was present and is given as w+(predictor), as suggested by Burnham & Anderson (2002).
Table 1.
Model selection statistics for the confidence set of models (relative likelihood>0.05) and the global model (*) for seasonal (W, winter; S, summer) survival (ϕ) and recapture (p) probabilities of mouse lemurs (1995–2005). (Factors considered are age (a; juv: juveniles, ad: adults), sex (s) and year (t). Model notation: *, interaction; +, additive effect (parallel lines on a logit scale). The number of estimable parameters (K), Akaike's information criterion for small samples (AIC_c_), the difference between the AIC_c_ of the top model and the model considered (Δ_i_) and Akaike weights (w i) are given for each model.)
| rank | model i | K | DEV | AIC_c_ | Δ_i_ | w i |
|---|---|---|---|---|---|---|
| 1 | ϕjuv(s)ad(·)Wϕs+tSptWpa*s+tS | 35 | 699.55 | 1484.04 | 0.00 | 0.222 |
| 2 | ϕjuv(s)ad(·)Wϕs+tSptWps+tS | 33 | 704.57 | 1484.67 | 0.63 | 0.162 |
| 3 | ϕjuv(s)ad(·)Wϕa*s+tSptWps+tS | 35 | 701.64 | 1486.13 | 2.09 | 0.078 |
| 4 | ϕa*sWϕs+tSptWpa*s+tS | 36 | 699.46 | 1486.16 | 2.12 | 0.077 |
| 5 | ϕjuv(s)ad(·)Wϕa*s+tSptWpa*s+tS | 37 | 697.33 | 1486.24 | 2.20 | 0.074 |
| 6 | ϕa*sWϕs+tSptWps+tS | 34 | 704.16 | 1486.46 | 2.42 | 0.066 |
| 7 | ϕjuv(s)ad(·)Wϕs+tSpjuv(s+t)ad(t)Wpa*s+tS | 37 | 698.14 | 1487.05 | 3.00 | 0.050 |
| 8 | ϕa*sWϕa*s+tSptWps+tS | 36 | 700.88 | 1487.58 | 3.54 | 0.038 |
| 9 | ϕjuv(s)ad(·)Wϕs+tSpjuv(s+t)ad(t)Wps+tS | 35 | 703.10 | 1487.60 | 3.56 | 0.038 |
| 10 | ϕa*sWϕa*s+tSptWpa*s+tS | 38 | 697.05 | 1488.18 | 4.13 | 0.028 |
| 11 | ϕjuv(s)ad(·)Wϕa*s+tSpjuv(s+t)ad(t)Wps+tS | 37 | 699.64 | 1488.55 | 4.51 | 0.023 |
| 12 | ϕjuv(s)ad(·)Wϕa*s+tSpjuv(s+t)ad(t)Wpa*s+tS | 39 | 695.39 | 1488.74 | 4.70 | 0.021 |
| 13 | ϕa*sWϕs+tSpjuv(s+t)ad(t)Wpa*s+tS | 38 | 698.10 | 1489.23 | 5.18 | 0.017 |
| 14 | ϕjuv(s)ad(·)Wϕs+tSptWpa*s+tS | 38 | 698.13 | 1489.26 | 5.22 | 0.016 |
| 15 | ϕa*sWϕs+tSpjuv(s+t)ad(t)Wps+tS | 36 | 702.81 | 1489.51 | 5.46 | 0.014 |
| 16 | ϕjuv(s)ad(·)Wϕs+tSpa*s+tWps+tS | 36 | 703.10 | 1489.80 | 5.76 | 0.012 |
| 91* | ϕa*s+tWϕa*s+tSpa*s+tWpa*s+tS | 51 | 684.76 | 1505.29 | 21.25 | 0.00001 |
Table 2.
Model selection statistics for breeding season survival of mouse lemurs in (a) 1999 and (b) 2000 (quasi-likelihood adjusted (Q)). (Given are rank, number of parameters (K), deviance (Q)DEV, (Q)AIC_c_, difference between the (Q)AIC_c_ of the top model and the model considered (Δ_i_) and Akaike weights (w i) for the three best-supported models of the candidate set, the highest ranked model for each survival hypothesis and the global model (*).)
| rank | model i | K | (Q)DEV | (Q)AIC_c_ | Δ_i_ | w i |
|---|---|---|---|---|---|---|
| (a) breeding season 1999 | ||||||
| 1 | ϕ s(MP)*T p m+s(MP)*t | 17 | 245.52 | 526.66 | 0 | 0.417 |
| 2 | ϕ s(MP)*T p(m+t)*s(MP) | 18 | 245.51 | 528.83 | 2.17 | 0.141 |
| 3 | ϕ s*T p m+s(MP)*t | 19 | 243.59 | 529.11 | 2.44 | 0.123 |
| 5 | ϕ s+T p m+s(MP)*t | 17 | 249.77 | 530.91 | 4.25 | 0.050 |
| 15 | ϕ T p m+s(MP)*t | 16 | 257.65 | 536.62 | 9.96 | 0.003 |
| 20* | ϕ s*T p(m+t)*s(MP) | 24 | 242.48 | 539.15 | 12.49 | 0.002 |
| (b) breeding season 2000 | ||||||
| 1 | ϕ s(MP)*T p m+s(MP)*t | 17 | 152.62 | 387.34 | 0 | 0.276 |
| 2 | ϕ s*T p m+s(MP)*t | 19 | 149.81 | 388.94 | 1.60 | 0.124 |
| 3 | ϕ s(MP)*T p(m+t)*s(MP) | 18 | 152.27 | 389.18 | 1.85 | 0.109 |
| 4 | ϕ s+T p m+s(MP)*t | 17 | 154.98 | 389.69 | 2.35 | 0.085 |
| 6 | ϕ T p m+s(MP)*t | 16 | 157.30 | 389.82 | 2.48 | 0.080 |
| 23* | ϕ s*T p(m+t)*s(MP) | 26 | 146.04 | 401.05 | 13.71 | 0.0003 |
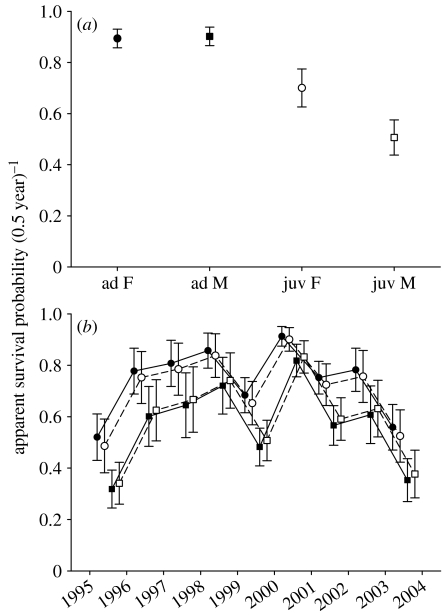
Figure 1.
