Neutrophils as sources of extracellular nucleotides: Functional consequences at the vascular interface (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 15.
Published in final edited form as: Trends Cardiovasc Med. 2008 Apr;18(3):103–107. doi: 10.1016/j.tcm.2008.01.006
Abstract
Nucleotide signaling is currently an area of intense investigation. Extracellular ATP liberated during hypoxia or inflammation can either signal directly to purinergic receptors or, following phosphohydrolytic metabolism, can activate surface adenosine (Ado) receptors. Given the association of polymorphonuclear leukocytes (PMN) with adenine nucleotide / nucleoside signaling in the inflammatory milieu, it was recently demonstrated that PMN actively release ATP via a connexin 43 (Cx43) hemichannel-dependent mechanism. Here we review the mechanisms of ATP release and subsequent functional implications of ATP metabolism at the interface between PMN and vascular endothelial cells during inflammation and in hypoxia.
Keywords: Nucleotide, nucleoside, adenosine, endothelia, inflammation, ATP, connexin, inflammation, hypoxia
Neutrophils as a source of extracellular nucleotides
Historically, activated platelets have been thought to serve as the primary source of extracellular adenine nucleotides (ATP, ADP, AMP) during inflammation (Marcus et al. 2003). Platelets have well-established mechanisms for activated degranulation of dense granule constituents (ATP, ADP) as a means to elevate extracellular nucleotides for a variety of physiologic and pathophysiologic functions.
The first studies implicating inflammatory cells other than platelets as a source of extracellular nucleotides dates back to the early 1990’s in work by Madara et.al., who identified a soluble factor from activated neutrophils (PMN, polymorphonuclear leukocytes) which elicited electrogenic chloride secretion (i.e. water transport) in cultured epithelial cells (Nash et al. 1991, Madara et al. 1992). They termed the active factor in the soluble fraction NDS for “neutrophil-derived secretagogue”. In subsequent studies, they were able to capture the activity, purify to homogeneity and scale the system for mass spectrometry. Based on structural, biophysical and functional evidence, NDS was identified as 5′-AMP (Madara et al. 1993).
These original studies demonstrating functional responses (e.g. electrogenic chloride secretion) to PMN-derived 5′-AMP were somewhat surprising, since no 5′-AMP receptor existed. Rigorous pharmacologic studies were employed to define potential signaling pathways through 5′-AMP. A breakthrough was made when Madara et. al. inhibited PMN-derived 5′-AMP activity by incubation with adenosine deaminase (Madara et al. 1993). These results implicated adenosine (Ado) as the active factor in PMN-derived 5′-AMP. Subsequent studies revealed that inhibitors of the ecto-5′-nucleotidase (CD73), a surface enzyme which converts 5′-AMP to adenosine, blocked activity found within PMN-derived 5′-AMP (Madara et al. 1993). These results suggested the necessity of CD73 as an intermediate step in the formation of active Ado. The Ado liberated by this enzymatic process is then made available to activate any of four subtypes of G-protein coupled Ado receptors (termed A1, A2A, A2B and A3). It was subsequently shown that the rate-limiting enzyme CD73 is more widely expressed than originally thought and may broadly contribute to a number of inflammatory conditions (Colgan et al. 2006), particularly in the setting of hypoxia and stabilization of the transcription factor hypoxia-inducible factor (HIF, see later).
