Major Role of Cathepsin L for Producing the Peptide Hormones ACTH, β-Endorphin, and α-MSH, Illustrated by Protease Gene Knockout and Expression (original) (raw)
Abstract
The pituitary hormones adrenocorticotropic hormone (ACTH), β-endorphin, and α-melanocyte stimulating hormone (α-MSH) are synthesized by proteolytic processing of their common proopiomelanocortin (POMC) precursor. Key findings from this study show that cathepsin L functions as a major proteolytic enzyme for the production of POMC-derived peptide hormones in secretory vesicles. Specifically, cathepsin L knock-out mice showed major decreases in ACTH, β-endorphin, and α-MSH that were reduced to 23, 18, and 7% of wild-type controls (100%) in pituitary. These decreased peptide levels were accompanied by increased levels of POMC consistent with proteolysis of POMC by cathepsin L. Immunofluorescence microscopy showed colocalization of cathepsin L with β-endorphin and α-MSH in the intermediate pituitary and with ACTH in the anterior pituitary. In contrast, cathepsin L was only partially colocalized with the lysosomal marker Lamp-1 in pituitary, consistent with its extralysosomal function in secretory vesicles. Expression of cathepsin L in pituitary AtT-20 cells resulted in increased ACTH and β-endorphin in the regulated secretory pathway. Furthermore, treatment of AtT-20 cells with CLIK-148, a specific inhibitor of cathepsin L, resulted in reduced production of ACTH and accumulation of POMC. These findings demonstrate a prominent role for cathepsin L in the production of ACTH, β-endorphin, and α-MSH peptide hormones in the regulated secretory pathway.
The peptide hormones ACTH2, β-endorphin, and α-MSH are produced by proteolytic processing of their common proopiomelanocortin (POMC) prohormone precursor (1,2). The mature peptide hormones are stored in pituitary secretory vesicles for regulated secretion and control of physiological functions in target organs. ACTH regulates the production of glucocorticoids in the adrenal cortex for control of metabolism (3,4). α-MSH is implicated in the regulation of appetite and the production of melanin (5,6). β-Endorphin is an endogenous opioid peptide involved in pain regulation (7,8). The proteolytic mechanisms that generate these functionally distinct peptide hormones from POMC are essential for these hormones to exert their biological functions.
The role of cysteine protease activity for POMC processing has been implicated by several studies (9–11), but the identity of the protease has not yet been achieved. In early studies of POMC processing activity in pituitary secretory vesicles, cysteine protease activity represented a significant portion of POMC cleaving activity, based on its inhibition by the thiol reagent p-chloromercuribenzoate (9). In anterior and intermediate pituitary cells in culture, treatment of cells with the cysteine protease inhibitor E64d reduced cell levels of POMC-derived ACTH, β-endorphin, and α-MSH (10,11). Thus, it is likely that a cysteine protease participates in POMC processing. Participation of a cysteine protease in POMC processing would represent a new protease pathway, in addition to the subtilisin-like prohormone convertase enzymes (PC2 and PC1/3) that participate in processing POMC (12–15). Therefore, the goal of this study was to identify the cysteine protease that produces ACTH, β-endorphin, and α-MSH peptide hormones derived from POMC.
Herein we provide evidence indicating a key role for the cysteine protease cathepsin L in the biosynthesis of ACTH, β-endorphin, and α-MSH in secretory vesicles. Cathepsin L gene knock-out mice showed major reductions in pituitary tissue levels of ACTH, β-endorphin, and α-MSH. Furthermore, cathepsin L KO mouse pituitaries displayed accumulation of POMC, consistent with proteolysis of POMC by cathepsin L. In vivo cellular localization of cathepsin L in pituitary cells demonstrated a high degree of colocalization with β-endorphin, α-MSH, and ACTH in secretory vesicles. Expression of the cathepsin L cDNA in pituitary AtT-20 cells resulted in increased production of ACTH and β-endorphin in the regulated secretory pathway. In addition, CLIK-148, a specific inhibitor of cathepsin (16), reduced ACTH production. These gene knock-out and gene expression studies indicate a significant role for cathepsin L in the production of POMC-derived peptide hormones in secretory vesicles.
EXPERIMENTAL PROCEDURES
_Analyses of POMC-derived Peptide Hormones in Cathepsin L Knock-out Mice_—Cathepsin L-deficient mice were generated by gene targeting in mouse embryonic stem cells, as described previously (17,18). Genotyping established cathepsin L gene knock-out (-/-) and wild-type (+/+) mice. Cathepsin L knock-out mice and age-matched wild-type control mice were generated in the C57BL/6J mouse strain. The absence of cathepsin L in the knock-out mice has been confirmed by the absence of cathepsin L mRNA (by Northern blots) and absence of cathepsin L enzyme protein (by Western blots) (18).
Brain tissues from adult mice (∼3 months of age) were collected and homogenized in 1 n acetic acid, heated at 95% for 10 min, and centrifuged (15,000 × g for 15 min), and the supernatant was analyzed for ACTH, β-endorphin, and α-MSH by radioimmunoassays as described previously (12). Protein concentrations in tissue extracts were measured (DC protein assay kit; Bio-Rad). Tissue content of peptide hormones per unit amount of protein was calculated.
Anti-ACTH Western blots were performed for pituitary from cathepsin L gene knock-out mice and wild type animals. Pituitaries were homogenized on ice in radioimmune precipitation buffer (50 mm Tris, 150 mm sodium chloride, 1.0 mm EDTA, 1% Nonidet P-40, and 0.25% sodium deoxycolate, pH 7.0) supplemented with a complete protease inhibitor mixture (Roche Applied Science) and phosphatase inhibitor mixture 1 (Sigma). After incubation at 4 °C for 1 h, followed by a centrifugation step at 10,000 × g at 4 °C for 5 min, protein concentration was measured (Bio-Rad). The homogenate preparation was subjected to SDS-PAGE, transferred to nitrocellulose, and incubated with 5% nonfat dry milk overnight at 4 °C with mouse anti-ACTH (N-terminal (residues 1–24) reactive; Cymbus Biotechnology, Eastleigh, UK) at a dilution of 1:400. The washed blots were then incubated with secondary antibodies conjugated to horseradish peroxidase (sheep anti-mouse; Amersham Biosciences) at a dilution of 1:10,000 for 1 h and developed with the KPL western luminescence ECL plus (Amersham Biosciences) chemiluminescent system.