Semi-annual apparent survival probabilities of M. murinus during (a) winter (non-breeding season) and (b) summer (breeding season; filled circles, ad F; filled squares, ad M; open circles, yrl F; open squares, yrl M). Depicted are model-averaged MLEs and unconditional SEs (ad, adult; juv, juvenile; yrl, yearling; F, female; M, male).
We assessed the goodness of fit (GOF) of our global models using a two-pronged approach: to detect potential structural problems in our recapture data such as transience or trap dependence we employed the program Ucare (Choquet et al. 2005). Additionally, to estimate the overdispersion factor in the case of non-systematic deviations (‘extra-binomial noise’), we used the median-cˆ approach implemented in the program Mark. Only one of the component tests in Ucare indicated a lack of fit of our global model for the seasonal survival dataset (test 3.SR, juvenile males: _Χ_2=31.89, _p_=0.007, all others _p_≥0.4). Component 3.SR provides statistics for the presence of transients, and because many of the juvenile males caught for the first time in autumn disperse before the spring capture, this was expected. These findings also indirectly confirm our observation of philopatry in juvenile females. For the global model of the seasonal survival dataset, the variance inflation factor cˆ was estimated to be only marginally above 1 (cˆseasonal=1.036), indicating an appropriate fit of our global model. We found evidence for trap dependence (‘trap happiness’) in both of the breeding season datasets (test 2.CT, 1999, females: _Χ_2=10.28, _p_=0.113, males: _Χ_2=9.90, _p_=0.042; 2000: females: _Χ_2=20.55, _p_=0.002, males: _Χ_2=11.13, _p_=0.084; all others _p_≥0.14). This effect is probably due to the combination of closely spaced trapping sessions and baited traps (Pradel 1993). Following Pradel (1993), we modelled trap dependence as an additive immediate trap effect on capture. The model incorporating the trap effect was strongly supported over the one without such an effect in both years (1999: Δ=11.4, 2000: Δ=26.4); thus we included the trap effect in all our recapture models (see below). The corresponding median-cˆ estimates for the breeding season datasets were cˆ1999=1.013 and cˆ2000=1.083, and thus, for 2000, we adjusted model selection statistics, MLEs and SEs, accordingly.
(d) Candidate set of models
Apart from the factor sex (s), we considered the factors age (a) and time (t) in our models for seasonal survival. Model notation follows Lebreton et al. (1992), with ‘*’ denoting an interactive effect and ‘+’ an additive one. Age was represented by three age classes: juveniles (juv: 3–9 months old, i.e. first winter); yearlings (yrl: 10–16 months old, i.e. first summer, first breeding season); and adults (ad: >16 months old, i.e. second winter and older). We included yearlings as a separate age class, because first-time breeders often fare worse in terms of survival, e.g. due to inexperience and low body condition (e.g. Murie & Dobson 1987). It seems possible that mouse lemurs experience such effects too, because yearlings have a lower body weight than older adults (M. Eberle 1999–2005, unpublished data). Owing to the high population turnover, very few older (5+ years) animals were caught to include them as an additional age group. Weather conditions, especially timing and levels of rainfall, differ strongly among years. We therefore expected temporal variation in recapture probabilities and annual survival, at least during summer. However, our sample size was too small to allow a saturated model with all predictors interacting. Hence, our global model includes interactions among age, sex and season (W, winter; S, summer), plus an additive effect of year for both survival and recapture probabilities (ϕa*s+tWϕa*s+tSpa*s+tWpa*s+tS).
All five-candidate models for winter survival included an age effect, because natal male dispersal in the Kirindy population takes place between April and September (Eberle & Kappeler 2004). With the CJS model, we cannot separate emigration and mortality; hence, estimates for juvenile males are ‘apparent survival’ probabilities. We do know that female dispersal and/or secondary male dispersal are at most very rare events in this population (Wimmer et al. 2002; Fredsted et al. 2004), and thus we feel confident that estimates for these sex–age classes represent ‘true survival’ probabilities. We were mainly interested in a potential sex effect on winter survival (model ϕa*s+tW versus ϕjuv(s+t)ad(t)W and model ϕa*sW versus ϕjuv(s)ad(·)W). Additionally, we considered the possibility that hibernation might buffer adult female survival against time variation (ϕjuv(s+t)adM(t)adF(·)W). Alternatively, because winter conditions are much more stable across years than summer conditions, we included models without year variation (model ϕa*sW versus ϕjuv(s)ad(·)W). The five-candidate models for summer survival were again built to allow the evaluation of an effect of sex (model ϕs+tS versus ϕtS and model ϕa*sS versus ϕaS). Additionally, we considered the possibility that first-time breeders' survival might differ somewhat from that of older individuals (model ϕa*s+tS versus ϕs+tS).
In order to limit the total number of models, we restricted the candidate set for recapture probabilities to three models per season. Apart from the global model (pa*s+tWpa*s+tS) and an only time-varying one (ptWptS), we added a model including a sex effect for juveniles only for early winter recapture probabilities (pjuv(s+t)ad(t)W) because juvenile male dispersal might be associated with higher recapture probabilities. Since summer trapping sessions were held usually at the onset of the mating period, we included a recapture model with an additive effect of sex on time (ps+tS).
