Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model (original) (raw)
Abstract
Grape polyphenols can act as antioxidants, antiangiogenics, and selective estrogen receptor (ER) modifiers and are therefore especially relevant for gynecological cancers such as breast cancer. The major polyphenols of red wine (resveratrol, quercetin, and catechin) have been individually shown to have anticancer properties. However, their combinatorial effect on metastatic breast cancers has not been investigated in vivo. We tested the effect of low dietary concentrations of resveratrol, quercetin, and catechin on breast cancer progression in vitro by analyzing cell proliferation and cell cycle progression. The effects of these compounds on fluorescently tagged breast tumor growth in nude mice were assessed using in situ fluorescence image analysis. Individual polyphenols at 0.5 µM neither decreased breast cancer cell proliferation nor affected cell cycle progression in vitro. However, a combination of resveratrol, quercetin, and catechin at 0.5, 5, or 20 µM each significantly reduced cell proliferation and blocked cell cycle progression in vitro. Furthermore, using in situ image analysis, we determined that combined dietary polyphenols at 0.5, 5, or 25 mg/kg reduced primary tumor growth of breast cancer xenografts in a nude mouse model. Peak inhibition was observed at 5 mg/kg. These results indicate that grape polyphenols may inhibit breast cancer progression.
Introduction
Grapes and red wine are thought to have cancer and cardiovascular health benefits due to polyphenolic compounds from grape skin [1]. Grape polyphenols can act as antioxidants, antiangiogenics, and selective estrogen receptor (ER) modifiers and are therefore especially relevant for gynecological cancers such as breast cancer [2–4]. The anticancer effects of grape polyphenols, which are concentrated in red wine, have been demonstrated in numerous in vitro and in vivo systems, including breast cancer models [5–13]. These compounds elicit increased growth-inhibitory effects on mouse mammary cells in culture and in vivo systems [7].
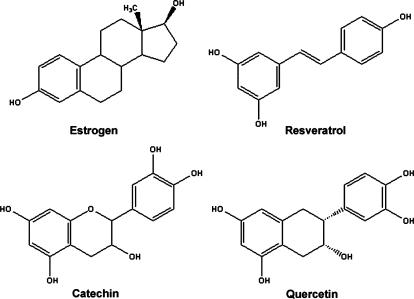
The compounds resveratrol, quercetin, and catechin selected for this study represent about 70% of the red wine polyphenols [6,14]. All three have antioxidant properties and, due to structural similarity to the steroid hormone estrogen, can have both estrogenic and antiestrogenic effects (Figure 1) [2,4,15]. Interestingly, resveratrol, quercetin, and catechin are the most effective anticancer compounds in red wine in a mouse skin cancer model [16]. Additionally, all three polyphenols are considered viable chemopreventives because they are absorbed in vivo and can be detected intact in plasma and urine samples in humans and rodent models. However, these compounds are metabolized rapidly following digestion and the plasma levels can be relatively low [5,17–21]. Individually, each of these compounds has been shown to induce cell cycle arrest and apoptosis in cancer cells [22–26], as well as prevent breast carcinogenesis and cancer progression in rodent models [27–31].
Figure 1.
Structures of red wine polyphenols. Structures of the stilbene resveratrol, flavan-3-ol catechin, and flavonol quercetin are compared with estrogen. These polyphenols can act as antioxidants and may have estrogenic/antiestrogenic activity due to similarity in distance between terminal hydroxyl groups of estrogen that can interact with estrogen receptors.
Herein, we report a study aimed at understanding the preventive and therapeutic efficacy of combined resveratrol, quercetin, and catechin on breast cancer. We show that dietary concentrations of these grape polyphenols can inhibit breast cancer cell proliferation in vitro and mammary tumor growth in vivo.
Materials and Methods
Cell Culture
MDA-MB-231 human breast cancer cells (American Type Culture Collection) were transfected with pMax-enhanced green fluorescent protein (GFP) plasmid by nucleofection according to the manufacturer, s instructions (Amaxa Inc., Gaithersburg, MD). Stable GFP-expressing clones were selected and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2.
Cell Proliferation
Semiconfluent cells seeded on six-well plates in phenol red-free DMEM and 5% FBS (charcoal-stripped) were treated either with vehicle or with polyphenols (0.5, 5, or 20 µM each of resveratrol, quercetin, or catechin or a combination of resveratrol, quercetin, and catechin at 0.5, 5, or 20 µM) every 48 hours for 96 hours. Stock solutions of resveratrol, quercetin, and catechin (LKT Laboratories, St. Paul, MN) in 100% ethanol were diluted in DMEM to a final concentration of 0.1% (v/v) ethanol before treatment. Cells were fixed in methanol, stained with propidium iodide (PI), and intact nuclei were counted from digital images acquired using a microscope (Model CKX41; Olympus America, Inc., Center Valley, PA). The total number of cells was quantified from 30 microscopic fields per well from experiments carried out on two separate days with each treatment done in triplicate (N = 6).
