Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium (original) (raw)
Summary
Encapsulation of prespore cells of Dictyostelium discoideum is controlled by several intercellular signals to ensure appropriate timing during fruiting body formation. Acyl-CoA-binding protein, AcbA, is secreted by prespore cells and processed by the prestalk protease TagC to form the 34 amino acid peptide SDF-2 that triggers rapid encapsulation. AcbA is secreted when γ-aminobutyric acid (GABA) is released from prespore cells and binds to GrlE, a G protein-coupled receptor (GPCR). Analysis of SDF-2 production in mutant strains lacking Gα subunits and GPCRs, either as pure populations or when mixed with other mutant strains, uncovered the non-cell-autonomous roles of GrlA, Gα4 and Gα7. We found that Gα7 is essential for the response to GABA and is likely to be coupled to GrlE. _GrlA_-null and _G_α_4_-null cells respond normally to GABA but fail to secrete it. We found that they are necessary for the response to a small hydrophobic molecule, SDF-3, which is released late in culmination. Pharmacological inhibition of steroidogenesis during development blocked the production of SDF-3. Moreover, the response to SDF-3 could be blocked by the steroid antagonist mifepristone, whereas hydrocortisone and other steroids mimicked the effects of SDF-3 when added in the nanomolar range. It appears that SDF-3 is a steroid that elicits rapid release of GABA by acting through the GPCR GrlA, coupled to G protein containing the Gα4 subunit. SDF-3 is at the head of the cascade that amplifies the signal for encapsulation to ensure the rapid, synchronous formation of spores.
Keywords: Steroids, SDF-2, SDF-3, G protein-coupled receptor, GrlA
INTRODUCTION
Following the initiation of development, vegetative Dictyostelium amoebae stream together to form multicellular aggregates, which transform into slug-shaped organisms containing up to 100,000 cells. Slugs migrate phototactically to visible light before constructing fruiting bodies. Most of the posterior cells encapsulate into resilient spores during culmination of fruiting bodies, while the anterior cells construct a stalk. Dispersal of the spores is facilitated by holding them as a sorus on the top of the stalk, which can be several millimeters high. Prestalk cells not only generate the stalk tube but also enter it and vacuolize to provide added strength. Prespore cells climb the rising stalk before encapsulating. Since spores are no longer able to move, the timing of sporulation must be carefully controlled to avoid premature encapsulation while only part way up the stalk.
Sporulation is triggered when the activity of the cAMP-dependent protein kinase PKA rapidly increases (Anjard et al., 1998a). Several intercellular signaling pathways control the internal concentrations of cAMP that activate PKA (Anjard and Loomis, 2005;Anjard and Loomis, 2006;Anjard and Loomis, 2008). SDF-1 is a phosphopeptide of ∼1.2 kDa secreted at the onset of culmination that induces spore formation after 90 minutes through a process that requires protein synthesis (Anjard et al., 1998b). Later on during development, other factors induce rapid spore differentiation independently of any protein synthesis. A 34 amino acid peptide, SDF-2, results in a decrease in cAMP phosphodiesterase activity and cytokinins stimulate the activity of the adenylyl cyclase ACR (Anjard and Loomis, 2005;Anjard and Loomis, 2008). Simultaneously removing the brake on the accumulation of cAMP and accelerating its rate of synthesis can lead to a jump in PKA activity.
The activity of the cAMP phosphodiesterase, RegA, is dependent on phosphorylation of its response regulator domain (Shaulsky et al., 1996;Thomason et al., 1999). Phosphates are relayed to RegA from the two-component histidine kinase, DhkA, via the H2 intermediate RdeA (Wang et al., 1999; Thomason et al., 1999; Anjard and Loomis, 2005). DhkA is the surface receptor for SDF-2 and its activity is controlled by ligand binding (Wang et al., 1999; Anjard and Loomis, 2005). When SDF-2 is present in the extracellular environment, phosphorelay from DhkA to RegA is blocked.
Production of SDF-2 is also finely controlled. It is generated from the acyl-CoA-binding protein, AcbA, which is released from prespore cells and processed extracellularly by the prestalk-specific protease TagC. When cells are exposed to low levels of SDF-2, AcbA is released and the TagC protease is exposed to the outside. Thus, low levels of SDF-2 `prime' the cells for production of more SDF-2. However, priming is inhibited by glutamate, which is present outside the cells at ∼1 mM (Anjard and Loomis, 2006). Glutamate binds to the G protein-coupled receptor (GPCR) GrlE, and the signal is transduced via a trimeric G protein containing Gα9. Priming by low levels of SDF-2 is unaffected by the presence of 10 mM glutamate in cells lacking either GrlE or Gα9 (Anjard and Loomis, 2006). Release of AcbA and cell surface exposure of the protease can also be triggered by 10 nM γ-aminobutyric acid (GABA) (Anjard and Loomis, 2006;Kinseth et al., 2007). Glutamate is a competitive inhibitor of GABA induction of AcbA release and both signals are dependent on GrlE (Anjard and Loomis, 2006). However, the affinity of GrlE for glutamate appears to be 100-fold lower than it is for GABA. Surprisingly,_G_α_9_-null mutants respond normally when GABA is added (Anjard and Loomis, 2006). Since we have evidence for direct binding of GABA to GrlE, it appears that this GABAB-type receptor, unlike its mammalian homologs, binds both glutamate and GABA, but is coupled only to Gα9 when it binds glutamate.
In an effort to determine which trimeric G protein is coupled to GrlE when it binds GABA, we surveyed all available mutations affecting Gα subunits. We found that cells lacking Gα7 failed to produce SDF-2 in response to GABA, although glutamate still inhibited priming by low levels of SDF-2. Since these cells develop well but fail to accumulate SDF-2, it is likely that the GABA signal transduction pathway is activated by GrlE when it is coupled to a Gα7. All the other Gα mutants responded normally to GABA, SDF-2 and glutamate. However, cells lacking Gα4 failed to accumulate SDF-2 in their sori, suggesting that there might be another GPCR-mediated signal transduction pathway in the sporulation cascade. To try to identify the receptor that might be coupled to Gα4, we screened mutant strains lacking specific GPCRs for the production of SDF-2, and found that fruiting bodies of a mutant strain lacking GrlA contained no measurable SDF-2. By analyzing material released by wild-type cells late in development, we were able to recognize a novel factor, SDF-3, that triggers GABA signaling in wild-type cells but not in cells lacking either GrlA or Gα4. SDF-3 stands at the head of the cascade as we now understand it.
MATERIALS AND METHODS
Strains and bioassay
The wild-type strain AX4 and the pkaC overexpressing strain KP have been described previously (Anjard et al., 1992). Most of the other strains were obtained from the_Dictyostelium_ Stock Center (http://dictybase.org). The strain identifiers are: _gpa1_- (DBS0236088);_gpa2_- (DBS0236094); _gpa3_- (DBS0235986); _gpa4_- (DBS0235984);_gpa5_- (DBS0236451); _gpa7_- (DBS0236106); _gpa8_- (DBS0236107);_gpa9_- (DBS0236109); _grlE_- (DBS0236112); _gadA_- (DBS0235989);_acbA_- (DBS0236991). The _grlA_- strain (Prabhu et al., 2007) was the kind gift of Angelica Noegel.