Modelling breeding season survival, we did not include an age effect, owing to the small sample sizes (see above), and because we knew that the age effect was rather small (figure 1b). Survival between trapping sessions within the periods (T) of interest (before, during and after the mating period) was constrained to be constant. Our main goal regarding breeding season survival was to compare support for the competing hypotheses that male survival was only lowered during the mating period (‘risky male behaviour’: ϕ s(MP)*T) versus the hypothesis that male survival was lower than that of females over the entire breeding season (‘adverse effects of androgens’: ϕ s+T). The global model (ϕ s*T) allowed a combination of these non-exclusive hypotheses as well as a potential reversal of the sex effect after the mating period due to costs of female reproduction, i.e. gestation and lactation. Finally, the time-varying model (ϕ T) was included to quantify the relative importance of the sex effect in these 2 years.
Owing to the trap dependence detected by the GOF (see above), in addition to the effects of sex and time, we included an additive trap effect (m) when modelling recapture probabilities during the breeding season. As with survival probabilities, our global model allowed sex and time to interact. Additionally, we considered the sex effect on recapture as either constant across the breeding season or restricted to the mating period (higher male activity). We allowed the trap effect to either differ between the sexes (p(m+t)*s and p(m+t)*s(MP)) or, alternatively, constrained it to be identical for males and females (p m+s*t, p m+s(MP)*t p m+s+t and p m+t).
3. Results
(a) Winter survival (non-breeding season)
Remaining more active throughout the dry winter did not result in a survival cost for male mouse lemurs (figure 1a). The two best-supported models (Δ_i_<2) suggested constant and equal survival for adults of both sexes (table 1), and multi-model inference strongly supported this survival model over competing ones (w+(_ϕ_juv(s)ad(·))=0.717). Still, 6 of the 16 models within the confidence set for the K-L best model (table 1) did provide some support for a sex effect on winter survival (w+(s)=0.282). Model-averaged MLEs, however, revealed that if survival is sex biased at all, it is, surprisingly, the males that enjoy marginally higher survival chances (_ϕ_females=0.894±0.036, _ϕ_males=0.902±0.036). As expected, apparent survival probabilities of juveniles were lower than those of adults and strongly sex dependent, probably due to natal dispersal of males (_ϕ_juv females=0.701±0.075, _ϕ_juv males=0.506±0.069). None of the models in the confidence set supported between-year variability of winter survival probabilities (w+(t)=0.0025).
Recapture probabilities varied among years, but there was little evidence for differences among age–sex classes (w+(p t)=0.76), suggesting that activity levels at the onset of the dry season were similar for all animals.
(b) Summer survival (breeding season)
Survival patterns in summer differed substantially from those in winter (table 1; figure 1b). Models containing a sex effect were 150 times more likely than those without (w+(s)=0.993). On average (geometric mean across years), chances of adult male survival over the summer were 16% lower than those of adult females (11% for yearlings). Survival probabilities of first-year breeders were similar to those of older consexuals. In general, survival probabilities in summer were lower than those in winter. We found virtually 100% support for yearly variation in summer survival (w+(t)=0.99993), with survival probabilities differing up to 50% between years.
Recapture probability at the onset of the breeding season was substantially higher for males than for females (w+(s)=0.993, geometric means: _p_ad F=0.565, _p_ad M=0.820, _p_yrl F=0.584, _p_yrl M=0.703), consistent with a higher activity level of males compared with females during the mating period.
(c) Survival before, during and after the mating period
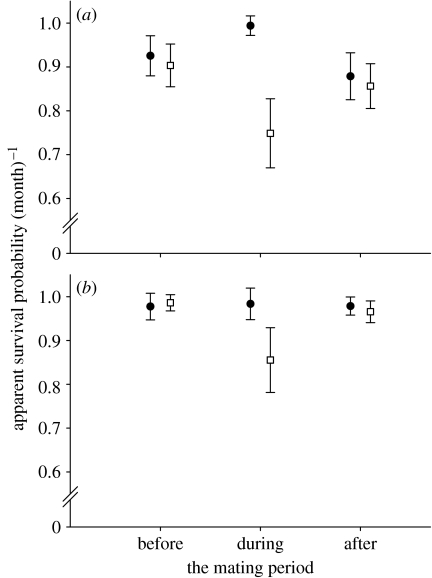
For the 1999 and 2000 datasets, we could confirm the existence of a sex gap in survival during the breeding season which we found in the long-term dataset, though the relative importance of the effect differed somewhat between years (1999: w+(s)=0.995, 2000: w+(s)=0.874). Model selection clearly favoured the hypothesis that the lowered survival of males was limited to the short mating period compared with the alternative of a constantly lowered male survival over the entire breeding season (table 2). Model ϕ s(MP)*T was eight times more likely than the competing model ϕ s+T for the 1999 dataset and three times more likely for the 2000 one. Model averaging estimated the sex gap in survival during the mating period as 24.6% in 1999 and 12.8% in 2000 (figure 2). Concurrent with decreased male survival probabilities, recapture probabilities of males were higher than those of females during the mating period in both years (table 2), and also the effect of trap dependence on recapture probabilities was similar for the sexes (table 2).
Figure 2.
Sex differentials in apparent survival probability of M. murinus over the breeding season: before, during and after the mating period in (a) 1999 and (b) 2000 (circles, females; squares, males). Depicted are model-averaged MLEs and unconditional SEs.
4. Discussion
Seasonal survival in our study population of grey mouse lemurs was characterized by high constant winter survival and lowered summer survival that varied substantially among years. We found clear evidence for a sex difference in survival in this sexually monomorphic primate. Surprisingly, sex-specific activity patterns during the dry winter did not contribute to the female survival advantage—adult winter survival did not differ between active males and hibernating females. Instead, males incurred higher mortality during the breeding season, consistent with the hypothesis that intrasexual selection imposes a survival cost on males. We could show that at least in 2 years lowered male survival was restricted to the four-week mating period when males roam widely in search of receptive females. Our findings provide strong support for the hypothesis that male roaming behaviour is indeed costly in terms of survival. This study also emphasizes the fact that in order to understand the proximate factors that drive sex differences in survival, we need to dissect annual survival into time slots that correspond to distinct processes and events in the life cycle of the population of interest.
