Genetic Basis for Kidney Cancer: Opportunity for Disease-Specific Approaches to Therapy (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 27.
Published in final edited form as: Expert Opin Biol Ther. 2008 Jun;8(6):779–790. doi: 10.1517/14712598.8.6.779
Abstract
Background
Kidney cancer is not a homogenous entity; it is comprised of many different tumor types, with different biology, molecular mechanisms leading to disease, and therefore different treatment approaches.
Objective
To describe the genetic basis and biochemical pathways underlying inherited forms of renal cancer, specifically in four described syndromes (von Hippel-Lindau [VHL], Hereditary Papillary Renal Cancer [HPRC], Birt-Hogg-Dubé [BHD], and Hereditary Leiomyomatosis Renal Cell Carcinoma [HLRCC]), and to elucidate how the understanding of these diseases enables the possibility for disease-specific approaches to therapy.
Methods
Systematic review of the published literature on inherited and sporadic forms of renal cancer.
Conclusion
Understanding of the biology and mechanisms of different forms of kidney cancer provides an opportunity for development of new treatment options.
Keywords: Birt-Hogg-Dubé, fumarate hydratase, kidney cancer, Hereditary Leiomyomatosis Renal Cell Carcinoma, Hereditary Papillary Renal Cancer, Met, renal cell carcinoma, Von Hippel-Lindau
1. Introduction
In 2007, about 3.5% of all cancer diagnoses in the USA were renal cancer, with 51,190 new cases and 12,890 deaths attributed to the malignancy(1). Until recently, there have been few chemotherapeutic options for metastatic renal cancer, with most patients dying from disease within a year (median survival about 10 months). Conventional systemic chemotherapy agents have been ineffective, and have not played a role in treatment of advanced renal cancer. Interleukin-2 (IL-2) and interferon have provided the only standard systemic treatment options for these patients, with response rates of 10-20%, and are still the only forms of therapy associated with durable long term complete remissions(2).
Although the majority of renal malignancies are sporadic and non-familial, about 4% are associated with hereditary cancer syndromes. Hereditary renal cancer syndromes provide a unique opportunity to study the molecular mechanisms and genes leading to several different types of renal malignancies. Insight into the pathways of these diseases has opened the door to development of targeted therapy approaches, not only for familial renal tumors, but for sporadic kidney cancer as well.
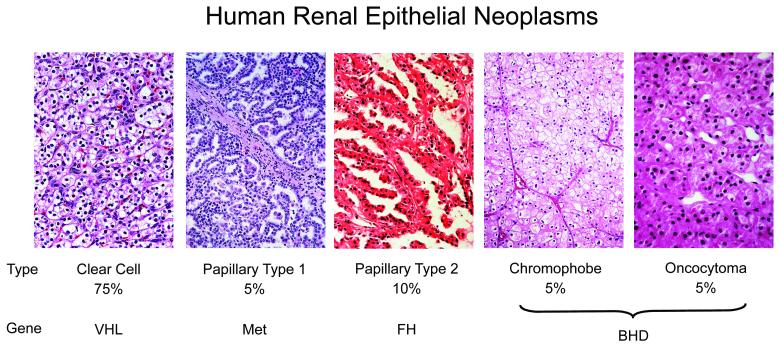
There are four main types of inherited epithelial renal cancer currently described: von Hippel-Lindau (VHL), Hereditary Papillary Renal Cancer (HPRC), Birt-Hogg Dubé (BHD), and Hereditary Leiomyomatosis Renal Cell Cancer (HLRCC). VHL is uniformly clear cell renal cancer due to a germline mutation of the tumor suppressor gene VHL. HPRC is uniformly papillary type I due to a germline mutation of the proto-oncogene MET. Patients affected with BHD are at risk of developing chromophobe, hybrid oncocytic tumors, oncocytomas, and clear cell renal cancer, and are found to have a germline mutation of the tumor suppressor BHD gene. HLRCC has unique characteristic histological features, most often described as papillary type 2, due to a germline mutation of gene for the Krebs cycle enzyme fumarate hydratase (FH), which acts as a tumor suppressor. (Figure 1)(3).
Figure 1.
Kidney cancer is not a single disease; it is made up of a number of different types of cancer that occur in the kidney, each with different histologic types, having a different clinical course, and associated with alteration of a different gene. Percentages represent the frequency of the renal carcinoma subtype. (From LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172.)[2]
2. Sporadic Kidney Cancer
2.1 Sporadic/Non-Inherited Renal Cancer
The tumor type of the majority of sporadic renal cancer is clear cell renal cell carcinoma (RCC). A mutation in the VHL gene has been found in a very high percentage of sporadic clear cell RCC, but is not found in papillary type 1 or 2, chromophobe, or oncocytomas(4,5). VHL inactivation through promoter hypermethylation or mutation has been identified in up to 75% of sporadic clear cell renal cancer(4,6). Patients who have sporadic clear cell renal carcinoma with a mutation in the VHL gene have been found to have an improved survival rates over patients with tumors that do not have mutated VHL(7). Considering the association of alteration of the VHL gene in sporadic clear cell RCC, insight into the molecular pathways leading to von Hippel-Lindau may have direct relevance to the treatment of sporadic RCC.
3. Hereditary Kidney Cancer Syndromes
3.1 von Hippel-Lindau
3.1.1 von Hippel-Lindau (VHL)
VHL is a hereditary cancer syndrome with a prevalence estimated at 1 in 36,000(8). Renal tumors are seen in 24–45% of mutation carriers, and 60% of patients have either renal tumors, cysts, or both(9,10). VHL can cause cysts and tumors in multiple organs beyond the kidneys: eye (retinal angiomas), pancreas (cysts and neuroendocrine islet cell tumors), adrenal (pheochromocytomas), epididymis and broad ligament (cystadenomas), inner ear (endolymphatic sac tumors), and central nervous system (brain and spine hemangioblastomas). Renal cysts and solid lesions corresponding to carcinoma are multi-focal, bilateral, early-onset (as early as the second decade), and solid lesions are uniformly clear cell carcinoma(3). VHL patients are at risk to develop an estimated 1,100 cysts and 600 tumors per kidney, most of which are incipient microscopic lesions(11,12). Due to the need for repeat surgical excision of lesions, these patients are often managed with surveillance with close observation of small renal tumors until the largest reaches 3cm. Parenchymal-sparing surgery, minimizing the risk of metastases while preserving renal function as long as possible, is often recommended for tumors 3 cm or larger(13,14,15,16,17).