To generate a strain lacking GABA transaminase, a 1593 bp genomic fragment starting 328 bp upstream of gabT coding sequence (Dictybase ID: DDB_G0268104) was amplified by PCR using the forward primer 5′-GTCAGATTGAAATTACCCACCCC-3′ and the reverse primer 5′-GAGGTGAGATTCCAATGTTCGTG-3′. The PCR product was cloned into pGEMT-EASY (Promega, A1360) as previously described (Anjard and Loomis, 2006) and sequenced. The BSR cassette from pBSR519 (Puta and Zeng, 1998) was cloned into the unique Hin_dIII site located at the beginning of the_gabT coding sequence. For gene disruption, 10 μg of the plasmid was linearized with _Not_I before electroporation into 1×107 AX4 or KP cells. Clones were selected with 5 μg/ml Blasticidin S for 2 weeks then subcloned before analysis. Disruption of the endogenous gabT gene in transformants was confirmed by a series of four PCR amplifications using primers located outside the cloned sequences and primers located within the BSR cassette. More than 50% of the tested clones were positive for gabT disruption.
The bioassay was carried out on KP cells and their derivatives after 18 hours development in monolayers as previously described (Anjard et al., 1998a). Cells were incubated in cAMP buffer (20 mM MES pH 6.2, 20 mM NaCl, 20 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 5 mM cAMP) at a density of 1×103 cells/cm2 in the wells of a 24-well dish at 23°C. After 18 hours incubation, samples or defined products were added and the numbers of spores and undifferentiated cells were counted 1 hour later unless otherwise indicated. The priming effect of SDF-2 results in a positive-feedback loop that gives an all-or-none response when SDF-2 is above threshold concentration. SDF-2 and SDF-3 activity was determined by serial dilution of the samples before addition to KP cells. Quantitation is only accurate within a factor of two. One unit corresponds to the lowest dilution giving full induction of spore formation. The number of units in the sample was standardized to 1×103 producing cells whenever applicable.
The response of cells from strains that are not sporogenous to SDF-3 and steroids was measured following dissociation of culminants that had developed on filters or non-nutrient agar (Anjard and Loomis, 2006). Filters (25 mm diameter) were each spread with 5×106 to 1×107 cells and allowed to develop for 20 hours at 22°C. Each filter was then examined under a dissecting microscope. Only those filters on which most of the structures were similar were used and any asynchronously developing culminants were removed from these filters with a needle. The _grlA_- strain developed better when spread at lower density (5×106 cells per filter), whereas the _gpa4_- strain does not develop synchronously on filters. The _gpa4_- strain was developed by plating on non-nutrient agar made with 2% agar in phosphate buffer, resulting in a more homogenous and synchronous development. The procedure to collect cells from_gpa4_- was otherwise identical to that for the other strains. The cells were allowed to develop and monitored every 15 minutes. When stalks became apparent under the rising sori, the cells were collected by vortexing the filters in 1 ml cAMP buffer followed by centrifugation at 4000 rpm (1250 g) for 1 minute in a microcentrifuge. The cells were counted and diluted to 3.6×104/ml. Because the window of development during which induction of sporulation can be assayed is only 15-30 minutes, only preparations that contained 10-20% spores were used. Five hundred microliters of the suspension was added to each well of a 24-well plate, resulting in a cell density of 104/cm2. Inducing compounds were added at various concentrations and the number of spores counted after 1 hour.
Cell growth and development
Cells were grown in HL5 medium at 22°C with shaking at 180 rpm for oxygenation (Sussman, 1987). Exponentially growing cells at a density of 2-5×106/ml were harvested by centrifugation. Cells were washed with PDF buffer (20 mM Na/K phosphate pH 6.5, 20 mM KCl, 1.2 mM MgSO4), centrifuged again at 1000 rpm (200 g) for 5 minutes and resuspended at a density of 1-2×108 cells/ml in PDF before being deposited on nitrocellulose filters placed on pads saturated with PDF or on non-nutrient agar plates made with 2% agar in PDF. Developing cells were then incubated at 22°C in a humid chamber for the indicated time. Spore viability assays were performed as described (Anjard and Loomis, 2006).
Purification of SDF-3
After a series of characterizations as described in the Results, a procedure was developed to obtain a stock of SDF-3 used for later experiments. A 20 ml suspension of Klebsiella aerogenes in 20 mM phosphate buffer (pH 6.5) was prepared using bacteria collected from four SM plates. About 2×107 AX4 cells were collected and mixed with the K. aerogenes suspension and plated on 22 SM plates. After 3 days incubation at 22°C, the Dictyostelium cells cleared the plate and proceeded to culminate. The fruiting bodies were collected in 20 ml water and SDF-3 was extracted using 20 ml chloroform. The chloroform was evaporated under vacuum and SDF-3 was dissolved in 20 ml 10% ethanol. A 2 ml suspension (50/50) of Amberlite XAD-2 was added. The resin was pelleted and washed once with 25 ml 10% ethanol then 30% and 50% ethanol before elution with four times 5 ml of 70% ethanol. After vacuum evaporation, the pellet was redissolved in 200 μl 10% methanol. A 100 μl sample was loaded on a Majic C-18 column (internal diameter 1 mm × length 150 mm) using an ultrafast HPLC apparatus (Microm BioResources). The LC mobile phase A was 2.5% methanol in water and the LC mobile phase B was 100% methanol. The LC flow rate was 50 μl/minute, and the LC gradient was 100% A to 95% B in 20 minutes then held at 95% B for 5 minutes. Fractions of 50 μl were collected and tested by bioassay. Most of the activity was found in fractions 20-21 (see Fig. S1 in the supplementary material). These fractions were pooled, evaporated, and resuspended in 100% methanol to create a main stock at 1000 units SDF-3/μl. This stock was kept at -20°C and appears very stable. Intermediate stocks at 100, 10 and 1 unit/μl were prepared in 10% methanol (final).
Chemicals
GABA, l-glutamate, vigabatrin, terbamine, cAMP and most steroids were supplied by Sigma. Progesterone and metyrapone were obtained from Aldrich, corticosterone from Fluka. Dexamethasone was supplied by BIOMOL International (Plymouth, MA, USA). Stock solutions were prepared as recommended by suppliers. For most steroids, 50 mM stocks were prepared in 100% methanol, with more dilute intermediate stocks prepared in 10% methanol. For terbamifine and aldosterone, DMSO was used for the 50 mM stock solution. Anti-GABA antibodies (AB5016) were purchased from Chemicon International (Temecula, CA, USA). A 1/10 intermediate stock of anti-GABA antibodies was prepared in PBS; a final dilution of 1/5000 is sufficient to block the induction effect of 20 nM GABA in the bioassay.
RESULTS
Gα7 is required for the GrlE response to GABA
Cells dissociated from fruiting bodies at the mid-culminant stage and plated at low density can be induced to form spores by the addition of 10 nM GABA or 1 pM SDF-2 (Anjard and Loomis, 2005; Anjard and Loomis, 2006). The GABA signal is transduced by the GPCR GrlE, which activates a pathway that is independent of PKA (Anjard and Loomis, 2006). GrlE also functions in the signal transduction pathway by which glutamate inhibits priming by SDF-2. The trimeric G protein subunit Gα9 is required for the glutamate response but not for the GABA response. To determine the Gα subunit involved in the GABA response, we analyzed all available Gα mutant strains that could develop at least to the mid-culmination stage. Null mutants in four putative Gα proteins (Gα6, Gα10, Gα11, Gα12) were not available and null mutants lacking Gα2 or Gα3 fail to develop, even to the aggregation stage (Kumagai et al., 1991;Brandon et al., 2002). Co-development of _G_α_2_-null with _acbA_-null cells resulted in the asynchronous formation of small fruiting bodies that contained SDF-2 (Table 1).
Table 1.
