Role of the estrogen receptors GPR30 and ERα in peripheral sensitization: relevance to trigeminal pain disorders in women (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 12.
Abstract
Estrogen increases facial allodynia through its actions on activation of the MAP kinase ERK in trigeminal ganglion neurons. This goal of study was to determine which estrogen receptor is required for behavioral sensitization. Immunohistochemical studies demonstrated the presence of estrogen receptor alpha (ERα) in nuclei of larger neurons and cytoplasm of smaller neurons, and the novel estrogen receptor G-protein coupled receptor 30 (GPR30) in small diameter neurons that also contained peripherin, a marker of unmyelinated C-fibers. Specific agonists for ERα (PPT) and GPR30 (G-1), but not ERβ (DPN), activated ERK in trigeminal ganglion neurons in vitro. Both G-1 and PPT treatment increased allodynia after CFA injections in to the masseter of ovariectomized Sprague-Dawley rats. Treatment with estrogen increased expression of ERα but not GPR30, while masseter inflammation increased GRP30 but not ERα. Differential modulation of these ERK-coupled receptors by estrogen and inflammation may play a role in painful episodes of TMD and migraine.
Keywords: GPR30, estrogen receptor α, allodynia, trigeminal ganglion, ERK, estrogen
Introduction
Trigeminal pain disorders such as migraine and temporomandibular disorder (TMD) are among the most common neurological conditions (1-3) and are two to three times more prevalent in women than in men (1, 4) The sex disparity in the prevalence of trigeminal pain has been attributed to fluctuations in estrogen (5), and trigeminal pain is concentrated during the reproductive years, often beginning at menarche and declining after menopause (6). Trigeminal pain varies across the menstrual cycle, increasing during the perimenstrual period (7), and in some cases at mid-cycle (8). Although clinical data suggest that estrogen increases susceptibility to migraine, the fall in serum estrogen levels at the time of menstruation may precipitate an attack, suggesting a complex relationship between estrogen levels and the severity of trigeminal pain.
Exogenous estrogens can also affect nociception in the trigeminal system. For example, estrogen replacement therapy decreases thermal pain thresholds (9) and may trigger migraine with aura (10). Oral contraceptive use increases the risk of TMD pain (11). These effects may result from estrogen acting directly on the trigeminal system, which is rich in estrogen receptors (12).
Estrogen may affect a cell through classical nuclear pathways or by signal transduction cascades initiated at cell surface membrane receptors. These estrogen receptors may include the well characterized estrogen receptor α (ERα), estrogen receptor β (ERβ), or the more recently described GPR30, a G-protein coupled receptor that has been identified as a novel estrogen receptor (13-15). In response to estrogen, GPR30 activates downstream second messenger pathways, including the MAPK extracellular-signal regulated kinase (ERK) (16). ERK activation is a specific marker of nociceptor activation (17) that is required for thermal and mechanical hyperalgesia in several models of neuropathic and inflammatory pain (18, 19). Estrogen increases facial allodynia through activation of ERK in trigeminal ganglion neurons (20), but the specific receptor mediating these changes has not been determined.
In order to better characterize the effects of estrogen on trigeminal nociception, we analyzed the distribution of GPR30 and the classical estrogen receptor ERα in the trigeminal ganglion and investigated the effects of selective agonists for ERα or GPR30 on ERK activation and facial allodynia. In order to determine how estrogen receptors may be regulated by estrogen and inflammation, we examined changes in protein expression of ERα and GPR30 in the presence and absence of estrogen and peripheral inflammation. Our results support the hypothesis that GPR30 is a mediator of estrogen-related trigeminal nociception and indicate that either GPR30 or ERα can mediate trigeminal sensitization. Furthermore, differential regulation of these receptors by estrogen and inflammation may contribute to migraine and TMD pain.
Methods
Animals
Female Sprague-Dawley rats aged 60-75 days (Harlan, Indianapolis, IN) were used for all studies. Rats were maintained on a daily 12h light, 12 h dark schedule with ad libitum access to water and food (Harlan Teklad 8604). All studies were conducted in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Ovariectomy
Rats were rapidly anesthetized with 4% isoflurane in compressed oxygen, and anesthesia was maintained on 1% isoflurane. Dorsal bilateral incisions were made midway between the lower ribs and the iliac crest, and both ovaries were isolated and removed. Both incisions were closed with suture clips. Animals then received Buprenex (0.1mg/kg) and were allowed to fully regain consciousness before returning to the animal facility.
Western blot
Trigeminal ganglia were harvested and homogenized in cold lysis buffer (20 mM Hepes buffer, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 5 mg/ml pepstatin A, 10 mg/ml leupeptin and 10 mg/ml aprotinin) using a Dounce homogenizer. The homogenate was centrifuged at 12,000 g (10 min, 4 ° C) and the supernatant collected. Total protein concentrations were measured with a bicinchoninic acid (BCA) assay kit using bovine serum albumin as a standard (Pierce Biotechnology, Inc., Rockford, IL). Proteins were separated using SDS-PAGE on 12%TRIS-HCl gels (BioRad, Hercules, CA) and electrophoretically transferred to polyvinylidene difluoride membranes. Membranes were subsequently probed with a rabbit anti-GPR30 antibody raised against the C-terminal domain of human GPR30 (1:500, LifeSpan) or rabbit anti-estrogen receptor α (MC-20, 1:300, Santa Cruz Biotechnology, Santa Cruz, CA). Goat anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 1:1000 (Santa Cruz Biotechnology, Santa Cruz, CA) was used to control for sample loading. Membranes were rinsed in TBS-Tween-20 and IRDye 800 conjugated anti-goat (Rockland Immunochemicals, Gilbertsville, PA) and Alexa 680 conjugated anti-rabbit (Invitrogen, Eugene, OR) secondary antibodies were applied 1:10,000 in blocking buffer for 1 hour at room temperature. Membranes were rinsed in TBST followed by TBS and visualized using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Band intensities were determined using Odyssey software version 1.0 (LI-COR, Lincoln, NE) and reported relative to GAPDH.