3.1.2 VHL Gene and Pathway
The VHL gene, on chromosome 3, was identified in 1993 through genetic linkage analysis, almost 100 years after the initial description of the disease(18). VHL is transmitted in an autosomal dominant fashion, with high penetrance (patients with a germline mutation are likely to develop manifestations of the disease). Gene mutation analysis is able to identify the VHL mutation in nearly 100% of affected families(19). Numerous types of mutations have been described, including intragenic mutations (frameshift, missense, and nonsense), partial and complete deletions, and splicing mechanism defects. Genotype-phenotype correlations have demonstrated that the type and location of VHL mutation is associated with the differing clinical manifestations exhibited among VHL families(20,21,22,23). For instance, VHL kindreds with a germline partial VHL deletion, large rearrangement, or truncated protein have a significantly higher incidence of renal cancer than kindreds with a complete deletion or missense changes(24,25). VHL is subtyped into 4 groups, depending upon risk of associated risk for pheochromocytoma and RCC: Type 1 and Type 2B having a higher risk for RCC than types 2A or 2C(26).
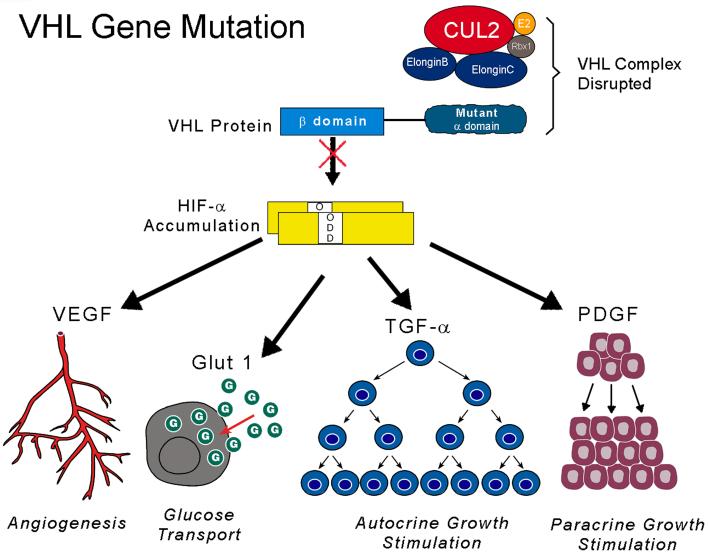
VHL is a tumor suppressor gene which also plays a role in the regulation of tumor angiogenesis. The VHL protein forms a multiprotein complex with proteins such as Cul2 and elongin c and b(27,28,29). The complex then targets the α subunits of the hypoxiainducible factors (HIF) HIF-1α and HIF2α for ubiquitin-mediated degradation, which is an oxygen-mediated process(30,31). Normoxia triggers oxygen to hydroxylate HIF through the catalyst HIF propyl hydroxylase (HPH), which permits the VHL complex to recognize, target, and subsequently lead to degradation of HIF. Conversely, hypoxia is associated with a build-up of HIF, since unhydroxylated HIF avoids detection by VHL and averts proteosomal decomposition. Inactivating mutations of both copies of VHL produce a similar effect as hypoxia, by inhibiting binding of the VHL complex and preventing HIF degradation, thus allowing HIF to overaccumulate(32,33). HIF subsequently acts as a transcription factor regulating genes for angiogenesis, metabolic changes, and unopposed growth stimuli, such as vascular endothelial growth factor (VEGF), transforming growth factor α (_TGF_α) (a ligand for the epidermal growth factor receptor [EGFR]), platelet derived growth factor (PDGF), erythropoietin (EPO), and glucose transporter-1 (GLUT-1)(34,35). (Figure 2)(36). Thus it is postulated that inactivation of VHL and overexpression of HIF can lead to tumorigenesis through dysregulation of these pathways.
Figure 2.
Molecular targeting of the VHL pathway in clear cell renal carcinoma: Mutation of the VHL gene in clear cell kidney cancer results in increased accumulation of HIF and the resulting increase in transcription of downstream targets such as VEGF, Glut-1, and TGFα (A). Mutation of the VHL gene in the α domain (demonstrated here) inhibits binding to elongin C and formation of the VHL complex.[31] Mutations in other parts of the gene, such as the β domain, prevents binding to and ubiquitin mediated degradation of HIF.[32] Potential disease-specific therapeutic approaches include agents which block the function of HIF (B), VEGFR, or EGFR (C). (From LINEHAN WM, ZBAR B: Focus on kidney cancer. Cancer cell (2004) 6(3):223-228.)[35]
3.1.3 Target for Therapeutics
Constitutive overexpression of downstream endproducts of HIF, such as VEGF, PDGF, and _TGF_α provide a feasible strategy in blocking the VHL pathway in renal tumors. Receptor tyrosine kinase inhibitors which target some of these pathways, such as sunitinib and sorafenib (both of whose spectrum covers some receptors for both VEGF and PDGF), have already been FDA approved as therapy for advanced sporadic clear cell renal cancer. Similarly, use of a pure VEGF antagonist alone has been evaluated in clear cell renal cancer. Yang et al. found in a randomized double-blind, placebo-controlled phase 2 trial in patients with advanced clear cell RCC that progression-free survival improved from treatment with bevacizumab, an anti-VEGF antibody(37). Other single agents and combinations of agents which block multiple downstream targets of the HIF pathway are under investigation. It is logical that this approach of targeted therapy used for sporadic clear cell RCC would also be efficacious for VHL clear cell renal tumors, for it could potentially overcome the effects from loss of VHL function. Clinical trials specifically in VHL patients with renal tumors using agents targeting VEGF and other HIF downstream products are currently underway.
3.2 Hereditary Papillary Renal Cell Carcinoma
3.2.1 Hereditary Papillary Renal Cell Carcinoma (HPRC)
Insight into the molecular pathways of VHL disease led the way to investigation of mechanisms underlying other forms of inherited kidney cancer associated with non-clear cell tumors. In 1994, a novel inherited kidney cancer syndrome was described by Zbar et al. called Hereditary Papillary Renal Cell Carcinoma (HPRC), in which affected individuals are at risk of developing bilateral, multifocal papillary type 1 renal cancer(38). Although HPRC is a highly penetrant, rare autosomal dominant syndrome, the papillary type 1 renal carcinomas are usually of late onset (fifth or sixth decade), but they can occur as early as the third decade(39,40,41,42). These patients are at risk of developing up to an estimated 3,000 tumors per kidney, and pathology usually demonstrates multiple incipient lesions alongside larger lesions(43).
Unlike the other familial renal cancer syndromes described, the kidney is the only organ known to be involved in HPRC. Lesions are usually well-differentiated, however, the tumors do have metastatic potential. Detection of lesions can be difficult, since they often are undetectable by ultrasonography, are can be small, with poor enhancement on IV contrast CT. However, CT is the preferred screening tool since it has greater sensitivity for detecting these small hypovascular lesions(44). Due to the multi-focal bilateral nature of these lesions, HPRC patients are often managed by surveillance and close observation with nephron-sparing surgery when the largest tumor reaches 3 cm (43,13,14,15,17,16).
