Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 29.
Published in final edited form as: Oncogene. 2008 Jul 21;27(47):6151–6163. doi: 10.1038/onc.2008.215
Abstract
In a breast tumor xenograft model, the MCT-1 oncogene increases the in vivo tumorgenicity of MCF7 cells by promoting angiogenesis and inhibiting apoptosis. Increases in the tumor microvascular density are accompanied by a strong reduction in the levels of the angiogenesis inhibitor thrombospondin-1 (TSP1), but the mechanisms underlying this process are unknown. We show that TSP1 expression is controlled, at least in part, by post-transcriptional events. Using RNA interference to knock down the expression of the RNA binding-protein HuR in MCF7 cells as well as HuR overexpression, we demonstrate that HuR plays an important role in translation of the TSP1 mRNA. Furthermore, employing the RIP-CHIP assay yielded 595 transcripts with significantly altered binding to HuR in the more tumorigenic breast cancer clones compared with the weakly tumorigenic clones. These mRNAs clustered in several pathways implicated in the transformed phenotype, such as the RAS pathway (involved in mitogenesis), the PI3K pathway (evasion of apoptosis), and pathways mediating angiogenesis and the cellular response to hypoxia. These findings demonstrate for the first time that global changes in HuR-bound mRNAs are implicated in the evolution to a more tumorigenic phenotype in an in vivo tumor model and underscore the role of global mRNA-protein interactions towards tumor progression.
Keywords: post-transcriptional operon, MCT-1 oncogene, translation, TSP1, HuR, RNA-binding protein, tumor angiogenesis
Introduction
In a human xenograft model, the MCT-1/MCTS-1 (Multiple copies in T-cell lymphoma 1) oncogene, hereafter named MCT-1 (Prosniak et al., 1998), promotes the transition to a more aggressive phase in breast cancer progression by enhancing invasiveness and decreasing apoptosis (thereby promoting the formation of larger tumors), and increases angiogenesis by reducing the expression of the angiogenesis inhibitor TSP1/THBS-1 (Thrombospondin 1) (Levenson et al., 2005), hereafter named TSP1. Given that natural angiogenesis inhibitors appear to have low toxicity, and that newly formed vessels are more sensitive to angiogenesis inhibitors than quiescent vessels, there is growing interest in developing TSP1-based cancer therapies (Ren et al., 2006). Previous data demonstrate that overall vascular density correlates to poor cancer prognosis (Weidner, 1998) and that TSP1 expression is inversely correlated with malignant progression of breast cancer (Zabrenetzky et al., 1994; Watnick et al., 2003). Thus, understanding the mechanism of action and regulation of TSP1 during angiogenesis and tumor progression has become important.
RNA turnover and translation are critical post-transcriptional mechanisms of gene regulation in mammalian cells, primarily governed by the interaction of mRNA with specific RNA-binding proteins (RBPs). Many ribonucleoprotein (RNP) associations implicating sequences present in the 3′-untranslated regions (3′UTRs) of mRNAs have been shown to regulate the transport, turnover and translation rates of the target mRNAs (Piecyk et al., 2000; Bevilacqua et al., 2003; Mazan-Mamczarz et al., 2006; Keene, 2007; Liao et al., 2007). Although the nucleotide composition of such regulatory sequences is very variable, often they are rich in adenines and uracils (AREs). The presence of AREs in the TSP1 3′-UTR suggest that regulation of TSP1 on the mRNA level may be an important mechanism involved in controlling TSP1 gene expression and plays a critical role in tumor progression.
In this investigation, we set out to formally examine the molecular mechanisms regulating the expression of TSP1 in a model of increased tumorigenicity elicited through enhanced expression of the MCT-1 oncogene (Levenson et al., 2005). Systematic study of several RBPs implicated in post-transcriptional gene regulation (collectively termed translation and turnover regulatory RNA-binding proteins or TTR-RBPs, Pullmann et al., 2007) to the TSP1 mRNA revealed that HuR/ELAVL1, hereafter named HuR specifically associated with TSP1 mRNA and that this interaction was dramatically decreased in highly tumorigenic clones (with increased MCT-1 levels). HuR regulates the stability and translation of many ARE-containing mRNAs and thereby controls the expression of the encoded proteins, often involved in the cell division cycle, carcinogenesis, senescence, and the immune and stress responses (Brennan and Steitz, 2001; Kullmann et al., 2002; Mazan-Mamczarz et al., 2003; Galban et al., 2003; Lal et al., 2005; Lopez de Silanes et al., 2004). In keeping with these functions, we found that HuR enhanced the translation of the TSP1 mRNA in this model system. Interestingly, however, in cells with a more aggressive phenotype (expressing high levels of MCT-1), HuR dissociated from TSP1 mRNA, in turn causing a reduction in TSP1 mRNA translation. Given the proposed function of HuR in cancer, we examined the global interaction of HuR with other target mRNAs in this model of increased tumorigenicity. We found that the MCT-1-enhanced breast cancer tumorigenicity was associated with significant alterations in the association of HuR with functional subsets of mRNAs. In accordance with the post-transcriptional operon model (Keene and Tenenbaum, 2002), our findings suggest that HuR functions as a downstream effector of MCT-1 regulated gene expression in cancer: HuR’s association with mRNAs that encode tumor-promoting proteins increases [as shown for cyclins and prothymosin α (Wang et al., 2000a; Lal et al., 2005)] and its association with mRNAs that inhibit tumorigenesis decreases, as shown here for TSP1 mRNA.
Results
Increased expression of MCT-1 reduces the translation of angiogenesis inhibitor TSP1
Mammary epithelial adenocarcinoma MCF7 cells were stably transfected with MCT-1, and two clones that overexpressed MCT-1 protein, N1 and N7, were isolated and described previously in a human xenograft mouse model (Levenson et al., 2005). Here, we used the N1 and N7 clones from the in vivo tumor progression model in order to examine the mechanism(s) underlying the decreased TSP1 production in the MCF7 cells with a more aggressive phenotype.
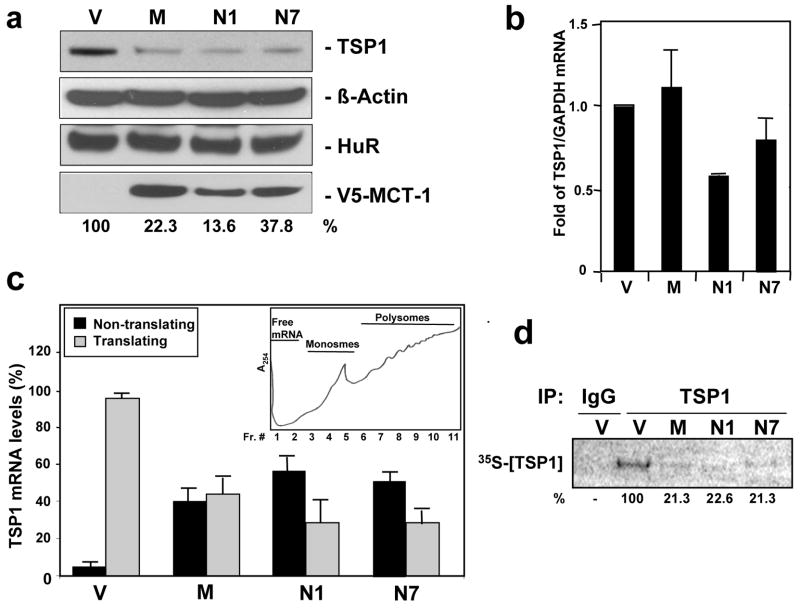
We first examined the expression levels of TSP1 from MCF7 cells transfected with empty vector (V), the pooled population overexpressing MCT-1 (M), and the two MCT-1-overexpressing clones (N1 and N7). The levels of TSP1 protein decreased markedly (~ 5 fold) in all MCT-1 overexpressing cell lines compared to empty vector cells (Fig. 1a). Although the possible contributions of altered trancription or TSP1 protein stability were not formally ruled out, the observed reduction in TSP1 protein abundance was not accompanied by a comparable decrease in TSP1 mRNA levels (Fig. 1b) or TSP1 mRNA stability (Supplementary information, Fig. S1), suggesting that the MCT-1 mediated decrease of TSP1 might be effected by altered translation.