Immunohistochemistry
Two weeks following ovariectomy, rats were deeply anesthetized with sodium pentobarbital (50mg/kg i.p.) and perfused transcardially with 0.1M PBS followed by 4% buffered paraformaldehyde. Trigeminal ganglia were removed, post-fixed overnight in 4% buffered paraformaldehyde and cryoprotected in 30% sucrose in 0.1 M phosphate buffer pH 7.2. Ganglia were frozen in Tissue Freezing Medium (Triangle Biomedical Science, Durham, NC) at −80° C. Frozen sections (20μm) through the horizontal plane of the trigeminal ganglion were prepared using a cryostat (Carl Zeiss, Inc., Oberkochen, Germany). Horizontal sectioning of the ganglion allows visualization of the three anatomic divisions in a single section. The sections were permeabilized and blocked for 1 hour in a solution of 0.2% Triton-X100 and 2% normal goat serum in 0.1M PBS. GPR30 was labeled by incubating overnight at 4°C with rabbit anti-GPR30 (1:300, LifeSpan Biosciences) followed by 1 hour in AlexaFluor 568 goat anti-rabbit in PBS (1:500, Molecular Probes, Carlsbad, CA). For double-labeling experiments, slides were incubated overnight in rabbit anti-GPR30 antibody and either chicken anti-neurofilament H antibody (NFH, 1:300, Chemicon, Temecula, CA), chicken anti-peripherin antibody (1:300, Chemicon), or mouse anti-ERα antibody (Abcam, 1:100). AlexaFluor 568 conjugated goat anti-rabbit was used as a secondary antibody with either AlexaFluor 488 goat anti-mouse antibody or AlexaFluor 488 goat anti-chicken antibody (1:500, Molecular Probes). Slides were coverslipped using ProLong Gold anti-fade reagent (Molecular Probes).
Microscopy and analysis
Fluorescent digital images were obtained using a Nikon Eclipse 90i upright microscope and a Nikon C1 confocal imaging system using a 20× objective and a frame size of 1024 × 1024 pixels. Sections processed omitting the primary antibody were used to control for non-specific fluorescence. Detector gain and laser intensity were initially set using control slides, and all images were subsequently collected under the same photomultiplier detector conditions and pinhole diameter. In double-label experiments, single-antibody labeled sections were used to control for channel bleed-through, and multi-channel images were acquired using sequential excitation. For cell counting, individual images were montaged into a single image using Adobe Photoshop CS2 to prevent double-counting at the image edges. The peak intensity of cells processed in the absence of primary antibody determined the threshold for counting a cell as GPR30 positive. At least 1000 cells were counted from each of 4 animals for each condition. At least 100 neurons were measured per trigeminal ganglion section.
Single and double-labeled neurons were counted from digital images to obtain a percent score for colocalization of GPR30 with each phenotype marker. The percentage of neurons labeled with both markers was determined by counting the total number of neurons containing GPR30 immunoreactivity and identifying the number of cells labeled with the second neuronal marker. The same procedure was repeated to count the Alexafluor 488 labeled cells. We determined the percentage of peripherin and NFH immunoreactive neurons that also contain GPR30 by dividing the number of double-labeled neurons by the total number of neurons that were immunoreactive for each marker. The experiment was repeated using two sets of independent samples.
Cell diameters were measured using the ruler function of Adobe Photoshop CS2. The length of the short and long axes of each neuron in every selected file was measured. The diameter of each cell was calculated as the average of length and width. Data are reported as mean ± standard error, with a P value less than 0.05 using a two-tailed t-test considered significant.
Tissue Culture
For each experiment, trigeminal ganglia from 4-5 cycling female rats were dissected from the base of the skull, rinsed in Hank’s balanced salt solution, minced and suspended in digestion medium containing Leibovitz L15 medium with 1 mg/mL bovine serum albumin (BSA), 250 U/mL of CLSPA collagenase, 1 U/mL of ESL elastase, 5 U/mL of PAPL papain (enzymes from Worthington Biochemical Corp., Lakewood, NJ, USA). An equal volume of 30% Stractan (Larex, White Bear Lake, MN, USA) in Leibovitz’s L15 was added to the digest. After mixing by inversion, samples were centrifuged in a swinging bucket rotor at 4°C or 10 minutes at 500_g_ to enrich the culture for neuronal cells. The pellet of trigeminal cells was suspended in a small volume of neurobasal A medium (Gibco/Invitrogen, Carlsbad, CA). The cells were plated onto poly-d-lysine coated glass coverslips (Becton Dickinson) and maintained in phenol red-free neurobasal A medium containing 10% fetal bovine serum, 50 ng/mL nerve growth factor, B27 supplement, and 20 _μ_M cytosine arabinoside. Serum and NGF were removed 24 hours prior to pharmacologic treatment to reduce baseline ERK phosphorylation. Experiments were repeated using at least 5 independent tissue cultures.
In vitro pharmacologic treatments
We used synthetic 17_β_-estradiol (Sigma E-2758, St. Louis, MO, USA), the most potent and receptor-specific form of estrogen, to study the effects of estrogen. The 17_β_-estradiol was initially dissolved in ethanol at a concentration of 10−1 M, then further dilutions were performed in phosphate-buffered saline (PBS) and 0.1% BSA to achieve a final concentration of 10−8 M, corresponding to a physiological range of plasma estrogen. Cells in the control wells received the diluent only. After 72 hours in culture, to allow endogenous estrogen to be metabolized, and 24 hours following replacement with serum-free medium, cells were treated with 10 nM 17J-estradiol (Sigma, St. Louis, MO), 0.1 M PBS, or selective agonists for ERα— 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT, 10 nM Sigma, St. Louis, MO) (21), GPR30—G-1 (1 μM, Chemical Diversity, San Diego, CA (22), or diarylpropionitrile DPN (100 nM, Sigma). G-1 is a recently characterized selective agonist for GPR30 (22). PPT is a potent ERα agonist that binds ERα with a 410-fold higher binding affinity than ERβ (21), and MDPN is a selective agonist for ERβ that binds ERβ with a 70 fold higher affinity than ERα.