3.2.2 MET Gene and Pathway
Genetic linkage analysis in 1997 led to the identification of the proto-oncogene MET (Mesenchymal Epithelial Transition factor), which is located on the long arm of chromosome 7, as the gene responsible for HPRC(45,46,38). Activating gain-of-function mutations of MET are present in both the germline of HPRC patients, and can also be mutated in a small subset of sporadic papillary type 1 renal carcinoma tumors(47,46). Additionally, trisomy of chromosome 7, which contains the genes for both MET and its ligand, hepatocyte growth factor (HGF), has been found to occur in 75% of sporadic papillary renal carcinomas(48). Trisomy 7 has also been found in HPRC-associated kidney cancers with a non-random duplication of the mutant MET allele.(49)
MET encodes for a tyrosine kinase membrane receptor. The ligand HGF activates the tyrosine kinase domain of the MET receptor, which initiates signaling cascades leading to multiple biologic events, such as proliferation, survival, motility, and morphogenesis of many different cell types(50). Activating gain-of-function mutations in the tyrosine kinase domain of the MET gene are believed to be responsible for tumorigenesis. The constitutively active tyrosine kinase allows for unregulated proliferation, transformation, and invasive potential(45,51). Hypoxia, through HIF-1, has also been shown to result in upregulation of both MET and VEGF, thus activating mutations of MET can indirectly promote angiogenesis and tumor growth through overexpression of VEGF(52,53).
3.2.3 Target for Therapeutics
Considering that MET and HGF are important genes in papillary type 1 renal cancer, both in HPRC and in sporadic cases, they also provide a potential pathway for novel therapeutic agents to target. Inhibition of MET tyrosine kinase activity, antagonism of the HGF-MET ligand/receptor interaction, and blockade of receptor/effector and downstream intracellular signaling effects are all potential therapeutic targets(54). Interest from the biotechnology industry has intensified in the past several years with development of multiple agents targeting these mechanisms. There are more than a half-dozen protein kinase inhibitors that block MET induced pathways currently in development. GSK1363089 (formerly XL880), an oral small molecule inhibitor of multiple receptor tyrosine kinases, primarily targeting MET and VEGF, is one such agent that holds promise. In a Phase I clinical trial with GSK1363089, 3 of the 5 partial responses (n=55) were in patients with papillary renal cancer, with duration of response being 5, 10+ and 23+ months(55). There are also several agents which are truncated variants of c-MET that act as decoys. Another approach is to use anti-HGF monoclonal antibodies to block the binding of HGF to its receptor MET. There are several in development, one of which is the monoclonal antibody against human HGF, AMG102. Both AMG102 and GSK1363089 are currently in Phase II clinical trials in renal cancer.
3.3 Birt-Hogg-Dubé Syndrome
3.3.1 Birt-Hogg-Dubé (BHD)
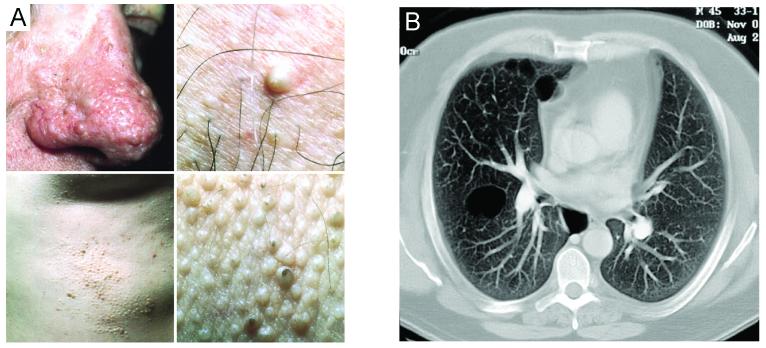
BHD is a rare autosomal dominant familial syndrome occurring in about 1/200,000 people(56). BHD Syndrome was described by Birt et al. in 1977 and initially characterized by its cutaneous manifestations (57). A BHD patient with renal cancer was initially reported in 1993 by Roth et al., and the association of multiple renal tumors in BHD patients noted by Toro et al. in 1999(58,59). The statistical association of renal tumors and pulmonary manifestations was established by Zbar et al. in 2002(60).It is now clear that BHD syndrome confers a susceptibility in affected patients to renal tumors of varying histologic subtypes, characteristic benign tumors of the hair follicle (fibrofolliculomas), and lung pulmonary cysts, which can manifest as spontaneous pneumothoraces(57,60). Fibrofolliculomas are highly penetrant (about 85% of patients develop skin lesions), develop after puberty (usually after the age of 25), and most often have a distribution on the face, neck, and anterior trunk. Although they can be a cosmetic concern to some patients, they are not malignant. Pulmonary cysts are a common manifestation of BHD, developing in over 80% of BHD affected patients, with nearly 25% having a history of pneumothorax(60,61). (Figure 3)(59,3).
Figure 3.
Non-renal manifestations of BHD: (A-D) Multiple fibrofolliculomas and (E) multiple pulmonary cysts. (Adapted from LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172. and TORO JR, GLENN G, DURAY P et al.: Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Archives of dermatology (1999) 135(10):1195-1202.)[2,55]
Renal tumors occur in 25-35% of BHD patients(60,59,62). Subsequent genotype/phenotype analysis by Schmidt et al. suggests that germline deletion of cytosine at exon 11 in the BHD gene may correlate with fewer renal tumors in BHD patients than those patients with insertion of cytosine at the same hotspot mutation(63). Renal lesions can be solitary or bilateral and multifocal, and can have variable histology within the same patient or even within the same kidney. Histology is most often characteristic oncocytic-hybrid (multifocal hybrid chromophobe-oncocytoma renal carcinoma) (50%), followed by chromophobe renal cancer (33%), clear cell renal cancer (9%), and benign oncocytomas (4%)(62). Due to the potential for repeated surgical procedures of multifocal and recurrent renal lesions, surveillance and close observation with nephron-sparing surgery when the largest tumor reaches 3cm is often recommended(16,17,64).
3.3.2 BHD Gene
The mutation most frequently found in the germline of BHD patients has been a truncating mutation of the BHD gene (also known as the FLCN gene)(63). The BHD gene, which has the characteristics of a tumor suppressor, was localized to the short arm of chromosome 17 (17p11.2) and encodes for a novel protein, folliculin(65,66,67). Although folliculin has no characteristic domains suggestive of function, coimmunoprecipitation studies by Baba et al. recently identified an interacting/binding protein, folliculin-interacting protein 1 (FNIP1)(68). Baba et al. found that FNIP1 facilitates the phosphorylation of folliculin. FNIP1 was also found to interact with 5’ AMP-activated protein kinase (AMPK), an important protein in energy sensing and a negative regulator of mTOR. Blockade of AMPK or mTOR was found to inhibit folliculin phosphorylation facilitated by FNIP1. This suggested that folliculin may be a downstream effector of the AMPK and mTOR signaling pathways(68).