Figure 1.
MCT-1 overexpression decreases translation and protein expression of TSP1 in MCF7 cells. (a) Representative Western blot analysis of cells stable transfected with the empty vector (V), and cells stable overexpressing MCT-1; mass culture (M) and to different clones (N1 and N7). 50 μg of total protein lysates were loaded and the abundance of TSP1, β-Actin, HuR and MCT-1 was assessed. (b) Changes in TSP1 total mRNA levels (relative to GAPDH mRNA) assessed by RT-qPCR for vector (V), and MCT-1 overexpressiong cells; mass culture (M) and two clones (N1 and N7). Graphs represent the means and standard errors of the means (SEM) from three independent experiments. Clone N1 showed less than 2-fold difference compared to vector (V); t-test value of p <0.01. (c) The relative association of the TSP1 mRNA with polysomes was tested by preparing cytoplasmic lysates from MCF7 stable cell lines (described in Fig. 1a), fractionating them through sucrose gradients and collecting 11 fraction for analysis. Representative polysome distribution profile obtained after centrifugation of cytopasmic lysates over sucrose gradient (inset); free mRNA, monosomes and polysomes of increasing molecular weight are indicated. The levels of TSP1 mRNA in the pooled 1–5 fractions comprised the nontranslating RNA, and pooled 6–11 fractions comprised the translating RNA were quantified by RT-qPCR and normalized to GAPDH mRNA. Graphs represent the means and SEM from six independent experiments; p <0.01 significantly different compared to vector (V) by t-test. (d) Newly translated TSP1 protein was assessed by 20 min incubation of each stable transfected MCF7 cell lines (described in Fig. 1a) with L-[35S]methionine and L-[35S]cysteine and immunoprecipitation (IP) of protein lysates with either anti-TSP1 antibody or control IgG. Samples were resolved by SDS-PAGE, transferred onto membranes and signal was visualized by PhosphoImager. Representative data are shown. In a and c, quantification of TSP1 signals is expressed as the percentage of the signal intensity relative to that in vector (V) cells.
To test the that TSP1 translation might be reduced in MCT-1-overexpressing cells, cytoplasmic extracts from vector and MCT-1 cell lines were subjected to sucrose density gradient analysis and the TSP1 mRNAs associated with the translational machinery were monitored. The fractions containing the nontranslating RNA (fractions 1–5) and containing polysomes (fractions 6–11) were pooled and TSP1 mRNA abundance was measured by RT-qPCR (Fig. 1c). As shown, TSP1 mRNA levels in polysomal fractions were decreased in all MCT-1-overexpressing populations compared to V cells: it was 60% lower in M cells, and 70% in both N1 and N7 clones. The reduced presence of TSP1 mRNA in the translating fractions suggested that the heightened expression of MCT-1 in MCF7 cells decreased the translation of TSP1 protein. Further evidence that translation of TSP1 was inhibited with increased expression of MCT-1 was obtained using a de novo translation assay that measured the rate of nascent TSP1 protein synthesis. Vector (V) and MCT-1 transfected lines (M, N1, N7) were briefly incubated in presence of L-[35S]methionine and L-[35S]cysteine, whereupon the newly translated TSP1 protein was visualized by immunoprecipitation with anti- TSP1 antibody. While the short (20 min) incubation period for labeling is inefficient and yields relatively weak signals, it allows the detection of newly translated protein and minimizes the potential contribution of protein degradation. These data revealed about five fold lower de novo protein biosynthesis of TSP1 in all MCT-1 overexpressing cell lines that was consistent with the decreased association of TSP1 mRNA with polysomes (Fig. 1d). Together, these data show that in the highly tumorigenic MCF7 clones expressing elevated levels of the MCT-1 oncogene, the translation of the TSP1 mRNA were diminished, leading to decreased TSP1 protein biosynthesis.
HuR binds the TSP1 mRNA
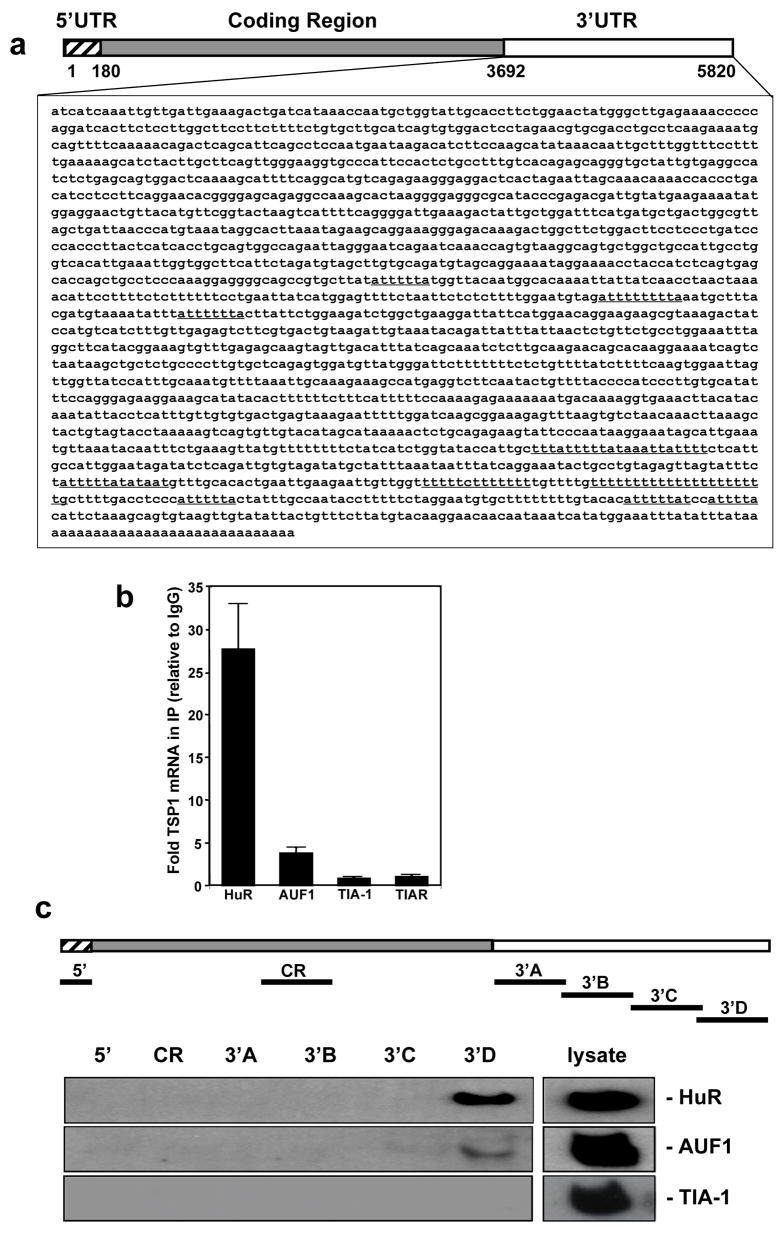
Given previous reports linking RNA-binding proteins (RBP) to post-transcriptional gene regulation (Keene, 2001; Wilusz and Wilusz, 2004), the existence of U-rich and AU-rich regions in the long (~2-kb) TSP1 3′UTR (Fig. 2a), and the fact that the TSP1 3′UTR contains 6 computationally predicted hits of a previously identified HuR motif (Lopez de Silanes et al., 2004), we directly examined whether this RBP might be involved in the post-transcriptional regulation of TSP1 expression. The association of several turnover and translation-regulatory (TTR)-RBPs with endogenous TSP1 mRNA was tested by immunoprecipitation (IP) analysis using MCF7 cell lysates and antibodies recognizing the major TTR-RBPs (HuR, AUF1/ hnRNPD, TIA-1 and TIAR). The association of RBPs with TSP1 mRNA was monitored by RT-(real time)qPCR analyses of mRNAs isolated from the IP material using TSP1 mRNA-specific primers (Fig. 2b). As shown, the TSP1 PCR product was highly enriched in the IP material obtained with anti-HuR antibody compared with the low-level of amplification seen in control IgG IP and in the IPs obtained using anti-AUF-1, anti-TIA-1 and anti-TIAR antibodies. To monitor the evenness of loading of the input material, we amplified GAPDH mRNA, which showed equal low-level background contamination in all of the IP samples tested (not shown).