Immunocytochemistry
Following pharmacologic treatments, cells were fixed for 10 min in 4% paraformaldehyde at room temperature, rinsed, then permeabilized for 15 minutes in 0.2% Triton X-100 and blocked for 1 h in 1× PBS containing 5% normal goat serum. Coverslips were incubated in rabbit anti-p-ERK (1:200, Cell Signaling Technology) and mouse anti-NeuN (1:1000, Chemicon, Temecula, CA) in 1X PBS containing 3% NGS overnight at 4°C followed by washing and secondary antibody staining with goat anti-rabbit Alexa Fluor 568 and goat anti-mouse Alexa Fluor 488 (1:200, Molecular Probes) for 30 min at 25°C. Images were acquired at 20× magnification using a Nikon confocal microscope. A minimum of 100 cells from three independent cultures were analyzed for each experimental group. Cells immunoreactive for NeuN were scored as p-ERK positive or negative by an observer who was unaware of the treatment group.
Orofacial sensitivity assay
Mechanical sensitivity of the orofacial region was assessed using the behavioral assay described previously (20). Briefly, rats were conditioned to traverse a clear plastic tunnel attached to a behavioral arena in order to gain access to a bottle containing 0.1M sucrose. During each session, the rats consume approximately 2 mL sucrose, less than 10% of their total daily water intake. This amount of sucrose consumption is less than 1% of the amount required to produce antinociceptive effects in adult rats, approximately 1M sucrose ad libitum over three to four weeks (23). Importantly, the acute effects of sucrose on analgesia, present in neonates, disappear by P21 (24). The rats were not restrained in any way, and the tunnel is large enough for the rat to turn around to reenter the arena at any time. Rats were coded and experimenters blinded to experimental groups. Withdrawal behaviors were assessed in response to stimulation of the whisker pad or skin overlying the masseter using 0.16 g and 4 g monofilaments, respectively. Preliminary studies established that these filaments produced withdrawal responses at or below threshold in control rats. The behavioral responses were scored as follows: no response (scored 0); flinch/orienting reflex (scored 1); partial retraction (scored 2); or complete retraction (scored 3). A flinch/orienting reflex was classified as a discontinuation in licking behaviors and orientation toward the stimulus. A partial retraction was classified as retraction of the head away from the reward. Escape back into the tunnel or arena was scored as a full retraction. A percent withdrawal score was calculated for each rat as the sum of response scores divided by the total possible score, a score of 3 on all trials. Mechanical withdrawal responses were measured 24, 48, and 72 hours following CFA in combination with selective agonist treatments. (31).
CFA and selective agonist treatment
Two weeks following ovariectomy, rats were given a single 50 μl injection of Complete Freund’s Adjuvant (CFA; Mycobacterium tuberculosis H37 Ra 1 mg/ml; Sigma, St. Louis, MO) emulsion, 1:1 in 0.1M phosphate buffer into the left masseter muscle under isoflurane anesthesia. This dose produces molecular and behavioral effects that last for 3 days (25, 26) when injected into the masseter. Control rats were injected with an equal volume of isotonic saline. Estradiol valerate (E2, Delestrogen, Monarch Pharmaceuticals, Bristol, TN, 10 μg/kg in 100μl sesame oil vehicle) was injected subcutaneously using a 22 gauge needle during the same surgery as CFA injection into the masseter. Estradiol valerate is an estradiol conjugate that provides prolonged stable serum estrogen levels (27). All control rats received sesame oil vehicle. Post-mortem examination of CFA-treated rats and hematoxylin and eosin staining of sectioned facial structures revealed granulomatous inflammation confined to the masseter muscle. We did not observe inflammatory pathology upon examination of cranial sites outside the masseter, including the whisker pad. This is supported by other studies using CFA injection of TMJ and masseter that demonstrate inflammation confined to the injection site (28-30). Behavioral experiments were conducted with three groups of 10-12 rats. Each rat was given a CFA injection into the masseter muscle and either the ERα agonist PPT, (Sigma, St. Louis, MO, 1mg/kg dissolved 1 μg/ul in sesame oil), the GPR30 agonist G-1 (Chemical Diversity, San Diego, CA, 1mg/kg dissolved 1 μg/ul in sesame oil), or sesame oil vehicle by subcutaneous injection between the scapulae using a 22 gauge needle. The PPT dose was selected according to Harris, 2002. G-1 has been shown to induce sensitization in vivo at a range of doses spanning several orders of magnitude (32).
Statistical analysis
Data were analyzed using Graph Pad Prism version 4.0 software (Graph Pad, San Diego, CA) Behavioral data and immunocytochemistry were analyzed using one-way ANOVA with a Bonferroni post-hoc test. Western blots and cell size measurements were compared using two-tailed unpaired t-tests. Data are presented as mean +/− SEM, with a P value less than 0.05 considered significant.