3.3.3 Target for Therapeutics
The presumed downstream effects on folliculin by mTOR suggest a therapeutic option for BHD patients with the use of agents such as mTOR inhibitors. Although potential advances in treatment for BHD patients are possible through this mechanism of mTOR inhibition, this has yet to be examined in clinical trials.
3.4 Hereditary Leiomyomatosis Renal Cell Cancer
3.4.1 Hereditary Leiomyomatosis Renal Cell Cancer (HLRCC)
As with BHD, discovery of a predisposition to renal cancer in HLRCC came later than the original description of the syndrome. Reed Syndrome, or Multiple Cutaneous and Uterine Leiomyomatosis (MCUL) Syndrome, was described 30 years prior to the association to renal cancer, first noted in 2001 by Launonen et al.(69,70). Renal cancer has low penetrance in HLRCC syndrome, with an estimated incidence from 2%-6% to 15%, and possibly as high as 32% of germline mutation affected families, depending upon selection bias and imaging utilized(69,71,72,73).
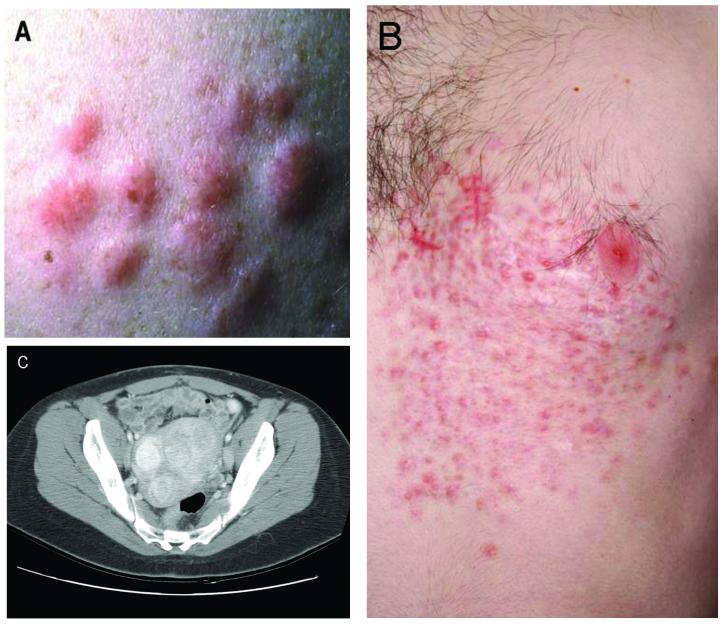
Individuals affected with HLRCC are at risk for renal carcinoma as well as cutaneous and uterine leiomyomas(69). Female HLRCC patients frequently report a history of having had large and multiple uterine fibroids at a young age, often requiring hysterectomy in their 20’s or early 30’s. Although the penetrance of uterine leiomyomas is very high (Toro et al. found >98 % of female HLRCC patients with cutaneous leiomyomas also had uterine fibroids), it is felt that the risk of uterine leiomyosarcoma is very low(69,72,71,73). Multiple cutaneous leiomyomas most often occur on the trunk or extremities, and are typically grouped, disseminated, or disseminated and segmental(73). (Figure 4)(72,3). These cutaneous lesions usually begin to appear in the third decade, and can be painful. Wei et al. found that 81% of HLRCC patients had cutaneous lesions, and of those that had cutaneous leiomyomas, 90% had sensitivity to light touch of the lesions(73).
Figure 4.
Non-renal clinical manifestations of HLRCC: (A,B) cutaneous leiomyomas in a grouped and segmental distribution, (C) multiple large uterine fibroids in a female patient. (Adapted from TORO JR, NICKERSON ML, WEI MH et al.: Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. American journal of human genetics (2003) 73(1):95-106 and LINEHAN WM, WALTHER MM, ZBAR B: The genetic basis of cancer of the kidney. The Journal of urology (2003) 170(6 Pt 1):2163-2172.)[2,69]
HLRCC renal lesions appear to have a different biology than other renal tumor types. Unlike other hereditary renal carcinoma syndromes, HLRCC renal cancer usually presents as a solitary lesion, can be highly aggressive and can metastasize early, often initially to regional and distant lymph nodes. HLRCC renal malignancies may have an early onset, with the youngest published metastatic patient reported at age 16, although there may be cases of even earlier onset(71). These patients also develop renal cysts. Lehtonen et al. found 42% of mutation positive patients had renal cysts on radiologic review(74). Further studies will be needed to detect the potential of these cysts to develop into renal tumors. Since papillary renal tumors can be isoechoic on ultrasound and can be missed, CT and MRI are the recommended imaging modalities(44). Considering the aggressive nature of these tumors, these patients should receive regular surveillance with early surgical intervention of solid lesions instead of observation.
Histopathologic diagnosis of HLRCC renal tumors has become more precise, with a distinctive architectural pattern described. The histopathology of HLRCC tumors has a characteristic morphology, demonstrating hallmark large orangiophilic nuclei and a clear perinuclear halo, with a variety of patterns such as cystic, tubulo-papillary, tubulo-solid, and are often mixed(69,75,76).
3.4.2 FH Gene and Pathway
Patients with HLRCC have a germline mutation localized to the long arm of chromosome 1 (1q42.3-q43)(69,77,78). The gene responsible for HLRCC was identified through linkage analysis, and found to encode for fumarate hydratase (FH), a Krebs cycle enzyme(72,79,80). Complete germline inactivation of FH through homozygous or compound heterozygous mutations produce an inborn error of metabolism, leading to an autosomal recessive disease, Fumarate Hydratase Deficiency, consisting of encephalopathy, neurologic impairment, and death within the first decade(81). The FH gene appears to act as a tumor suppressor gene, following Knudson’s “two hit” model of carcinogenesis(82,78). Functional inactivation of the remaining second copy has been found in most HLRCC papillary type-2 renal tumors, cutaneous leiomyomas, and uterine leiomyomas(78,80,79). Correspondingly, tumors in HLRCC patients have been found to have extremely low or absent FH activity(80). Germline mutations are generally due to missense, frameshift, nonsense, or splice site mutations, and are distributed throughout the gene(73). Sequencing has detected a germline mutation detection rate of 93%(73). However, no definitive genotype-phenotype association of location/type of mutation and risk of renal cancer has been noted to date in HLRCC(73). There is currently no conclusive evidence that somatic mutations of FH play a significant role in sporadic kidney cancer tumorigenesis(83,84).