More recent studies revealed that the active fraction originally defined as PMN-derived 5′-AMP may actually be ATP. Indeed, studies directed at understanding vascular barrier function during conditions of inflammation or hypoxia identified the existence of a soluble fraction derived from activated PMN which amplified the resealing of vascular barrier function following subjection of cultured endothelial cells to periods of hypoxia. A screen of HPLC purified fractions identified this activity as PMN-derived ATP (Eltzschig et al. 2003). Like 5′-AMP, this activity was functionally linked to CD73 and to Ado receptors. This was puzzling, since CD73 was not known to utilize ATP as a substrate. Rather, these studies identified the ecto-nucleoside triphosphate diphosphohydrolase (NTPDase)(Wang and Guidotti 1996), previously identified as ecto-ATPase, ecto-ATPDase or CD39 (Gendron et al. 2002, Mizumoto et al. 2002) as the central surface enzyme in this reaction. CD39 is expressed on the endothelium (Eltzschig et al. 2003) and it’s primary role had been to modulate platelet purinoreceptor activity by the sequential hydrolysis of extracellular ATP / ADP to AMP (Qawi and Robson 2000, Gendron et al. 2002). It is now appreciated that CD39 and CD39-like molecules (NTPDases) are widely distributed in most tissues and have various orientations within the cell. For example, the catalytic site of NTPDases faces the extracellular milieu (NTPDase1-3) and/or the lumen of intracellular organelles such as the Golgi apparatus and endoplasmic reticulum (NTPDase4-7). NTPDase5 and 6 may also be found on the plasma membrane and secreted following proteolytic cleavage. Studies in both cd73- and cd39-null mice have confirmed many of the original in vitro studies and strongly implicate this metabolic pathway in a variety of inflammatory conditions, particularly as they relate to inflammatory hypoxia (Eltzschig et al. 2003, Thompson et al. 2004).
Mechanisms of PMN-derived ATP release
Studies directed at understanding mechanisms of ATP release by activated PMN considered several potential mechanisms, including exocytosis of ATP containing vesicles, transport via connexin hemichannels, transport through nucleoside transporters, or direct transport through ATP-binding cassette (ABC) proteins (Novak 2003). Initially, it was determined that ATP does not localize with known granule markers in PMN. While isolated granules from resting PMNs contained greater than 95% of proteolytic enzyme activity markers, ATP levels within the isolated granules were nearly undetectable. Moreover, PMN fractions containing cytosolic markers contained ATP concentrations that were higher than 5 mM, thereby suggesting that activation-dependent ATP release likely occurs independent of classical PMN degranulation. Based on these findings, a pharmacologic approach was employed to examine potential mechanisms. Brefeldin A (BFA), a general vesicular secretion inhibitor did not influence activated PMN ATP secretion. Likewise, neither the nucleoside transport inhibitor dipyridamole nor the general ABC transport inhibitor verapamil significantly influenced PMN ATP secretion (Eltzschig et al. 2006).
Based on previous reports suggesting that connexin hemichannels may serve as ATP release channels (Goodenough and Paul 2003) and the observation that PMN express surface connexins (Zahler et al. 2003), the non-specific gap junction inhibitor 18-α-glycyrrhetinic acid (18αGA) was examined. These studies revealed that 18αGA inhibited ATP release in a concentration-dependent manner. Likewise, connexin-mimetic peptides specifically directed against Cx43 (Goodenough and Paul 2003), but not Cx40, significantly blocked ATP liberation from activated PMN. Cx43 molecules can assemble as hexadimers (so called “connexons”) that form junctional connections between different cell types. In addition to their role as gap-junction proteins, recent studies indicate that Cx43 connexons are also active in single plasma membranes and can function in intercellular signaling as ATP release channels (Goodenough and Paul 2003). The conductance and permeability of such Cx43 hemichannels is regulated by modification of their cytoplasm domain, with phosphorylation of Ser-368 causing a conformational change resulting in decreased connexon permeability (Bao et al. 2004). Studies addressing Cx43 Ser-368 phosphorylation in intact PMN showed prominent phosphorylation in resting PMN and protein phosphatase 2A-dependent dephosphorylation within minutes of PMN activation.
As proof of principle, PMN were isolated from tamoxifen-inducible Cx43 conditionally deleted mice (Eckardt et al. 2004) and examined for ATP release. These studies revealed that ATP release correlated with the degree of Cx43 expression. In total, these studies provide strong evidence that ATP release occurs through a conformational opening of membrane Cx43 hemichannels in response to PMN activation (Eltzschig et al. 2006).