Figure 2.
HuR binds the TSP1 mRNA. (a) Sequence of TSP1 mRNA depicting AREs sequences (underline) in TSP1 3′-untranslated region (3′UTR). (b) The association of TSP1 mRNA with ARE binding proteins (HuR, AUF1/HRNDP, TIA-1, and TIAR) was checked by immunoprecipitation (IP) of MCF7 cell lysates using either control (IgG) or specific antibodies. The obtained RNA was reverse transcribed and measured by qPCR. Shown are means and SEM from three independent experiments; GAPDH mRNA background amplification in the IP material was used as normalization control. (c) Schematic of TSP1 mRNA, representing the biotynolated transcripts (5′UTR, CR, and 3′UTR - A, B, C and D fragments) used for biotin pull-down analysis (top). Two ug of each biotinylated transcript were incubated with 40 μg of MCF7 cytoplasmic lysates. The presence of HuR, AUF1/HRNDP and TIA-1 in the pull-down material was detected by Western blotting; representative picture of couple repeats is shown.
In order to precisely determine the region of the TSP1 mRNA that interacted with HuR, we synthesized several biotinylated transcripts spanning the TSP1 mRNA (Fig. 2c, schematic) and used them in pull down assays (Materials and methods). HuR prominently bound the terminal region of 3′UTR (“3′D” fragment) but not the CR, 5′UTR transcripts and other parts of 3′UTR. AUF1 was found to bind the same “3′D” TSP1 sequence, although binding appeared to be significantly weaker, while TIA-1 did not bind any TSP1 transcripts (Fig. 2c). These data indicate that TSP1 mRNA is a specific target of RBP HuR both when assessing the endogenous mRNA and when testing a recombinant transcript in vitro, and the binding occurs in the terminal region of the TSP1 3′UTR.
Binding of the HuR to the TSP1 mRNA decreased when using lysates from cells with increased MCT-1 levels
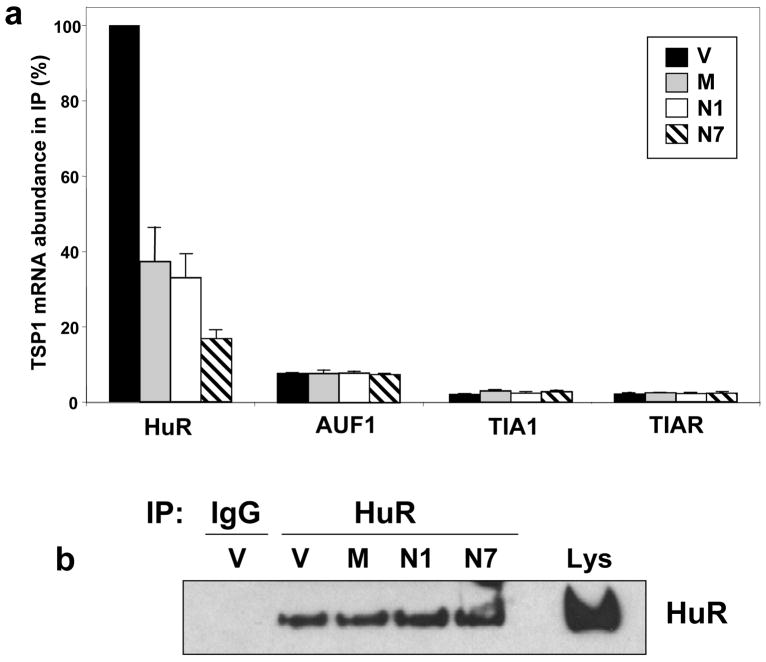
Given the reported ability of HuR and others ELAV proteins to influence mRNA stability and/or translation (Brennan and Steitz, 2001; Gorospe, 2003) we set out to directly investigate the potential contribution of HuR to the reduction of TSP1 expression in MCT-1-expressing MCF7 cells. First, we examined the association of TSP1 mRNA with HuR by carrying out RNP IP reactions similar to those described in Fig. 2b, using lysates from V, M, N1, and N7 cells. The abundance of mRNAs encoding TSP1 or the housekeeping GAPDH mRNA (included in order to monitor background binding and as a loading control) in the IP samples was quantified by RT-qPCR. The association of TSP1 mRNA with HuR was 65 to 85% reduced in the IP material obtained from all three cell lines with elevated MCT-1 levels (M, N1, N7) compared with the parental cells (V) (Fig. 3a). The reduced abundance of HuR-TSP1 mRNA complex was not a result of different HuR IP efficiency (Fig. 3b) or changes in HuR levels (Fig. 1a). However, we did not find any differences in the association of the other RBPs studied (AUF1, TIA-1 and TIAR) to the TSP1 mRNA when comparing cells that expressed different MCT-1 levels (Fig. 3a, Supplementary information, Fig. S3).
Figure 3.
MCT-1 overexpression decreased the association of HuR with TSP1 mRNA. (a) Cytoplasmic lysates from stable transfected MCF7 cell lines (described in Fig. 1a) were employed for IP assays, using antibodies that recognized HuR, AUF1/hnRNP D, TIA-1 or TIAR. RNA was isolated and RT following qPCR was performed. Graph represents the mean and SEM from three independent assays. p < 0.02 versus vector (V) in IPs with HuR antibody. (b) Representative IP assays performed as described for Fig. 3a but followed by Western blotting analysis to assess the abundance of HuR protein in the IP material.
Modulation of HuR expression influences TSP1 translation
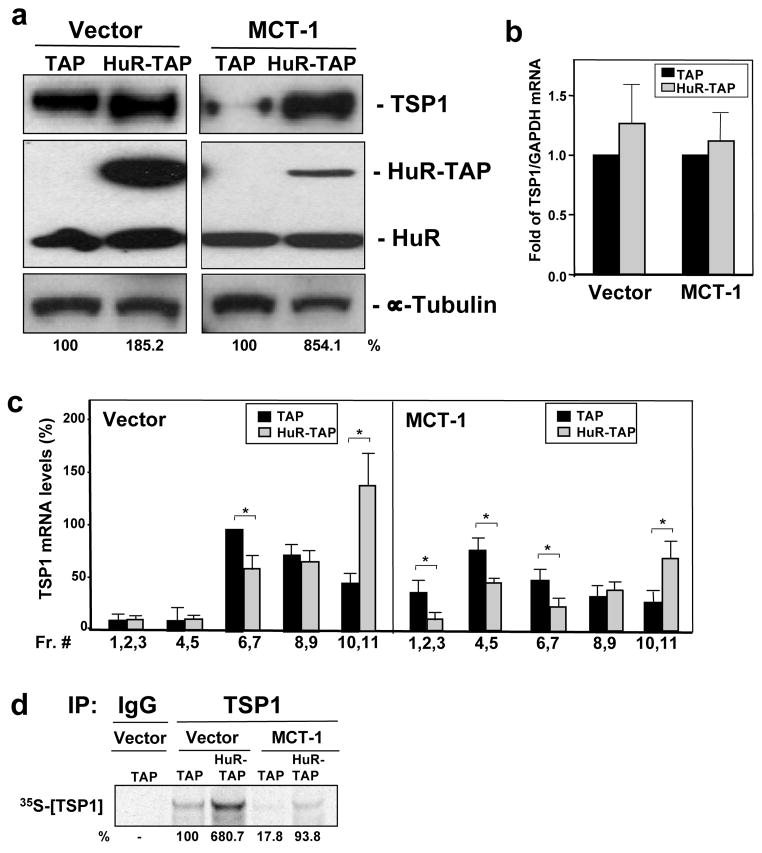
In order to study the functional consequences of decreased HuR-TSP1 mRNA interaction on TSP1 mRNA and protein levels, we carried out experiments in which HuR expression in MCF7 cells were modified (Material and methods). Overexpression of HuR increased TSP1 protein abundance in both the Vector and MCT-1-overexpressing (M) populations (Fig. 4a). HuR overexpression in Vector cells had lower effect (~2 fold) on TSP1 production (the cells might have reached the maximum levels of TSP1 expression possible), while MCT-1 cells showed strikingly heightened (>8 fold) TSP1 levels compared with cells transfected with the empty vector (TAP) despite the fact that they consistently showed a reduced magnitude of HuR overexpression. TSP1 mRNA levels, as measured by RT-qPCR analysis, was modestly elevated after HuR overexpression in both cell lines (Fig. 4b). Taking into consideration earlier reports showing that HuR can promote the translation of target mRNAs (Kullmann et al., 2002; Mazan-Mamczarz et al., 2003; Galban et al., 2003; Lal et al., 2005), and our findings that TSP1 mRNA was much less abundant in translating fractions and that TSP1 protein was less actively translated in the more malignant lines (Fig. 1c and 1d), it was important to investigate if the TSP1 translation was regulated by HuR. First, we examined if the positive effect of HuR on TSP1 expression levels in MCF7 cells was due to an enhanced association of TSP1 mRNA with actively translating polysomes. When pooling sucrose gradient fractions according to translational activity (no translation, low translation or active translation), cells expressing elevated levels of HuR (HuR-TAP) shifted the distribution of TSP1 mRNA towards the heavier polysomal fractions (fractions 10 and 11) in both the Vector and MCT-1-overexpressing populations (Fig. 4c). The translational status of the TSP1 mRNA was also monitored by directly assessing de novo protein synthesis assay (Fig. 4d). These data revealed more than five-fold increase in TSP1 translation in cells overexpressing HuR (HuR-TAP) relative to control transfection (TAP) in both cell lines (Vector, MCT-1), providing additional in vivo evidence that HuR enhances TSP1 mRNA translation.