Cell Cycle Analysis
Cells were seeded at a density of 5 x 105 on 10-cm plates in 5% FBS, phenol red-free medium. Cells were treated either with vehicle (0.1% ethanol) or with polyphenols (0.5, 5, or 20 µM each of resveratrol, quercetin, or catechin or a combination of resveratrol, quercetin, and catechin at 0.5, 5, or 20 µM) every 48 hours for 96 hours. Cells were harvested, fixed in 70% ethanol, and stained with 40 µg/ml PI. At least 40,000 cells were analyzed per sample using a FACS-Calibur machine (Becton Dickinson, San Jose, CA). Flow cytometry data analysis was performed using a software (FlowJo 7.2.1; Tree Star, Inc., San Carlos, CA). Samples from each treatment were gated similarly and the mean percentage of cells in the G0/G1, S, and G2/M phases was quantified from FL2-A histograms using the Dean-Jett-Fox Model. One-way analysis of variance with posttest was done for each cell cycle stage using a software (GraphPad InStat 3 Version 3.06; GraphPad, San Diego, CA).
In Vivo Experiments
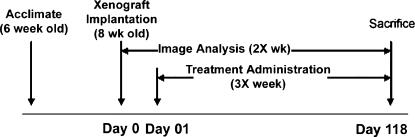
As diagrammed in Figure 2, animal experiments were designed to determine the effect of dietary polyphenols on established mammary tumors.
Figure 2.
Schematic design of the animal experiments. Six-week-old female nude mice were acclimated for 2 weeks and GFP-MDA-MB-231 xenografts were inoculated at the mammary fat pad at 8 weeks of age (day 0). On day 1, mice were administered vehicle, 0.5, 5, or 25 mg/kg each of resveratrol, quercetin, and catechin thrice a week for 117 days. Fluorescence image analysis of tumors commenced on day 0 and continued twice a week until the end of the study at 118 days (32 weeks).
Animals. Female athymic nu/nu mice, 5 to 6 weeks old (Charles River Laboratories, Inc., Wilmington, MA), were maintained in high-efficiency particulate air-filtered cages in a pathogen-free facility. Mice were fed autoclaved diet (Tek Global; Harlan Teklad, Madison, WI) with 14% protein and 3.5% fat and minimal alfalfa or soybean meal that may contain phytoestrogens.
Tumor establishment. GFP-MDA-MB-231 cells (∼ 2 x 106) in Matrigel (BD Biosciences, San Jose, CA) were injected into the mammary fat pad of three female nude mice according to established protocols [32]. When tumors reached 20 to 30 mm2, they were harvested, pooled, and segmented into ∼ 2 mm3 xenografts which were immediately implanted into the mammary fat pad of 40 female nude mice as previously described [33]. A small incision was made at the mammary fat pad and the xenograft was implanted under the skin, followed by closure of incision using staples. The efficiency of xenograft establishment was 100% as determined by palpation and measurement of fluorescence intensity at the site of implantation.
Polyphenol administration. Nude mice (10 mice per treatment group) were gavaged either with vehicle (90% corn oil and 10% ethanol) or with combined resveratrol, quercetin, and catechin at 0.5, 5, and 25 mg/kg body weight in a 100-µl volume thrice a week starting at 1 day after xenograft implantation. Treatments continued until sacrifice at day 118.
Macroscopic fluorescence image analysis. Hand-restrained mice were imaged immediately following xenograft implantation and twice a week thereafter. A 300-W power source (LT99D2; Lightools Research, Encinitas, CA) with two optical delivery systems fitted with excitation filters (470/40 nm) was used for whole body imaging of GFP fluorescence. Images were captured with a charge-coupled device camera (Spot II; Diagnostic Instruments, Sterling Heights, MI) mounted with a 530/25 nm emission filter (Chroma Technology, Rockingham, VT). GFP fluorescence was quantified as a measure of tumor growth as per our previously described methods [34]. Tumor area was manually traced as delineated by fluorescence and fluorescence intensities were analyzed using Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) and ImageJ software (National Institutes of Health (NIH), Bethesda, MD). Relative tumor area for each mouse was calculated as the tumor area at each imaging session divided by the tumor area directly after xenograft implantation.