Characteristics of Gpa mutants
| Strain | SDF-2 in sori | Response to SDF-2 | Response to GABA | Inhibition by glutamate |
|---|---|---|---|---|
| Wild type | + | + | + | + |
| gpa1 null | + | + | + | + |
| gpa2 null | +* | No dev | No dev | No dev |
| gpa3 null | No dev | No dev | No dev | No dev |
| gpa4 null | − | + | + | + |
| gpa5 null | + | + | + | + |
| gpa7 null | − | + | − | + |
| gpa8 null | + | + | + | + |
| gpa9 null | + | + | + | − |
The remaining six Gα mutants were developed and tested for the presence of SDF-2 in the sorus. Both _gpa4_-null and _gpa7_-null strains failed to accumulate SDF-2 (Table 1). Dissociated cells collected at the mid-culmination stage were plated in multitest wells at 104 cells/cm2 and presented with 1 pM SDF-2 or 10 nM GABA. One hour later, the increase in the number of spores was determined. All of the mutant strains responded to SDF-2 by rapid sporulation, as expected because the SDF-2 receptor is a histidine kinase rather than a GPCR (Table 1). However, the _gpa_7-null strain failed to respond to GABA. This Gα protein may function in trimeric G proteins that are coupled to GrlE when it has bound GABA. Activation of GrlE is required both for the release of AcbA precursor by prespore cells and for exposure of the TagC protease on the cell surface of prestalk cells. Defects in either aspect were tested independently (Anjard and Loomis, 2006). The presence of secreted but unprocessed AcbA was tested by digesting the extracellular media with trypsin, which mimics TagC activity. Trypsin treatment did not generate any activity (<0.2 units/103 cells) from the supernatant of _gpa7_-null cells primed with GABA, indicating that AcbA was not released. The activation of TagC protease activity by GABA can be tested by adding recombinant AcbA and measuring the production of SDF-2. Even after addition of recombinant AcbA, no SDF-2 (<0.2 units/103 cells) was obtained from the supernatant of_gpa7_-null cells primed by GABA. Thus, Gα7 is required to transduce GABA activation in both cell types.
We also tested for inhibition of SDF-2 priming by 10 mM glutamate. As previously described, the strain lacking Gα9 is insensitive to inhibition by glutamate (Anjard and Loomis, 2006). By contrast, glutamate inhibited priming in the strain lacking Gα7, showing that GrlE discriminates among trimeric G proteins depending on which ligand is bound.
Surprisingly, we found that cells lacking Gα4 had no measurable SDF-2 in their sori although dissociated cells responded normally to SDF-2, GABA and glutamate (Table 1). The simplest explanation for this phenotype is a defect in the initiation of GABA signaling, resulting in the lack of AcbA release and subsequent processing to SDF-2.
Synergy among strains
Mutants that do not produce an intercellular signal are often rescued when they develop together with the wild type or other strains that provide the signal (Sussman, 1954). We used this synergy assay systematically. Six mutants unable to generate SDF-2 were co-developed with each of the other mutants. The production of SDF-2 by each of these chimeras was tested, allowing us to establish synergy groups based on their ability to rescue each other (Fig. 1). Mutants lacking Gα4 produced normal levels of SDF-2 if allowed to develop together with an equal number of cells of strains that are defective in production of SDF-2 but nonetheless able to release GABA (Fig. 1). However, SDF-2 production was not rescued in_G_α_4_ mutants when they were developed together with_gadA_-null cells that are compromised in the synthesis of GABA as the result of loss of glutamate decarboxylase (GadA). Although Gα4 is expected to act in an intracellular manner, its role is bypassed when the cells are presented with GABA. Mutants lacking components in the same signal transduction pathway leading to release of an intercellular signal fail to synergize.
Fig. 1.
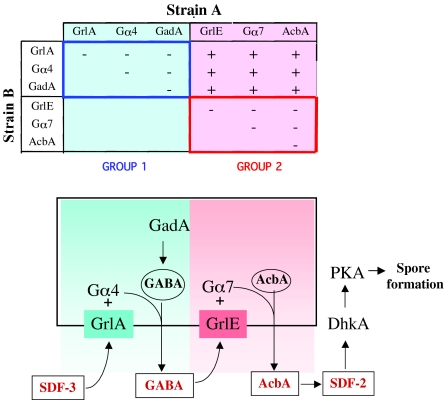
Synergy between Dictyostelium discoideum strains unable to produce SDF-2. (Above) Mutant strains lacking one of six proteins essential for production of SDF-2 were mixed in equal proportions with cells lacking another of the indicated proteins. After developing together for 24 hours, fruiting bodies were collected in cAMP buffer and the supernatant tested for SDF-2 activity. Minus indicates that less than 0.2 units of SDF-2/103 cells were recovered, whereas plus indicates that more than 5000 units of SDF-2/103 cells were produced. Strains that failed to synergize to produce SDF-2 constituted a synergy group. (Below) A signaling cascade is proposed to connect the two groups. GrlA and GrlE are GPCRs and GadA is glutamate decarboxylase. Gα4 and Gα7 are trimeric G protein components. AcbA, acyl-CoA-binding protein. GABA, γ-aminobutyric acid. SDF-2 is a 34 amino acid signaling peptide.
The synergistic relationships can be seen in a schematic of the various pathways leading to rapid sporulation (Fig. 1). The observed synergy between cells lacking Gα4 and cells lacking any of the components of the GABA response (group 2) suggests that the rescuing cells are responding to a factor that triggers GABA release. This factor is likely to act through a GPCR because a trimeric G protein subunit, Gα4, is implicated in the pathway. Therefore, we considered which of the genes encoding GPCRs might be candidates for the receptor.
GrlA is coupled to Gα4
Prabhu et al. (Prabhu et al., 2007) recently characterized a GPCR encoded by grlA that is dispensable for growth and early development but necessary for post-aggregative events. grlA is expressed at low levels in vegetative cells and at high levels after 12 hours of development. It encodes a seven-transmembrane protein of 90 kDa with well-conserved residues in the intracellular loops where G proteins bind. By contrast, the N-terminal ligand-binding domain displays little homology to characterized proteins. Using a construct in which GFP was fused to the C-terminus of GrlA, Pradhu et al. showed that the protein was present on the plasma membrane (Prabhu et al., 2007). Disruption of the gene resulted in a delay in culmination and a reduced efficiency of sporulation. Activation of PKA by addition of 8-Br-cAMP to_grlA_- cells dissociated from early culminants was found to rescue sporulation, showing that the defect lay in the pathway leading to activation of PKA late in development (Prabhu et al., 2007). We found that _grlA_- cells do not accumulate measurable SDF-2 in their sori, which could account for the defects in sporulation. However,_grlA_- cells synergize with cells of any of the strains lacking components of the GABA response (_grlE_-,_gpa_7-, _acbA_-). These strains did not accumulate more than 0.2 units SDF-2/103 cells when developed as pure populations, whereas chimeric fruiting bodies had >5000 units/103 cells (Fig. 1).
The _grlA_- cells failed to synergize when developed together with cells lacking either Gα4 or GadA; no SDF-2 was found in these chimeric fruiting bodies. Taken together, these results suggest that a novel secreted factor activates the receptor GrlA coupled to Gα4, resulting in release of GABA synthesized by GadA (Fig. 1).
Characterization of SDF-3
We isolated a factor from the supernatant of developed_gadA_- cells that was able to stimulate rapid sporulation of KP cells developing as monolayers. We used _gadA_- cells as the source of the factor to avoid having GABA or SDF-2 in the supernatant. Moreover, we removed SDF-1 from the supernatant by passage over cationic resin. Doing so allowed us to detect a novel factor that we named SDF-3. Wild-type cells were used later on as the source of SDF-3 after it became clear that the new activity could be easily separated from other factors.