Results
GPR30 expresssion in rat trigeminal ganglion
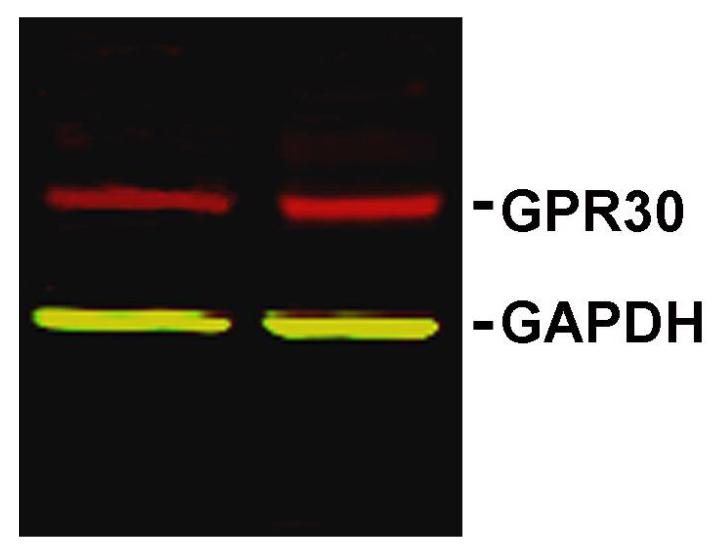
In order to determine whether GPR30 protein is expressed in the trigeminal ganglion, we performed Western blots on homogenates of trigeminal ganglia from ovariectomized female Sprague-Dawley rats using a GPR30-specific antibody. Membranes showed a single band of an appropriate molecular weight (Figure 1) that was eliminated by preincubation of the antibody with the immunizing peptide, indicating that the antibody is specific for GPR30 (data not shown).
Figure 1.
GPR30 protein is present in rat trigeminal ganglion. Western blot of trigeminal ganglion lysates from ovariectomized female rats labeled with antibodies to GPR30 (red) and the loading control GAPDH (green).
GPR30 expression in trigeminal neuron subpopulations
To determine the trigeminal ganglion cell types that express GPR30, we performed immunohistochemistry on frozen sections using the same GPR30 antibody used in Western blots. GPR30 immunoreactivity was localized to neurons (Figure 2). We observed immunofluorescence throughout the neuronal cytoplasm of positively labeled cells. Immunofluorescence was also specific for GPR30, as preincubation of the antibody with the immunogen peptide eliminated staining (data not shown).
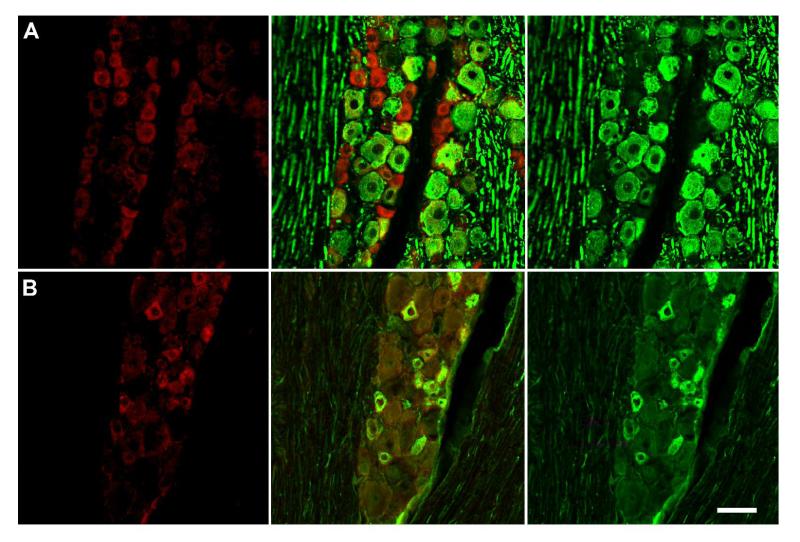
Figure 2.
GPR30 is localized to unmyelinated neurons in the trigeminal ganglion A) Double-label immunohistochemistry for GPR30 and NFH, a marker of myelinated neurons.
There is a small degree of overlap between GPR30 and NFH. B) Double-label immunohistochemistry for GPR30 and peripherin. GPR30 and peripherin are highly co-localized. Panels on the left are single-labeled for GPR30. The middle row of panels contains merged images of the left and right columns. The right row of panels are single-labeled neurofilament H (top) or peripherin (bottom). Scale bar (50 μm) applies to all images.
To characterize the expression of GPR30 within trigeminal ganglion sub-populations, we used double-label immunohistochemistry to determine the colocalization of GPR30 with peripherin, a marker of neurons with unmyelinated axons and neurofilament H (neurofilament 200), a marker of neurons with myelinated axons (Figure 2). The localization of GPR30 expression was primarily restricted to neurons with unmyelinated axons. Seventy-six percent of GPR30-positive neurons were peripherin positive, and 73% of peripherin-positive neurons were immunoreactive for GPR30. Thirty four percent of GPR30 positive neurons were NFH positive, and GPR30 was present in 32% of NFH positive neurons.
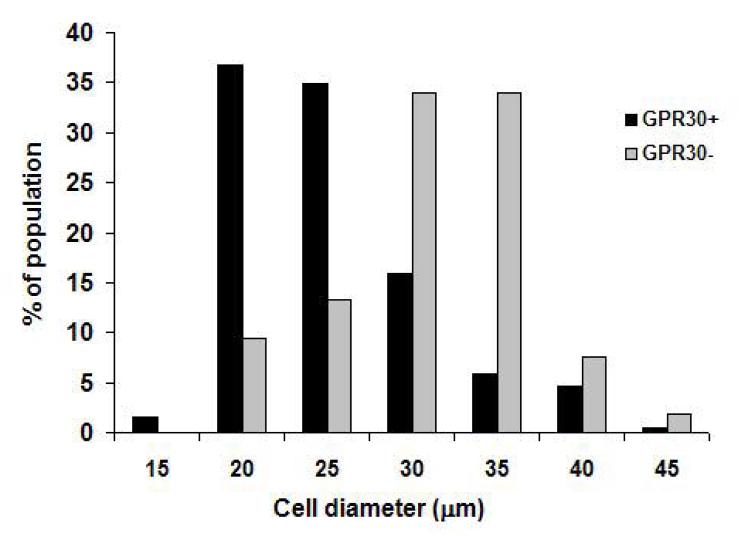
In order to investigate the neuronal phenotypes that express GPR30 in the trigeminal ganglion, we measured the diameters of cells that were immunoreactive and non-immunoreactive for GPR30. GPR30 immunoreactivity was skewed toward small diameter neurons (Figure 3), although GPR30 was expressed across a broad range of cell sizes. The average diameter of GPR30-positive cells (23.9 ± 0.8 _μ_m) was significantly smaller (p<0.01) than the average diameter of GPR30-negative cells (28.9 ± 1.04 _μ_m).