Results
Grape Polyphenols Inhibit Breast Cancer Cell Proliferation and Cell Cycle Progression In Vitro
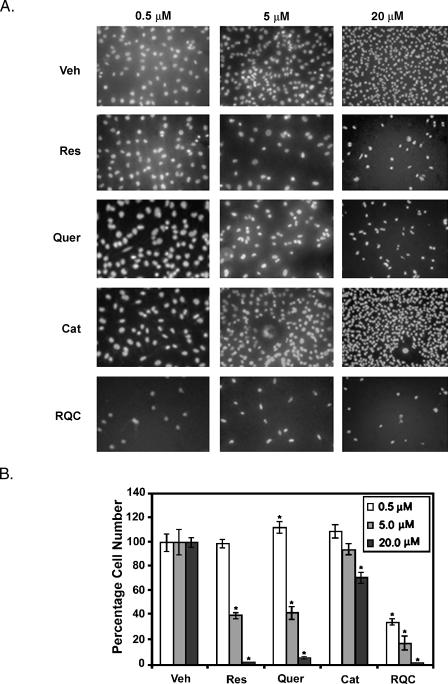
The effect of grape polyphenols on cell proliferation was analyzed in vitro using ERα (-) ERβ (+) GFP-MDA-MB-231. Cells in 5% charcoal-stripped serum and phenol red-free media were treated with individual resveratrol, quercetin, or catechin at 0.5, 5, or 20 µM, or a combination of 0.5, 5, or 20 µM resveratrol, quercetin, and catechin for 96 hours. At 48 hours, the old media was removed and fresh compounds and media were added to the cells. Therefore, the concentrations of compounds that were perceived by the cells at end point may be slightly higher because of the potential for unmetabolized compounds inside the cells from the initial treatment. At 0.5 µM, treatment with resveratrol, quercetin, or catechin alone did not demonstrate any changes in cell number or nuclear morphology. However, combined resveratrol, quercetin, and catechin at 0.5 µM dramatically reduced cell number (Figure 3_A_). Individual resveratrol or quercetin at 5 or 20 µM, catechin at 20 µM, or a combination of resveratrol, quercetin, and catechin at 0.5, 5, or 20 µM each reduced cell number significantly compared to control (Figure 3_B_). Quercetin demonstrated a biphasic regulation of cell proliferation with a significant increase at 0.5 µM and decrease at 5 and 20 µM. Catechin alone was not as effective as resveratrol or quercetin at all concentrations tested. However, at 5 and 20 µM catechin, there was a disproportionate number of larger cells with larger segmented nuclei, indicating a different mechanism of regulation by catechins (Figure 3_A_). Combination of all three compounds at 0.5, 5, or 20 µM reduced cell number by 65%, 83%, and 98%, respectively, and cells demonstrated apoptotic nuclei (Figure 3).
Figure 3.
Effect of grape polyphenols on MDA-MB-231 cell proliferation. MDA-MB-231 cells at a density of 5 x 104 in 5% charcoal-stripped FBS and phenol red-free media were plated on six-well plates. After 4 hours and every 48 hours for 96 hours, cells were treated with vehicle (Veh), 0.5, 5, or 20 µM resveratrol (Res), quercetin (Quer), or catechin (Cat), or a combination of 0.5, 5, or 20 µM each Res, Quer, and Cat (RQC). Cells were fixed and nuclei were stained with PI. (A) Representative micrographs of cells stained with PI. (B) Cell proliferation as analyzed by quantification of intact (nonapoptotic) nuclei. Percent viable cells ± SEM for 10 microscopic fields per triplicate treatments (N = 30) were calculated.
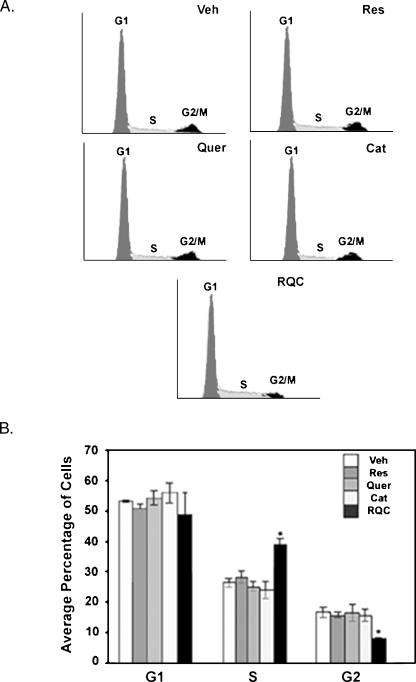
The synergistic effect of a low (0.5 µM) concentration of polyphenols on cell proliferation was also confirmed by analysis of cell cycle progression using flow cytometry of PI-stained cells. Individual treatment of 0.5 µMeach resveratrol, quercetin, or catechin did not affect cell cycle progression. However, a combination of resveratrol, quercetin, and catechin at 0.5 (Figure 4) or 5 µM (data not shown) each increased the percentage of cells at S phase with a concomitant decrease at G2/M phase of the cell cycle in a statistically significant manner, when compared to control. At 5 µM, resveratrol was the most potent polyphenol with reduced cell numbers at G1, a statistically significant increase in the average number of cells at S phase, and a concomitant decrease in cell numbers at G2/M, when compared to control. At 20 µM each, consistent with the large decrease in cell numbers, resveratrol, quercetin, or combined polyphenol treatment resulted in cell cycle arrest at G0/G1 by demonstrating increased percentage of cells at G1 with drastically reduced numbers at S and G2 phases. The effect of catechin on cell proliferation and cell cycle progression was different by demonstrating an increase in cells at S phase at 20 µM consistent with the observed difference in nuclear morphology compared to resveratrol or quercetin at similar concentrations (Figure 3).