_Colocalization of Cathepsin L with POMC-derived Peptides in Mouse Pituitary by Immunofluorescence Confocal Microscopy_—Tissues in mice (CD1 Swiss male, adult mice; Charles River Laboratories) were perfused with phosphate-buffered saline (PBS) (intracardially) and then with 4% paraformaldehyde. Pituitary tissue was dissected and fixed in 4% paraformaldehyde overnight and cryoprotected with 30% sucrose. Tissue sections (30 μm) were then incubated and permeabilized with PBS containing 3% bovine serum albumin (BSA) and 0.1% Triton for 1 h at room temperature. Double immunostaining of tissue sections with cathepsin L and each POMC-derived peptide hormone was conducted to assess their colocalization. The extent of colocalization of cathepsin L with the lysosomal marker Lamp-1 was also assessed.
Incubation with primary antibodies consisted of anti-cathepsin L-goat (15 μg/ml; R & D Systems), anti-β-endorphin-guinea pig (1:1000; Bachem-Peninsula Laboratories), anti-ACTH-rabbit (1:1000; NIDDK, National Institutes of Health, Bethesda, MD), anti-α-MSH-rabbit (1:500; Phoenix Pharmaceuticals), or monoclonal anti-Lamp-1-rat (1:100; Abcam). Cathepsin L was detected with secondary anti-goat IgG labeled with Alexa Fluor 488 (green fluorescence; 1:200; Molecular Probes, Eugene, OR); β-endorphin, ACTH, α-MSH, or Lamp-1 was detected with antisera (directed to IgGs from guinea pig, rabbit, or rat, respectively) labeled with Alexa Fluor 594 (red fluorescence; 1:200; Molecular Probes). Tissue sections were incubated overnight at 4 °C with primary antibodies, washed with PBS, and incubated with secondary antibodies for 2 h at room temperature. Sections were mounted on glass slides, coverslipped with mounting medium, and examined with spectral deconvolution confocal microscope FV1000 (Olympus). Images were analyzed with the Olympus Fluoview software.
Information for antibody specificity and controls conducted for immunofluorescence staining were utilized to confirm the specificity of immunostaining. With respect to primary antisera, immunostaining of cathepsin L was blocked by preincubation of anti-cathepsin L with cathepsin L enzyme protein. The antisera to ACTH and α-MSH used in our immunochemical studies have been well characterized for specificity in our radioimmunoassays (12). Anti-ACTH (NIDDK, National Institutes of Health) specifically detects ACTH and does not cross-react with α-MSH or β-endorphin. Anti-α-MSH (Phoenix, CA) specifically detects α-MSH and does not cross-react with ACTH, β-endorphin, or (Met)enkephalin. The specificity of anti-β-endorphin has been determined (by Bachem-Peninsula Laboratories), and it detects β-endorphin and does not cross-react with ACTH, α-MSH, or (Met)enkephalin. In addition, the anti-Lamp-1 is a monoclonal antibody that is directed to mouse Lamp-1 (Abcam).
With respect to secondary antisera, specificities have been determined by assessing immunofluorescence with incubation with secondary antisera alone (i.e. without primary antisera), which results in lack of immunofluorescence staining. For double immunostaining procedures to detect two antigens (“a” and “b”), the secondary antisera to antigen “a” has been tested for lack of cross-reactivity to antigen “b”, and the secondary antisera to antigen “b” has been tested for lack of cross-reactivity to antigen “a”. These controls have been conducted for all colocalization experiments of cathepsin L colocalization with POMC-derived peptide hormones and controls that compared cathepsin L and Lamp-1 localization.
_Evaluation of Cathepsin L Colocalization with ACTH in Mouse Pituitary AtT-20 Cells by Immunofluorescence Confocal Microscopy_—AtT-20 cells were cultured as previously described (19,20) on polylysine-coated glass chamber slides (Nalge Nunc International) and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After permeabilization with Triton X-100 (0.1%), cells were incubated with PBS containing 3% BSA for 30 min at room temperature. Cells were then double-stained for 2 h at room temperature in PBS plus 3% BSA with primary antibodies anti-cathepsin L-rabbit (1:50; Athens Research and Technology, Athens, GA) and anti-ACTH-mouse (1:100; Abcam). Primary antibodies were revealed by the secondary antibodies in PBS plus 3% BSA with goat anti-rabbit Alexa Fluor 488 (green fluorescence; 1:200; Molecular Probes) and goat anti-mouse Alexa Fluor 594 (red fluorescence; 1:200; Molecular Probes) for 45 min at room temperature. Slides were mounted with Aqua Poly/Mount (Polysciences) and images were taken with the microscope Delta Vision Spectris Image Deconvolution System on an Olympus IX70 using the software Softworx Explorer from Applied Precision.
Expression of Cathepsin L in AtT-20 Cells and Analyses of ACTH and β_-Endorphin Production in the Regulated Secretory Pathway_—Cathepsin L cDNA was expressed in AtT-20 cells to assess effects on production of ACTH and β-endorphin in the regulated secretory pathway. Effective expression of transfected cathepsin L in a neuroendocrine cell line has been demonstrated (21).
Cells were plated 1 day before transfection in 6-well plates (Corning Glass) and were at ∼70% confluence when transfected with the preprocathepsin L cDNA/pcDNA3.1 (bovine) (21) or pcDNA3.1 vector alone (at 2 μg of DNA/well) with transfection reagent Fugene HD (8 μl; Roche Applied Science). Two days after transfection, cells were incubated with BaCl2 (1 mm) for 2 h to stimulate regulated secretion of ACTH and β-endorphin, measured by radioimmunoassays as previously described (12). Expression conditions were each conducted in replicate wells (six replicates), and experiments were repeated two times. Results are expressed as the mean ± S.E. of ACTH or β-endorphin secreted/well. Cell extracts were also analyzed by anti-ACTH Western blots (anti-ACTH from NIDDK), performed as described previously (12).
Expression of cathepsin L activity in cell extracts was confirmed by monitoring benzyloxycarbonyl-Phe-Arg-MCA cleaving activity that is inhibited by the specific cathepsin L inhibitor CLIK-148 (16). Cells were harvested by homogenization in cathepsin L assay buffer (0.1 m sodium acetate, pH 5.5, 1 mm EDTA, and 4 mm dithiothreitol). Homogenate was sonicated and centrifuged (13,000 × g). Cathepsin L activity was monitored as the amount of activity (37 °C for 15 min) detected with the substrate benzyloxycarbonyl-Phe-Arg-MCA (16) that is inhibited by CLIK-148 (50 μm).
_Expression of Cathepsin L/EGFP Fusion Protein_—To demonstrate expression from the CMV promoter-driven plasmid vector in AtT-20 cells, expression of cathepsin L was monitored as a fusion protein with EGFP to distinguish it from endogenous cathepsin L. Expression of the cathepsin L/EGFP protein allowed its detection by anti-EGFP in Western blot to demonstrate expression.