Figure 4.
Overexpression of HuR in MCF7 cells increases translation and TSP1 protein expression. (a) MCF7 cells with normal (Vector) and overexpressing MCT-1 (MCT-1) levels were transiently transfected with either a control plasmid (TAP) or a plasmid overexpressing a tagged HuR (HuR-TAP), and lysate 72 hrs later. Ectopically expressed HuR (HuR-TAP), endogenous HuR, TSP1, and α-tubulin (loading control) levels were analyzed by Western blotting; representative data from three independent assays are shown. (b) The abundance of total TSP1 mRNA in cells expressing normal HuR levels (TAP) or overexpressing HuR (HuR-TAP) from both Vector and MCT-1 cell lines were measured by RT-qPCR in two separate experiments (mean values and SEM are shown). (c) MCF7 cells were transfected as in (a). mRNAs were extracted from each fraction, each of two or three consecutive fractions were pooled together (fractions 1, 2 and 3, fractions 4 and 5, fractions 6 and 7, fractions 8 and 9, fractions 10 and 11) and the TSP1 mRNAs were measured by RT-qPCR. Data were normalized to the GAPDH mRNA. Graphs represent the means and SEM from three independent experiments. *, p <0.05. (d) De novo TSP1 translation in MCF7 cell lines (Vector and overexpressing MCT-1) transfected as in (a) was assessed by cells incubation in L-[35S]methionine and L[35S]cysteine, IP reaction using anti-TSP1 antibody or IgG, protein electrophoresis by SDS-PAGE and detection of incorporated radiolabeled amino acids into newly synthesized TSP1 protein by PhosphorImager. Representative results are shown. In a and d, TSP1 signals were quantified and are represented as the percentage of the signal intensity in the empty vector (TAP) group.
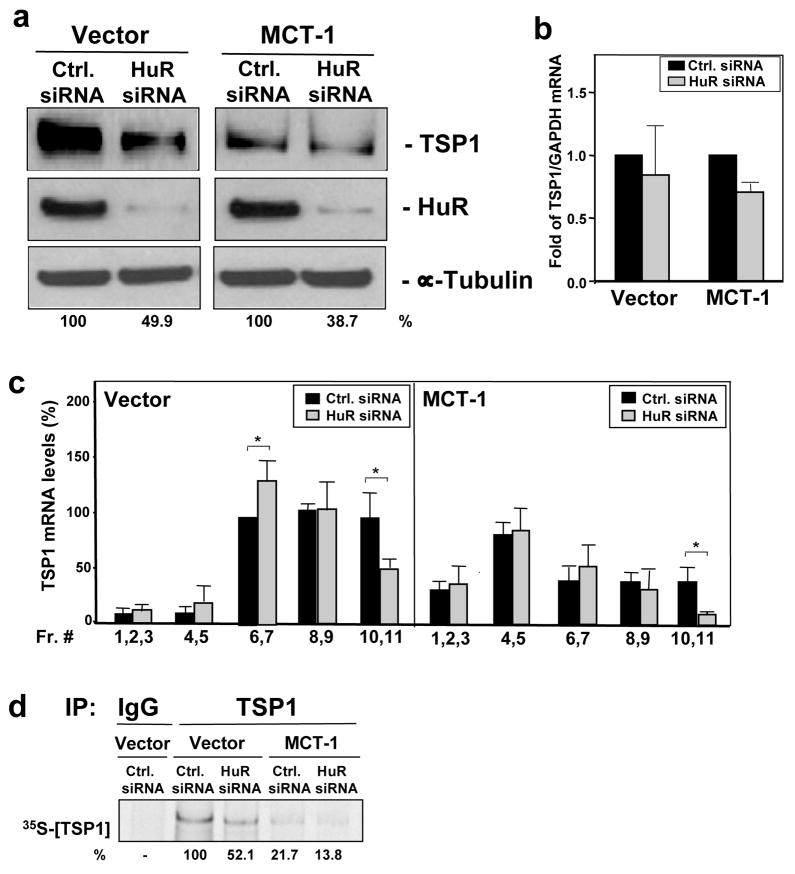
Reduction of HuR levels by transfecting MCF7 cells with targeting HuR siRNA effectively decreased the HuR levels in both Vector cells and MCT-1-overexpressing populations to <10% of the HuR levels seen in control transfection populations. Decreasing HuR expression (HuR siRNA) caused more than two fold decrease in TSP1 protein abundance in both cell lines studied (Fig. 5a). As measured by RT-qPCR, the reduction was not influenced significantly by lower total TSP1 mRNA levels in HuR siRNA cells (Fig. 5b). As in the HuR overexpression experiments, the translational status of TSP1 mRNA was tested by monitoring by distribution of TSP1 mRNA in polysomal fractions along a sucrose gradient in control and HuR-silenced cell populations. HuR-silenced groups (HuR siRNA) showed that TSP1 mRNA was more abundant in the low translating fractions (fractions 6 and 7) and less abundant in the actively translating fractions (10 and 11) in both Vector and MCT-1 cells (Fig. 5c). Importantly, nascent TSP1 translation (as measured by in vivo incorporation of radiolabeled amino acids into newly synthesized TSP1) was two fold lower in both Vector and MCT-1 MCF-7 populations with silenced HuR (HuR siRNA) (Fig. 5d). Taken together, our findings support the concept that HuR associates with the TSP1 mRNA and enhances its translation. Furthermore, in MCT-1 expressing cells, HuR association with TSP1 mRNA is reduced, and consequently TSP1 mRNA and protein levels decrease.
Figure 5.
HuR silencing reduces the expression of TSP1 in MCF7 cells. (a) MCF7 cells (Vector and overexpressing MCT-1) were transiently transfected either with control siRNA (Ctrl. siRNA) or siRNA targeting HuR (HuR siRNA). 72 hrs later Western blot analysis of HuR, TSP1, and the loading control α-tubulin was performed. Assays were repeated three times; representative pictures are shown. (b) RT-PCR analysis to monitor total TSP1 mRNA levels by 72 hrs after siRNA transfection. Analysis was performed three times independently. (c) 72 hrs after transfection cells with HuR-targeting (HuR siRNA) or control (Ctrl. siRNA) siRNAs, TSP1 mRNA levels in sucrose fractions were measured as described in legend of Fig. 4c. Graphs depict the means and SEM from three independent experiments. *, p < 0.05. (d) Changes in newly translated TSP1 in cells transfected as in (a) were measured as described for Fig. 4d. Shown are representative results from three separate assays.