In addition to connexin-mediated ATP transport, an interesting but under-studied family of gap junction proteins are the pannexins (Shestopalov and Panchin 2007). Pannexins were originally described in invertebrates and at least three pannexins (PANX1-3) are now cloned in mammals. These membrane channels appear to be well suited for ATP transport and experimental evidence now suggest that pannexons synergize with metabotropic purinergic receptors to drive ATP release (Shestopalov and Panchin 2007). It is currently not known whether PMN express pannexons.
Functional implications of extracellular ATP derived from PMN
Studies directed at understanding extracellular metabolism of nucleotides in cell and tissue responses now suggest that a number of different cells can release ATP in an active manner, particularly when oxygen levels are limited (Burnstock 2002). Most studies have suggested that ATP liberated by activated PMN is “auto-hydrolyzed” to AMP through PMN surface CD39. Further metabolism of AMP to Ado requires an additional cell type to contribute CD73 (or other phosphatase activity such as alkaline phosphatase) activity as a means to generate adenosine. As such, PMN CD39 may function as an immunomodulatory control point, requiring close special relationship to CD73-positive cells (such as endothelia, epithelia, or lymphocytes). Alternatively, other phosphatases (e.g. alkaline phosphatase) also have AMP hydrolyzing activity. A direct comparison of CD73 and alkaline phosphatase activity in the mucosa revealed that while both enzymes hydrolyze AMP, CD73 appears to predominate at physiologically-relevant concentrations of AMP (Picher et al. 2003). Nonetheless, PMN express plasma membrane alkaline phosphatase (Pellme et al. 2007), and as such, PMN as a single cell population have the capacity to generate Ado from ATP. It is not currently known how relevant this latter pathway is to overall ATP metabolism.
Ado exerts paracrine and autocrine functions on most cell types. Pathophysiologic conditions of hypoxia / ischemia result in numerous adenine nucleotide metabolic changes, and Ado has a demonstrated role in organ function under such conditions. While the source of interstitial Ado in hypoxic tissue has been the basis of much debate, it is generally accepted that the dephosphorylation of AMP by CD73 represents the major pathway of Ado formation during oxygen supply imbalances (Minamino et al. 1996). Ado production in the ischemic myocardium, for example, is attributable to activity of CD73 (Minamino et al. 1996), and both CD73 activity and Ado metabolism have been demonstrated in cardiac pre-conditioning by brief periods of ischemia (McCallion et al. 2004). Specifically, Ado is made and liberated into the extracellular milieu through both transcriptional and non-transcriptional mechanisms (Linden 2005). It is appreciated that the Ado made during intermittent hypoxia (HPC) occurs through non-transcriptional metabolic changes [e.g. inhibition of Ado kinase, release through nucleoside transporters (Mubagwa and Flameng 2001, Linden 2005)]. By contrast, chronic (non-intermittent) hypoxia results in the coordinated transcriptional induction of surface enzymes which metabolize extracellular nucleotides (Linden 2001, Colgan et al. 2006).
Increased CD73 activity in ischemic preconditioning has been attributed to a variety of acute activation pathways (Minamino et al. 1996), and recent studies provide direct evidence that CD73 is transcriptionally regulated by hypoxia in a variety of cells in vitro (Boeck et al. 2000, Ledoux et al. 2003, Napieralski et al. 2003). Once liberated in the extracellular space, Ado is either taken up into the cell (through dipyridamole-sensitive carriers) or interacts with cell surface Ado receptors (Hasko and Cronstein 2004, McCallion et al. 2004). Currently, four subtypes of G protein-coupled Ado receptors exist, designated A1, A2A, A2B, and A3. These receptors are classified according to their G-protein coupling to either Gαi (A1 and A3) or Gαs (A2A and A2B) (Hasko and Cronstein 2004). Endothelial cells of many origins express Ado receptors constitutively, primarily of the A2A and A2B subtypes (Montesinos et al. 1997), wherein vascular endothelial cells are one of the most enriched cell populations of A2BR.