Specifically, the bovine cDNA encoding preprocathepsin L was amplified by reverse transcription-PCR from preprocathepsin L cDNA/cDNA3.1 with Pfx DNA polymerase (Invitrogen) utilizing primers consisting of 5′-AAAAAGCTAGCATGAATCCTTCATTCTTCCTGACTGTCCTTTGCTTG-3′ (with the NheI site underlined) for the sense primer and 5′-AAAAACTCGAGAACAGTTGGATAGCTGGCTGCTGTGGCAATT-3′ (with XhoI site underlined) for the antisense primer, under PCR cycle conditions of 94 °C for 45 s, 55 °C for 1 min, and 68 °C for 1 min, for a total of 35 cycles. The amplified preprocathepsin L cDNA fragment (size of about 1.0 kb) was double-digested with NheI/XhoI and ligated to NheI/XhoI-digested pEGFP-N1 plasmid expression vector (Clontech). This construct was subjected to DNA sequencing (Davis DNA Sequencing Inc.) to verify the nucleotide sequence and deduced primary amino acid sequence of the bovine preprocathepsin L cDNA.
Preprocathepsin L/EGFP fusion protein plasmid expression vector was transfected in AtT-20 cells with the same conditions as for preprocathepsin L cDNA, described above. Two days after transfection, cells were harvested, and homogenates were prepared for Western blots, as described previously (12). Samples were subjected to electrophoresis on 4–12% BisTris gels (Invitrogen), and proteins were transferred electrophoretically to nitrocellulose membranes (Amersham Biosciences). Membranes were blocked in 3% BSA in TBST buffer (20 mm Tris-HCl, pH 7.4, 0.5 m NaCl, 0.05% Tween 20) and were incubated with a mouse anti-EGFP antibody (Abcam) at dilution of 1:500 in TBST overnight at 4 °C. After washing in TBST and incubation with sheep anti-mouse-horseradish peroxidase (GE Healthcare) for 1 h followed by washing, the membrane was developed by ECL plus Western blotting detection systems (GE Healthcare).
_Treatment of AtT-20 Cells with CLIK-148, Inhibitor of Cathepsin L_—AtT-20 cells were treated with the specific inhibitor of cathepsin L, CLIK-148 (16) (50 μm) (from Enzyme Systems Products, Livermore, CA). Triplicate cell samples of CLIK-148-treated and -untreated conditions were prepared. After treatment, cells were harvested by preparation of acetic acid extracts (as described previously (12)) for measurements of ACTH content by radioimmunoassay. Protein content in extracts was determined (DC protein assay kit; Bio-Rad). The amount of ACTH (pg) per unit amount of protein was calculated. This experiment was repeated three times. Analyses of cells included anti-ACTH Western blots, performed as described previously (12).
_Statistical Evaluation of Results_—Experiments were conducted with an appropriate number of replicate samples, consisting of 8–10 cathepsin L knock-out mice and 8–10 wild-type age-matched control mice. This experiment was repeated twice. AtT-20 cell experiments were conducted in triplicate samples for each condition, and experiments were repeated two or three times. Differences in peptide hormone levels were evaluated for statistical significance with Student's t test, with p < 0.05.
RESULTS
_Cathepsin L Gene Knock-out Mice Show Substantial Reduction of POMC-derived Peptide Hormones in Pituitary_—The peptide hormones ACTH, β-endorphin, and α-MSH are derived from the common POMC prohormone precursor by proteolytic processing. They are especially important as pituitary hormones and are highly expressed in this tissue. To determine the role of cathepsin L in the production of these POMC-derived peptide hormones, cathepsin L gene knock-out (-/- genotype) mice were analyzed for pituitary levels of these peptide hormones, with comparison with wild-type controls (+/+ genotype).
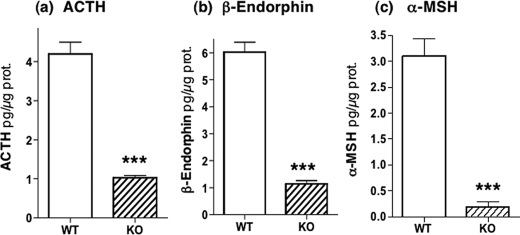
Notably, all POMC-derived peptide hormones were substantially reduced in the cathepsin L knock-out mice compared with controls (Fig. 1). ACTH levels in cathepsin L KO mice were reduced to 23% of control levels (100%). β-Endorphin levels in pituitaries of cathepsin L KO mice were reduced to 18% of control levels (100%), and α-MSH was reduced in KO mice to 7% of control levels (100%). These data demonstrate a key role for cathepsin L in the production of POMC-derived peptide hormones.
FIGURE 1.
Cathepsin L knock-out mice show substantial reduction of POMC-derived ACTH, β-endorphin, and α-MSH peptide hormones in pituitary. Pituitaries from cathepsin L knock-out mice (eight mice) and from wild-type control mice (10 mice) were dissected and prepared as acid extracts for radioimmune assay quantitation of the peptide hormones ACTH, β-endorphin, and α-MSH with results shown in a, b, and c, respectively. The mean ± S.E. are shown. Comparisons of cathepsin L KO with wild-type (WT) mice showed highly significant differences (indicated by ***) between these groups for ACTH, β-endorphin, and α-MSH, which showed p < 0.001 (by Student's t test).
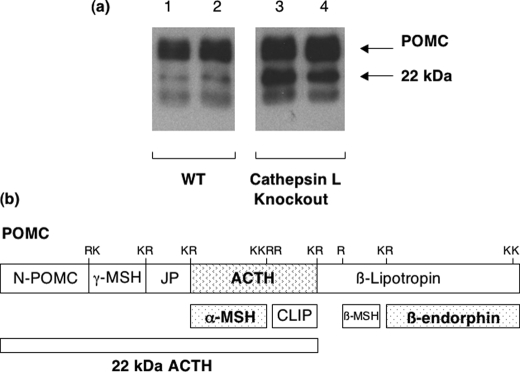
In parallel to the decreased levels of POMC-derived peptide hormones, cathepsin L KO mice showed increased levels of POMC, detected by anti-ACTH Western blots, compared with wild-type controls (Fig. 2). Accumulation of POMC in the cathepsin L knock-out is consistent with its role as a substrate for cathepsin L. The increase in POMC was not due to changes in its mRNA, since POMC mRNA levels in pituitaries of cathepsin L knock-out compared with wild-type controls were the same.3 The accumulation of POMC in the cathepsin L KO condition (compared with control wild-type mice) suggests a role for proteolysis of POMC by cathepsin L.
FIGURE 2.