Functionally related subsets of mRNAs are regulated by HuR in the MCF7 cells exhibiting an aggressive phenotype
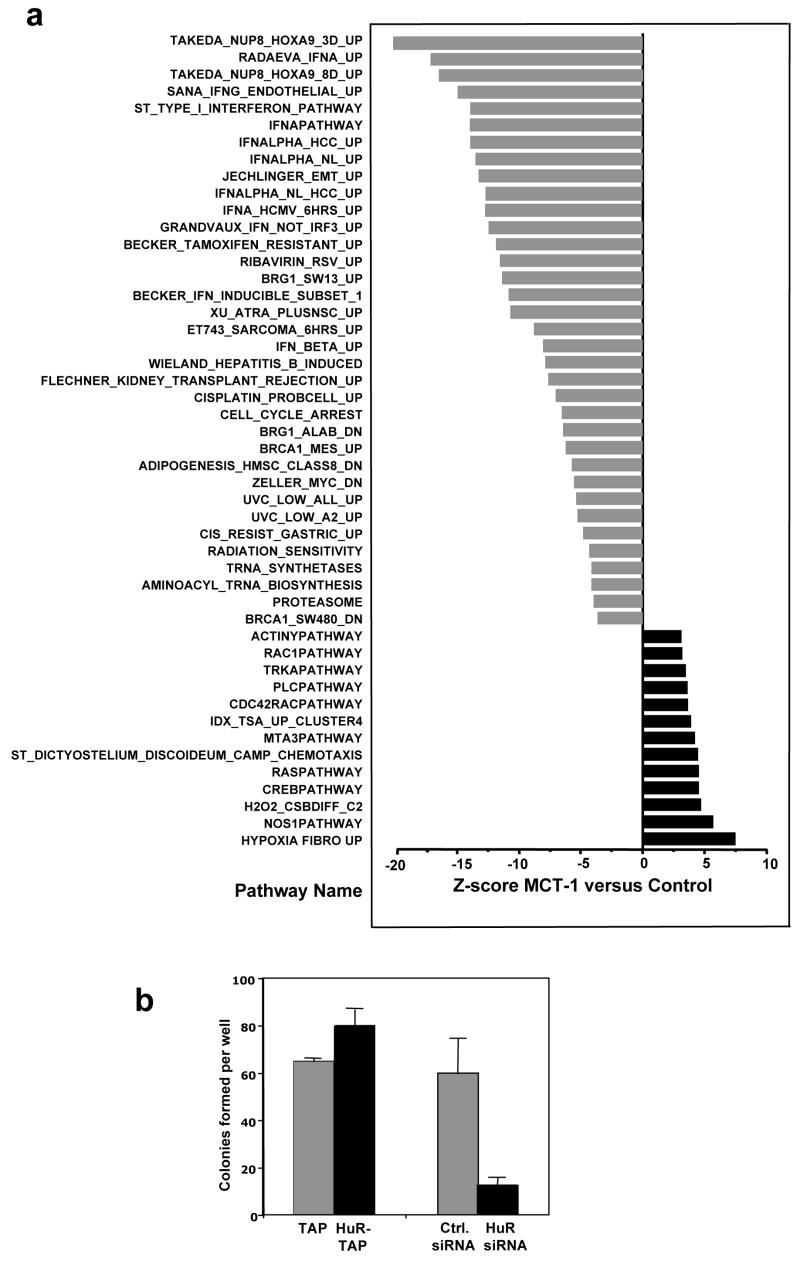
According to the proposed post-transcriptional operon model, functionally related genes are coordinately regulated at the post-transcriptional level through the action of specific RBPs that recognize sequence elements shared among the mRNAs that encode such genes (Keene and Tenenbaum, 2002; Keene et al., 2006). Therefore we sought to investigate if MCT-1 elicited a similar modulation of binding of HuR to target mRNAs associated with the MCT-1 induced tumorigenic phenotype. To this end, we studied the subsets of mRNAs that were co-regulated with TSP1 mRNA through modulation of their association with the HuR in the MCT-1-expressing cells. We performed RNP-IP analysis of MCF7 vector and MCT-1 overexpressing cell lysates using anti-HuR antibody. The identification of mRNAs in each IP group was achieved by reverse transcription followed by cDNA array hybridization (Materials and methods). Microarray analysis showed that out of 24,000 transcript probes available on the microarray ~9,000 mRNAs were associated with HuR and the association of 595 transcripts with HuR was significantly changed (269 reduced, 326 elevated) in more tumorigenic MCT-1 overexpressing cells (Z-ratio difference values greater or less than ±1.5, p value < 0.05). Functional analysis clustered those 595 genes into numerous different biological pathways. In Fig. 6a gray bars correspond to pathways exhibiting genes whose binding to HuR significantly decrease in the MCT-1 overexpression group (Z-score less than −1.5). Several of the pathways represented in these populations are signal transduction cascades implicated in neoplastic transformation, including genes involved in cell cycle arrest, angiogenesis and apoptosis (such as p21/CDKN1A, TSP1 and Bax). Black bars represent pathways containing genes whose association with HuR significantly increases in the MCT-1 overexpression group (Z-score >1.5). Such pathways are involved in survival signaling and proliferation (as PIK3R1/AKT, RAS/MEK/ERK). Importantly, TSP1 mRNA was found among the genes with significantly decreased association with HuR in MCT-1-overexpressing cells compared to vector control populations (Table 1, Supplementary information Table S1). The microarray data provided additional evidence that HuR-TSP1 mRNA complexes dissociated in the more tumorigenic MCF7 populations and further supported the view that HuR is a downstream effector of the pro-tumorigenic gene expression program triggered by MCT-1. See http://www.grc.nia.nih.gov/branches/rrb/dna/index/dnapubs.htm#2 for complete microarray data.
Figure 6.
Influence of HuR on MCT-1-overexpressing MCF7 cells. (a) Top 48 biological pathways with the highest percentage of genes showing significantly altered association with HuR in response to overexpression of MCT-1 in MCF7 cells. Microarray analysis was performed in triplicate and the resulting data were normalized by Z score transformation and tested for significant differences in signal intensity. Differentially expressed genes (with Z ratio >1.5 or <-1.5, and fdr <0.3) were organized by the Ingenuity Pathway Analysis into known biological pathways. Probability scores for each functional grouping were calculated based on the chance of mRNA abundance changes predicting these interactions. The significance of each pathway was calculated by using the right-tailed Fisher’s Exact Test. The p-value was calculated by comparing the number of user-specified genes of interest involved in a given pathway relative to the total number of occurrences of these genes in all pathway annotations stored in the knowledge base. Graph shows pathways in which association with HuR significantly decreased with Z-score <−1.5 (gray bars) or increased with Z-score >1.5 (black bars), p <0.05. Pathways and genes involved are described in Table 1 and Supplementary information (Table S1). (b) Modulation of HuR levels alters anchorage-independent colony growth in MCT-1 overexpressing MCF7 cells. 24 h after transfection cells were cultured in agarose/medium, one week later colonies were counted. Graphs represent the mean and SEM of three independent experiments, p <0.03.
Table 1.