In addition to regulating endothelial and epithelial barrier function, a recent study reported a role for PMN-dependent ATP release in directed movement of PMN (Chen et al. 2006). These studies demonstrated that human PMN release ATP at the leading edge of migrating cells and amplify chemotactic signals for directed cell orientation by feedback through P2Y2 nucleotide receptors. These studies suggested that PMN rapidly hydrolyze released ATP to adenosine that then acts through A3-type adenosine receptors, which are recruited to the leading edge and thereby promote cell migration. As such, ATP release and autocrine feedback through P2Y2 and A3 receptors provide signal amplification, controlling gradient sensing and migration of neutrophils. Such studies highlight an additional role of extracellular nucleotide release in modulating inflammatory functions of innate immune responses.
Control of extracellular nucleotide metabolism: critical role HIF
Adenosine is liberated in at high levels in the setting of hypoxia (Linden 2005, Colgan et al. 2006). At the tissue and cellular level, hypoxia induces an array of genes pivotal to survival in low oxygen states. As a global regulator of oxygen homeostasis, the αβ heterodimeric transcription factor hypoxia-inducible factor-1 (HIF-1) facilitates both oxygen delivery and adaptation to oxygen deprivation (Semenza et al. 2000).
HIF-1 is a member of the Per-ARNT-Sim (PAS) family of basic helix-loop-helix (bHLH) transcription factors. HIF-1 activation is dependent upon stabilization of an O2-dependent degradation domain of the α subunit and subsequent nuclear translocation to form a functional complex with HIF-1β and cofactors such as CBP and its ortholog p300 (Semenza 2001). Under conditions of adequate oxygen supply, iron- and oxygen-dependent hydroxylation of two prolines (Pro564 and Pro 402) within the oxygen-dependent degradation domain (ODD) of HIF-1α initiates the association with the von Hippel-Lindau protein (pVHL) and rapid degradation via ubiquitin-E3 ligase proteasomal targeting (Semenza et al. 2000). A second hypoxic switch operates in the carboxy terminal transactivation domain of HIF-1α, where hypoxia blocks the hydroxylation of asparagine-803 and facilitates the recruitment of CBP/p300 (Lando et al. 2002).
For a number of years, it remained poorly understood how hypoxia might stabilize the expression of HIF. In the past several years, the molecular mechanisms of HIF activation have become clarified. These studies have defined three HIF-selective iron- and oxygen-dependent hydroxylation enzymes (on HIF prolines 564 and 402) within the oxygen-dependent degradation domain (ODD) of the HIF-α subunit (Bruick and McKnight 2001, Jaakkola et al. 2001). The three enzymes have different tissue distributions and, at least under conditions of over-expression, have distinct patterns of sub-cellular localization (Schofield and Ratcliffe 2004). PHD1 mRNA is expressed in many tissues, with especially high expression in the testes. Likewise, PHD2 mRNA is widely expressed, with particularly abundant expression in adipose tissue (Bruick 2003, Schofield and Ratcliffe 2004). PHD3 mRNA is also expressed in many tissues, but is most abundant in the heart and placenta (Bruick 2003, Schofield and Ratcliffe 2004).
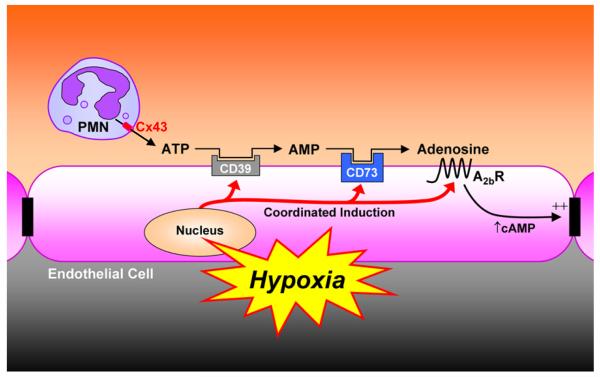
When levels of O2 fall below a critical threshold (hypoxia), the lack of PHD substrate (O2) results in the accumulation of HIF—α, which then associates with HIF—β. The HIF heterodimer translocates to the nucleus where it is made available to activate HIF-bearing gene promoters. Genes induced by HIF-1 include those necessary for cell, tissue and whole animal adaptive responses to hypoxia (Semenza et al. 2000). These proteins include enzymes involved in anaerobic metabolism, the angiogenic cytokine vascular endothelial growth factor (VEGF), and inducible nitric oxide synthase (Semenza et al. 2000). Important for this review, and as depicted in Figure 1, HIF coordinates extracellular nucleotide metabolism (CD73) (Synnestvedt et al. 2002), adenosine signaling (A2BR) (Kong et al. 2006) and the extracellular nucleoside transporter (ENT) (Eltzschig et al. 2005).