Unlike SDF-1 or SDF-2, the novel activity does not bind to cationic or anionic resin, but was found to bind strongly to hydrophobic resin and could only be eluted by ethanol at greater than 50% concentration (see Table S1 in the supplementary material). Consistent with its hydrophobicity, ∼40-fold more SDF-3 was found in the organic phase than in the aqueous phase upon chloroform extraction. SDF-3 passed through an ultrafiltration membrane with a 3 kDa cut-off, indicating that it is a relatively small molecule. The partially purified SDF-3 was found to be resistant to heating at 100°C or treatment with proteinase K, indicating that it is not a peptide (see Table S1 in the supplementary material). SDF-3 was further purified by HPLC on a C-18 column using a water-methanol gradient (see Fig. S1 in the supplementary material). The biological assays to define the effects of SDF-3 were performed using a stock of this HPLC-purified activity.
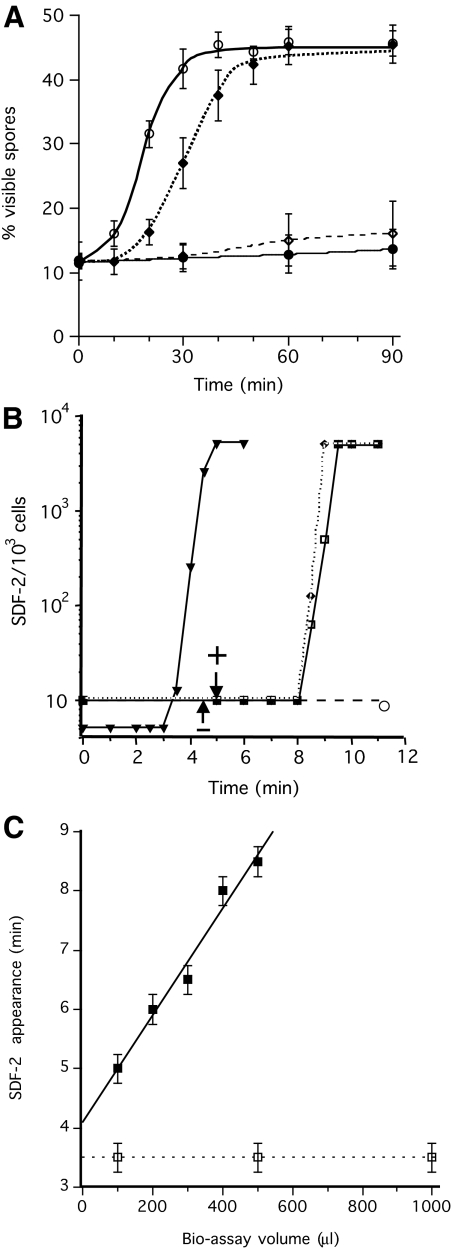
SDF-3 rapidly induced high levels of spore formation in KP cells but the response was ∼10 minutes slower than with SDF-2 (Fig. 2A). Antibodies against GABA or AcbA blocked the induction effects of SDF-3, indicating that SDF-3 acts upstream of GABA and SDF-2. Direct assay of SDF-2 showed that it was released ∼9 minutes after the addition of SDF-3 (Fig. 2B). This induction was also prevented by the addition of anti-GABA antibodies. During optimization of the bioassay for response to SDF-3, it became clear that the delay between addition of the factor and the appearance of SDF-2 increased linearly with the volume of the assay. This linearity held up to 500 μl, the volume normally used in the bioassay (Fig. 2C). The delay increased to 11 minutes for 600 μl and no SDF-2 production was observed, even after 1 hour, for volumes above 750 μl (data not shown). When GABA was used directly to induce SDF-2 production, the delay of 3.5 minutes was independent of the volume used in the bioassay (Fig. 2C).
Fig. 2.
Time course of spore formation and SDF-2 production after induction by SDF-3. (A) Induction of spore formation by SDF-2 or SDF-3. KP cells were plated in 24-well plates at 1×103 cells/cm2 in cAMP buffer. After overnight incubation at 22°C, 10 pM SDF-2 or 10 units SDF-3 were added and the number of spores was scored every 10 minutes for 1 hour and at 90 minutes. Antibodies against GABA or AcbA were added prior to induction. Open circle, induction by SDF-2; black diamond, induction by SDF-3; open diamond, induction by SDF-3 in the presence of anti-AcbA antibodies (1/500); black circle, induction by SDF-3 in presence of anti-GABA antibodies (1/5000). Each experiment was repeated at least three times. (B) Induction of SDF-2 production by SDF-3, hydrocortisone or GABA. 10 nM GABA (triangle), 10 nM hydrocortisone (diamond) or 10 units SDF-3 (square) were added to KP cells and aliquots were taken at the indicated times. SDF-2 production was measured after purification over anion-exchange resin. In another set of experiments, anti-GABA antibodies were added just prior to SDF-3 (circle) or at the indicated time after SDF-3 addition (arrows). In these experiments, SDF-2 production was measured 11 minutes after SDF-3 addition. The arrow with a minus sign indicates the time when addition of anti-GABA antibodies prevented SDF-2 production, and the arrow with a plus sign indicates the time when addition of anti-GABA antibodies did not prevent SDF-2 production. The data for GABA are derived from Anjard and Loomis (Anjard and Loomis, 2005). (C) The delay between SDF-3 addition and SDF-2 production increases with the volume of the bioassay. KP cells were starved overnight in 500 μl cAMP buffer (standard conditions). Just before being used in the assay the volume was adjusted to that indicated, by adding or removing cAMP buffer. SDF-2 production was stimulated by addition of 2 units SDF-3/100 μl (black square) or 10 nM GABA (open square). Aliquots were taken every 30 seconds and tested for SDF-2 production. The earliest time SDF-2 could be detected for a given volume was plotted. Error bars corresponding to ±15 seconds are given. Each experiment was repeated two to three times.
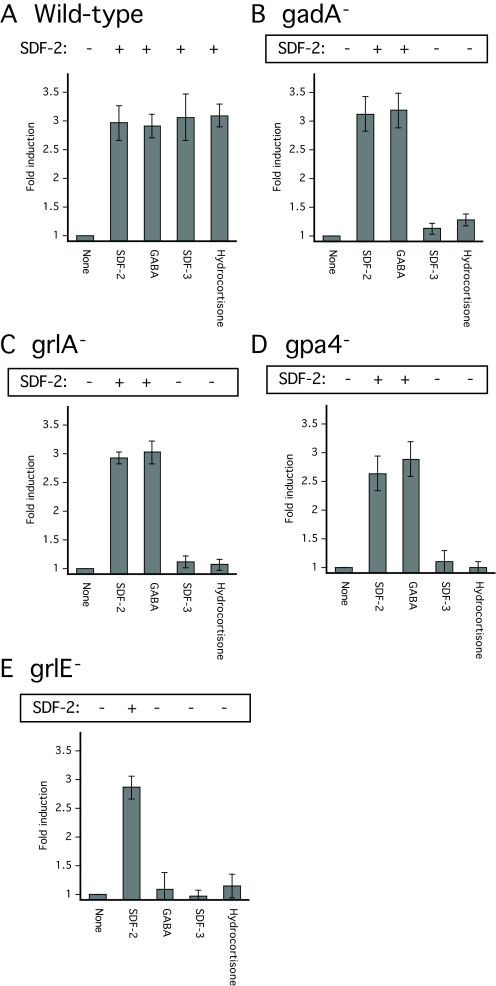
Although partially purified SDF-3 was able to induce rapid encapsulation of wild-type cells dissociated from culminants, it had no effect on culminating cells lacking GrlA, Gα4, GrlE or GadA (Fig. 3). These mutant cells all responded normally to induction by SDF-1 or the cytokinin isopentenyladenine, which act through SDF-2-independent pathways (data not shown).
Fig. 3.