(a) Sex differences in survival in a monomorphic primate
Sexually monomorphic species are expected to show a small sex differential in survival compared with dimorphic species (e.g. Promislow 1992; Moore & Wilson 2002). Combining seasonal survival estimates resulted in a (geometric) mean sex gap of 16% (_ϕ_females=0.65, _ϕ_males=0.49) in annual adult survival (11% in yearlings: _ϕ_females=0.62, ϕ_males=0.51) over the 10-year study period. Assuming no age dependence in survival after 2 years of age, our estimates translate into a sex differential in life expectancy at first reproduction (e_1) of 0.8 years (efemales1=2.3 years, emales1=1.5 years). Sex differences in life expectancy at birth are likely to be even higher due to survival costs of natal dispersal in males. The magnitude of the sex differential in survival (Δ_ϕ s) in M. murinus is within the range of those found in some of the sexually dimorphic squirrels that share several aspects of mouse lemur life history, in particular hibernation/torpor and a promiscuous mating system characterized by competitive male mate searching (Spermophilus townsendi: Δ_ϕ s_=13%, Smith & Johnson 1985; Spermophilus citellus: Δ_ϕ_=18%, Millesi et al. 1999; Spermophilus columbianus: Δ_ϕ_=9%, Neuhaus & Pelletier 2001). Sex-biased survival has been reported for several other monomorphic small mammals (Tamias amoenus: Δ_ϕ s_=21%, Schulte-Hostedde et al. 2002; Zapus hudsonicus: Δ_ϕ _s_=15%, Meaney et al. 2003). On the other hand, no evidence for sex differential survival was found in a number of promiscuous monomorphic small mammals (Eliomys quercinus: Schaub & Vaterlaus-Schlegel 2001; Dipodomys spectabilis: Skvarla et al. 2004; Sciurus vulgaris: Wauters et al. 2004), documenting that there is large variability within this group.
The survival patterns in our study population partially diverge from those published for the Ampijoroa population of M. murinus. Lutermann et al. (2006) also reported high mortality in their study population in northwestern Madagascar. However, they concluded that males do actually outlive females on average. Unfortunately, they did not account for recapture probabilities. If, as in our study, males have higher capture probabilities during mating periods, male survival estimates will be inevitably biased towards higher values. Thus, comparative inference drawn from these studies has to be regarded as preliminary. If these distinct patterns in sex-specific survival at the two study sites are indeed real, they would provide a unique opportunity to study behavioural and, ultimately, environmental conditions shaping intraspecific sex differences in survival. Population differences may be due to the fact that females do not hibernate in the Ampijoroa population and that they produce up to two birth cohorts per year, which might entail an increased survival cost of reproduction (Radespiel et al. 1998; Radespiel et al. 2003; Schmelting et al. 2007).
Only limited information on sex differences in survival in other wild lemur populations exists. Longitudinal observations suggest that in two species at least females might enjoy a longer lifespan (Propithecus verreauxi: Richard et al. 2002; Lemur catta: Gould et al. 2003). Further circumstantial evidence comes from studies on the adult sex ratio in primates (Clutton-Brock & Iason 1986; Mitani et al. 1996; Kappeler 2000). Whereas in anthropoid primate groups the adult sex ratio is usually strongly female biased suggesting excess male mortality, the sex ratio in lemur groups is balanced or even slightly male biased (Kappeler 2000; Ostner & Kappeler 2004). Hence, both mortality and sex ratio at birth are male biased or are unbiased. The latter would be consistent with the hypothesis that sexually monomorphic species should show low sex differentials in mortality. There is some evidence for a male-biased sex ratio at birth in captive and wild lemur populations (Debyser 1995; Kappeler 1997). Our results also support the first scenario of male-biased sex ratios at births and clearly show that the observed sex ratio can easily lead to erroneous inference regarding sex differences in survival.
(b) Winter survival: safe sleeping?
Contrary to our prediction, hibernating adult female mouse lemurs did not survive better than males that only undergo daily torpor bouts of less than 24 hours throughout the dry winter. Thus, remaining active does not seem to impose an additional survival risk on males. Equal or even male-biased winter survival has been reported for other small mammalian species with annual male-biased mortality (e.g. Michener & Locklear 1990; Millesi et al. 1999; Neuhaus & Pelletier 2001). Juvenile mouse lemurs of both sexes had substantially lower survival chances (age differential: Δ_ϕ_females=19%, Δ_ϕ_males=40%). Unfortunately, it is not possible to estimate unbiased sex differentials for juvenile survival without additional information on natal dispersal rates in males.
Various potential benefits have been proposed to explain tropical torpor and hibernation in general and female hibernation in the Kirindy mouse lemur population in particular (Schülke & Ostner 2007). Since females actually emerge from hibernation with a lower body mass than males (Schmid 1999) as Schülke & Ostner (2007) already pointed out, resource scarcity (food and/or water) is unlikely to be the ultimate cause why females choose to sleep through a large part of the dry season. Indeed, a constant and high level of overwinter survival relative to breeding season survival has been reported for many species where both sexes undergo obligate hibernation (Michener & Locklear 1990; Millesi et al. 1999; Neuhaus & Pelletier 2001; Schaub & Vaterlaus-Schlegel 2001; Meaney et al. 2003; Sendor & Simon 2003; Smith & Nichols 2003).