Figure 1.
Model of PMN — endothelial crosstalk by extracellular nucleotides. At sites of hypoxia or ongoing inflammation, activated PMN provide an extracellular source of ATP through membrane Cx43 hemichannels. ATP released in this fashion is metabolized through two enzymatic steps and results in the liberation of extracellular adenosine. Adenosine generated through this pathway is available for activation of surface endothelial adenosine receptors, particularly the AdoA2BR. Diminished oxygen supply (hypoxia) at sites of inflammation coordinates the induction of CD39, CD73 and AdoA2BR. At such sites, post-receptor increases in intracellular cyclic AMP result in enhanced barrier function. This biochemical crosstalk mechanism may provide an innate mechanism to preserve vascular integrity and attenuate vascular leak. This model is adapted with permission from published work (Eltzschig et al. 2003).
The discovery of HIF-selective PHD’s as central regulators of HIF expression has now provided the basis for potential development of PHD-based molecular tools and therapies (Mole et al. 2003). Pharmacological inactivation of the PHDs by 2-OG analogues is sufficient to stabilize HIF-α (Mole et al. 2003), but this action is nonspecific with respect to individual PHD isoforms. In vitro studies do suggest significant differences in substrate specificity. For example, PHD3 does not hydroxylate proline 564 on HIF-α, and comparison of enzyme activity in vitro showed that the ODD sequence is hydroxylated most efficiently by PHD2 (Bruick 2003, Schofield and Ratcliffe 2004). These observations have generated an interest in identifying enzyme-modifying therapeutics. Indeed, a number of PHD inhibitors have been described, including direct inhibitors of the prolyl-hydroxylase (Nwogu et al. 2001), analogs of naturally occurring cyclic hydroxymates (Schlemminger et al. 2003), as well as antagonists of α-keto-glutarate (Mole et al. 2003). Based on extensive work in vitro and in vivo, mucosal diseases are excellent targets for PHD-based therapeutics (Taylor and Colgan 2008). In particular, most evidence indicates that HIF activation results in an inflammatory-protective phenotype, including nucleotide metabolic pathways that promote tissue barrier function and stimulate the resolution of inflammation.
Conclusion
The dynamic interplay of PMN with endothelial and epithelial cells defines a complex and elegant lesson in cell biology. Studies of model systems incorporating cultured cells and purified PMN have allowed for the identification of functional determinants now well accepted in the scientific literature. The identification of soluble metabolites, such as adenine nucleotides, which in turn are metabolized to active nucleosides will continue to contribute important information regarding the regulation of cell-cell interactions, and such information may provide previously unappreciated insight into the pathogenesis of inflammatory diseases. Targeting the mechanisms by which PMN liberate adenine nucleotides may permit insights into new approaches to influence inflammatory cascades.