SDF-2 production and spore formation in wild-type and mutant_Dictyostelium_ strains. Cells of the indicated wild-type (A) and mutant (B-E) strains were developed on filters (non-nutrient agar for _gpa4_-) and harvested at the mid-culmination stage. The fruiting bodies were dissociated and the cells washed before plating at 1×104 cells/cm2 in cAMP buffer. The cells were then treated with no addition (none), 10 pM synthetic SDF-2, 10 nM GABA, 10 units of SDF-3 or 100 nM hydrocortisone. Spores were counted after 1 hour. An aliquot of the supernatant was harvested for each sample to measure the amount of SDF-2 produced after purification using anion-exchange resin. Minus indicates production of less than 0.2 units of SDF-2/103 cells, whereas plus indicates more than 5000 units of SDF-2/103 cells.
A time course of SDF-3 accumulation was performed, using the hydrophobic resin Amberlite XAD-2 for purification. Wild-type cells developed for up to 20 hours yielded no detectable SDF-3 activity. Approximately 10-2 units/103 cells were detected in the supernatant of wild-type cells developed for 22 or 24 hours (see Table S2 in the supplementary material). When the cells were extracted with chloroform or methanol, 20- to 40-fold more SDF-3 activity was obtained than from the cell supernatant, indicating that only a small proportion was secreted (see Table S2 in the supplementary material).
We considered the possibility that SDF-3 might correspond to one of the known small hydrophobic morphogens secreted by Dictyostelium during development. DIF-1 has been characterized mostly for its role in the regulation of stalk cell differentiation (Saito et al., 2008). MPBD (4-methyl 5-pentylbenzene 1,3 diol) was recently shown to affect both stalk and spore formation (Saito et al., 2006). Both DIF-1 and MPBD were tested at various concentrations but failed to mimic the effects of SDF-3 (data not shown). Cytokinins are small and somewhat hydrophobic molecules that induce spore formation by activating the adenylyl cyclase ACR (Anjard and Loomis, 2008). However, unlike SDF-3, they act independently of GABA or SDF-2. Moreover, we found that a strain lacking the enzyme that synthesizes cytokinins, IptA, produces normal levels of SDF-3 (data not shown).
SDF-3 activities are mimicked by steroids
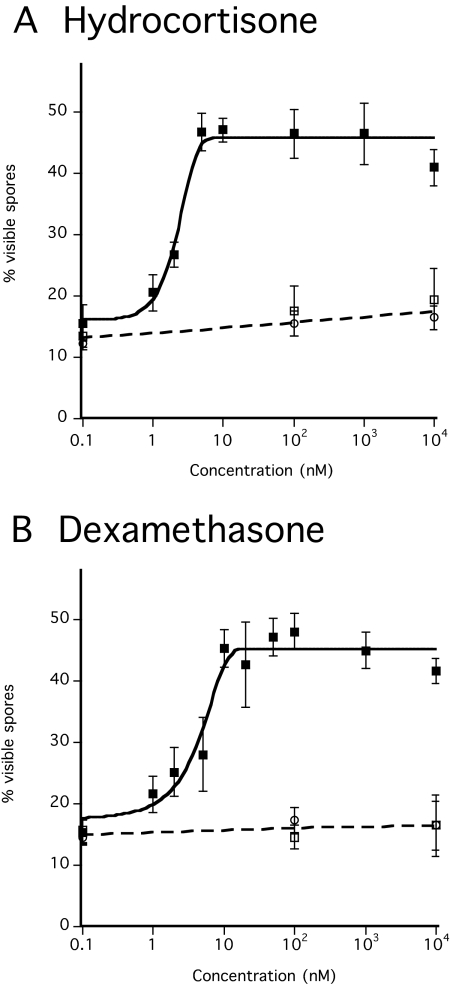
Among the molecules used by organisms for intercellular communication, few are hydrophobic yet water soluble enough to diffuse between cells. Steroids are the only major type of widely used signaling molecule that fit this description. Moreover, several steroids co-elute from the C-18 HPLC column with the SDF-3 activity (see Fig. S1 in the supplementary material). We tested a dozen steroids and steroid precursors, first randomly then more systematically when positive results were obtained. Hydrocortisone and the synthetic steroid dexamethasone are able to induce spore formation in KP cells down to 5 nM and 10 nM, respectively (Table 2; Fig. 4A,B). As was the case for SDF-3, the induction by these steroids was prevented by addition of anti-GABA or anti-AcbA antibodies. We also showed that hydrocortisone induces the production of SDF-2 with the same kinetics as does SDF-3 (Fig. 2B). Hydrocortisone carries three hydroxyl groups on positions 11, 17 and 21 of the sterol backbone. Steroids from the same family but with fewer hydroxyl groups were less efficient, whereas sex steroids had little or no activity (Table 2; see Fig. S2 in the supplementary material). Some of the steroids, such as corticosterone, had some inhibitory activity at the higher concentrations tested. Aldosterone, a derivative of corticosterone, did not induce spore formation and inhibited the activity of SDF-3 (not shown). Precursors of steroids such as cholesterol or stigmasterol failed to induce spore formation. Since hydrocortisone was the most efficient steroid found, it was tested on wild-type and mutant strains in parallel to SDF-3 (Fig. 3). Like SDF-3, hydrocortisone induced spore formation and SDF-2 in cells harvested from culminating wild-type cells but was inactive on_gadA_-, _grlA_-,_grlE_- or _gpa4_- mutant strains.
Table 2.
Steroid concentration required to induce spore formation
| Steroid | Minimal concentration |
|---|---|
| Hydrocortisone | 5 nM |
| Dexamethasone | 10 nM |
| 11-deoxycortisol, corticosterone | 20 nM |
| 17α progesterone, deoxycorticosterone | 50 nM |
| 11α progesterone | 50-100 nM |
| Pregnenolone | 100 nM |
| Progesterone | 200 nM |
| Estradiol | Weak induction at 10 μM |
| Cholesterol, stigmasterol, testosterone | No effect at 10 μM |
| Aldosterone | Competitive inhibitor |
Fig. 4.
Sporulation induced by steroids. Developed KP cells were incubated with various concentrations of hydrocortisone or dexamethasone. The number of spores was determined after 1 hour of incubation. Each experiment was repeated three to five times. Antibodies against GABA (circle) or AcbA (open square) were added prior to the addition of (A) dexamethasone (black square) or (B) hydrocortisone (black square).
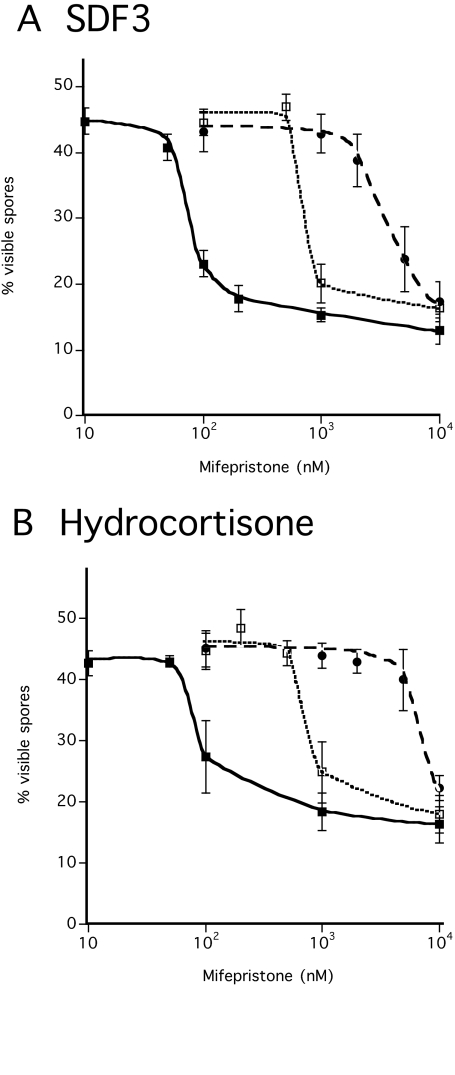
Mifepristone is a synthetic steroid analog with inhibitory activity (Schreiber et al., 1983). Mifepristone acted as a competitive inhibitor for both hydrocortisone and SDF-3 (Fig. 5). By contrast, 1 μM mifepristone failed to block spore induction by 1 nM GABA or 1 pM SDF-2 (data not shown), indicating that the inhibitory effect against steroid and SDF-3 is specific.