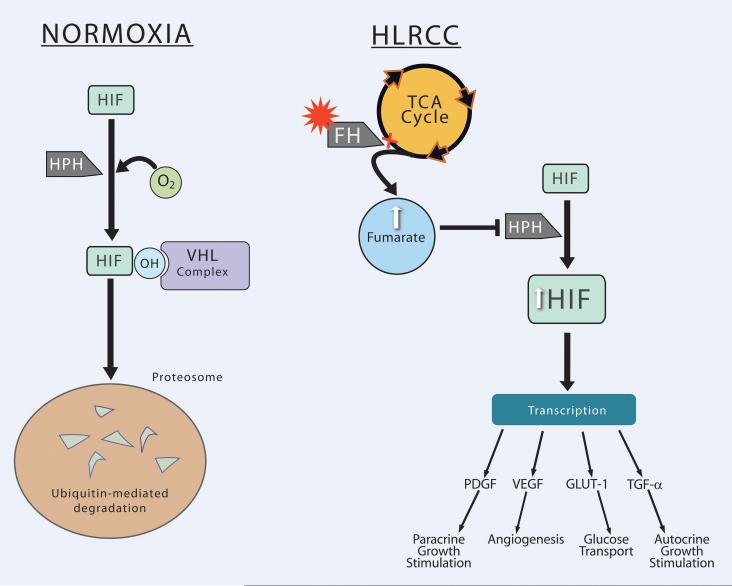
FH is an essential enzyme catalyzing the conversion of fumarate to malate in the mitochondrial tricarboxylic acid (TCA) cycle leading to the oxidation of pyruvate for the production of cellular energy. Loss of FH shunts the cycle to overaccumulate intracellular fumarate, leading to a state of pseudohypoxia through overexpression of HIF. Overexpression of HIF and upregulation of HIF-regulated genes is postulated to be the mechanism by which a mutation in FH leads to renal malignancy(85,86,87). Isaacs et al. found that inhibition of FH and excess intracellular fumarate competitively inhibit HIF propyl hydroxylase (HPH) function, which alters hydroxylation of HIF and subsequent recognition of HIF by the VHL complex, thus preventing VHL-dependent proteosomal degradation of HIFs(85). Similarly, Pollard et al. demonstrated strong HIF-1 expression in HLRCC renal tumors(87). The resulting accumulation of HIF was also shown to increase the HIF-regulated transcripts of downstream target genes VEGF and Glut-1 in HLRCC tumors(85,86). (Figure 5).
Figure 5.
Normoxia: In the presence of oxygen (as a substrate), HIF is hydroxylated by HPH, which allows the VHL complex to recognize and target it for ubiquitin-mediated degradation in the proteosome. HLRCC: Loss of FH shunts the TCA cycle to produce excess fumarate. Fumarate stabilizes HIF through competitive inhibition of HPH, allowing HIF to remain unhydroxylated and thus avoiding degredation. Elevated HIF drives transcription products involved with angiogenesis (VEGF), glucose transport (GLUT-1), and growth stimulation (TGF-α, PDGF).
3.4.3 Target for Therapeutics
The hypothesized mechanism of FH mutations leading to tumorigenesis through pseudohypoxia and angiogenic pathways suggests an approach to treating HLRCC renal cancer targeting HIF-regulated factors, such as VEGF. As in VHL, there are several currently available therapeutic options facilitating this tactic. One such agent is the anti-VEGF monoclonal antibody bevacizumab. Similarly, other agents such as sorafenib and sunitinib, a combination of agents, or multi-targeted agents currently under investigation against different downstream products of the HIF pathway may also be potential therapeutic options. However, to date, there are no clinical trials supporting this approach.
3.5 Familial Renal Cancer of Undetermined Genetic Significance
Diagnostic DNA testing is available for the four types of inherited renal cancer, and presence of a germline mutation helps to determine whether the patient and their family members may be at risk for developing renal carcinoma. However, there are limitations to the sensitivity of the mutation analysis assays, especially when the mutation is due to a partial or complete deletion. There are also families with presumed familial renal cancer (FRC) who are apparently not affected with one of the four characterized syndromes described above. The known hereditary kidney cancer syndromes VHL, BHD, HPRC and HLRCC are each characterized by an autosomal dominant inheritance pattern, and each appears to have a high penetrance where affected individuals are likely to develop a clinical manifestation of the disease; however, there is a possibility that seemingly sporadic cases may also have an inherited basis from other not-yet identified FRC syndromes with low penetrance. Gudbjartsson et al. studied records of kidney cancer patients in Iceland spanning 44 years, and found that 58% of patients had a relative (as distant as second cousin in some cases) who also had kidney cancer, suggesting the existence of a low penetrance kidney cancer susceptibility gene(88). Future study of these families and genetic linkage analysis will hopefully elucidate the genetic basis of these forms of familial renal cancer.
CONCLUSION
Kidney cancer is not a single disease; it is comprised of multiple different histopathologies, genetic alterations, molecular pathways, clinical manifestations, and treatment options. Studies of inherited renal cancer have led to a better understanding of the mechanisms underlying sporadic kidney cancer, and holds promise for better therapies for treatment of all types of renal cancer. Insights into the molecular pathways underlying carcinogenesis in familial kidney cancer syndromes provide rationales for new molecular therapeutic approaches.
EXPERT OPINION
The lack of promising treatment options for the majority of RCC patients has led to intensive study into the mechanisms and genes underlying the array of kidney cancer types, as well as development of novel agents to target them. Therapies able to affect specific aberrant cellular pathways, while potentially causing minimal damage to normal cells, has been the focus of research over the past two decades in multiple cancer types, including renal cancer.
One approach to targeted therapy in RCC has been to focus on effectors of the HIF pathway. (Figure 6). HIF is implicated in the molecular pathways leading to tumorigenesis in most sporadic clear cell RCC, as well as directly and possibly indirectly in all four hereditary kidney cancer syndromes. Thus targeting the HIF pathway and its downstream products makes this appear to be a promising approach for many different kidney cancer tumor types.
Although there have been advancements in treatment of RCC with single agents such as bevacizumab, sorafenib, sunitinib, and newly approved temsirolimus, these agents have only shown to have only modest, if any, improvement in overall survival in advanced RCC. Currently underway are also randomized placebo-controlled trials using sorafenib or sunitinib in the adjuvant setting, since prior studies with adjuvant cytokine therapy have not demonstrated benefit. Multifaceted treatment by inhibiting parallel pathways (horizontal blockade) or maximally inhibiting a single pathway at different levels (vertical blockade), thus overcoming potential redundancy of growth-factor signaling cascades and escape to alternate angiogenic pathways, may lead to additional benefit in the future, as more insight into the mechanisms underlying the different types of RCC is gained. However, although there is excellent rationale and preclinical data for using combinations of agents or broad-spectrum agents targeting multiple different pathways, no combination regimen has yet produced mature data to prove safety and increased efficacy in randomized trials.
Clinical research trials in RCC with a horizontal blockade approach, using agents or combinations which target some, but not all, downstream products of HIF, have to date not shown benefit over blocking one endproduct alone, such as VEGF. A randomized phase 2 comparison by Bukowski et al. in clear cell RCC of the anti-VEGF antibody bevacizumab with or without the EGFR tyrosine kinase inhibitor erlotinib found progression free survival rates, response rates, and overall survival unchanged with the addition of erlotinib compared to bevacizumab alone, and greater toxicity with the combination(89). The rationale was that both VEGF and TGF- α (an activating ligand for EGFR) are downstream effectors of HIF, and this combination can affect several endproducts resulting from loss of VHL. However, it did not appear that addition of an EGFR inhibitor to a VEGF inhibitor, at least in this combination, was of significant benefit. Agents such as sunitinib and sorafenib, which block both VEGF and PDGF, have not yet been compared directly in a randomized trial in RCC to bevacizumab, which blocks VEGF alone.