Examples of pathways and input genes with altered association with HuR in more tumorigenic phenotype. Ingenuity Pathway Analysis identified altered functional pathways by categorizing the genes differentially associated with HuR. Arrows indicate an increased or decreased association with HuR in more tumorigenic MCF7 cells.
| Pathway Name | Ontology | Impact Genes | Z-score | % impact genes in pathway |
|---|---|---|---|---|
| ZELLER_MYC_DN | Genes responsive to MYC oncogenic transcription factor | ↓CDKN1A, DUSP1, GADD45A, THBS1, TPM1 | −5.35 | 60.00 |
| PLCPATHWAY | Phospholipase C/PKC/IP3 increase in intracellular calcium levels leading to activation of PI3K/AKT and MAPK | ↑AKT1, PIK3R1, PRKCA | 3.58 | 50.00 |
| TRKAPATHWAY | Promotion of survival and proliferation by activation PI3K/AKT, Ras, and the MAP kinase pathway | ↑AKT1, PIK3R1, PRKCA | 3.58 | 42.86 |
| UVC_LOW_A2_UP | DNA damage response pathways cell cycle arrest and apoptosis after UV irradiation | ↓CDKN1A, GDF15, GPRC5A, SOX4 | −5.18 | 42.86 |
| RADAEVA_IFNA_UP | Regulation of genes contributing to the antiviral and anti-tumor activities of IFN | ↓ADAR, BAX, BST2, EIF2AK2, HLA- E, IFI27, IFI35, IFI44, IFI44L, IFITM1, IFITM2, IFITM3, IRF1, IRF7, MXD4, OAS1, PLSCR1, RSAD2, STAT1, UBE2L6↑UBE2D3 | −17.08 | 32.50 |
| CISPLATIN_PROBCELL_UP | Chemotherapy induced apoptotic pathways | ↓CDKN1A, DHRS3, TMEM49, TP53INP1 | −6.94 | 30.77 |
| CREBPATHWAY | Activation of transcription in response to extra-cellular signaling | ↑AKT1, PIK3R1, PRKCA, RPS6KA5 | 4.54 | 26.67 |
| CELL_CYCLE_ARREST | Halting of the cell cycle during one of the normal phases (G1, S, G2, M) | ↓CDKN1A, EIF4G2, GADD45A, MYC, PPP1R15A↑CDKN3 | −6.36 | 25.00 |
| BRCA1_SW480_DN | BRCA1 induced cell cycle arrest(CDK inhibition) and DNA repair | ↓ACTB, BAX, ZYX | −3.29 | 25.00 |
| TAKEDA_NUP8_HOXA9_8D_UP | Proliferation and differentiation changes that underlie the leukemic transformation | ↓BIRC4BP, DHRS3, FHL2, GDF15, HERC5, HERC6, HOXA5, IFI27, IFI44, IFI44L, IFIH1, IFIT2, IFIT3, IFIT5, IFITM1, IRF7, OAS1, OAS2, OAS3, PALLD, SOX4, STAT1, THBS1↑LOC134147, RP3-477H, RPS6KA5 | −16.47 | 24.00 |
| RAC1PATHWAY | Stimulation of formation of actin- dependent structures | ↑LIMK1, PIK3R1, RPS6KB1↓MYLK | 3.25 | 23.08 |
| RADIATION_SENSITIVITY | Genes related to radiation sensitivity | ↓ACTB, BAX, CDKN1A, GADD45A | −4.11 | 21.43 |
| RASPATHWAY | Ras induced stimulation of signaling cascades, including PI3K/AKT activation to inhibit apoptosis | ↑AKT1, RALA, ELK1, PIK3R1 | 4.51 | 21.05 |
Finally, to demonstrate that altered HuR binding to target mRNAs affects the phenotype of MCT-1 overexpressing MCF7 cells, we carried out soft agar colony formation assays after modulation of HuR levels. As shown, overexpression of HuR increased, while HuR knockdown reduced the ability of MCF7 MCT-1-overexpressing cells to form anchorage-independent colonies (Fig. 6b).
Discussion
We recently demonstrated that MCT-1 promotes the transition to a more aggressive phase in breast cancer progression by increasing tumor angiogenesis. This effect was achieved, at least in part, through the MCT-1-triggered downregulation of an endogenous angiogenesis inhibitor, thrombospondin-1 (TSP1) (Levenson et al., 2005). However, the mechanism(s) underlying the regulation of TSP1 in this model remains unknown. In light of accumulating evidence that post-transcriptional events critically determine gene expression patterns (Orphanides and Reinberg, 2002), we set out to study if the levels of TSP1 were governed by post-transcriptional mechanisms. Using MCF7 cells, we showed that the reduced TSP1 expression in the more aggressive (MCT-1-overexpressing) clones is due to the suppression of TSP1 translation levels, and identify HuR as a major regulatory factor in this process. HuR associated with the 3′UTR of the TSP1 mRNA, which bears U- and AU-rich sequences (ARE) (Fig. 2a), as revealed by IP followed by either RT-qPCR or microarray analysis. The abundance of the [HuR-TSP1 mRNA] complex decreased dramatically in MCT-1-overexpressing more aggressive cells, in turn decreasing TSP1 translation, and leading to a reduction in TSP1 protein abundance in these cells. Experiments in which the entire TSP1 3′UTR was linked to the coding region of the heterologous reporter GFP revealed decreased expression of GFP protein in overexpressing MCT-1 MCF7 cells compared to cells transfected with plasmid containing the GFP coding region alone (Supplementary information, Fig. S2), suggesting that the TSP1 3′UTR functions to suppress TSP1 expression levels specifically in MCT-1-overexpressing cells. Similar results were obtained for the c-fos and c-myc 3′UTR ARE, which destabilized the c-fos and c-myc mRNAs: deletion of this sequence led to increases in mRNA stability, protein abundance, and cellular transformation (Meijlink et al., 1985; Hollis et al., 1988; Aghib et al., 1990).
HuR has been described as a protein that stabilizes many important target mRNAs (i.e. VEGF, c-myc, c-fos, TNF-α, cyclin A, cyclin B1, p21, EGF) (as reviewed Brennan and Steitz, 2001). HuR has also been shown to promote the translation of numerous target transcripts independent of its effect on mRNA stability, p53, ProTα, Cytochrome c, p27Kip1, and TIA-1 (Mazan-Mamczarz et al., 2003; Lal et al., 2005; Millard et al., 2000; Kawai et al., 2006; Pullmann et al., 2007), although in some cases, it has also been shown to repress target mRNA translation (p27Kip1, IGF-IR, Wnt5a, and various cytokines) (Kullmann et al., 2002; Meng et al., 2005; Katsanou et al., 2005; Leandersson et al., 2006). Most of those reports show enhanced binding of HuR to target transcripts (e.g. p21, p53, ProTα, mRNAs) following exposure to different stress stimuli (Wang et al., 2000b; Mazan-Mamczarz et al., 2003; Lal et al., 2005). Here, we found instead a reduction in the association of HuR and the TSP1 mRNA in MCT-1 overexpressing MCF7 cell lines. Importantly, the dissociation of [HuR-TSP1 mRNA] complexes caused TSP1 mRNA to be less efficiently recruited to translationally active polysomes (Fig. 1c), and less actively translated into protein (Fig. 1d). One recent report demonstrated that oxidative stress-triggered dissociation of the [HuR-SIRT1 mRNA] complex promoted SIRT1 mRNA decay (Abdelmohsen et al., 2007). Some reports have begun to emerge documenting the dynamic association of RBPs from target mRNAs under a variety of conditions (Lal et al., 2006; Kim et al., 2007). Tenenbaum et al. (2000) demonstrated the association and dissociation of specific mRNAs from the HuB RNP in embryonic carcinoma cells following treatment with retinoic acid. A recent study showed that UVC irradiation triggered a decrease in the binding of HuR to cyclin D1 mRNA, and instead promoted the binding of AUF1/hnRNP D to this mRNA (Lal et al., 2004). Similarly, HuR transiently dissociated from the cytochrome c mRNA, while the binding of TIA-1 to this transcript increased following endoplasmic reticulum stress (Kawai et al., 2006). Bhattacharyya et al. (2006) demonstrated that the microRNA miR-122 can bind to the CAT-1 3′UTR and repress its translation; HuR was proposed to function by displacing miR-122 from the CAT 3′UTR and thereby relieved the miRNA-imposed translational repression. These studies underscore the existence of a complex and dynamic association of RBPs (as well as miRNAs) with a given target mRNA, which can be modulated by different genotoxic stresses and growth conditions, and can vary from transcript to transcript. We hypothesized that another TTR-RBP(s), likely one that represses translation, might displace HuR from the TSP1 in MCT-1-overexpressing cells and suppress TSP1 expression. Other TTR-RBPs studied showed comparatively less enrichment in TSP1 mRNA (Fig. 2b), although the different relative affinities of the antibodies used preclude a direct comparison of the relative ability of each TTR-RBP to associate with the TSP1 mRNA.