Fig. 5.
Competition between mifepristone and either hydrocortisone or SDF-3. KP cells were plated at low-density in cAMP buffer and incubated overnight. The indicated amounts of mifepristone and either SDF-3 (A) or hydrocortisone (B) were added. The number of spores was scored 1 hour later. One unit (black square), 10 units (open square), or 100 units (circle) of SDF-3 were added together with the indicated amounts of mifepristone. Hydrocortisone was added to 10 nM (black square), 100 nM (open square) or 1 μM (circle) together with the indicated amounts of mifepristone. Each experiment was repeated at least three times.
We also explored the effects of various inhibitors of the steroid biosynthetic pathway. The antifungal agent, terbinafine, inhibits squalene epoxidase such that conversion of squalene to lanosterol is blocked and steroids cannot be synthesized (Petranyi et al., 1984; Jandrositz et al., 1991). Dictyostelium has a single gene encoding a homolog of squalene epoxidase, sqlE, that is 50% identical to plant and 40% identical to animal squalene epoxidases. Addition of 1 μM terbinafine to developing KP cells completely blocked accumulation of SDF-3 (Table 3). Likewise, we tested aminoglutethimide (AGI), metyrapone and etomidate, which are inhibitors of the cholesterol side-chain cleavage enzyme and the P450 hydroxylases that are necessary for conversion of cholesterol to steroids. Each of these drugs blocked accumulation of SDF-3 when added to developing KP cells (Table 3). By contrast, the addition of cerulinin, which blocks the polyketide synthase responsible for synthesis of DIF-1 and similar compounds (Serafimidis and Kay, 2005), failed to block the production of SDF-3. KP cells in monolayers incubated overnight with terbinafine, AGI, metyrapone or etomidate were still able to respond to SDF-3 by rapid sporulation. Likewise, addition of terbinafine or AGI to wild-type cells developed on filters did not significantly affect morphogenesis but blocked the production of SDF-3 and SDF-2 (data not shown). The spore viability of the drug-treated wild-type cells was ∼20% of that of untreated wild-type cells. Taken together, these results indicate that SDF-3 is likely to be a steroid.
Table 3.
Inhibition of SDF-3 production by steroid synthesis inhibitors
| Conditions | % SDF-3 recovered |
|---|---|
| No addition | 100 |
| 100 μM cerulinin | 100 |
| 10 μg/ml AGI | ≤1 |
| 5 μM terbinafine | ≤1 |
| 500 μM metyrapone | 1-2.5 |
| 100 nM etomidate | 1-2 |
Steroids modulate GABA level and sensitivity to glutamate inhibition
The induction of SDF-2 production and spore formation by SDF-3 or steroids were prevented by anti-GABA antibodies (Fig. 3A,B;Fig. 4A,B). Furthermore, the_gadA_-null strain failed to respond to SDF-3, indicating that extracellular GABA is required to transduce SDF-3 effects. Since we could not directly measure GABA production, we used an indirect approach to evaluate extracellular GABA in response to SDF-3 or steroid. Anti-GABA antibodies were added at various times after induction by SDF-3 or hydrocortisone to better define when sufficient GABA has been produced to activate GrlE and SDF-2 production. Addition of anti-GABA antibodies 4.5 minutes after induction still blocked SDF-2 production, whereas addition at 5 minutes or later failed to prevent SDF-2 accumulation (Fig. 2B, arrows). Thus, extracellular GABA reaches threshold levels between 4.5 and 5 minutes after stimulation by SDF-3 or hydrocortisone. This accounts for the temporal difference in the effects of SDF-3 and GABA (8 and 3 minutes until induction of SDF-2 production, respectively).
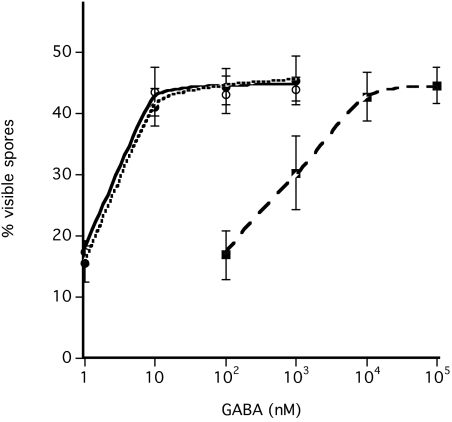
We also investigated the role of SDF-3 in GABA induction of sporulation and its inhibition by glutamate. Glutamate has been detected in_Dictyostelium_ sori in the mM range (Kelly et al., 1979;Klein et al., 1990). We found that 10 μM GABA is sufficient to overcome inhibition by 1 mM or 10 mM glutamate, suggesting that the concentration of glutamate is saturating in this range. When 2 units of SDF-3 or 10 nM hydrocortisone was added to the cells, as little as 10 nM GABA was sufficient to bypass glutamate inhibition (Fig. 6).
Fig. 6.
SDF-3 and hydrocortisone decrease the level of GABA required to overcome glutamate inhibition. Low-density KP cells were incubated overnight in cAMP buffer before addition of 1 mM glutamate. The indicated amount of GABA was then added with 2 units of SDF-3 (black circle) or 10 nM hydrocortisone (open circle) or left untreated (black square). Spores were counted after 1 hour. Each experiment was repeated at least three times. Almost identical results were obtained in the presence of 10 mM glutamate.
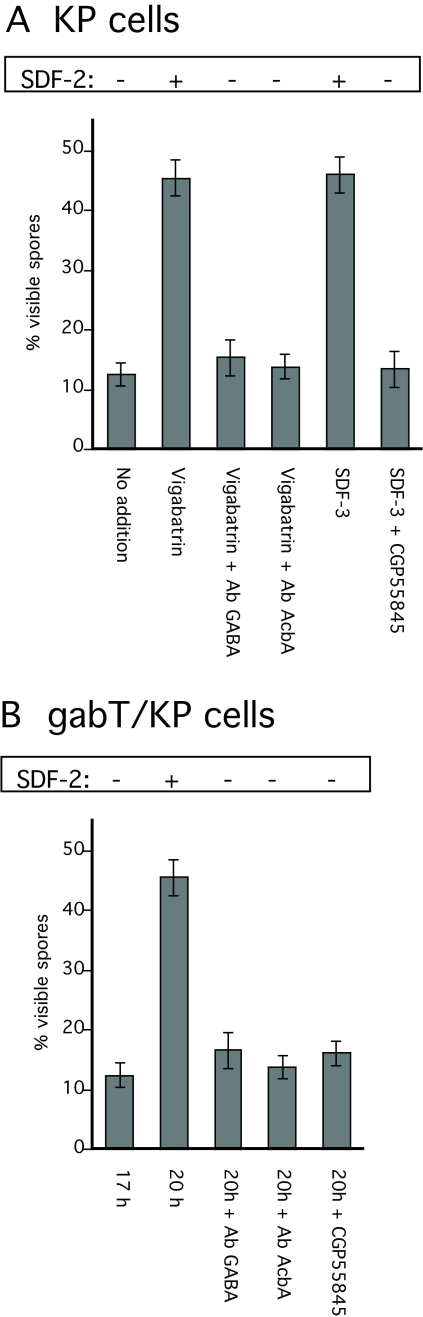
The level of GABA is determined by its rates of synthesis and degradation. Since the main enzyme known to degrade GABA is GABA transaminase, we investigated its role in regulating GABA levels. The Dictyostelium genome contains only one GABA transaminase gene (gabT). The predicted protein is highly similar to mammalian homologs. The role of gabT was investigated by inactivation using the non-reversible GABA transaminase inhibitor vigabatrin or by disruption of gabT. When vigabatrin was added to low-density KP cells, it induced SDF-2 release after 1 hour, followed by an induction of spore formation (Fig. 7A). This induction was prevented by anti-GABA or anti-AcbA antibodies, as well as by the GABA antagonist CGP 55845. The inactivation of_gabT_ in wild-type cells has little consequence for the development cycle. The _gabT_-null strain finished development 1-2 hours earlier than the wild-type strain and was less synchronous (not shown). Inactivation of gabT in KP cells resulted in a strain that secretes SDF-2 spontaneously. At a cell density of 2×104/cm2, SDF-2 appeared within 16 hours (data not shown), whereas it took 19-20 hours at a density of 1×103 cells/cm2 (Fig. 7B). Addition of CGP 55845, anti-GABA or anti-AcbA antibodies to the _gabT_-null KP cells after 17 hours of incubation prevented SDF-2 production and the resulting induction of spore formation.