Figure 4.
Effect of grape polyphenols on MDA-MB-231 cell cycle progression. MDA-MB-231 cells at a density of 5 x 105 in 5% charcoal-stripped FBS and phenol red-free media were plated on 10-cm plates. After 4 hours and every 48 hours for 96 hours, cells were treated with vehicle (Veh), or with 0.5 µM each resveratrol (Res), quercetin (Quer), or catechin (Cat), or a combination of 0.5 µM each Res, Quer, and Cat (RQC). Cells were fixed and nuclei were stained with PI. Percentage of cells at each cell cycle stage was quantified by flow cytometry of PI-stained cells. (A) Representative histograms (FL-2A) for cells treated with vehicle or polyphenols. (B) Average percentage of cells at each cell cycle stage for vehicle or polyphenol treatment. Results shown are for N = 3 ± S.D. Asterisks indicate statistical significance (P < .05) when compared to Veh controls.
Grape Polyphenols Inhibit Breast Cancer Growth In Vivo
To investigate the physiological relevance of the observed reduction in breast cancer cell number and the inhibition of cell cycle progression in response to grape polyphenols, we tested the effect of combined resveratrol, quercetin, and catechin treatment on tumor growth from MDA-MB-231 cells in vivo. Xenografts of GFP-MDAMB-231 cells, ∼ 2 mm2 in size, were implanted in the mammary fat pads of female athymic nude mice and tumor growth was monitored using fluorescence image analysis for 118 days.
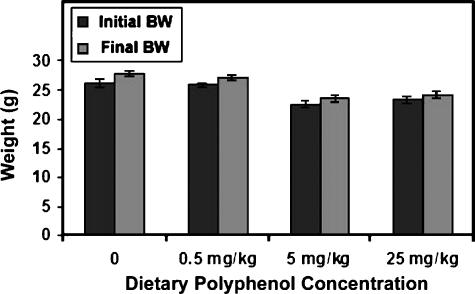
During the course of the study, nude mice implanted with GFP-tagged MDA-MB-231 xenografts did not show a difference in body weight (Figure 5), indicating that polyphenol treatment, ethanol intake, and/or tumor burden did not significantly affect weight.
Figure 5.
Effect of grape polyphenols on mouse body weight. Mice were weighed weekly. Initial and final body weights (BW) are shown. Data are represented as weight ± SEM for 10 mice per treatment group.
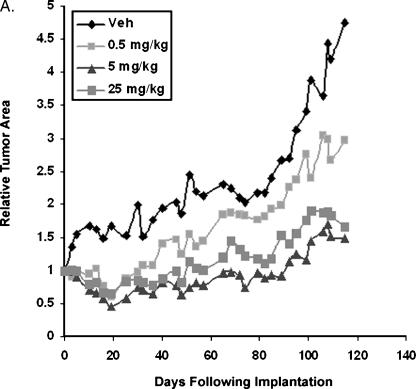
Each mouse was imaged twice a week and the relative tumor area was calculated as a function of the fluorescence intensity of the tumor on day 1 directly after xenograft implantation. Therefore, data represent the change in tumor intensity from time of implantation for each individual mouse in all of the groups tested. Figure 6 demonstrates that treatment with 0.5, 5, or 25 mg/kg body weight of resveratrol, quercetin, and catechin decreased tumor growth compared to controls. Tumor growth increased in a linear fashion in response to 0.5 and 5 mg/kg combined polyphenol treatments. From the concentrations tested, a maximum inhibitory effect was achieved using 5 mg/kg combined polyphenols. At all concentrations tested, the polyphenol treatments resulted in an initial lag in tumor establishment compared to controls where the slope for increase of control tumor intensity from day 1 to 20 was 0.09, whereas 0.5, 5, or 25 mg/kg body weight treatments resulted in a slope of - 0.03. At 20 days post xenograft implantation, tumors from mice treated with 0.5 mg/kg treatments increased in intensity with a slope of 0.028, tumors from 5 mg/kg treatments with a slope of 0.017, and tumors from 25 mg/kg treatments with a slope of 0.014 (Figure 6_A_).