Figure 3.
GPR30 is present in small diameter neurons. Histogram shows cell diameter measurements for trigeminal ganglion neurons that were GPR30 immunoreactive (black bars) and GPR30 non-immunoreactive (gray bars).
ERα immunohistochemistry
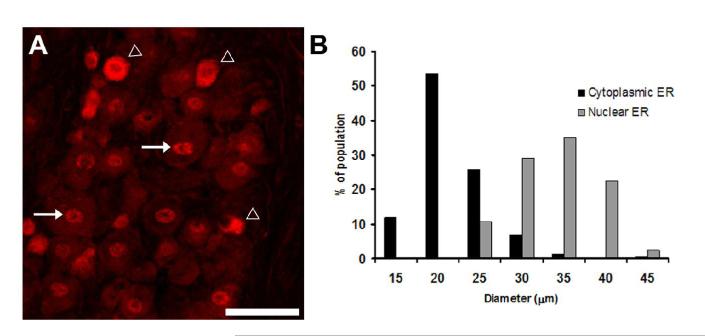
Because ERα is a putative mediator of estrogen-modified nociception, we used immunohistochemistry to label ERα in the trigeminal ganglion. We observed prominent ERα immunoreactivity throughout the trigeminal ganglion that was localized to neurons. ERα immunoreactivity was present in both nuclear and cytoplasmic compartments (Figure 4A). By visual inspection, cells with a predominately cytoplasmic ERα immunoreactivity pattern were prinicipally small neurons, while cells with a predominately nuclear ERα staining pattern had larger diameters. In order to quantify the distribution of immunoreactivity, we measured the diameters of neurons with cytoplasmic-predominant and nuclear-predominant staining patterns. Figure 4B shows the diameters of these neuronal populations. Cells containing prominent cytoplasmic ERα immunoreactivity had a mean diameter of 19.1 +/− 0.3 μm, which was significantly smaller (p<0.05) than cells expressing predominately nuclear ERα, which had a mean diameter of 31.2 +/− 0.4 μm.
Figure 4.
Cytoplasmic and nuclear ERERα immunoreactivity is present in the trigeminal ganglion. A) Immunohistochemistry for ERα in trigeminal ganglion from ovariectomized female rat. ERα staining was present in neurons in both cytoplasmic and nuclear locations B. Histogram showing cell diameter measurements for neurons that were immunoreactive for nuclear and cytoplasmic ERα. Cytoplasmic ERα was present in predominately small neurons and nuclear ERα was present in larger neurons.
Colocalization of GPR30 and ER α
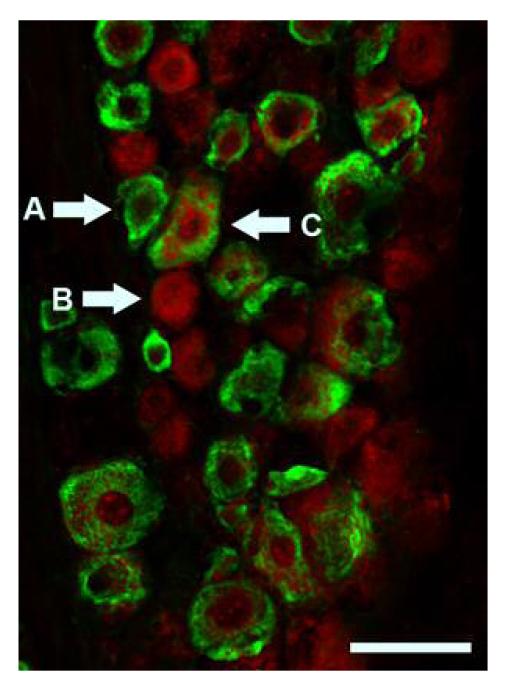
We used double-label immunohistochemistry to determine the co-localization of GPR30 and ERα. ERα and GPR30 were present in distinct but partially overlapping populations. We observed neurons that expressed both GPR30 and ERα, and neurons that expressed only ERα or GPR30 (Figure 5). Ten percent of trigeminal ganglion neurons expressed both GPR30 and ERα, 22% expressed ERα, and 35% expressed GPR30.
Figure 5.
GPR30 and ERα are present in distinct but overlapping populations in the rat trigeminal ganglion. Immunohistochemistry for GPR30 (red) and ERα (green) shows ERα positive (A), GPR30 positive (B), and ERα /GPR30 double-positive (C) neurons. Scale bar (50 μm)
ERK activation by selective agonists for ER and GPR30
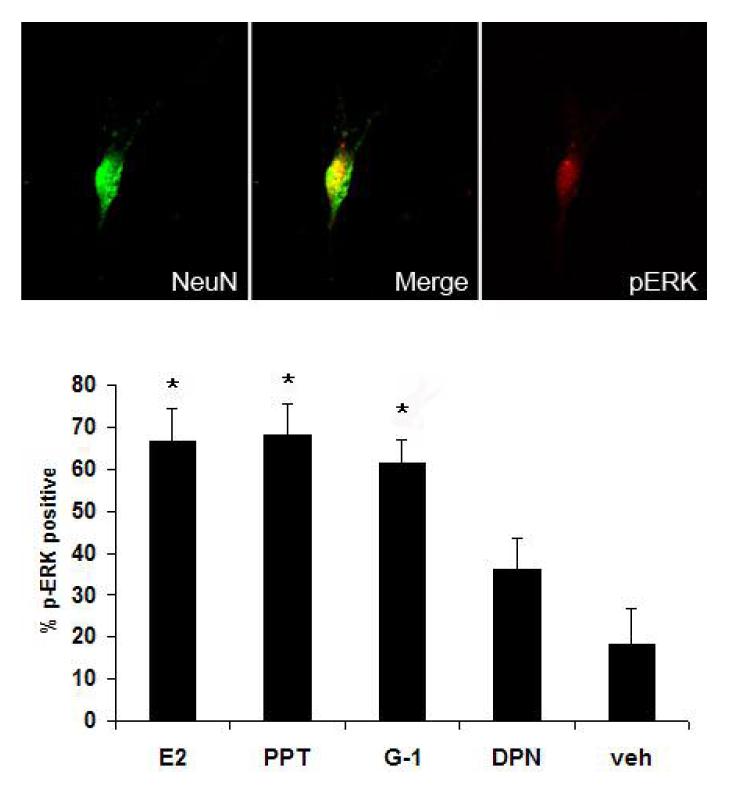
Estrogen has been demonstrated to activate ERK in primary cultures of dorsal root ganglion and trigeminal ganglion neurons (33, 34) and in the rat trigeminal ganglion in vivo (20). In order to determine which estrogen receptor mediates ERK activation, we assessed ERK activation in primary trigeminal ganglion cultures following application of agonists selective for GPR30, ERα, or ERβ. G-1 or PPT increased the percentage of p-ERK positive neurons compared to vehicle (Figure 6). Activated ERK immunoreactivity was present in 67% of PPT-treated neurons and 64% of G-1 treated neurons compared to 18% with vehicle treatment. DPN treatment did not significantly increase ERK activation.