Escaping or at least lowering a high predation pressure has been hypothesized as the decisive benefit of prolonged female inactivity (Schmid & Kappeler 1998; Goodman 2003; Schülke & Ostner 2007). Differences in activity level have often been causally linked to selective predation (e.g. Norrdahl & Korpimaki 1998; Roth & Lima 2007), and in Kirindy Forest, grey mouse lemurs are regularly preyed upon by various predators, including owls and snakes, carnivores (Eberle & Kappeler 2008) and a lemur, Coquerel's dwarf lemur Mirza coquereli (Kappeler & Rasoloarison 2003). However, the high overwinter survival in both sexes argues against an increased predation risk due to higher levels of activity at this time of the year. Hence, with respect to direct survival consequences, our findings suggest that daily torpor and hibernation are equivalent alternative strategies to cope with energetic hazards and hungry predators during the dry winter.
So why do female mouse lemurs in Kirindy Forest doze away a good part of the year or, the other way round, why do males forego a good winter's sleep? Even if female hibernation does not result in a direct survival benefit, delayed effects, i.e. a later onset or a reduced pace of the ageing process, are conceivable (Clutton-Brock & Isvaran 2007). Hibernation has been theoretically and empirically linked to a long lifespan (Calder 1985; Brunet-Rossinni & Austad 2004; Holliday 2006); for example, in captive Turkish hamsters, lifespan was shown to be directly correlated with the length of hibernation (Lyman et al. 1981). The answer to the second question might be that males have to prepare themselves for the mating season by accumulating fat reserves for roaming and direct male–male competition (Schülke & Ostner 2007).
(c) Summer survival: risky roaming?
Male mouse lemurs in our population seem to pay a high price for reproduction. High breeding season mortality in males has been reported in many small mammals, but only a few studies have used a temporal scale fine enough to explore the potential proximate causes of this phenomenon in more detail (e.g. Boonstra et al. 2001; Neuhaus & Pelletier 2001). We could not detect an effect of sex on survival outside the short mating period, which strongly supports the risky male behaviour hypothesis over the ‘adverse effects of androgens’ hypothesis, since roaming and physical male competition only occur during the short mating period (Eberle & Kappeler 2002, 2004), while testosterone levels are increased over a much longer period of time than the brief mating season (Perret & Aujard 2001). However, the balance between these processes might differ across years, e.g. dependent on yearly survival (‘good years’ versus ‘bad years’) and parasite dynamics.
Sperm competition plays a large role in male–male competition in mouse lemurs (Eberle & Kappeler 2007), and competitive mate searching leads to higher activity levels that were reflected in increased male recapture probabilities during the mating period. Predation pressure might actually reach its maximum when both prey and predator are active and breeding. The dangers of increased activity combined with reduced vigilance might render males especially vulnerable to predation. Hoogland et al. (2006) documented that predation risk in Utah prairie dogs was higher for roaming males than for any other functional category during the mating period. Additionally, the body condition of male mouse lemurs deteriorates substantially over the mating period (Schmid & Kappeler 1998), which might further increase their susceptibility to predation and possibly disease.
Acknowledgments
All research reported in this manuscript is in compliance with animal care regulations and applicable national laws of Germany and Madagascar.We thank Prof. Berthe Rakotosamimanana (late), Prof. Olga Ramilijaona, Dr Daniel Rakotondravony (Université d'Antananarivo), Dr Lucien Rakotozafy (Parc Botanique et Zoologique Tsimbazaza Antananarivo), the Commission Tripartite of the Direction des Eaux et Forêts and the CFPF Morondava for their authorization or support of this study. Thanks to Emilien Marc and Tiana Andrianjanahary for assistance in the field and Alex Scheuerlein for helpful discussions. Comments by Marco Festa-Bianchet and an anonymous referee improved the previous version of this paper. The research was conducted with financial support from the German Primate Center (DPZ) and German Research Foundation (DFG, Ka 1082/1 and Ka 1082/5).
References
- Alberts S.C, Altmann J. Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 1995;145:279–306. doi:10.1086/285740 [Google Scholar]
- Austad S.N. Why women live longer than men: sex differences in longevity. Gend. Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. doi:10.1016/S1550-8579(06)80198-1 [DOI] [PubMed] [Google Scholar]
- Boonstra R, McColl C.J, Karels T.J. Reproduction at all costs: the adaptive stress response of male Arctic ground squirrels. Ecology. 2001;82:1930–1946. [Google Scholar]
- Brei B, Fish D. Comment on “Parasites as a viability cost of sexual selection in natural populations of mammals”. Science. 2003;300:55A. doi: 10.1126/science.1081430. doi:10.1126/science.1079746 [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni A.K, Austad S.N. Ageing studies on bats: a review. Biogerontology. 2004;5:211–222. doi: 10.1023/B:BGEN.0000038022.65024.d8. doi:10.1023/B:BGEN.0000038022.65024.d8 [DOI] [PubMed] [Google Scholar]