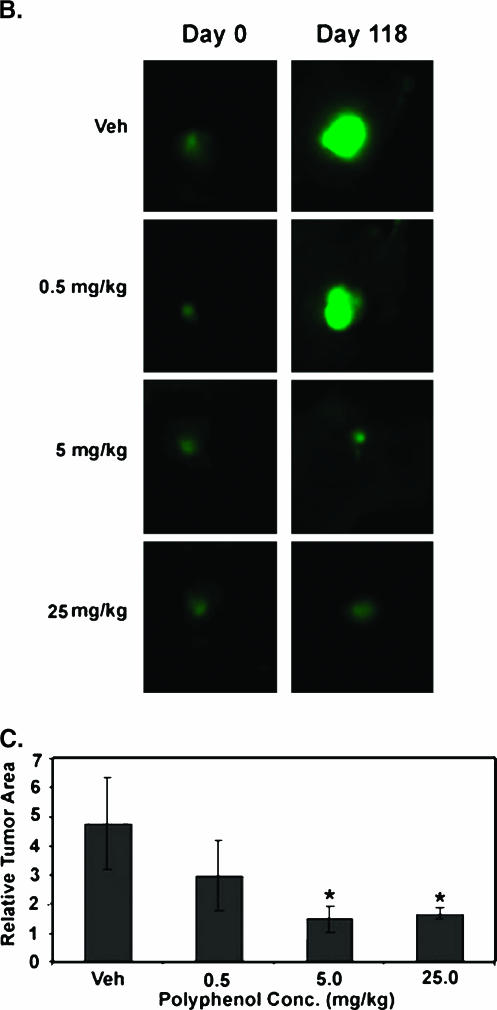
Figure 6.
Effect of grape polyphenols on mammary tumor growth. Mice were administered with vehicle (10% ethanol in oil) or with 0.5, 5 or 25 mg/kg resveratrol, quercetin, and catechin (RQC) thrice a week. GFP-MDA-MB-231 xenografts (∼ 2 mm2) were inoculated in the right mammary fat pad of female athymic nude mice. Tumor growth was analyzed from digital fluorescent images and made relative to each image acquired on day 0 immediately following inoculation. (A) Average relative tumor growth as analyzed by the increase in pixel intensity for 10 mice per experimental group. Regions of green fluorescence from unsaturated digital images were outlined to define tumor area. Tumor area (in pixels) and average intensity were acquired using ImageJ software (NIH). (B) Representative digital images of primary GFP mammary tumors from mice treated with dietary polyphenol treatments on days 0 and 118. (C) Quantification of mammary tumor growth from digital images acquired twice a week for 118 days. Relative tumor area from mice treated with dietary treatments on day 118. Asterisks denote a statistically significant difference (P < .05) from control.
At 118 days postimplantation, the average mammary tumor area increased 4.8-fold compared to day 0 in control mice treated with vehicle alone; for mice treated with grape polyphenols at 0.5, 5, and 25 mg/kg, this average increase was 3-fold, 1.5-fold, and 1.6-fold, respectively. Decrease in tumor growth was observed visually on inspection of change in tumor fluorescence area and intensity. Treatments at 5 and 25 mg/kg showed a statistically significant reduction in mammary tumor growth relative to control mice treated with vehicle alone (Figure 6, B and C).
Discussion
This study was designed to determine the combinatorial effect of polyphenols at dietary concentrations on established mammary tumors. Most investigations on environmental and dietary chemopreventives focus on breast cancer initiation and not on established mammary tumors. Studies conducted with chemically induced as well as spontaneous carcinogenic transgenic mouse models have demonstrated the use of red wine solids, grape juice, or grape seed extract in the prevention of mammary tumor initiation [9–13]. However, many studies on the potential anticancer properties of grape polyphenols have been conducted using concentrations too high to be achieved using dietary consumption. Moreover, a majority of these reports investigates the effect of individual polyphenols. Previous studies in rodents including breast cancer models have demonstrated the cancer-preventive efficacy of individual treatments of resveratrol, quercetin, or catechins at pharmacological concentrations [28–31, 35–37]. Because the majority of dietary compounds contain a mixture of polyphenols, it is important to understand their combinatorial effect at dietary concentrations.
The MDA-MB-231 low metastatic human breast cancer cell line was used to represent an aggressive breast cancer cell line that will illustrate the utility of using red wine polyphenols as preventives/therapeutics for advanced breast cancer. This cell line has been used in vitro to demonstrate the inhibitory effect of red wine polyphenols at low concentrations [6]. Moreover, our previously published data using this cell line has demonstrated that resveratrol can have biphasic concentration-dependent effects on cell functions relevant to breast cancer metastasis such as migration, invasion, and actin cytoskeletal changes as well as Akt and focal adhesion kinase activities that regulate cancer cell survival and invasion [38–40].