Figure 6.
Selective activation of GPR30 or ERα activates ERK in trigeminal ganglion neurons in vitro. Cultured trigeminal ganglion neurons were treated with specific agonists for ERα (PPT, 10 nM), GPR30 (G-1, 1 μM), ERβ (DPN, 100 nM), 17β–estradiol (E2, 10nM), or vehicle. Following agonist treatment, cultures were stained with antibodies raised against the dually-phosphorylated form of ERK and the neuron-specific marker NeuN and the percentage of neurons immunoreactive for activated ERK was assessed. Data are presented as the mean percentage of NeuN positive neurons that were p-ERK positive from three independent experiments. (*=p<0.05 compared to vehicle, one-way ANOVA).
Selective agonists for GPR30 and ERα increase orofacial sensitivity
We previously demonstrated that estrogen increases facial allodynia through activation of ERK (20). To better characterize which estrogen receptor mediates increased trigeminal sensitization, we examined the effects of PPT and G-1 on facial mechanical nociception in the presence of orofacial inflammation using a rat behavioral model. We used Complete Freund’s Adjuvant (CFA), an established model of inflammatory pain (19, 35, 36) that can induce widespread mechanical hyperalgesia (37), to induce inflammation of the masseter.
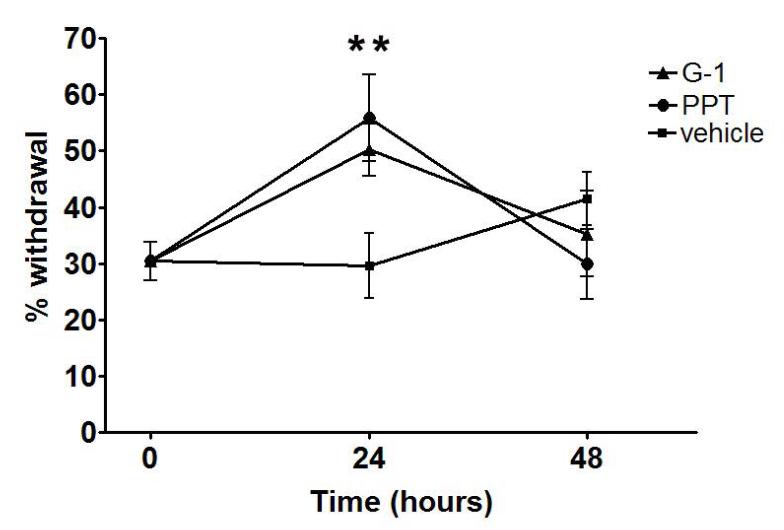
In order to assess facial mechanical sensitivity, we developed a behavioral model based on operant conditioning that allows monofilament testing of the orofacial region. We conducted a preliminary study to determine the monofilament force resulting in a 50% withdrawal threshold in ovariectomized rats. A sub-threshold filament was subsequently used for the testing of whisker pad in order to measure allodynia. In order to assess secondary allodynia, mechanical sensitivity of the whisker pad was measured using a 0.16g monofilament 24, 48, and 72 hours following CFA injection and selective agonist treatment. Injection of either PPT or G-1 enhanced withdrawal responses in the setting of peripheral inflammation at 24 hours compared to vehicle-treated controls (Figure 7).
Figure 7.
Selective activation of GPR30 or ERα enhances orofacial allodynia. Specific agonists for ERα (PPT) and GPR30 (G-1) increased withdrawal response to stimulation of the whisker pad in CFA-treated ovariectomized female rats. For each animal, the left masseter of was injected intra-muscularly with 50 μl CFA (1:1 in saline) and PPT (1 mg/kg), G-1 (1 mg/kg) or vehicle were injected subcutaneously. Withdrawal response to stimulation of the whisker pad with a von Frey filament (0.16g) was assessed at 24 hours after agonist administration. Data are shown as mean+/− SEM. N=8 per group; *=p<0.05 compared to vehicle, one-way ANOVA.