HuR was proposed to play a central role in cancer by binding to mRNAs encoding proteins involved in malignant transformation and altering their expression via post-transcriptional mechanisms (Nabors et al., 2001; Lopez de Silanes et al., 2005). Through its association with target mRNAs, HuR was found to enhance the expression of many of growth-promoting, proliferative, and proto-oncogenic factors like EGF, cyclin A, cyclin B1, and c-myc, (Sheflin et al., 2004; Wang et al., 2000a). It has been postulated that HuR can expand the cell’s invasiveness and metastatic ability by elevating the expression of mRNAs encoding MMP-9 (Akool et al., 2003) and MTA1 (Lopez de Silanes et al., 2004). Finally, HuR was shown to regulate angiogenesis through its binding to mRNAs and elevating of expression of pro-angiogenic genes such as VEGF and HIF-1 (Levy et al., 1998; Sheflin et al., 2004, Galban et al., 2008). Our findings that the dissociation of HuR negatively impacts upon the expression of the anti-angiogenic gene TSP1 are in keeping with the groups of cancer-related mRNAs whose association changes with a more tumorigenic phenotype. Together, an exciting model emerges whereby HuR functions as a downstream effector of cancer-related gene expression. According to this model, in cells with a less tumorigenic phenotype, HuR associates with mRNAs encoding pro-apoptotic proteins (such as TSP1, CDKN1A, and Bax). In cells with a more malignant phenotype, however, HuR switches its mRNA interaction partners, and associates instead with other mRNAs encoding proteins that favor a more tumorigenic phenotype (such as PIK3R1/AKT, RAS/MEK/ERK). A corollary of this model is that MCT-1 functions as a ‘master’ upstream regulator of the subsets of mRNAs with which HuR associates in cells of each phenotype.
Our findings lend strong support to the “post-transcriptional operon” model first proposed by Keene and Tenenbaum (2002). According to this model, RBPs can jointly regulate subsets of mRNAs encoding functionally related proteins. Our microarray analysis yielded 595 transcripts with significantly altered binding to HuR in the more aggressive breast cancer cell lines. These mRNAs clustered in several pathways implicated in malignant transformation/progression, such as the RAS pathway (implicated in mitogenesis), the PI3K pathway (evasion of apoptosis), the hypoxic response, and angiogenesis (Hanahan and Weinberg, 2000). These findings are in keeping with the hypothesis that MCT-1 mediates a more aggressive phenotype in breast carcinoma cells, partially through its ability to re-direct the subsets of mRNAs to be expressed in the cell. Our results reported here demonstrate for the first time that global changes in HuR-bound mRNAs are implicated in the progression to a more malignant phenotype in an in vivo model.
In summary, our work reveals a novel mechanism of regulation of cancer-related genes in MCF7 cells. HuR associates with TSP1 mRNA and enhances its translation, however, in cells overexpressing MCT-1, HuR dissociates from the TSP1 mRNA linked to decreases in TSP1 protein synthesis. Importantly, we show herein that knocking-down HuR has a significant effect on the ability of MCF7 cells to grow in an anchorage-independent manner. These studies underscore the role of global mRNA-protein interactions towards the development of a more malignant phenotype and suggest that interventions targeting mRNA-protein associations may represent novel treatment strategies for cancer therapy. Additional work will provide a better understanding of the role that post-transcriptional gene regulation plays in tumor development and progression.
Materials and methods
Cell culture, plasmids and transient transfections
Mammary epithelial adenocarcinoma MCF7 cells stably transfected with the pLXSN-empty vector (Vector) or pLXSN-MCT-1-V5-histidine (MCT-1) (Levenson et al., 2005), were cultured in Dulbecco’s modified essential medium (Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum and antibiotics.
Plasmids used for HuR overexpression (pcDNA3-TAP) were previously described (Lal et al., 2005). For HuR RNAi transfections siRNA targeting the HuR coding region (AAGAGGCAATTACCAGTTTCA) and a control siRNA (Qiagen) were used at 20 nM. Cells were transfected with Lipofectamine 2000 (Invitrogen) when plasmids were used and with Oligofectamine (Invitrogen) when siRNA were used. Seventy-two hrs after transfection cells were collected for analysis; experiments were performed three times independently.
Cell fractionation
For the whole-cell protein lysates ~3 × 106 cells were trypsinized and prepared in RIPA buffer (10 mM Tris-HCL (pH 7.4), 1% NP-40, 1 mM EDTA, 0.1% SDS, 150 mM NaCl) as described (Lal et al., 2004).
Linear sucrose gradient fractionation was prepared as previously described (Galban et al., 2003) with minor modifications. Briefly, ~ 6 × 106 cells were incubated for 15 min with 100 mg/ml cycloheximide. Cells were lysed in cytoplasmic lysis buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 5 mM MgCl2, 0.3% IGEPAL CA-630, RNaseOUT (Invitrogen) and, protease inhibitor cocktail for 5 min on ice and centrifuged (10,000 × g, 10 min, 4°C). Cytoplasmic extracts were loaded onto the 10–50% sucrose gradients and after centrifugation (Beckman SW41, 35,000 rpm, 3 h, 4°C) the material was fractionated into 1 ml aliquots using a gradient fractionator (Brandel) and monitored by optical density measurement (A254). RNA from fractions was extracted using Trizol reagent.
Western blotting
For Western blot analysis, 50 μg of protein lysates were resolved by SDS-PAGE (Invitrogen, Carlsbad, CA) and transferred onto PVDF membranes. Blots were probed with monoclonal antibodies recognizing TSP1 (NeoMarkers, Fremont, CA), HuR (Santa Cruz Biotechnology, Inc.), β-Actin (Abcam, Cambridge, UK), α-Tubulin (Sigma), V5 (MCT-1-V5) (Invitrogen), or polyclonal AUF1/HRNDP antibody from Upstate Biotech., Lake Placid, NY. Following incubation with appropriate secondary antibodies, signals were detected by enhanced chemiluminescence (Pierce, Rockford, IL) and quantified by using VisionWorksLS Software (UVP, Upland, CA).
Binding assays; immunoprecipitation (IP) of ribonucleoprotein (RNP) complexes and biotin pull-down
IP of endogenous RNA-protein complexes were carried out as previously described (Mazan-Mamczarz et al., 2006) and are explained in detail in Supplementary information. In vitro transcription of TSP1 transcripts was done as described (Mazan-Mamczarz et al., 2006). Oligomers used for amplification of TSP1 fragments for pull-down assay are listed in Supplementary information. After incubating biotinylated transcripts (2 μg) with 40 μg of cytoplasmic lysate, the RBP complexes were isolated with paramagnetic streptavidin-conjugated Dynabeads (Dynal), and the pull-down material was analyzed by Western blotting.
RT-PCR analysis of RNA
RNA from total, polysome or IP material, was reverse transcribed by using iScript cDNA synthesis kit (BioRad, Hercules, CA) following the manufacture’s protocol and subjected to real-time qPCR analysis using gene-specific primer pairs: CAGAGGCCAAAGCACTAAGG and TGAGTAAGGGTGGGGATCAG for TSP1 mRNA and, CGGAGTCAACGGATTTGGTCGTAT and AGCCTTCTCCATGGTGGTGAAGAC GAPDH mRNA. qPCR analysis was performed using iQ™SYBR Green Supermix (BioRad) and BioRad iCycler instrument. Each reaction was carried out in triplicate and three independent experiments were run. GAPDH cDNA served as the internal control.
In vivo translation
Analysis of de novo translation was carried out using methodology previously described (Mazan-Mamczarz et al., 2006). Briefly, 106 cells were incubated with 1 mCi L-[35S]methionine and L[35S]cysteine (Easy Tag ™ EXPRESS, NEN/Perkin Elmer) per 60-mm plate for 20 min, whereupon cells were lysed using RIPA buffer. Immunoprecipitation was carried out overnight at 4°C using TSP1 antibody and IgG as a control. Following extensive washes in TNN buffer (50 mM Tris (pH 7.5), 250 mM NaCl, 5 mM EDTA, 0.5% NP-40) the IP material was resolved by SDS–PAGE, transferred onto PVDF membranes, and visualized by PhosphorImager (Molecular Dynamics).