Data presented herein illustrate the utility of using a combination of grape polyphenols as breast cancer chemopreventives and therapeutics. The grape polyphenol concentrations used in the in vitro studies fall within the range of 0.1 to 10 µM that may be accumulated in the circulation following consumption of products rich in grapes such as red wine [5,19–21,38–42]. However, caution must be used with the interpretation of in vitro data because the compounds that were added to the tissue culture cells are relatively stable in their aglycone forms compared to the dietary polyphenols that accumulate in the blood following oral administration.
Our in vitro data demonstrate that, individually, resveratrol, quercetin, or catechin at a dietary concentration of 0.5 µM does not exert a significant inhibitory effect on ERα (-) ERβ (+) MDA-MB-231 breast cancer cell proliferation or on cell cycle progression. Interestingly, combined resveratrol, quercetin, and catechin each at 0.5 µM exerted a synergistic effect to result in cell cycle arrest and reduced cell proliferation. Decreased cell proliferation and G2/M phase arrest in response to a similar combination of polyphenols have been reported for breast cancer cells but not for normal mammary epithelial cells, indicating the utility of grape polyphenols as breast cancer therapeutics [6,23,43]. The subtle but statistically significant increase in cell numbers in response to low concentrations of quercetin has been previously reported for other cell types [44]. As demonstrated previously [24,45–47], resveratrol and quercetin were potent inhibitors of cell proliferation at high concentrations. The effect of catechin on cell proliferation and cell cycle progression was modest even at 20 µM. Therefore, catechin may not have contributed to the anticancer properties of grape polyphenols at the concentrations tested. However, because catechin is the major monomeric polyphenol in red wine [31,48], catechin was included in the in vivo study on the effect of grape polyphenols on breast cancer progression.
The in vivo effect of combined dietary treatment of resveratrol, quercetin, and catechin was quantified by intravital image analysis of fluorescently tagged breast tumor growth in nude mice. We and others have demonstrated the utility of such noninvasive imaging modalities for in situ tumor analysis from the time of tumor cell inoculation to the formation of distant metastases from fluorescently tagged cancer cells in rodent models [34,49,50].
In mice with an average bodyweight of 20 g, 5 mg/kg bodyweight of each compound in a 100-µl gavage volume equates to 4.38 µM resveratrol, 3.3 µM quercetin, and 3.48 µM catechin. These concentrations are found in dietary components rich in grape polyphenols such as red wine [14]. A growing debate on the cancer-preventive properties of natural compounds is that dietary consumption is insufficient to achieve cancer inhibitory concentrations at target tissues [17–21]. However, resveratrol, quercetin, and catechins are all considered viable chemopreventives because they are absorbed and metabolized rapidly in vivo and can be detected in plasma and urine samples in the intact form in humans and rodent models [5,17–21]. Data on bioavailability of grape polyphenols following consumption in animal models vary depending on the route of administration, animal models, and method of detection. For example, administration of as little as 50 µg/kg body weight of resveratrol per day for 3 months to rats achieved serum levels as high as 8.0 µM [39]. However, others have shown that administration of 20 mg/kg resveratrol to rats, mice, or rabbits demonstrated a very short half-life (10 minutes) and reached circulating levels of less than 1 µM very rapidly [20]. Similarly, following 20 mg/kg body weight administration of quercetin to mice, at concentrations that inhibited metastatic melanoma growth, the quercetin levels in the plasma decreased to ∼ 1 µM after 120 minutes [21]. Therefore, the combined polyphenols that demonstrated reduced mammary tumor growth in this study at 5 mg/kg body weight must be effective at the cellular level at very low concentrations.
As has been reported previously for this cell line [32,51], MDA-MB-231 cells did not form significant metastases during the 4-month study period. Isolated lungs from mice that received only vehicle control demonstrated more micrometastases (2–10 cells) compared to mice treated with polyphenols. However, these differences were not statistically significant due to the small sample size of the mice that developed metastases. Therefore, we were unable to perform a comprehensive analysis of the effect of resveratrol, quercetin, and catechin on breast cancer metastasis. Resveratrol at similar concentrations has been shown to reduce lung metastasis in other systems [52,53]. A recent study demonstrated that grape polyphenols, including resveratrol, epicatechin, and epigallocatechin, can inhibit cancer cell invasion [54]. The contribution of combined grape polyphenols on prevention of metastasis or progression of established breast cancers will be a focus of future investigations using more efficient metastatic breast cancer cell lines. Overall, the data presented indicate a preventive/therapeutic role for dietary grape polyphenols in primary breast cancer. However, more animal and clinical studies are needed before dietary recommendations of grape products for breast cancer patients, for those at risk, or for survivors of breast cancer can be made.
Acknowledgments
We thank Rafael Vasquez and Celia Venza for excellent technical assistance.