- Buckland S.T, Burnham K.P, Augustin N.H. Model selection: an integral part of inference. Biometrics. 1997;53:603–618. doi:10.2307/2533961 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information-theoretic approach. [Google Scholar]
- Calder W.A. The comparative biology of longevity and lifetime energetics. Exp. Gerontol. 1985;20:161–170. doi: 10.1016/0531-5565(85)90033-6. doi:10.1016/0531-5565(85)90033-6 [DOI] [PubMed] [Google Scholar]
- Choquet, R., Reboulet, A. M., Lebreton, J.-D., Gimenez, O. & Pradel, R. 2005 U-Care 2.2 User's manual. Montpellier, France: CEFE. See http://ftp.cefe.cnrs.fr/biom/Soft-CR/
- Clutton-Brock T.H, Iason G.R. Sex-ratio variation in mammals. Q. Rev. Biol. 1986;61:339–374. doi: 10.1086/415033. doi:10.10.1086/415033 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 1992;67:437–456. doi:10.1086/417793 [Google Scholar]
- Clutton-Brock T.H, Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B. 2007;274:3097–3104. doi: 10.1098/rspb.2007.1138. doi:10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack R.M. Estimates of survival from the sighting of marked animals. Biometrika. 1964;51:429–438. [Google Scholar]
- Debyser I.W.J. Prosimian juvenile mortality in zoos and primate centers. Int. J. Primatol. 1995;16:889–907. doi:10.1007/BF02696109 [Google Scholar]
- de Magalhaes J.P, Costa J, Church G.M. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol. Biol. Med. Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle M, Kappeler P.M. Mouse lemurs in space and time: a test of the socioecological model. Behav. Ecol. Sociobiol. 2002;51:131–139. doi:10.1007/s002650100409 [Google Scholar]
- Eberle M, Kappeler P.M. Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 2004;57:77–90. doi:10.1007/s00265-004-0826-1 [Google Scholar]
- Eberle M, Kappeler P.M. Family insurance: kin selection and cooperative breeding in a solitary primate (Microcebus murinus) Behav. Ecol. Sociobiol. 2006;60:582–588. doi:10.1007/s00265-006-0203-3 [Google Scholar]
- Eberle M, Kappeler P.M. The early male catches the egg: sperm competition and optimal timing of matings in gray mouse lemurs (Microcebus murinus) Int. J. Primatol. 2007;28:1267–1278. doi:10.1007/s10764-007-9220-y [Google Scholar]
- Eberle, M. & Kappeler, P. M. 2008 Mutualism, reciprocity, or kin selection? Cooperative rescue of a conspecific from a boa in a nocturnal solitary forager the gray mouse lemur. Am. J. Primatol 70, 410–414. (doi:10.1002/ajp.20496) [DOI] [PubMed]
- Fredsted T, Pertoldi C, Olesen J.M, Eberle M, Kappeler P.M. Microgeographic heterogeneity in spatial distribution and mtDNA variability of grey mouse lemurs (Microcebus murinus, Primates: Cheirogaleidae) Behav. Ecol. Sociobiol. 2004;56:393–403. doi:10.1007/s00265-004-0790-9 [Google Scholar]
- Fredsted T, Pertoldi C, Schierup M.H, Kappeler P.M. Microsatellite analyses reveal fine-scale genetic structure in grey mouse lemurs (Microcebus murinus) Mol. Ecol. 2005;14:2363–2372. doi: 10.1111/j.1365-294X.2005.02596.x. doi:10.1111/j.1365-294X.2005.02596.x [DOI] [PubMed] [Google Scholar]
- Goodman S.M. Predation on lemurs. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; Chicago, IL; London, UK: 2003. pp. 1221–1228. [Google Scholar]
- Gould L, Sussman R.W, Sauther M.L. Demographic and life-history patterns in a population of ring-tailed lemurs (Lemur catta) at Beza Mahafaly Reserve, Madagascar: a 15-year perspective. Am. J. Phys. Anthropol. 2003;120:182–194. doi: 10.1002/ajpa.10151. doi:10.1002/ajpa.10151 [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. doi:10.1002/bies.20524 [DOI] [PubMed] [Google Scholar]
- Holliday R. Food, fertility and longevity. Biogerontology. 2006;7:139–141. doi: 10.1007/s10522-006-9012-3. doi:10.1007/s10522-006-9012-3 [DOI] [PubMed] [Google Scholar]
- Hoogland J.L, Cannon K.E, DeBarbieri L.M, Manno T.G. Selective predation on Utah prairie dogs. Am. Nat. 2006;168:546–552. doi: 10.1086/507714. doi:10.1086/507714 [DOI] [PubMed] [Google Scholar]
- Johnson M.L, Gaines M.S. Evolution of dispersal—theoretical models and empirical tests using birds and mammals. Annu. Rev. Ecol. Systemat. 1990;21:449–480. doi:10.1146/annurev.es.21.110190.002313 [Google Scholar]
- Jolly G.M. Explicit estimates from capture–recapture data with both death and immigration-stochastic model. Biometrika. 1965;52:225–247. [PubMed] [Google Scholar]
- Kappeler P.M. The evolution of sexual size dimorphism in prosimian primates. Am. J. Primatol. 1990;21:201–214. doi: 10.1002/ajp.1350210304. doi:10.1002/ajp.1350210304 [DOI] [PubMed] [Google Scholar]
- Kappeler P.M. Determinants of primate social organization: comparative evidence and new insights from Malagasy lemurs. Biol. Rev. 1997;72:111–151. doi: 10.1017/s0006323196004999. doi:10.1017/S0006323196004999 [DOI] [PubMed] [Google Scholar]
- Kappeler P.M. Causes and consequences of unusual sex ratios among lemurs. In: Kappeler P.M, editor. Primate males. Cambridge University Press; Cambridge, UK: 2000. pp. 55–63. [Google Scholar]
- Kappeler P.M, Rasoloarison R.M. Microcebus, mouse lemurs, Tsidy. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. University of Chicago Press; Chicago, IL: 2003. pp. 1310–1315. [Google Scholar]
- Kappeler P.M, van Schaik C.P. Evolution of primate social systems. Int. J. Primatol. 2002;23:707–740. doi:10.1023/A:1015520830318 [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. doi:10.1111/j.0141-9838.2004.00710.x [DOI] [PubMed] [Google Scholar]