Currently being studied are several combinations of targeted agents using a more vertical blockade approach, often combining a VEGF ± PDGF inhibitor with an mTOR inhibitor. One such study is a phase I/II trial by Merchan et al. investigating the combination of bevacizumab plus temsirolimus. Full doses of both agents could be given with acceptable toxicity, and initial phase I data is encouraging: 8 patients with partial responses and 3 patients with stable disease of 12 evaluable patients, with median number of 6 months treated(90). Nevertheless, these are very small cohorts and there are currently no randomized cohorts validating this approach.
Although drug development of MET inhibitors lags behind inhibitors of VEGF, PDGF, EGFR, and mTOR, horizontal blockade could hypothetically be enhanced through the addition of a MET inhibitor to other agents as well, especially in patients with papillary type 1 renal cancer. Regardless of approach, the future of treatment of all forms of RCC, both hereditary and sporadic, is encouraging. Further research into the pathways underlying the different forms of RCC and hereditary renal cancer syndromes will help direct the use of new agents and combinations that may prove to be of greater benefit than currently available options for RCC.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflicts of interest:
The authors have no conflicts of interest to declare.
Contributor Information
Elizabeth Cartwright Pfaffenroth, Medical Oncology, Urologic Oncology Branch National Cancer Institute 10 Center Drive MSC 1107 BLDG 10 CRC Room 1-5942 Bethesda, Maryland 20892-1107 Tel: 301-451-8135 Fax: (301) 402-0922 e-mail: cartwrie@mail.nih.gov
W. Marston Linehan, Chief, Urologic Oncology Branch National Cancer Institute 10 Center Drive MSC 1107 BLDG 10 CRC Room 1-5942 Bethesda, Maryland 20892-1107 Tel: (301) 496-6353 Fax: (301) 402-0922 e-mail: WML@nih.gov
Annotated Bibliography
- 1.JEMAL A, SIEGEL R, WARD E, MURRAY T, XU J, SMIGAL C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.YANG JC, SHERRY RM, STEINBERG SM, TOPALIAN SL, SCHWARTZENTRUBER DJ, HWU P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. Journal of Clinical Oncology. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LINEHAN WM, WALTHER MM, ZBAR B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–2172. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 4.GNARRA JR, TORY K, WENG Y, SCHMIDT L, WEI MH, LI H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Gen. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 5.SHUIN T, KONDO K, TORIGOE S, KISHIDA T, KUBOTA Y, HOSAKA M, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–2855. [PubMed] [Google Scholar]
- 6.HERMAN JG, LATIF F, WENG Y, LERMAN MI, ZBAR B, LIU S, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proceedings of the National Academy of Sciences USA. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. * Establishes VHL is inactivated in the majority of sporadic clear cell RCC
- 7.YAO M, YOSHIDA M, KISHIDA T, NAKAIGAWA N, BABA M, KOBAYASHI K, et al. VHL Tumor Suppressor Gene Alterations Associated With Good Prognosis in Sporadic Clear-Cell Renal Carcinoma. J Natl Cancer Inst. 2002;94:1569–1575. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 8.MAHER ER, ISELIUS L, YATES JR, LITTLER M, BENJAMIN C, HARRIS R, et al. Von Hippel-Lindau disease: a genetic study. Journal of Medical Genetics. 1991;28:443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LONSER RR, GLENN GM, WALTHER MM, CHEW EY, LIBUTTI SK, LINEHAN WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. * Excellent review of VHL
- 10.MAHER ER, YATES JR, HARRIES R, BENJAMIN C, HARRIS R, MOORE AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 11.POSTON CD, JAFFE GS, LUBENSKY IA, SOLOMON D, ZBAR B, LINEHAN WM, et al. Characterization of the renal pathology of a familial form of renal cell carcinoma associated with von Hippel-Lindau disease: clinical and molecular genetic implications. J Urol. 1995;153:22–26. doi: 10.1097/00005392-199501000-00009. [DOI] [PubMed] [Google Scholar]
- 12.WALTHER MM, LUBENSKY IA, VENZON D, ZBAR B, LINEHAN WM. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel-Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. J Urol. 1995;154:2010–2014. [PubMed] [Google Scholar]
- 13.WALTHER MM, CHOYKE PL, WEISS G, MANOLATOS C, LONG J, REITER R, et al. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma. J Urol. 1995;153:913–916. [PubMed] [Google Scholar]
- 14.WALTHER MM, THOMPSON N, LINEHAN WM. Enucleation procedures in patients with multiple hereditary renal tumors. World J Urol. 1995;13:248–250. doi: 10.1007/BF00182972. [DOI] [PubMed] [Google Scholar]
- 15.JAMIS-DOW CA, CHOYKE PL, JENNINGS SB, LINEHAN WM, THAKORE KN, WALTHER MM. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996;198:785–788. doi: 10.1148/radiology.198.3.8628872. [DOI] [PubMed] [Google Scholar]
- 16.HERRING JC, ENQUIST EG, CHERNOFF A, LINEHAN WM, CHOYKE PL, WALTHER MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001;165:777–781. * Establishes the role of partial nephrectomy in hereditary kidney cancer syndromes with multiple recurrent lesions ≥3cm
- 17.CHOYKE PL, PAVLOVICH CP, DARYANANI KD, HEWITT SM, LINEHAN WM, WALTHER MM. Intraoperative ultrasound during renal parenchymal sparing surgery for hereditary renal cancers: a 10-year experience. J Urol. 2001;165:397–400. doi: 10.1097/00005392-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 18.LATIF F, TORY K, GNARRA JR, YAO M, DUH F-M, ORCUTT ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. * Initial discovery of the VHL gene
- 19.STOLLE C, GLENN GM, ZBAR B, HUMPHREY JS, CHOYKE P, WALTHER MM, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12:417–423. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.NEUMANN HP, BENDER BU. Genotype-phenotype correlations in von Hippel-Lindau disease. J Intern Med. 1998;243:541–545. doi: 10.1046/j.1365-2796.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 21.ZBAR B, KLAUSNER R, LINEHAN WM. Studying cancer families to identify kidney cancer genes. Annu Rev Med. 2003;54:217–233. doi: 10.1146/annurev.med.54.101601.152514. * Excellent review of genes leading to RCC