Fig. 7.
Involvement of GABA transaminase in SDF-2 production. (A) Low-density KP cells were incubated overnight (∼18 hours) before the addition of the indicated compounds. The following concentrations were used: 1 μM vigabatrin, 10 units of SDF-3, 10 nM CGP55845, 1/5000 anti-GABA antibodies (final dilution), 1/500 anti-AcbA antibodies (final dilution). The number of spores was scored 1 hour after the addition of SDF-3 or 2 hours after the addition of vigabatrin. An aliquot of the cell supernatants was harvested and SDF-2 purified using anion-exchange resin before quantification on fresh KP cells. (B) Low-density _gabT_-null KP cells lacking functional GABA transaminase were incubated for 17 hours in cAMP buffer. The number of spores was scored before and again 3 hours after addition of 1/5000 anti-GABA antibodies (final dilution), 1/500 anti-AcbA antibodies (final dilution), or 10 nM CGP55845. Aliquots of the cell supernatants were harvested and SDF-2 purified using anion-exchange resin before quantification on fresh KP cells. Minus indicates production of less than 0.2 units of SDF-2/103 cells, whereas plus indicates more than 5000 units of SDF-2/103 cells.
DISCUSSION
Multiple lines of evidence indicate that SDF-3 is a steroid produced late in development of Dictyostelium that induces GABA release. Unlike SDF-1 and SDF-2, SDF-3 does not bind to either anionic or cationic resin but binds strongly to the hydrophobic resin Amberlite XAD-2. It is a heat-stable, protease-resistant small molecule that partitions into chloroform. Polyketide synthases are a known source of small hydrophobic morphogens such as DIF-1 or MPBD (Saito et al., 2006;Saito et al., 2008). However, inhibition of polyketide synthase by cerulinin failed to prevent SDF-3 accumulation and neither DIF-1 nor MPBD mimics SDF-3 activity. By contrast, drugs that inhibit cholesterol side-chain cleavage and the P450 hydroxylases that are necessary for conversion of cholesterol to steroids block accumulation of SDF-3. Various steroids were found to mimic SDF-3 by inducing SDF-2 production, with hydrocortisone showing the highest activity. Finally, the most convincing evidence that SDF-3 is a steroid is that mifepristone blocks its effects as effectively as it blocks those of hydrocortisone. We do not know the exact structure of SDF-3 because the necessary analytical techniques require relatively large amounts of purified material. Assuming that the affinity of SDF-3 for cells is similar to that of hydrocortisone, we estimate that at most 1 picomole of SDF-3 can be recovered and purified from 1×105 cells (Table 4).
Table 4.
Properties of known spore inducers
| Inducer | Time produced (hours) | Units produced/103 cells | Minimum effective concentration (M) |
|---|---|---|---|
| SDF-3 | 22 | 1 × 10−2 | Unknown |
| GABA | 22 | 0.5 | 1 × 10−9 |
| SDF-2 | 22 | 1 × 104 | 2 × 10−15 |
| SDF-1 | 18-20 | 1 × 103 | 1 × 10−12 |
| Discadenine | 24-30 | 1.5 × 10−3 | 1 × 10−8 |
Steroids, such as estrogen and testosterone, are well-known transcriptional regulators that diffuse into cells and enter the nucleus where they bind to specific transcription factors. However, steroid hormones have also been found to elicit rapid cellular responses by binding to surface GPCRs (Thomas et al., 2006). We found that GrlA, a plasma membrane-localized GPCR, is necessary for the rapid response to SDF-3. This receptor appears to be coupled to G proteins containing Gα4 because mutants lacking Gα4 also fail to respond to SDF-3. Gα4 had been shown to be required for folate sensing during growth, presumably coupled to a different GPCR because folate chemotaxis is unimpaired in cells lacking GrlA (Prabhu et al., 2007).
SDF-3 induces the production of SDF-2 by stimulating the release of sufficient GABA to induce secretion of AcbA within 5 minutes. Addition of antibodies to GABA blocked SDF-2 production if added within the first 4.5 minutes after addition of SDF-3, but not after 5 minutes (Fig. 2B). When the volume used in the bioassay was reduced from 500 μl to 100 μl, the difference in the time of appearance of SDF-2 following addition of SDF-3 and addition of GABA was reduced to ∼1 minute, indicating that GABA is constantly secreted for several minutes, rather than coming as a burst as more time is required to reach the threshold for SDF-2 production in the larger volume (Fig. 2C). The response to threshold levels of GABA appears to be very rapid because addition of 1 nM GABA to KP cells for only 10 seconds, followed by removal and addition of fresh buffer, was sufficient to trigger SDF-2 production (data not shown). Similar studies with mammalian cells have shown that activation of GPCRs by their agonists can activate downstream signaling within 500 milliseconds (Lohse et al., 2008).
SDF-3 fails to act in a strain lacking the late glutamate decarboxylase GadA, in which GABA levels are less than 20% of those in KP cells. SDF-3 signaling might stimulate GadA or could inhibit the GABA-degrading enzyme GABA transaminase, or both. We found that low-density cultures of a KP strain lacking GABA transaminase spontaneously produce SDF-2 and form many more spores than the parental KP strain (Fig. 7). This spontaneous sporulation was inhibited by addition of antibodies to GABA or the GABA antagonist CGP55845 to the low-density cultures. The levels of intercellular GABA appear to be in kinetic equilibrium determined by the relative rates of secretion and degradation. It is possible that GrlE only requires transient binding of GABA to trigger AcbA release.
SDF-3 also removes the inhibitory effect of glutamate on AcbA release, allowing a sharper induction by GABA. Since glutamate blocks both AcbA secretion by prespore cells and TagC exposure in prestalk cells, SDF-3 has to remove these inhibitions in both cell types. Gα9 and GrlE are required to transduce glutamate inhibition (Anjard and Loomis, 2006). It is possible that the observed desensitization by SDF-3 results from a modification to the relative affinity of GrlE for GABA and glutamate.
The results from the synergy experiments clearly put GrlA, Gα4 and GadA in one group and GrlE, Gα7 and AcbA in another (Fig. 1). None of these mutant strains produces SDF-2 when developed as pure populations. Mutant strains lacking a component in one group are able to cooperate with strains lacking a component of the other group, but not with strains lacking a component in their own group. The genes mutated in these strains almost all code for proteins that act internally and are not secreted. Therefore, it is somewhat surprising that their phenotypes are non-cell-autonomous. However, when viewed as a signaling cascade in which the internal components generate intercellular signals connecting the synergy groups, the results are all consistent. The results also imply that the pathway leading to SDF-2 production is a serial cascade rather than comprising the integration of converging signals.