Accumulation of POMC in pituitary of cathepsin L KO mice. a, anti-ACTH Western blots reveal accumulation of POMC and 22-kDa product in cathepsin L KO mice. Analyses by anti-ACTH Western blots of pituitary extracts from cathepsin L KO and wild-type mice were performed to investigate relative levels of POMC. Representative blots are shown for two samples of pituitary extracts from two KO mice and from two wild-type (WT) mice (25 μg of protein/lane). Cathepsin L KO mice showed increases in intact POMC and the POMC-derived 22-kDa ACTH intermediate.b, POMC-derived 22-kDa intermediate that contains ACTH. An illustration of the POMC prohormone shows the predicted domains for ACTH, β-endorphin, α-MSH, and the 22-kDa ACTH intermediate.
In addition, increased levels of the POMC-derived 22-kDa ACTH intermediate were observed in pituitaries of cathepsin L KO mice (compared with wild type). Such results implicate cathepsin L in modifying ACTH production from POMC.
In the cathepsin L KO mice, there was no change in levels of PC1/3 or PC2 protein or enzyme activities in pituitaries of cathepsin L KO mice. These controls indicated that there was no detectable compensatory change in PC1/3 or PC2 enzymes.3 Thus, the significant reduction of ACTH, β-endorphin, and α-MSH is consistent with a role of cathepsin L for production of ACTH, β-endorphin, and α-MSH peptide hormones.
Cathepsin L Colocalizes in Vivo with β_-Endorphin,_ α_-MSH, and ACTH in Secretory Vesicles of Mouse Pituitary; Comparison with the Lamp-1 Marker for Lysosomes_—A role for cathepsin L in the production of POMC-derived peptide hormones requires localization of cathepsin L with these peptide hormones in secretory vesicles, the primary subcellular site for peptide hormone production, storage, and secretion. Therefore, colocalization studies of cathepsin L with POMC-derived peptide hormones in pituitary (normal wild-type mouse) were conducted by immunofluorescence confocal microscopy.
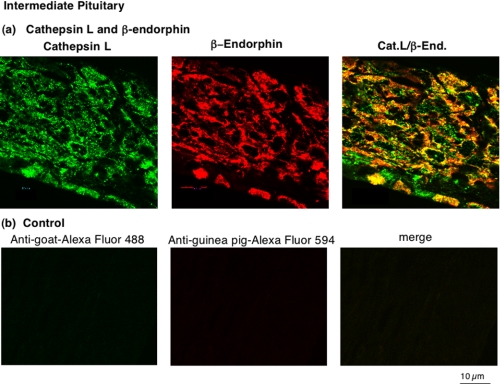
In intermediate pituitary, cathepsin L was colocalized with β-endorphin that is present in secretory vesicles (Fig. 3_a_). Cathepsin L immunofluorescence staining was observed as a punctate pattern of staining in the perinuclear areas of intermediate pituitary cells. The colocalization of cathepsin L with β-endorphin was illustrated by the merged images showing areas of colocalization as yellow fluorescence. All β-endorphin subcellular areas showed the presence of cathepsin L. Controls with only secondary antisera resulted in the absence of immunofluorescence staining (Fig. 3_b_). These results show selective immunofluorescence detection of cathepsin L and β-endorphin by primary antisera.
FIGURE 3.
In vivo colocalization of cathepsin L with β-endorphin present in secretory vesicles of intermediate pituitary. a, colocalization of cathepsin L with β-endorphin. The colocalization of cathepsin L with β-endorphin in intermediate pituitary (mouse) was demonstrated by immunofluorescence confocal microscopy. Cathepsin L immunoreactivity (green fluorescence) showed excellent overlapping colocalization with β-endorphin (red fluorescence), shown by the_yellow fluorescence_ of merged cathepsin L/β-endorphin fluorescent immunostaining. The majority of β-endorphin, contained in secretory vesicles, was colocalized with cathepsin L. b, controls show lack of immunostaining with only secondary antibody. As control, tissue sections were incubated only secondary antibody, without primary antisera to peptide hormones. The secondary anti-goat-Alexa Fluor 488 alone (without anti-cathepsin L) resulted in a lack of immunofluorescence staining. The secondary anti-goat-Alexa Fluor 594 (without anti-β-endorphin) also resulted in lack of immunofluorescence staining. The merged image of these two secondary antisera showed lack of immunofluorescence staining. These controls illustrate specific immunofluorescence staining by the primary antisera to the peptide hormones (a).
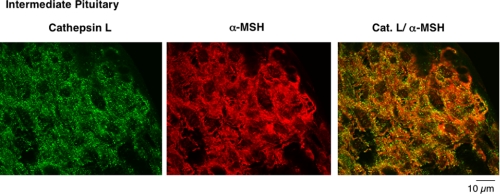
Results also showed excellent colocalization of cathepsin L with α-MSH in intermediate pituitary (Fig. 4). Cathepsin L localization (green fluorescence) with α-MSH (red fluorescence) was illustrated by their merged images showing areas of colocalization by yellow fluorescence.
FIGURE 4.
Colocalization of cathepsin L with α-MSH present in secretory vesicles of intermediate pituitary. The overlapping colocalization of cathepsin L with α-MSH peptide hormone in intermediate pituitary was illustrated by immunofluorescence confocal microscopy. The majority of cathepsin L (green fluorescence) and α-MSH (red fluorescence) was colocalized, shown by the merged areas of yellow fluorescence. Controls with only secondary antisera showed lack of immunofluorescence staining (like the results ofFig. 3_b_). Thus, primary antisera detected cathepsin L colocalization with α-MSH.
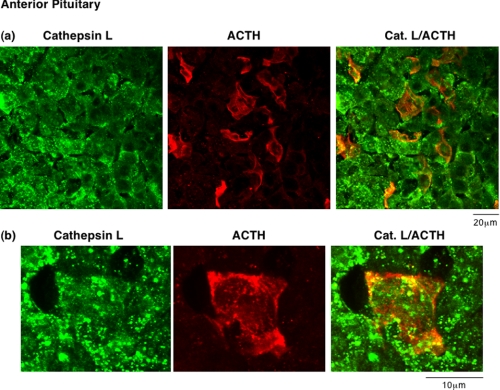
In mouse anterior pituitary, cathepsin L was colocalized with ACTH-containing cells (Fig. 5). The pattern of ACTH immunostaining was consistent with the knowledge that ACTH corticotroph cells comprise 3–5% of cells in anterior pituitary (3,4). Immunostaining showed that all ACTH-containing cells show colocalization of cathepsin L and ACTH. Furthermore, cathepsin L was present in other cell types of anterior pituitary that are known to produce other anterior pituitary hormones (3,4). The overlapping localization of ACTH and cathepsin L indicated the presence of cathepsin L in ACTH-containing secretory vesicles.
FIGURE 5.