Estrogen effects on expression of GPR30 and ERα
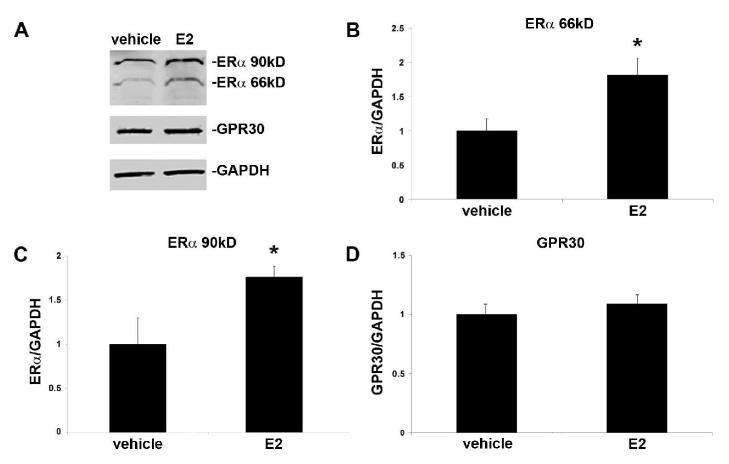
In order to determine whether estrogen treatment changes the protein content of estrogen receptors in the trigeminal ganglion, we used Western blot to compare ERα and GPR30 in trigeminal ganglion samples from OVX and OVX+E2 rats. We observed a significant increase in ERα in ganglia from estrogen-treated animals compared to vehicle-treated controls (Figure 8). Two size variants of ERα were present in the trigeminal ganglion, at 66 kD and 90 kD. Both variants were upregulated by estrogen, 1.81-fold and 1.76-fold respectively. GPR30 protein content was not significantly altered in the trigeminal ganglion by estrogen replacement.
Figure 8.
Estrogen treatment increases expression levels of ERα, but not GPR30, in the trigeminal ganglion of ovariectomized female rats. Estradiol valerate (10 μg/kg in sesame oil) or vehicle was administered by subcutaneous injection and trigeminal ganglia collected 24 hours later. A) Representative Western blots of trigeminal ganglion from ovariectomized rats treated with estrogen or vehicle. B) Effects of estrogen on protein level of the 66kD ERα isoform C) Effects of estrogen on the protein level of the 90kD ERα isoform D) Effects of estrogen on GPR30 protein levels. Data are shown as mean integrated intensity relative to GAPDH +/− SEM. (N=6 per group; *=p<0.05 compared to vehicle, t-test).
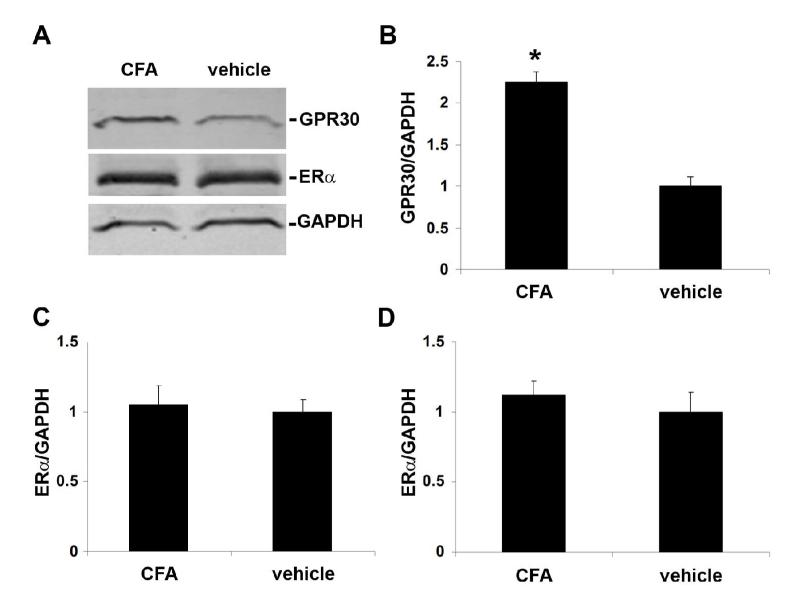
Inflammation effects on expression of GPR30 and ERα
In order to determine whether peripheral inflammation of the trigeminal nerve alters expression of ERα and GPR30 in the trigeminal ganglion, we compared ERα and GPR30 protein levels using Western blots of trigeminal ganglion samples taken from groups of ovariectomized rats with inflamed or normal masseter muscles. Inflammation of the masseter increased GPR30 expression 2.25 fold in the trigeminal ganglion compared to vehicle-treated controls, whereas it did not significantly change ERα expression in the trigeminal ganglion (Figure 9).
Figure 9.
Peripheral inflammation increases expression of GPR30 but not ERα in trigeminal ganglion from ovariectomized female rats. CFA (1:1 in saline) or vehicle was administered intra-muscularly into the masseter muscle and trigeminal ganglia were collected 24 hours later. A) Representative Western blots of trigeminal ganglion from rats with or without masseter inflammation. B) Effects of inflammation of the masseter muscle on GPR30 protein level. C and D) Effects of inflammation of the masseter muscle on ERα protein level for the 66kD (C) and the 90 kD (D) isoforms. Data are shown as mean integrated intensity relative to GAPDH +/− SEM (N=6 per group; *=p<0.05 compared to vehicle, two-tailed t-test).
Discussion
Although it is well established that estrogen modifies orofacial pain, the receptors mediating these changes have remained unclear. Results of the current study support roles for both the novel estrogen receptor GPR30 and estrogen receptor α in estrogen-modified nociception. Furthermore, our data show that these receptors are differentially regulated by estrogen and inflammation, which may have important implications for the modulation of trigeminal pain during the menstrual cycle.
GPR30 is present in the trigeminal ganglion
Our results show that GPR30 is present in the trigeminal ganglion of female rodents. GPR30 expression was localized to small, unmyelinated, peripherin-positive neurons, suggesting that GPR30 is localized to nociceptors in the trigeminal ganglion. Identification of a novel estrogen receptor in the sensory pathway is potentially significant to estrogen-modified trigeminal sensitization. Female animals are more sensitive to trigeminal nociception than males (38, 39) especially during the high estrogen phase of the estrous cycle (40, 41). Furthermore, estrogen replacement increases excitability of trigeminal afferents and enhances inflammatory sensitization of the trigeminal system (20, 42, 43). The presence of GPR30 in cells likely to be nociceptors suggests that GPR30 is a possible mediator of estrogen-modulated pain sensitization.