- Kraus C, Heistermann M, Kappeler P.M. Physiological suppression of sexual function of subordinate males: a subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiol. Behav. 1999;66:855–861. doi: 10.1016/s0031-9384(99)00024-4. doi:10.1016/S0031-9384(99)00024-4 [DOI] [PubMed] [Google Scholar]
- Kvarnemo C, Ahnesjo I. The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol. 1996;11:404–408. doi: 10.1016/0169-5347(96)10056-2. doi:10.1016/0169-5347(96)10056-2 [DOI] [PubMed] [Google Scholar]
- Lebreton J.-D, Burnham K.P, Clobert J, Anderson D.R. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 1992;62:67–118. doi:10.2307/2937171 [Google Scholar]
- Leigh S.R, Terranova C.J. Comparative perspectives on bimaturism, ontogeny, and dimorphism in lemurid primates. Int. J. Primatol. 1998;19:723–749. doi:10.1023/A:1020381026848 [Google Scholar]
- Lutermann H, Schmelting B, Radespiel U, Ehresmann P, Zimmermann E. The role of survival for the evolution of female philopatry in a solitary forager, the grey mouse lemur (Microcebus murinus) Proc. R. Soc. B. 2006;273:2527–2533. doi: 10.1098/rspb.2006.3603. doi:10.1098/rspb.2006.3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman C.P, Obrien R.C, Greene G.C, Papafrangos E.D. Hibernation and longevity in the Turkish hamster Mesocricetus brandti. Science. 1981;212:668–670. doi: 10.1126/science.7221552. doi:10.1126/science.7221552 [DOI] [PubMed] [Google Scholar]
- Magnhagen C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 1991;6:183–185. doi: 10.1016/0169-5347(91)90210-O. doi:10.1016/0169-5347(91)90210-O [DOI] [PubMed] [Google Scholar]
- Meaney C.A, Ruggles A.K, Lubow B.C, Clippinger N.W. Abundance, survival, and hibernation of Preble's meadow jumping mice (Zapus hudsonius preblei) in Boulder County, Colorado. SW Nat. 2003;48:610–623. doi:10.1894/0038-4909(2003)048<0610:ASAHOP>2.0.CO;2 [Google Scholar]
- Michener G.R, Locklear L. Differential costs of reproductive effort for male and female Richardson's ground squirrels. Ecology. 1990;71:855–868. doi:10.2307/1937357 [Google Scholar]
- Millesi E, Strijkstra A.M, Hoffmann I.E, Dittami J.P, Daan S. Sex and age differences in mass, morphology, and annual cycle in European ground squirrels, Spermophilus citellus. J. Mammal. 1999;80:218–231. doi:10.2307/1383222 [Google Scholar]
- Mitani J.C, GrosLouis J, Richards A.F. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am. Nat. 1996;147:966–980. doi:10.1086/285888 [Google Scholar]
- Moore S.L, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. doi:10.1126/science.1074196 [DOI] [PubMed] [Google Scholar]
- Murie J.O, Dobson F.S. The costs of reproduction in female Columbian ground squirrels. Oecologia. 1987;73:1–6. doi: 10.1007/BF00376969. doi:10.1007/BF00376969 [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Pelletier N. Mortality in relation to season, age, sex, and reproduction in Columbian ground squirrels (Spermophilus columbianus) Can. J. Zool. 2001;79:465–470. doi:10.1139/cjz-79-3-465 [Google Scholar]
- Norrdahl K, Korpimaki E. Does mobility or sex of voles affect risk of predation by mammalian predators? Ecology. 1998;79:226–232. [Google Scholar]
- Ostner J, Kappeler P.M. Male life history and the unusual adult sex ratios of redfronted lemur, Eulemur fulvus rufus, groups. Anim. Behav. 2004;67:249–259. doi:10.1016/j.anbehav.2003.05.012 [Google Scholar]
- Ostner J, Kappeler P.M, Heistermann M. Seasonal variation and social correlates of androgen excretion in male redfronted lemurs (Eulemur fulvus rufus) Behav. Ecol. Sociobiol. 2002;52:485–495. doi: 10.1007/s00265-007-0487-y. doi:10.1007/s00265-002-0532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens I.P.F. Sex differences in mortality rate. Science. 2002;297:2008–2009. doi: 10.1126/science.1076813. doi:10.1126/science.1076813 [DOI] [PubMed] [Google Scholar]
- Perret M, Aujard F. Regulation by photoperiod of seasonal changes in body mass and reproductive function in gray mouse lemurs (Microcebus murinus): differential responses by sex. Int. J. Primatol. 2001;22:5–24. doi:10.1023/A:1026457813626 [Google Scholar]
- Pradel R. Flexibility in survival analysis from recapture-data: handling trap-dependence. In: Lebreton J.-D, North P.M, editors. Marked individuals in the study of bird populations. Birkhäuser; Basel, Switzerland: 1993. pp. 29–37. [Google Scholar]
- Promislow D.E.L. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. B. 1992;247:203–210. doi:10.1098/rspb.1992.0030 [Google Scholar]
- Radespiel U. Sociality in the gray mouse lemur (Microcebus murinus) in northwestern Madagascar. Am. J. Primatol. 2000;51:21–40. doi: 10.1002/(SICI)1098-2345(200005)51:1<21::AID-AJP3>3.0.CO;2-C. doi:10.1002/(SICI)1098-2345(200005)51:1<21::AID-AJP3>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Radespiel U, Cepok S, Zietemann V, Zimmermann E. Sex-specific usage patterns of sleeping sites in grey mouse lemurs (Microcebus murinus) in northwestern Madagascar. Am. J. Primatol. 1998;46:77–84. doi: 10.1002/(SICI)1098-2345(1998)46:1<77::AID-AJP6>3.0.CO;2-S. doi:10.1002/(SICI)1098-2345(1998)46:1<77::AID-AJP6>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- Radespiel L, Lutermann H, Schmelting B, Bruford M.W, Zimmermann E. Patterns and dynamics of sex-biased dispersal in a nocturnal primate, the grey mouse lemur, Microcebus murinus. Anim. Behav. 2003;65:709–719. doi:10.1006/anbe.2003.2121 [Google Scholar]