In vivo colocalization of cathepsin L with ACTH present in secretory vesicles of anterior pituitary. ACTH-containing cells of anterior pituitary were indicated by ACTH immunofluorescence staining (red fluorescence). The areas of ACTH showed colocalization with cathepsin L (green fluorescence), shown by the merged areas of yellow fluorescence. It is known that only a few percent of anterior pituitary cells are represented by the corticotroph cells that contain ACTH. The_upper_ and lower sections (a) and (b) represent images of different magnification (see distance bars), with_b_ showing a larger magnification. Controls with only secondary antisera showed lack of immunofluorescence staining. Thus, results show colocalization of cathepsin L with ACTH.
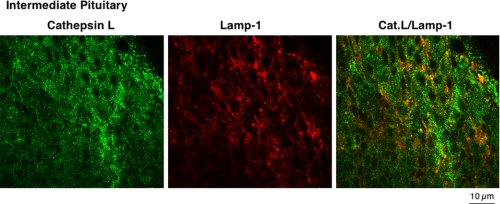
It was of interest that a large portion of cathepsin L was colocalized with β-endorphin and α-MSH in intermediate pituitary, suggesting perhaps greater localization of cathepsin L with peptide hormone-containing secretory vesicles compared with the presence of cathepsin L in lysosomes. Direct assessment of the localization of cathepsin L with the lysosomal marker Lamp-1 showed that cathepsin L partially colocalized with Lamp-1 in intermediate pituitary (Fig. 6). Similar results for partial colocalization of cathepsin L with Lamp-1 in anterior pituitary were also observed (data not shown). These findings support results of this study showing a high degree of cathepsin L localization in peptide hormone-containing secretory vesicles in pituitary.
FIGURE 6.
Lack of complete localization of cathepsin L with the lysosomal marker Lamp-1 in pituitary. Comparison of the cellular distribution of cathepsin L in intermediate lobe pituitary cells was compared with that of the lysosomal marker Lamp-1. Punctate-like immunofluorescence staining of cathepsin L (green fluorescence) was only partially colocalized with Lamp-1 (red fluorescence). This subcellular distribution of cathepsin L is consistent with the localization of cathepsin L in secretory vesicles as well as in lysosomes. Controls with only secondary antisera showed lack of immunofluorescence staining. Results show cathepsin L localization to extralysosomal areas of the cell, consistent with its observed localization in secretory vesicles.
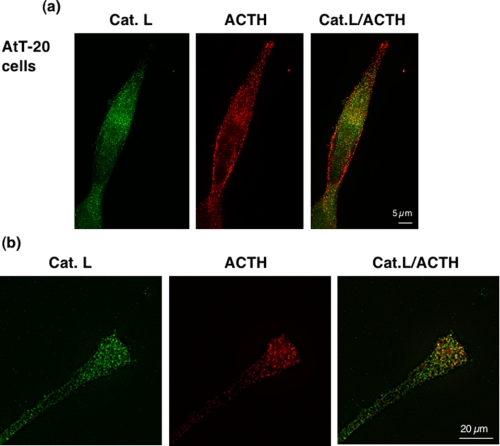
Expression of Cathepsin L Increases ACTH and β_-Endorphin Production in the Regulated Secretory Pathway of Pituitary AtT-20 Cells_—The mouse pituitary AtT-20 cell line produces ACTH and β-endorphin from POMC (19,20). The homogeneous nature of a clonal cell line allowed further assessment of the colocalization of cathepsin L with ACTH. The punctate immunostaining of cathepsin L was colocalized with ACTH (Fig. 7_a_). In neuritic-like extensions of AtT-20 cells, ACTH-containing secretory vesicles contained cathepsin L (Fig. 7_b_).
FIGURE 7.
Cathepsin L colocalization with ACTH in AtT-20 mouse pituitary cells. a, colocalization of cathepsin L with ACTH in the AtT-20 pituitary cell line. Cathepsin L in the AtT-20 mouse pituitary cell line was colocalized with ACTH, demonstrated by dual imunofluorescence confocal microscopy (a and b). Cathepsin L (green fluorescence) showed a punctate pattern of subcellular immunostaining that showed overlapping localization with areas of ACTH immunostaining (red fluorescence), shown by the merged images, with yellow fluorescence indicating colocalization. Controls with only secondary antisera showed the absence of immunofluorescence staining. b, cathepsin L localization with ACTH in neuritic extensions of AtT-20 cells. Examination of the neuritic extensions of AtT-20 cells shows the presence of cathepsin L colocalized with ACTH. Controls with only secondary antisera showed lack of immunofluorescence staining.
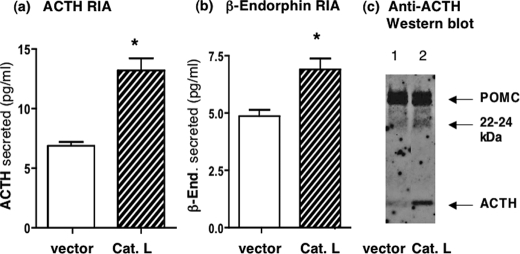
Expression of cathepsin L in AtT-20 cells was conducted to demonstrate the role of cathepsin L for production of ACTH and β-endorphin. Expression of cathepsin L resulted in significantly increased amounts of ACTH and β-endorphin secreted from the regulated secretory pathway (Fig. 8, a and_b_). Secretion from the regulated secretory pathway was conducted in the presence BaCl2, which is known to stimulate regulated secretion (22). Analyses of cell extracts with anti-ACTH Western blots showed that expression of cathepsin L resulted in elevated levels of ACTH (Fig. 8_c_).
FIGURE 8.
Expression of cathepsin L increases production of ACTH andβ-endorphin in the regulated secretory pathway of AtT-20 cells. a and b, ACTH and β-endorphin production by cathepsin L. Expression of cathepsin L (bovine) in AtT-20 cells by transfection resulted in increased amounts of ACTH (a) and β-endorphin (b) produced in regulated secretory vesicles whose contents were released in the presence of BaCl2. Cells were transfected at 70% confluence with cathepsin L/pcDNA3.1 or pcDNA3.1 vector alone, and 2 days later, cells were incubated in BaCl2 for 2 h, and the medium was collected for radioimmune assay measurements of ACTH and β-endorphin. BaCl2 is a stimulator of regulated secretion (22). Expression of cathepsin L resulted in increased production of ACTH and β-endorphin in regulated secretory vesicles, whose secretion was observed in the presence of BaCl2. Results are shown as the mean ± S.E.(n = 6 for each group, and the experiment was repeated twice) with significant increases of ACTH and β-endorphin produced after expression of cathepsin with p < 0.05 (by Student's t test). c, cathepsin L expression and analysis by anti-ACTH Western blots. Western blots with anti-ACTH of AtT-20 cells transfected with the cathepsin L cDNA (lane 2) or with control vector without cDNA (lane 1) showed that cathepsin L-transfected cells displayed increased amounts of ACTH (lane 2)(2μg of protein/lane).