Acknowledgments
The authors acknowledge Melanie Scully for technical assistance. This work was supported by NIH grants HL60569, DE016191, DK50189 and by a grants from the Crohn’s and Colitis Foundation of America and the Deutsche Forschungsgemeinschaft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. Epub 22004 Feb 20017. [DOI] [PubMed] [Google Scholar]
- Boeck CR, Bronzatto MJ, Souza DG, et al. The modulation of ecto-nucleotidase activities by glutamate in cultured cerebellar granule cells. Neuroreport. 2000;11:709–712. doi: 10.1097/00001756-200003200-00011. [DOI] [PubMed] [Google Scholar]
- Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Eltzschig HK, Eckle T, et al. Physiologic roles for ecto-5′-nucleotidase (CD73) Purinergic Signalling. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt D, Theis M, Degen J, et al. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–110. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Abdulla P, Hoffman E, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. Epub 2006 Oct 1112. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Ex. Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron FP, Benrezzak O, Krugh BW, et al. Purine signaling and potential new therapeutic approach: possible outcomes of NTPDase inhibition. Curr Drug Targets. 2002;3:229–245. doi: 10.2174/1389450023347713. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Kong T, Westerman KA, Faigle M, et al. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, et al. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Runembert I, Koumanov K, et al. Hypoxia enhances Ecto-5′-Nucleotidase activity and cell surface expression in endothelial cells: role of membrane lipids. Circ Res. 2003;92:848–855. doi: 10.1161/01.RES.0000069022.95401.FE. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. Epub 2005 Feb 1389. [DOI] [PubMed] [Google Scholar]
- Madara JL, Parkos CA, Colgan SP, et al. Cl- secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J. Clin. Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Patapoff TW, Gillece-Castro B, et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J. Clin. Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JH, et al. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J Pharmacol Exp Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- McCallion K, Harkin DW, Gardiner KR. Role of adenosine in immunomodulation: review of the literature. Crit Care Med. 2004;32:273–277. doi: 10.1097/01.CCM.0000098026.12020.45. [DOI] [PubMed] [Google Scholar]
- Minamino T, Kitakaze M, Morioka T, et al. Cardioprotection due to preconditioning correlates with increased ecto- 5′-nucleotidase activity. Am J Physiol. 1996;270:H238–244. doi: 10.1152/ajpheart.1996.270.1.H238. [DOI] [PubMed] [Google Scholar]
- Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- Mole DR, Schlemminger I, McNeill LA, et al. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–2680. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- Napieralski R, Kempkes B, Gutensohn W. Evidence for coordinated induction and repression of ecto-5′- nucleotidase (CD73) and the A2a adenosine receptor in a human B cell line. Biol Chem. 2003;384:483–487. doi: 10.1515/BC.2003.054. [DOI] [PubMed] [Google Scholar]
- Nash S, Parkos CA, Nusrat A, et al. In vitro model of intestinal crypt abcess: a novel neutrophil-derived secretagogue activity. J. Clin. Invest. 1991;87:1474–1477. doi: 10.1172/JCI115156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- Nwogu JI, Geenen D, Bean M, et al. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation. 2001;104:2216–2221. doi: 10.1161/hc4301.097193. [DOI] [PubMed] [Google Scholar]
- Pellme S, Dahlgren C, Karlsson A. The two neutrophil plasma membrane markers alkaline phosphatase and HLA class I antigen localize differently in granule-deficient cytoplasts. An ideal plasma membrane marker in human neutrophils is still lacking. J Immunol Methods. 2007;325:88–95. doi: 10.1016/j.jim.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Picher M, Burch LH, Hirsh AJ, et al. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. Epub 12003 Jan 13430. [DOI] [PubMed] [Google Scholar]
- Qawi I, Robson SC. New developments in anti-platelet therapies: potential use of CD39/vascular ATP diphosphohydrolase in thrombotic disorders. Curr Drug Targets. 2000;1:285–296. doi: 10.2174/1389450003349173. [DOI] [PubMed] [Google Scholar]
- Schlemminger I, Mole DR, McNeill LA, et al. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett. 2003;13:1451–1454. doi: 10.1016/s0960-894x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Feldser D, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2007 Nov.5:1–19. doi: 10.1007/s00018-007-7200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 (HIF-1) mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2008 doi: 10.1007/s00109-007-0277-z. In Press. [DOI] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leak during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TF, Guidotti G. CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J Biol Chem. 1996;271:9898–9901. [PubMed] [Google Scholar]
- Zahler S, Hoffmann A, Gloe T, et al. Gap-junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J Leukoc Biol. 2003;73:118–126. doi: 10.1189/jlb.0402184. [DOI] [PubMed] [Google Scholar]