- 22.CHEN F, KISHIDA T, YAO M, HUSTAD T, GLAVAC D, DEAN M, et al. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat. 1995;5:66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- 23.FRIEDRICH CA. Genotype-phenotype correlation in von Hippel-Lindau syndrome. Hum Mol Genet. 2001;10:763–767. doi: 10.1093/hmg/10.7.763. [DOI] [PubMed] [Google Scholar]
- 24.MARANCHIE JK, AFONSO A, ALBERT PS, KALYANDRUG S, PHILLIPS JL, ZHOU S, et al. Solid renal tumor severity in von Hippel Lindau disease is related to germline deletion length and location. Hum Mutat. 2004;23:40–46. doi: 10.1002/humu.10302. [DOI] [PubMed] [Google Scholar]
- 25.GALLOU C, CHAUVEAU D, RICHARD S, JOLY D, GIRAUD S, OLSCHWANG S, et al. Genotype-phenotype correlation in von Hippel-Lindau families with renal lesions. Hum Mutat. 2004;24:215–224. doi: 10.1002/humu.20082. [DOI] [PubMed] [Google Scholar]
- 26.SIMS KB. Von Hippel-Lindau disease: gene to bedside. Curr Opin Neurol. 2001;14:695–703. doi: 10.1097/00019052-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 27.DUAN DR, PAUSE A, BURGESS WH, ASO T, CHEN DY, GARRETT KP, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269:1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 28.KIBEL A, ILIOPOULOS O, DECAPRIO JA, KAELIN WG., JR. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 29.PAUSE A, LEE S, WORRELL RA, CHEN DY, BURGESS WH, LINEHAN WM, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proceedings of the National Academy of Sciences USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LONERGAN KM, ILIOPOULOS O, OHH M, KAMURA T, CONAWAY RC, CONAWAY JW, et al. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KONDO K, KICO J, NAKAMURA E, LECHPAMMER M, KAELIN W. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 32.STEBBINS CE, KAELIN WG, JR., PAVLETICH NP. Structure of the VHL-ElonginCElonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–461. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- 33.OHH M, PARK CW, IVAN M, HOFFMAN MA, KIM TY, HUANG LE, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 34.TYERS M, ROTTAPEL R. VHL: a very hip ligase. Proceedings of the National Academy of Sciences USA. 1999;96:12230–12232. doi: 10.1073/pnas.96.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SEAGROVES T, JOHNSON RS. Two HIFs may be better than one. Cancer Cell. 2002;1:211–213. doi: 10.1016/s1535-6108(02)00048-x. [DOI] [PubMed] [Google Scholar]
- 36.LINEHAN WM, ZBAR B. Focus on kidney cancer. Cancer Cell. 2004;6:223–228. doi: 10.1016/j.ccr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 37.YANG JC, HAWORTH L, SHERRY RM, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ZBAR B, TORY K, MERINO MJ, SCHMIDT L, GLENN GM, CHOYKE P, et al. Hereditary papillary renal cell carcinoma. J Urol. 1994;151:561–566. doi: 10.1016/s0022-5347(17)35015-2. ** Original description of HPRC
- 39.LUBENSKY IA, SCHMIDT L, ZHUANG Z, WEIRICH G, PACK S, ZAMBRANO N, et al. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol. 1999;155:517–526. doi: 10.1016/S0002-9440(10)65147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SCHMIDT LS, NICKERSON ML, ANGELONI D, GLENN GM, WALTHER MM, ALBERT PS, et al. Early onset Hereditary Papillary Renal Carcinoma: germline missense mutations in the tyrosine kinase domain of the Met proto-oncogene. J Urol. 2004;172:1256–1261. doi: 10.1097/01.ju.0000139583.63354.e0. [DOI] [PubMed] [Google Scholar]
- 41.ZBAR B, LINEHAN WM. Hereditary papillary renal cell carcinoma: clinical studies in 10 families. J Urol. 1996;156:1781. doi: 10.1016/s0022-5347(01)65520-4. [DOI] [PubMed] [Google Scholar]
- 42.ZBAR B. von Hippel-Lindau disease and sporadic renal cell carcinoma. Cancer Surv. 1995;25:219–232. [PubMed] [Google Scholar]
- 43.ORNSTEIN DK, LUBENSKY IA, VENZON D, ZBAR B, LINEHAN WM, WALTHER MM. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. J Urol. 2000;163:431–433. [PubMed] [Google Scholar]
- 44.CHOYKE PL, WALTHER MM, GLENN GM, WAGNER JR, VENZON DJ, LUBENSKY IA, et al. Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr. 1997;21:737–741. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 45.SCHMIDT L, DUH FM, CHEN F, KISHIDA T, GLENN GM, CHOYKE P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Gen. 1997;16:68–73. doi: 10.1038/ng0597-68. * Original description linking MET mutation to HPRC
- 46.SCHMIDT L, JUNKER K, WEIRICH G, GLENN G, CHOYKE P, LUBENSKY I, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998;58:1719–1722. [PubMed] [Google Scholar]
- 47.SCHMIDT L, JUNKER K, NAKAIGAWA N, KINJERSKI T, WEIRICH G, MILLER M, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 48.KOVACS G. Molecular cytogenetics of renal cell tumors. Adv Cancer Res. 1993;62:89–124. doi: 10.1016/s0065-230x(08)60316-4. [DOI] [PubMed] [Google Scholar]
- 49.ZHUANG Z, PARK WS, PACK S, SCHMIDT L, PAK E, PHAM T, et al. Trisomy 7 - harboring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Gen. 1998;20:66–69. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]
- 50.MICHALOPOULOS GK, DEFRANCES MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 51.JEFFERS M, SCHMIDT L, NAKAIGAWA N, WEBB CP, WEIRICH G, KISHIDA T, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proceedings of the National Academy of Sciences USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.PENNACCHIETTI S, MICHIELI P, GALLUZZO M, MAZZONE M, GIORDANO S, COMOGLIO PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 53.BOTTARO DP, LIOTTA LA. Cancer: Out of air is not out of action. Nature. 2003;423:593–595. doi: 10.1038/423593a. [DOI] [PubMed] [Google Scholar]
- 54.PERUZZI B, BOTTARO DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–3660. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 55.EDER JP, APPLEMAN L, HEATH E, MALBURG L, ZHU AX, LAEDER T, et al. Phase I experience with c-MET inhibitor XL880 administered orally to patients (pts) with solid tumors. ASCO Annual Meeting Proceedings. 2007;25:3526. [Google Scholar]
- 56.Atlas of Genetics and Cytogenetics in Oncology and Haematology: Birt-Hogg-Dubé Syndrome (BHD) Atlas Genet Cytogenet Oncol Haematol. 2006 [Google Scholar]
- 57.BIRT AR, HOGG GR, DUBE WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 58.ROTH JS, RABINOWITZ AD, BENSON M, GROSSMAN ME. Bilateral renal cell carcinoma in the Birt-Hogg-Dube syndrome. J Am Acad Dermatol. 1993;29:1055–1056. doi: 10.1016/s0190-9622(08)82049-x. [DOI] [PubMed] [Google Scholar]