Footnotes
1
This work was supported by grants from the American Institute of Cancer Research (AICR) (IIG 03-31-06) and the National Institutes of Health (NIH)/National Cancer Institute (NCI) (5RO3CA109913) to S.F.D., a grant from the National Centers of Competence in Research (NCCR)/NIH (2G12RR003035) to Universidad Central del Caribe (UCC), and by funds from Centro Universitario de Medicina Integral y Complementaria (CUMIC) to S.F.D., and NIH/Research Centers in Minority Institutions (RCMI) G-12-RR03051 to University of Puerto Rico-Medical Sciences Campus.
References
- 1.de Lorimier AA. Alcohol, wine, and health. Am J Surg. 2000;180:357–361. doi: 10.1016/s0002-9610(00)00486-4. [DOI] [PubMed] [Google Scholar]
- 2.Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or beta-mediated responses in transfected breast cancer cells. Exp Biol Med. 2005;230:558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 3.Klinge CM, Risinger KE, Watts MB, Beck V, Eder R, Jungbauer A. Estrogenic activity in white and red wine extracts. J Agric Food Chem. 2003;51:1850–1857. doi: 10.1021/jf0259821. [DOI] [PubMed] [Google Scholar]
- 4.Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G. Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem. 2003;51:5497–5503. doi: 10.1021/jf030113c. [DOI] [PubMed] [Google Scholar]
- 5.Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2002;35:119–124. doi: 10.1016/s0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 6.Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–441. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Morre DM, Morre DJ. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2005;238:202–209. doi: 10.1016/j.canlet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85:65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- 9.Clifford AJ, Ebeler SE, Ebeler JD, Bills ND, Hinrichs SH, Teissedre PL, Waterhouse AL. Delayed tumor onset in transgenic mice fed an amino acid-based diet supplemented with red wine solids. Am J Clin Nutr. 1996;64:748–756. doi: 10.1093/ajcn/64.5.748. [DOI] [PubMed] [Google Scholar]
- 10.Singletary KW, Stansbury MJ, Giusti M, van Breemen RB, Wallig M, Rimando A. Inhibition of rat mammary tumorigenesis by concord grape juice constituents. J Agric Food Chem. 2003;51:7280–7286. doi: 10.1021/jf030278l. [DOI] [PubMed] [Google Scholar]
- 11.Martinez C, Vicente V, Yanez J, Alcaraz M, Castells MT, Canteras M, Benavente-Garcia O, Castillo J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol Histopathol. 2005;20:1121–1129. doi: 10.14670/HH-20.1121. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Hall P, Smith M, Kirk M, Prasain JK, Barnes S, Grubbs C. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004;134:3445S–3452S. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]
- 13.Jung KJ, Wallig MA, Singletary KW. Purple grape juice inhibits 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 2006;233:279–288. doi: 10.1016/j.canlet.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Faustino RS, Sobrattee S, Edel AL, Pierce GN. Comparative analysis of the phenolic content of selected Chilean, Canadian and American Merlot red wines. Mol Cell Biochem. 2003;249:11–19. [PubMed] [Google Scholar]
- 15.Ratna WN, Simonelli JA. The action of dietary phytochemicals quercetin, catechin, resveratrol and naringenin on estrogen-mediated gene expression. Life Sci. 2002;70:1577–1589. doi: 10.1016/s0024-3205(01)01531-4. [DOI] [PubMed] [Google Scholar]
- 16.Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2006;39:492–497. doi: 10.1016/s0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 17.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 18.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 19.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans: I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 20.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer P, Asensi M, Segarra R, Ortega A, Benlloch M, Obrador E, Varea MT, Asensio G, Jorda L, Estrela JM. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nifli AP, Kampa M, Alexaki VI, Notas G, Castanas E. Polyphenol interaction with the T47D human breast cancer cell line. J Dairy Res. 2005;72:44–50. doi: 10.1017/s0022029905001172. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 24.Kim YA, Choi BT, Lee YT, Park DI, Rhee SH, Park KY, Choi YH. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol Rep. 2004;11:441–446. [PubMed] [Google Scholar]
- 25.Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas MM, Arunakaran J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J Cancer Res Clin Oncol. 2005;131:765–771. doi: 10.1007/s00432-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 26.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–1181. [PubMed] [Google Scholar]