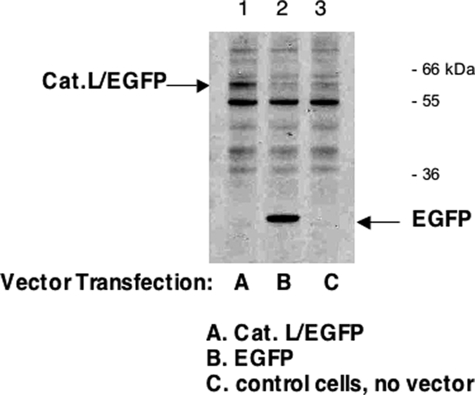
As a control experiment, cathepsin L expression was demonstrated by transfection of the cathepsin L/EGFP fusion protein (Fig. 9). Western blot detection of the cathepsin L/EGFP fusion protein confirmed expression with the CMV promoter plasmid expression vector. In addition, transfection of cathepsin L cDNA by CMV plasmid expression vector resulted in elevated levels of cathepsin L activity. Overall, expression of cathepsin L resulted in increased production of ACTH and β-endorphin.
FIGURE 9.
Expression of cathepsin L/EGFP fusion protein. Expression of cathepsin L by the CMV-driven promoter of the expression plasmid was monitored by demonstrating expression of the cathepsin L/EGFP fusion protein in the pEGFP-NI plasmid expression vector, which distinguishes expressed cathepsin L from endogenous cathepsin L. Two days after transfection, expression of cathepsin L/EGFP was monitored by Western blots with anti-EGFP. Western blots showed expression of cathepsin L/EGFP of an apparent molecular mass (∼60 kDa) (lane 1) that is consistent with the combined molecular masses of cathepsin L and EGFP (60 kDa) (indicated by an arrow). The ∼60 kDa band was absent in cells transfected with pEGFP-NI plasmid alone (no cathepsin L) (lane 2); these cells demonstrated expression of EGFP (indicated by an arrow). Cells that were not subjected to transfection did not show cathepsin L/EGFP or EGFP bands (lane 3). It is noted that a band at ∼55 kDa probably represents a nonspecific band detected by anti-EGFP, since it is present in untransfected and transfected cells in all three lanes. Comparison of lane 1 with lanes 2 and 3 demonstrates expression of cathepsin L/EGFP (lane 1).
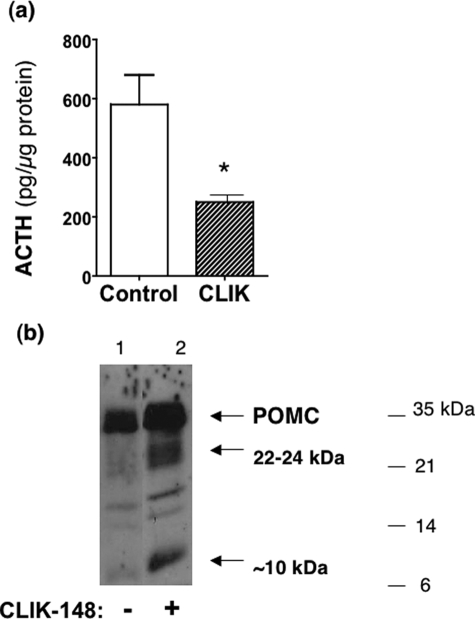
Treatment of AtT-20 cells with CLIK-148, inhibitor of cathepsin L, reduced ACTH production. The specific inhibitor of cathepsin L, known as CLIK-148, was utilized to assess its effects on ACTH production in AtT-20 cells. After treatment of cells with CLIK-148, ACTH content in cells was reduced by ∼60% compared with untreated controls (Fig. 10_a_). Furthermore, POMC processing was inhibited by CLIK-148, indicated by accumulation of POMC in cells treated with CLIK-148 (Fig. 10_b_). Treatment with CLIK-148 also resulted in accumulation of the 22–24-kDa POMC-derived intermediate. These results are consistent with proteolytic processing of POMC by cathepsin L.
FIGURE 10.
CLIK-148, inhibitor of cathepsin L, reduces ACTH production in AtT-20 cells. a, reduction of ACTH after treatment with CLIK-148. After treatment of AtT-20 cells with and without CLIK-148 (for 5 days), ACTH content in cells (in acetic acid extracts) was measured by a radioimmune assay.*, statistical significance between inhibitor and control groups (p < 0.05, based on Student's t test). b, CLIK-148 treatment results in accumulation of POMC and a 22-kDa intermediate. Anti-ACTH Western blots of cells (5 μg of protein/lane) of CLIK-148-treated cells showed accumulation of POMC (lane 2) compared with untreated control cells (lane 1). In addition, 22- and 10-kDa intermediates also accumulated in CLIK-148-treated cells compared with untreated controls.
DISCUSSION
The biosynthesis of the peptide hormones ACTH, β-endorphin, and α-MSH from their common POMC precursor is critical for targeted regulation of neuroendocrine systems. Herein we provide evidence that the cysteine protease cathepsin L plays a key role in the production of pituitary ACTH, β-endorphin, and α-MSH from the POMC precursor. In vivo localization studies of intermediate and anterior pituitary showed that cathepsin L possesses a high degree of colocalization with these POMC-derived peptide hormones that are produced and stored within secretory vesicles. In fact, a higher portion of cathepsin L in these regions of pituitary was localized with peptide hormone-containing secretory vesicles, compared with lysosomes. On a functional level, cathepsin L gene knock-out mice showed substantial reduction of POMC-derived peptide hormones in pituitary that were reduced to 23, 18, and 7% of control levels (100%) for ACTH, β-endorphin, and α-MSH, respectively. The increase in POMC and changes in the 22-kDa ACTH-containing intermediate are consistent with a role for cathepsin L in proteolysis of POMC to generate peptide hormones. Expression of cathepsin L in the mouse corticotroph pituitary cell line AtT-20 resulted in increased production of ACTH and β-endorphin in the regulated secretory pathway. Furthermore, inhibition of cathepsin L by the specific inhibitor CLIK-148 (16) resulted in decreased ACTH and accumulation of POMC in AtT-20 cells. These novel findings demonstrate that cathepsin L functions as a significant cysteine protease pathway for production of ACTH, β-endorphin, and α-MSH from their common POMC precursor.
Combined in vivo mouse studies and in vitro cellular studies suggested that POMC undergoes proteolysis by cathepsin L. Furthermore, treatment of pituitary AtT-20 cells with CLIK-148, a specific inhibitor of cathepsin L (16), resulted in accumulation of POMC that is consistent with inhibition of POMC processing by the inhibitor. These findings provide evidence for a role of cathepsin L in processing POMC that produces the peptide hormones ACTH, β-endorphin, and α-MSH.