- 59.TORO JR, GLENN G, DURAY P, DARLING T, WEIRICH G, ZBAR B, et al. Birt-Hogg-Dube syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195–1202. doi: 10.1001/archderm.135.10.1195. ** Important article confirming link of renal malignancies to BHD
- 60.ZBAR B, ALVORD WG, GLENN GM, TURNER M, PAVLOVICH CP, SCHMIDT L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 61.TORO JR, PAUTLER SE, STEWART L, GLENN GM, WEINREICH M, TOURE O, et al. Lung Cysts, Spontaneous Pneumothrorax and Genetic Associations in 89 Families with Birt-Hogg-Dube Syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.PAVLOVICH CP, WALTHER MM, EYLER RA, HEWITT SM, ZBAR B, LINEHAN WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 63.SCHMIDT LS, NICKERSON ML, WARREN MB, GLENN GM, TORO JR, MERINO MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.PAVLOVICH CP, GRUBB RL, HURLEY K, GLENN GM, TORO J, SCHMIDT LS, et al. Evaluation and Management of Renal Tumors in the Birt-Hogg-Dube Syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 65.NICKERSON ML, WARREN MB, TORO JR, MATROSOVA V, GLENN GM, TURNER ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. *Original description of BHD gene encoding for folliculin
- 66.SCHMIDT LS, WARREN MB, NICKERSON ML, WEIRICH G, MATROSOVA V, TORO JR, et al. Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 2001;69:876–882. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.KHOO SK, BRADLEY M, WONG FK, HEDBLAD MA, NORDENSKJOLD M, TEH BT. Birt-Hogg-Dube syndrome: mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2. Oncogene. 2001;20:5239–5242. doi: 10.1038/sj.onc.1204703. [DOI] [PubMed] [Google Scholar]
- 68.BABA M, HONG SB, SHARMA N, WARREN MB, NICKERSON ML, IWAMATSU A, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proceedings of the National Academy of Sciences USA. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103.*Initial description linking BHD gene to the mTOR pathway
- 69.LAUNONEN V, VIERIMAA O, KIURU M, ISOLA J, ROTH S, PUKKALA E, et al. Inherited Susceptibility to uterine leiomyomas and renal cell cancer. Proceedings of the National Academy of Sciences USA. 2001;98:3387–3382. doi: 10.1073/pnas.051633798. ** Initial description of HLRCC, linking renal cancer to MCUL (Reed’s Syndrome)
- 70.REED WB, WALKER R, HOROWITZ R. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53:409–416. [PubMed] [Google Scholar]
- 71.ALAM NA, OLPIN S, LEIGH IM. Fumarate hydratase mutations and predisposition to cutaneous leiomyomas, uterine leiomyomas and renal cancer. Br J Dermatol. 2005;153:11–17. doi: 10.1111/j.1365-2133.2005.06678.x. [DOI] [PubMed] [Google Scholar]
- 72.TORO JR, NICKERSON ML, WEI MH, WARREN MB, GLENN GM, TURNER ML, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WEI MH, TOURE O, GLENN GM, PITHUKPAKORN M, NECKERS L, STOLLE C, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. Journal of Medical Genetics. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LEHTONEN HJ, KIURU M, YLISAUKKO-OJA SK, SALOVAARA R, HERVA R, KOIVISTO PA, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523–526. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MERINO MJ, TORRES-CABALA CA, ZBAR B, CHIAN-GARCIA CA, LINEHAN WM. Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome (HLRCC); Clinical, Histopathological and Molecular Features of the First American Families Described. Mod Pathol. 2003;16:739. [Google Scholar]
- 76.MERINO MJ, TORRES-CABALA C, PINTO P, LINEHAN WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 77.ALAM NA, BEVAN S, CHURCHMAN M, BARCLAY E, BARKER K, JAEGER EE, et al. Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. Am J Hum Genet. 2001;68:1264–1269. doi: 10.1086/320124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.KIURU M, LAUNONEN V, HIETALA M, AITTOMAKI K, VIERIMAA O, SALOVAARA R, et al. Familial cutaneous leiomyomatosis is a two-hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol. 2001;159:825–829. doi: 10.1016/S0002-9440(10)61757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.TOMLINSON IP, ALAM NA, ROWAN AJ, BARCLAY E, JAEGER EE, KELSELL D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Gen. 2002;30:406–410. doi: 10.1038/ng849. * Original description of FH mutation involved with HLRCC
- 80.ALAM NA, ROWAN AJ, WORTHAM NC, POLLARD PJ, MITCHELL M, TYRER JP, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 81.GELLERA C, UZIEL G, RIMOLDI M, ZEVIANI M, LAVERDA A, CARRARA F, et al. Fumarase deficiency is an autosomal recessive encephalopathy affecting both the mitochondrial and the cytosolic enzymes. Neurology. 1990;40:495–499. doi: 10.1212/wnl.40.3_part_1.495. [DOI] [PubMed] [Google Scholar]
- 82.KNUDSON AG., JR. Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.KIURU M, LEHTONEN R, AROLA J, SALOVAARA R, JARVINEN H, AITTOMAKI K, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–4557. [PubMed] [Google Scholar]
- 84.MORRIS MR, MAINA E, MORGAN NV, GENTLE D, ASTUTI D, MOCH H, et al. Molecular genetic analysis of FIH-1, FH, and SDHB candidate tumour suppressor genes in renal cell carcinoma. J Clin Pathol. 2004;57:706–711. doi: 10.1136/jcp.2003.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ISAACS JS, JUNG YJ, MOLE DR, LEE S, TORRES-CABALA C, CHUNG YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. * Important study describing the role of FH in regulation of HIF
- 86.POLLARD P, WORTHAM N, BARCLAY E, ALAM A, ELIA G, MANEK S, et al. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2005;205:41–49. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 87.POLLARD PJ, BRIERE JJ, ALAM NA, BARWELL J, BARCLAY E, WORTHAM NC, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 88.GUDBJARTSSON T, JONASDOTTIR TJ, THORODDSEN A, EINARSSON GV, JONSDOTTIR GM, KRISTJANSSON K, et al. A population-based familial aggregation analysis indicates genetic contribution in a majority of renal cell carcinomas. Int J Cancer. 2002;100:476–479. doi: 10.1002/ijc.10513. [DOI] [PubMed] [Google Scholar]
- 89.BUKOWSKI RM, KABBINAVAR F, FIGLIN RA, FLAHERTY K, SRINIVAS S, VAISHAMPAYAN U, et al. Bevacizumab with or without erlotinib in metastatic renal cell carcinoma (RCC) Journal of Clinical Oncology (Meeting Abstracts) 2006;24:4523. [Google Scholar]
- 90.MERCHAN JR, LIU G, FITCH T, PICUS J, QIN R, PITOT HC, et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: Phase I safety and activity results. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part 1. 2007;25:5034. [Google Scholar]