- Rasoazanabary E. Male and female activity patterns in Microcebus murinus during the dry season at Kirindy Forest, western Madagascar. Int.J. Primatol. 2006;27:437–464. doi:10.1007/s10764-006-9017-4 [Google Scholar]
- Rasoloarison R.M, Rasolonandrasana B.P.N, Ganzhorn J.U, Goodman S.M. Predation on vetebrates in the Kirindy Forest, western Madagascar. Ecotropica. 1995;1:59–65. [Google Scholar]
- Reynolds J.D. Animal breeding systems. Trends Ecol. Evol. 1996;11:A68–A72. doi: 10.1016/0169-5347(96)81045-7. doi:10.1016/0169-5347(96)81045-7 [DOI] [PubMed] [Google Scholar]
- Richard A.F, Dewar R.E, Schwartz M, Ratsirarson J. Life in the slow lane? Demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi) J. Zool. 2002;256:421–436. [Google Scholar]
- Ricklefs R.E, Scheuerlein A. Comparison of aging-related mortality among birds and mammals. Exp. Gerontol. 2001;36:845–857. doi: 10.1016/s0531-5565(00)00245-x. doi:10.1016/S0531-5565(00)00245-X [DOI] [PubMed] [Google Scholar]
- Rödel H.G, Bora A, Kaetzke P, Khaschei M, Hutzelmeyer H, von Holst D. Over-winter survival in subadult European rabbits: weather effects, density dependence, and the impact of individual characteristics. Oecologia. 2004;140:566–576. doi: 10.1007/s00442-004-1616-1. doi:10.1007/s00442-004-1616-1 [DOI] [PubMed] [Google Scholar]
- Roth T.C, Lima S.L. The predatory behavior of wintering Accipiter hawks: temporal patterns in activity of predators and prey. Oecologia. 2007;152:169–178. doi: 10.1007/s00442-006-0638-2. doi:10.1007/s00442-006-0638-2 [DOI] [PubMed] [Google Scholar]
- Schaub M, Vaterlaus-Schlegel C. Annual and seasonal variation of survival rates in the garden dormouse (Eliomys quercinus) J. Zool. 2001;255:89–96. doi:10.1017/S0952836901001133 [Google Scholar]
- Schmelting B, Zimmermann E, Berke O, Bruford M.W, Radespiel U. Experience-dependent recapture rates and reproductive success in male grey mouse lemurs (Microcebus murinus) Am. J. Phys. Anthropol. 2007;133:743–752. doi: 10.1002/ajpa.20566. doi:10.1002/ajpa.20566 [DOI] [PubMed] [Google Scholar]
- Schmid J. Sex-specific differences in activity patterns and fattening in the gray mouse lemur (Microcebus murinus) in Madagascar. J. Mammal. 1999;80:749–757. doi:10.2307/1383244 [Google Scholar]
- Schmid J, Kappeler P.M. Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus) Behav. Ecol. Sociobiol. 1998;43:125–132. doi:10.1007/s002650050474 [Google Scholar]
- Schülke O, Ostner J. Physiological ecology of cheirogaleid primates: variation in hibernation and torpor. Acta Ethol. 2007;10:13–21. doi:10.1007/s10211-006-0023-5 [Google Scholar]
- Schulte-Hostedde A.I, Millar J.S, Gibbs H.L. Female-biased sexual size dimorphism in the yellow-pine chipmunk (Tamias amoenus): sex-specific patterns of annual reproductive success and survival. Evolution. 2002;56:2519–2529. doi: 10.1111/j.0014-3820.2002.tb00176.x. doi:10.1111/j.0014-3820.2002.tb0017.x [DOI] [PubMed] [Google Scholar]
- Seber G.A.F. A note on the multiple-recapture census. Biometrika. 1965;52:249–259. [PubMed] [Google Scholar]
- Sendor T, Simon M. Population dynamics of the pipistrelle bat: effects of sex, age and winter weather on seasonal survival. J. Anim. Ecol. 2003;72:308–320. doi:10.1046/j.1365-2656.2003.00702.x [Google Scholar]
- Setchell J.M, Kappeler P.M. Selection in relation to sex in primates. Adv. Stud. Behav. 2003;33:87–173. [Google Scholar]
- Skvarla J.L, Nichols J.D, Hines J.E, Waser P.M. Modeling interpopulation dispersal by banner-tailed kangaroo rats. Ecology. 2004;85:2737–2746. doi:10.1890/03-0599 [Google Scholar]
- Smith G.W, Johnson D.R. Demography of a Townsend ground squirrel population in southwestern Idaho. Ecology. 1985;66:171–178. doi:10.2307/1941317 [Google Scholar]
- Smith W.P, Nichols J.V. Demography of the Prince of Wales flying squirrel, an endemic of southeastern Alaska temperate rain forest. J. Mammal. 2003;84:1044–1058. doi:10.1644/BBa-033 [Google Scholar]
- Sorg, J.-P. & Rohner, U. 1996 Climate and phenology of the dry deciduous forest at Kirindy. In Ecology and economy of a tropical dry forest in Madagascar, Primate report 46-1 (eds J. U. Ganzhorn & J.-P. Sorg), pp. 57–80. Göttingen, Germany: Goltze.
- Sorg J.-P, Ganzhorn J.U, Kappeler P.M. Forestry and research in the Kirindy Forest/Centre de Formation Professionelle Forestière. In: Goodman S.M, Benstead J.P, editors. The natural history of Madagascar. The University of Chicago Press; Chicago, IL: 2003. pp. 1512–1519. [Google Scholar]
- Toigo C, Gaillard J.M. Causes of sex-biased adult survival in ungulates: sexual size dimorphism, mating tactic or environment harshness? Oikos. 2003;101:376–384. doi:10.1034/j.1600-0706.2003.12073.x [Google Scholar]
- Wauters L.A, Matthysen E, Adriaensen F, Tosi G. Within-sex density dependence and population dynamics of red squirrels Sciurus vulgaris. J. Anim. Ecol. 2004;73:11–25. doi:10.1111/j.1365-2656.2004.00792.x [Google Scholar]
- White G.C, Burnham K.P. Program Mark: survival estimation from populations of marked animals. Bird Study. 1999;46:120–139. [Google Scholar]
- Wimmer B, Tautz D, Kappeler P.M. The genetic population structure of the gray mouse lemur (Microcebus murinus), a basal primate from Madagascar. Behav. Ecol. Sociobiol. 2002;52:166–175. doi:10.1007/s00265-002-0497-8 [Google Scholar]
- Zuk M, McKean K.A. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1023. doi:10.1016/S0020-7519(96)00086-0 [PubMed] [Google Scholar]