Microarray analysis after IP
RNA from IP material was labeled using the Illumina Total Prep RNA Amplification Kit (Ambion; Austin, TX). Biotin-labeled cRNA was hybridized to HumanRef-8 v2 Expression BeadChips (Illumina, San Diego, CA) containing 22,000 transcripts probes per sample. The data were normalized by Z-score transformation (Cheadle et al., 2003) and tested for significant differences in signal intensity. Transcripts were considered changed when Z ratios were more then 1.5 or less then −1.5. Ingenuity Systems Pathways Analysis (Ingenuity Systems; Redwood City, CA) was used to identify top network functions involving differentially expressed genes. See Supplementary information and Calvano et al. (2005) for details in array analysis.
Anchorage-independent growth assay
Twenty hours after transfection, cells were plated at 7500 cells/ml in a 1:3 agarose/medium mixture on top of a solidified layer (1:1 agarose/medium) and covered with medium. One week later, colonies were counted under the microscope. Experiments were performed three times independently.
Supplementary Material
Figs S1-S3
Table S1
text
Acknowledgments
We thank Dr Y. Zhang for assistance with the statistical analysis of the RIP–chip array data and Dr J. Hasday for technical support. A Merit Review Award from the Department of Veterans Affairs to RBG supported this work. MG and SS were supported by the National Institute on Aging Intramural Research Program, NIH.
References
- Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;23:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghib DF, Bishop JM, Ottolenghi S, Guerrasio A, Serra A, Saglio G. A 3′ truncation of MYC caused by chromosomal translocation in a human T-cell leukemia increases mRNA stability. Oncogene. 1990;5:707–711. [PubMed] [Google Scholar]
- Akool el-S, Kleinert H, Hamada FM, Abdelwahab MH, Forstermann U, Pfeilschifter J, et al. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol Cell Biol. 2003;23:4901–4916. doi: 10.1128/MCB.23.14.4901-4916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol. 2003;195:356–372. doi: 10.1002/jcp.10272. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. Inflamm and Host Response to Injury Large Scale Collab. Res. Program. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J Mol Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galban S, Martindale JL, Mazan-Mamczarz K, Lopez de Silanes I, Fan J, Wang W, et al. Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Mol Cell Biol. 2003;23:7083–7095. doi: 10.1128/MCB.23.20.7083-7095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbán S, Kuwano Y, Pullmann R, Jr, Martindale JL, Kim HH, Lal A, et al. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1α. Mol Cell Biol. 2008;28:93–107. doi: 10.1128/MCB.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M. HuR in the mammalian genotoxic response: post-transcriptional multitasking. Cell Cycle. 2003;2:412–414. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hollis GF, Gazdar AF, Bertness V, Kirsch IR. Complex translocation disrupts c-myc regulation in a human plasma cell myeloma. Mol Cell Biol. 1988;8:124–129. doi: 10.1128/mcb.8.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, et al. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Kawai T, Lal A, Yang X, Galban S, Mazan-Mamczarz K, Gorospe M. Translational control of cytochrome c by RNA-binding proteins TIA-1 and HuR. Mol Cell Biol. 2006;26:3295–3307. doi: 10.1128/MCB.26.8.3295-3307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JD. Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc Natl Acad Sci USA. 2001;98:7018–7024. doi: 10.1073/pnas.111145598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- Keene JD, Komisarow JM, Friedersdorf MB. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nature Protocols. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
- Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell. 2002;9:1161–1167. doi: 10.1016/s1097-2765(02)00559-2. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kuwano Y, Zhan M, Pullmann R, Jr, Mazan-Mamczarz K, Li H, et al. Elucidation of a C-rich signature motif in target mRNAs of RNA-binding protein TIAR. Mol Cell Biol. 2007;27:6806–6817. doi: 10.1128/MCB.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann M, Gopfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 2002;16:3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Abdelmohsen K, Pullmann R, Kawai T, Galban S, Yang X, et al. Posttranscriptional derepression of GADD45alpha by genotoxic stress. Mol Cell. 2006;22:117–128. doi: 10.1016/j.molcel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Lal A, Kawai T, Yang X, Mazan-Mamczarz K, Gorospe M. Anti-apoptotic function of RNA-binding HuR effected through prothymosin α. EMBO J. 2005;24:1852–1862. doi: 10.1038/sj.emboj.7600661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1/HRNDP to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandersson K, Riesbeck K, Andersson T. Wnt-5a mRNA translation is suppressed by the Elav-like protein HuR in human breast epithelial cells. Nucleic Acids Res. 2006;34:3988–3999. doi: 10.1093/nar/gkl571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. Biol Chem. 1998;273:6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Levenson AS, Thurn KE, Simons LA, Veliceasa D, Jarrett J, Osipo C, et al. MCT-1 oncogene contributes to increased in vivo tumorigenicity of MCF7 cells by promotion of angiogenesis and inhibition of apoptosis. Cancer Res. 2005;65:10651–10656. doi: 10.1158/0008-5472.CAN-05-0845. [DOI] [PubMed] [Google Scholar]
- Liao B, Hu Y, Brewer G. Competitive binding of AUF1/HRNDP and TIAR to MYC mRNA controls its translation. Nat Struct Mol Biol. 2007;14:511–518. doi: 10.1038/nsmb1249. [DOI] [PubMed] [Google Scholar]
- Lopez de Silanes I, Fan J, Galban CJ, Spencer RG, Becker KG, Gorospe M. Global analysis of HuR-regulated gene expression in colon cancer systems of reducing complexity. Gene Expr. 2004;12:49–59. doi: 10.3727/000000004783992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. 2005;2:11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Lal A, Martindale JL, Kawai T, Gorospe M. Translational repression by RNA-binding protein TIAR. Mol Cell Biol. 2006;26:2716–2727. doi: 10.1128/MCB.26.7.2716-2727.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Galban S, Lopez de Silanes I, Martindale JL, Atasoy U, Keene JD, et al. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci USA. 2003;100:8354–8359. doi: 10.1073/pnas.1432104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijlink F, Curran T, Miller AD, Verma IM. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci USA. 1985;82:4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, et al. The ELAV RNA-stability factor HuR binds the 5′-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res. 2005;33:2962–2979. doi: 10.1093/nar/gki603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SS, Vidal A, Markus M, Koff A. AU-richelement in the 50 untranslated region is necessary for the translation of p27 mRNA. Mol Cell Biol. 2000;20:5947–5959. doi: 10.1128/mcb.20.16.5947-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001;61:2154–2161. [PubMed] [Google Scholar]
- Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosniak M, Dierov J, Okami K, Tilton B, Jameson B, Sawaya BE, et al. A novel candidate oncogene, MCT-1, is involved in cell cycle progression. Cancer Res. 1998;58:4233–4237. [PubMed] [Google Scholar]
- Pullmann R, Jr, Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, et al. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27:6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sheflin LG, Zou AP, Spaulding SW. Androgens regulate the binding of endogenous HuR to the AU-rich 3′UTRs of HIF-1alpha and EGF mRNA. Biochem Biophys Res Commun. 2004;322:644–651. doi: 10.1016/j.bbrc.2004.07.173. [DOI] [PubMed] [Google Scholar]
- Tenenbaum SA, Carson CC, Lager PJ, Keene JD. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc Natl Acad Sci USA. 2000;97:14085–14090. doi: 10.1073/pnas.97.26.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000a;19:1–12. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, et al. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000b;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol. 1998;184:119–122. doi: 10.1002/(SICI)1096-9896(199802)184:2<119::AID-PATH17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wilusz CJ, Wilusz J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. doi: 10.1016/j.tig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zabrenetzky V, Harris CC, Steeg PS, Roberts DD. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int J Cancer. 1994;59:191–195. doi: 10.1002/ijc.2910590209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs S1-S3
Table S1
text