- 27.Le Corre L, Chalabi N, Delort L, Bignon YJ, Bernard-Gallon DJ. Resveratrol and breast cancer chemoprevention: molecular mechanisms. Mol Nutr Food Res. 2005;49:462–471. doi: 10.1002/mnfr.200400094. [DOI] [PubMed] [Google Scholar]
- 28.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Dechsupa S, Kothan S, Vergote J, Leger G, Martineau A, Beranger S, Kosanlavit R, Moretti JL, Mankhetkorn S. Quercetin, Siamois 1 and Siamois 2 induce apoptosis in human breast cancer MDA-MB-435 cells xenograft in vivo. Cancer Biol Ther. 2007;6:3–4. doi: 10.4161/cbt.6.1.3548. [DOI] [PubMed] [Google Scholar]
- 31.Ebeler SE, Brenneman CA, Kim GS, Jewell WT, Webb MR, Chacon-Rodriguez L, MacDonald EA, Cramer AC, Levi A, Ebeler JD, et al. Dietary catechin delays tumor onset in a transgenic mouse model. Am J Clin Nutr. 2002;76:865–872. doi: 10.1093/ajcn/76.4.865. [DOI] [PubMed] [Google Scholar]
- 32.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 33.Hoffman RM. Orthotopic metastatic (MetaMouse) models for discovery and development of novel chemotherapy. Methods Mol Med. 2005;111:297–322. doi: 10.1385/1-59259-889-7:297. [DOI] [PubMed] [Google Scholar]
- 34.Carlson AL, Hoffmeyer MR, Wall KM, Baugher PJ, Richards-Kortum R, Dharmawardhane SF. In situ analysis of breast cancer progression in murine models using a macroscopic fluorescence imaging system. Lasers Surg Med. 2006;38:928–938. doi: 10.1002/lsm.20409. [DOI] [PubMed] [Google Scholar]
- 35.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 36.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- 37.Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans-resveratrol—a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. 2006;27:1038–1046. doi: 10.1093/carcin/bgi286. [DOI] [PubMed] [Google Scholar]
- 38.Brownson DM, Azios NG, Fuqua BK, Dharmawardhane SF, Mabry TJ. Flavonoid effects relevant to cancer. J Nutr. 2002;132:3482S–3489S. doi: 10.1093/jn/132.11.3482S. [DOI] [PubMed] [Google Scholar]
- 39.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7:128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia. 2007;9:147–158. doi: 10.1593/neo.06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 42.Bottner M, Christoffel J, Jarry H, Wuttke W. Effects of long-term treatment with resveratrol and subcutaneous and oral estradiol administration on pituitary function in rats. J Endocrinol. 2006;189:77–88. doi: 10.1677/joe.1.06535. [DOI] [PubMed] [Google Scholar]
- 43.Hakimuddin F, Paliyath G, Meckling K. Treatment of MCF-7 breast cancer cells with a red grape wine polyphenol fraction results in disruption of calcium homeostasis and cell cycle arrest causing selective cytotoxicity. J Agric Food Chem. 2006;54:7912–7923. doi: 10.1021/jf060834m. [DOI] [PubMed] [Google Scholar]
- 44.van der Woude H, Gliszczynska-Swiglo A, Struijs K, Smeets A, Alink GM, Rietjens IM. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003;200:41–47. doi: 10.1016/s0304-3835(03)00412-9. [DOI] [PubMed] [Google Scholar]
- 45.Conklin CM, Bechberger JF, Macfabe D, Guthrie N, Kurowska EM, Naus CC. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis. 2006;28:93–100. doi: 10.1093/carcin/bgl106. [DOI] [PubMed] [Google Scholar]
- 46.Devipriya S, Vani G, Ramamurthy N, Shyamaladevi CS. Regulation of intracellular calcium levels and urokinase activity in MDA MB 231 cells by quercetin. Chemotherapy. 2006;52:60–65. doi: 10.1159/000091306. [DOI] [PubMed] [Google Scholar]
- 47.Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- 48.Ebeler SE, Dingley KH, Ubick E, Abel S, Mitchell AE, Burns SA, Steinberg FM, Clifford AJ. Animal models and analytical approaches for understanding the relationships between wine and cancer. Drugs Exp Clin Res. 2005;31:19–27. [PubMed] [Google Scholar]
- 49.Yang M, Baranov E, Li XM, Wang JW, Jiang P, Li L, Moossa AR, Penman S, Hoffman RM. Whole-body and intravital optical imaging of angiogenesis in orthotopically implanted tumors. Proc Natl Acad Sci USA. 2001;98:2616–2621. doi: 10.1073/pnas.051626698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 51.Rose DP, Connolly JM, Liu XH. Effects of linoleic acid on the growth and metastasis of two human breast cancer cell lines in nude mice and the invasive capacity of these cell lines in vitro. Cancer Res. 1994;54:6557–6562. [PubMed] [Google Scholar]
- 52.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, Lopez-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2006;245:144–148. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer. 2005;115:36–45. doi: 10.1002/ijc.20874. [DOI] [PubMed] [Google Scholar]
- 54.Gunther S, Ruhe C, Derikito MG, Bose G, Sauer H, Wartenberg M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett. 2006;250:25–35. doi: 10.1016/j.canlet.2006.09.014. [DOI] [PubMed] [Google Scholar]