As previously mentioned, SDF-3 levels in fruiting bodies are extremely low. When a unit is defined as the lowest dilution giving full induction of spore formation in the KP cell bioassay, only 10-2 units SDF-3/103 cells are recovered (Table 4). However, SDF-3 triggers release of 50-fold more GABA in terms of units per cell. SDF-2 production occurs in a single burst upon stimulation by GABA and generates 104 units SDF-2/103 cells (Anjard et al., 1998b;Anjard and Loomis, 2006). Thus, the signaling cascade results in a million-fold amplification of the original signal. Since the bioassay is typically performed in a 500 μl volume with 2000 cells, the activity of SDF-3 and GABA is below the detection limit, unless the samples have been previously concentrated. This is in contrast to SDF-2 or SDF-1, which can easily be detected in the extracellular buffer. The advantages of having such a multistep cascade regulating encapsulation are that most cells are required to produce SDF-3 such that it can accumulate to the threshold for GABA induction, and that GABA then elicits a large burst of SDF-2 production ensuring that all prespore cells are synchronously induced to encapsulate. Since exposure of the protease domain of TagC on the outside of prestalk cells, where it can process AcbA into SDF-2, also requires GABA signaling through GrlE, the signaling cascade coordinates differentiation of the cell types.
Supplementary material
Supplementary material for this article is available athttp://dev.biologists.org/cgi/content/full/136/5/803/DC1
Supplementary Material
[Supplementary Material]
We thank Angelika Noegel for providing the _grlA_-null strain; Dale Hereld and the Dictyostelium Stock Center for the other mutants; Danny Fuller for technical help; Jean-Philippe Pin for discussion of GPCRs; Serge Picaud for useful discussions about GABA transaminase and for providing the initial stock of vigabatrin. This work was supported by a grant from the NIH (GM78175). Deposited in PMC for release after 12 months.
References
- Anjard, C. and Loomis, W. F. (2005). Peptide signaling during terminal differentiation of Dictyostelium. Proc. Natl. Acad. Sci. USA 102, 7607-7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjard, C. and Loomis, W. F. (2006). GABA induces terminal differentiation of Dictyostelium through a GABAB type receptor. Development 113, 2253-2261. [DOI] [PubMed] [Google Scholar]
- Anjard, C. and Loomis, W. F. (2008). Cytokinins induce sporulation in Dictyostelium. Development 135, 819-827. [DOI] [PubMed] [Google Scholar]
- Anjard, C., Chang, W. T., Gross, J. and Nellen, W. (1998a). Production and activity of spore differentiation factors (SDFs) in Dictyostelium. Development 125, 4067-4075. [DOI] [PubMed] [Google Scholar]
- Anjard, C., Zeng, C., Loomis, W. F. and Nellen, W. (1998b). Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol. 193, 146-155. [DOI] [PubMed] [Google Scholar]
- Anjard, C., Pinaud, S., Kay, R. R. and Reymond, C. D. (1992). Overexpression of Dd PK2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development 115, 785-790. [DOI] [PubMed] [Google Scholar]
- Brandon, M. A., Mahadeo, D. C. and Podgorski, G. J. (2002). Galpha3 and protein kinase A represent cross-talking pathways for gene expression in Dictyostelium discoideum. Dev. Growth Differ. 44, 457-465. [DOI] [PubMed] [Google Scholar]
- Jandrositz, A., Turnowsky, F. and Högenauer, G. (1991). The gene encoding squalene epoxidase from_Saccharomyces cerevisiae_: cloning and characterization. Gene 107, 155-160. [DOI] [PubMed] [Google Scholar]
- Kelly, P. J., Kelleher, J. K. and Wright, B. E. (1979). The tricarboxylic acid cycle in Dictyostelium discoideum: metabolite concentrations, oxygen uptake and 14c-labelled amino acid labelling patterns. Biochem. J. 184, 581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinseth, M., Anjard, C., Fuller, D., Guizzunti, G., Loomis, W. F. and Malhotra, V. (2007). The Golgi associated protein GRASP is required for unconventional protein secretion during development. Cell 130, 524-534. [DOI] [PubMed] [Google Scholar]
- Klein, G., Cotter, D. A., Martin, J. B. and Satre, M. (1990). A natural-abundance 13C-NMR study of Dictyostelium discoideum metabolism: monitoring of the spore germination process. Eur. J. Biochem. 193, 135-142. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., Hadwiger, J. A., Pupillo, M. and Firtel, R. A. (1991). Molecular genetic analysis of two Galpha protein subunits in Dictyostelium. J. Biol. Chem. 266, 1220-1228. [PubMed] [Google Scholar]
- Lohse, M. J., Hein, P., Hoffmann, C., Nikolaev, V. O., Vilardaga, J. P. and Bünemann, M. (2008). Kinetics of G-protein-coupled receptor signals in intact cells. Br. J. Pharmacol. 153, 125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petranyi, G., Ryder, N. S. and Stütz, A. (1984). Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 224, 1239-1241. [DOI] [PubMed] [Google Scholar]
- Prabhu, Y., Mondal, S., Eichinger, L. and Noegel, A. A. (2007). A GPCR involved in post aggregation events in Dictyostelium discoideum. Dev. Biol. 312, 29-43. [DOI] [PubMed] [Google Scholar]
- Puta, F. and Zeng, C. (1998). Blasticidin resistance cassette in symmetrical polylinkers for insertional inactivation of genes in Dictyostelium. Folia Biol. 44, 185-188. [PubMed] [Google Scholar]
- Saito, T., Taylor, G. W., Yang, J. C., Neuhaus, D., Stetsenko, D., Kato, A. and Kay, R. R. (2006). Identification of new differentiation inducing factors from Dictyostelium discoideum. Biochim. Biophys. Acta 1760, 754-761. [DOI] [PubMed] [Google Scholar]
- Saito, T., Kato, A. and Kay, R. R. (2008). DIF-1 induces the basal disc of the Dictyostelium fruiting body. Dev. Biol. 317, 444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, J. R., Hsueh, A. J. and Baulieu, E. E. (1983). Binding of the anti-progestin RU-486 to rat ovary steroid receptors. Contraception 128, 77-85. [DOI] [PubMed] [Google Scholar]
- Serafimidis, I. and Kay, R. R. (2005). New prestalk and prespore inducing signals in Dictyostelium. Dev. Biol. 282, 432-441. [DOI] [PubMed] [Google Scholar]
- Shaulsky, G., Escalante, R. and Loomis, W. F. (1996). Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl. Acad. Sci. USA 93, 15260-15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman, M. (1954). Synergistic and antagonistic interactions between morphogenetically deficient variants of the slime mould Dictyostelium discoideum. J. Gen. Microbiol. 10, 110-120. [DOI] [PubMed] [Google Scholar]
- Sussman, M. (1987). Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28, 9-29. [DOI] [PubMed] [Google Scholar]
- Thomas, P., Dressing, G., Pang, Y., Berg, H., Tubbs, C., Benninghoff, A. and Doughty, K. (2006). Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids 71, 310-316. [DOI] [PubMed] [Google Scholar]
- Thomason, P. A., Traynor, D., Stock, J. B. and Kay, R. R. (1999). The RdeA-RegA system, a eukaryotic phospho-relay controlling cAMP breakdown. J. Biol. Chem. 274, 27379-27384. [DOI] [PubMed] [Google Scholar]
- Wang, N., Soderbom, F., Anjard, C., Shaulsky, G. and Loomis, W. F. (1999). SDF-2 induction of terminal differentiation in_Dictyostelium discoideum_ is mediated by the membrane-spanning sensor kinase DhkA. Mol. Cell. Biol. 19, 4750-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Material]