The proteolytic processing of POMC has been studied as a model prohormone for investigating protease pathways for its conversion into active peptide hormones. The finding of secretory vesicle cathepsin L in this study for the production of POMC-derived peptide hormones indicates cathepsin L as a newly identified protease pathway for POMC processing in addition to the known functions of the subtilisin-like proprotein convertases PC2 and PC1/3 for POMC processing (12–15). Notably, cathepsin L knock-out mice displayed greater reduction in ACTH and β-endorphin compared with that in PC2 or PC1/3 knock-out mice (12,13,15,23). The cathepsin L knock-out and the PC2 knock-out mice (12,13,24) both show substantial reductions in α-MSH. Clearly, cathepsin L plays a major role in the production of all three peptide hormones derived from POMC.
In PC2 KO mice, reduction of α-MSH levels in PC2 KO mice (12,13,24) suggests that PC2 is necessary for α-MSH production. However, elevated levels of ACTH (12,13) and β-endorphin-(1–31) (12,23) in the PC2 knock-out indicate that these peptides serve as substrates for PC2. In PC1/3 knock-out mice, minimal effects on POMC-derived peptide hormones were observed (15). Comparison of protease gene knock-out studies suggests the joint participation of cathepsin L and PC2 as processing proteases for production of ACTH, β-endorphin, and α-MSH peptide hormones derived from POMC.
One of the notable findings from this study is the demonstration that cathepsin L functions in secretory vesicles for producing peptide hormones, which contrasts with the well known role of cathepsin L in lysosomes for protein degradation (25,26). Results from this study demonstrate colocalization of cathepsin L with all three POMC-derived peptide hormones consisting of ACTH, β-endorphin, and α-MSH in pituitary. Furthermore, cathepsin L was only partially colocalized with the lysosomal marker Lamp-1 in intermediate pituitary, which indicates function of cathepsin L in organelles other than lysosomes (i.e. in secretory vesicles). Furthermore, cathepsin L expression in AtT-20 cells resulted in increased production of ACTH and β-endorphin in the regulated secretory pathway.
In addition to the biosynthesis of the POMC-derived peptide hormones, the role of cathepsin L in the production of peptide neurotransmitters has been demonstrated for conversion of proenkephalin into active enkephalin opioid peptide neurotransmitters (21,27). In keeping with this notion, brains of cathepsin L gene knock-out mice show reduced levels of (Met)enkephalin that are 50% lower compared with wild-type controls (27). In addition, coexpression of cathepsin L with proenkephalin in PC12 neuroendocrine cells resulted in production of processed enkephalin (21). In addition to cathepsin L, PC2 also participates in enkephalin production, as demonstrated in PC2 knock-out mice that show reduction of brain (Met)enkephalin by 50%. These results implicate dual roles for both cathepsin L and PC2 in enkephalin peptide neurotransmitter production. However, little change in enkephalin levels has been observed in brains of PC1/3 knock-out mice.4 These findings indicate that selected protease components of the dual cathepsin L and PC protease pathways may be utilized for proteolytic processing of proenkephalin or other proneuropeptides and prohormones.
Cathepsin L possesses specificity for processing paired basic residue-processing sites of prohormones and proneuropeptides. Notably, cleavage site studies with peptide-MCA substrates containing dibasic residue-processing sites indicated that cathepsin L prefers to cleave at the N-terminal side of dibasic residues as well as between the dibasic residues (28). In contrast, similar evaluation of PC1/3 and PC2 with dipeptide-MCA substrates showed that these PC proteases prefer to cleave at the C-terminal side of dibasic residue-processing sites (29). These data indicate that, following cathepsin L cleavage, removal of the remaining basic residue extensions at the N terminus of peptide products can be accomplished by Arg/Lys aminopeptidase activity. Arg/Lys aminopeptidase activity is present in pituitary secretory vesicles (30), and this activity has been identified as aminopeptidase B in neuropeptide-containing secretory vesicles of neuroendocrine chromaffin cells (31).
In contrast to cathepsin L, evaluation of PC1/3 and PC2 with dipeptide-MCA substrates showed that these PC proteases prefer to cleave at the C-terminal side of dibasic residue-processing sites (29). Thus, processing of prohormones by PC2 or PC1/3 results in peptide intermediates with basic residue extensions at the C terminus, which are then removed by the neuroendocrine-specific carboxypeptidase E/H enzyme (32–35). The different exopeptidase steps following prohormone processing by cathepsin L or the PC enzymes implicate extended diversity in proteolytic pathways that may allow several steps for protease regulation in peptide hormone production.
In summary, this study demonstrates that cathepsin L in secretory vesicles functions as a key protease for the production of POMC-derived peptide hormones ACTH, β-endorphin, and α-MSH. These findings indicate the distinct function of cathepsin L in secretory vesicles for production of active peptide hormones, which contrasts with its presence in lysosomes for protein degradation. Significantly, results from this study identify cathepsin L as a novel cysteine protease pathway in secretory vesicles for production of peptide hormones derived from POMC.
Acknowledgments
We thank B. Brinkman (Facility Manager) and J. Gleeson (Director) of the University of California, San Diego, Neuroscience Microscopy Shared Facility for confocal microscopy resources (supported by a grant P30 NS047101 from the National Institutes of Health). The authors also thank the laboratory of Dr. M. Tuszynski (Department of Neurosciences, University of California, San Diego) for assistance in tissue preparation for confocal microscopy.
*
This work was supported, in whole or in part, by National Institutes of Health Grants ROl MH 077305; ROl DA 04271, and ROl NS 24553 (to V. H.). This work was also supported by grants from the Deutsche Forschungsgemeinschaft (to T. R. and C. P.) and from the Landesstiftung Baden-Wuerttemberg (to F. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
2
The abbreviations used are: ACTH, adrenocorticotropic hormone; POMC, proopiomelanocortin; α-MSH, α-melanocyte stimulating hormone; PBS, phosphate-buffered saline; BSA, bovine serum albumin; EGFP, enhanced green fluorescence protein; CMV, cytomegalovirus; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; KO, knock-out; MCA, methylcoumarinamide.
3
L. Funkelstein, T. Toneff, C. Mosier, S.-R. Hwang, F. Beuschlein, U. D. Lichtenauer, T. Reinheckel, C. Peters, and V. Hook, unpublished observations.
4
V. Hook, and T. Toneff, unpublished observations.
References
- 1.Nakanishi, S., lnoue, A., Kita, T., Nakamura, M., Chang, A. C. Y., Cohen, S. N., and Numa, S. (1979) Nature 278 423-424 [DOI] [PubMed] [Google Scholar]
- 2.Boileau, G., Barbeau, G., Jeannotte, L., Chretien, M., and Drouin, J. (1983) Nucleic Acids Res. 11 8063-8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohman, L. A. (1981) in Endocrinology and Metabolism (Felig, P., Baxter, J. D., and Frohman, L. A., eds) pp. 293-312, McGraw-Hill, Inc., New York
- 4.Norris, D. O. (1997) Vertebrate Endocrinology, pp. 106-166, Academic Press, Inc., San Diego
- 5.Lechan, R. M. and Fekete, C. (2006) Peptides 27 310-325 [DOI] [PubMed] [Google Scholar]
- 6.Adan, R. A., Hillebrand, J. J., De Rijke, C., Jijenhuis, W., Vink, T., Garner, K. M., and Kas, M. J. (2003) Ann. N. Y. Acad. Sci. 994 267-274 [DOI] [PubMed] [Google Scholar]
- 7.Dalayeun, J. G., Nores, J. M., and Bergal, S. (1993) Biomed. Pharmacother. 47 311-320 [DOI] [PubMed] [Google Scholar]
- 8.Hartwig, A. C. (1991) Anesth. Prog. 38 75-78 [PMC free article] [PubMed] [Google Scholar]
- 9.Loh, Y. P., and Gainer, H. (1982) Proc. Natl. Acad. Sci. U. S. A. 79 108-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sei, C., Toneff, T., Aaron, W., and Hook, V. Y. H. (2002) Peptides 23 1409-1418 [DOI] [PubMed] [Google Scholar]
- 11.Sei, C., Toneff, T., Aaron, W., and Hook, V. Y. H. (2003) Peptides 24 717-725 [DOI] [PubMed] [Google Scholar]
- 12.Miller, R., Aaron, W., Toneff, T., Vishnuvardhan, D., Beinfeld, M., and Hook, V. Y. H. (2003) J. Neurochem. 86 556-563 [DOI] [PubMed] [Google Scholar]
- 13.Laurent, V., Jaubert-Miazza, L., Desejardinsm, R., Day, R., and Lindberg, I. (2004) Endocrinology 145 519-528 [DOI] [PubMed] [Google Scholar]
- 14.Benjannet, S., Rondeau, N., Day, R., Chretien, M., and Seidah, N. G. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 3564-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan, H., Nanno, D., Che, F. Y., Zhu, X., Salton, S. R., Steiner, D. F., Fricker, L. D., and Devi, L. A. (2005) Biochemistry 44 4939-4948 [DOI] [PubMed] [Google Scholar]
- 16.Katunuma, N., Murata, E., Kakegawa, H., Matsui, A., Tsuzuki, H., Tsuge, H., Turk, D., Turk, V., Fukushima, M., Tada, Y., and Aso, T. (1999) FEBS Lett. 458 6-10 [DOI] [PubMed] [Google Scholar]
- 17.Reinheckel, T., Deussing, J., Roth, W., and Peters, C. (2001) Biol. Chem. 382 735-741 [DOI] [PubMed] [Google Scholar]
- 18.Roth, W., Deussing, J., Botchkarev, V. A., Pauley-Evers, M., Saftig, P., Hafner, A., Schmidt, P., Schmahi, W., Scherer, J., Anton-Lamprecht, I., Von Figura, K., Paus, R., and Peters, C. (2000) FASEB J. 14 2075-2086 [DOI] [PubMed] [Google Scholar]
- 19.Hook, V. Y., Heisler, S., and Axelrod, J. (1982) Proc. Natl. Acad. Sci. U. S. A. 79 6220-6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hook, V. Y. H., Heisler, S., Sabol, S. L., and Axelrod, J. (1982) Biochem. Biophys. Res. Commun. 106 1364-1371 [DOI] [PubMed] [Google Scholar]
- 21.Hwang, S.-R., Garza, C., Mosier, C., Toneff, T., Wunderlich, E., Goldsmith, P., and Hook, V. (2007) J. Biol. Chem. 282 9556-9563 [DOI] [PubMed] [Google Scholar]
- 22.Ciccotosto, G. D., Schiller, M. R., Eipper, B. A., and Mains, R. E. (1999) J. Cell Biol. 144 459-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen, R. G., Peng, B., Pellegrino, M. J., Miller, E. D., Grandy, D. K., Lundblad, J. R., Washbrun, C. L., and Pintar, J. E. (2001) J. Neurosci. 21 5864-5870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan, H. K., Che, F. Y., Peng, B., Steiner, D. F., Pintar, J. E., and Fricker, L. D. (2006) J. Neurochem. 98 1763-1777 [DOI] [PubMed] [Google Scholar]
- 25.Collette, J., Bocock, J. P., Ahn, K., Chapman, R. L., Godbold, G., Yeyeodu, S., and Erickson, A. H. (2004) Int. Rev. Cytol. 241 1-51 [DOI] [PubMed] [Google Scholar]
- 26.Ishidoh, K., and Kominami, E. (2002) Biol. Chem. 383 1827-1831 [DOI] [PubMed] [Google Scholar]
- 27.Yasothornsrikul, S., Greenbaum, D., Medzihradszky, K. F., Toneff, T., Bundey, R., Miller, R., Schilling, B., Petermann, I., Dehnert, J., Logvinova, A., Goldsmith, P., Neveu, J. M., Lane, W. S., Gibson, B., Reinheckel, T., Peters, C., Bogyo, M., and Hook, V. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 9590-9595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azaryan, A. V., and Hook, V. Y. H. (1994) FEBS Lett. 341 197-202 [DOI] [PubMed] [Google Scholar]
- 29.Azaryan, A. V., Krieger, T. J., and Hook, V. Y. H. (1995) J. Biol. Chem. 270 8201-8208 [DOI] [PubMed] [Google Scholar]
- 30.Gainer, H., Russell, J. T., and Loh, Y. P. (1984) FEBS Lett. 175 135-139 [DOI] [PubMed] [Google Scholar]
- 31.Hwang, S.-R., O'Neill, A., Bark, S., Foulon, T., and Hook, V. (2007) J. Neurochem. 100 1340-1350 [DOI] [PubMed] [Google Scholar]
- 32.Hook, V., Funkelstein, L., Lu, D., Bark, S., Wegrzyn, J., and Hwang, S.-R. (2007) Annu. Rev. Pharmacol. Toxicol. 48 393-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fricker, L. D. (1988) Annu. Rev. Physiol. 50 309-321 [DOI] [PubMed] [Google Scholar]
- 34.Seidah, N. G., and Prat, A. (2002) Essays Biochem. 38 79-94 [DOI] [PubMed] [Google Scholar]
- 35.Zhou, A., Webb, G., Zhu, X., and Steiner, D. F. (1999) J. Biol. Chem. 274 20745-20748 [DOI] [PubMed] [Google Scholar]