ERα is present in both cytoplasmic and nuclear compartments
ERα immunoreactivity was present in both cytoplasmic and nuclear compartments of trigeminal ganglion neurons. Previous studies of ERα in the rat trigeminal ganglion have restricted analyses to cells with nuclear labeling, discounting the potential importance of cytoplasmic estrogen receptor immunoreactivity (12, 33). These results are intriguing in light of the dual role of ERα in both classical nuclear pathways and ‘non-genomic’ actions through signal transduction pathways. ERα-dependent activation of signal transduction molecules is thought to originate from receptors sequestered in cytoplasmic signaling complexes (44). The current results suggest that this mechanism predominates in nociceptive neurons, while, in larger diameter neurons, where ERα immunoreactivity is localized to the nucleus, estrogen may preferentially function through direct genomic mechanisms. Previous studies have shown that estrogen modifies nociception through non-genomic mechanisms by activating ERK in the trigeminal ganglion (20).
ERα and GPR30 are expressed in distinct populations
Because the present data show that cytoplasmic ERα and GPR30 are expressed in small-diameter neurons, the co-localization of ERα and GPR30 in the trigeminal ganglion was analyzed. Results show that ERα and GPR30 are present in distinct, but partially overlapping, neuronal populations, and that the majority of small trigeminal neurons express one of these receptors. Expression of either ERα or GPR30 by the majority of nociceptors in the trigeminal ganglion suggests a basis for the high sensitivity of the trigeminal system to estrogen.
ERK is activated through GPR30 and ERα
Results show that selective agonists for either GPR30 or ERα induce rapid ERK activation in trigeminal ganglion neurons, suggesting that ERK activation mediated by estrogen (20, 33) can occur through either estrogen receptor α or GPR30. Furthermore, these data show that trigeminal GPR30 is functional in activating signal transduction pathways in trigeminal ganglion neurons, which may be relevant to estrogen’s modification of nociception.
There is considerable precedent for the activation of ERK by both ERα and GPR30 in many estrogen sensitive cell types (45-47), and signaling through either receptor has been demonstrated in the same cell type (45).
Estrogen receptor-evoked activation of ERK may be significant to the pathogenesis of estrogen-modified sensitization. ERK phosphorylation in sensory neurons is a specific marker of nociceptive stimulation (17) and a mediator of mechanical hyperalgesia in pain models of peripheral nerve injury and inflammation (48, 49). As estrogen increases mechanical allodynia of the orofacial region through activation of ERK (20), ERK phosphorylation resulting from ERα or GPR30 activation may be important to the pathogenesis of trigeminal pain. Activation of ERK in the unmyelinated neurons expressing ERα or GPR30 could lead to increases in neuronal excitability resulting in allodynia.
GPR30 and ERα participate in trigeminal sensitization
The results of the current study demonstrate that selective agonists for either GPR30 or ERα enhance secondary mechanical allodynia of the orofacial region, suggesting that both GPR30 and ERα are capable of mediating pro-nociceptive responses to estrogen in the trigeminal system. The in vitro data showing ERK activation in trigeminal ganglion neurons by selective estrogen receptor agonists suggest direct activation of ERK through ERα and GPR30 as a possible mechanism for the in vivo effects on allodynia. An additional possibility is that estrogen receptor agonists increase allodynia indirectly by modifying the inflammatory response. Knowledge of the in vivo properties of G-1 is also currently limited. Although in vitro studies have reported it to be a selective agonist for GPR30, the possibility that G-1 binds another orphan receptor cannot be excluded.
By identifying GPR30 as a putative modulator of sensitization in the trigeminal system, the current results reveal an increased complexity of pro-nociceptive effects of estrogen in the trigeminal system. The presence of GPR30 in the trigeminal ganglion presents a novel pathway through which estrogen may modulate the activity of sensory neurons, and which may be relevant to effects of hormonal fluctuations on migraine and facial pain.
ERα is increased by estrogen while GPR30 is increased by inflammation
Results show that inflammation in the trigeminal distribution upregulates GPR30 protein in the trigeminal ganglion. Although the effect of inflammation on GPR30 expression has not been previously addressed, estrogen and inflammation have been shown to have additive effects on trigeminal excitability and nociceptive responses (38, 40, 43). Increased expression of GPR30 in the presence of inflammation may lead to enhanced estrogen signaling. The current data suggest that peripheral inflammation during episodes of trigeminal pain may increase GPR30 and lead to enhanced modulation of nociception by estrogen. Furthermore, since inflammation did not modify expression levels of ERα, peripheral trigeminal inflammation may shift the balance of estrogen signaling in trigeminal neurons toward GPR30.
Data show that the presence of estrogen increases levels of ERα protein in the trigeminal ganglion. The relationship between serum estrogen and ERα receptor expression is complex and appears to be highly dependent on both the estrogen level and tissue studied, but these data are consistent with several previous studies showing that estrogen increases ERα in sensory neurons (12, 50). GPR30 expression, by contrast, was not changed by estrogen replacement. This result is consistent with studies of hamster ovary showing that GPR30 expression was not changed by estrogen replacement (51).
In conclusion, the results of this study demonstrate that both GPR30 and ERα pathways can modulate trigeminal nociception, providing new insight into the complex relationship between estrogen and trigeminal pain.
Acknowledgments
This project was supported by NIH Grant P20 RR016475 from the INBRE Program of the National Center for Research Resources, R21 DE01582, National Headache Foundation, KUMC Lied Foundation and Biomedical Research Training Program.
References
- 1.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Scher AI. Epidemiology and economic impact of migraine. Curr Med Res Opin. 2001;17(Suppl 1):s4–12. doi: 10.1185/0300799039117005. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 5.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 6.LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118:201–209. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63:351–353. doi: 10.1212/01.wnl.0000133134.68143.2e. [DOI] [PubMed] [Google Scholar]
- 8.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain. 2001;92:229–234. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor A. Estrogen replacement and migraine aura. Headache. 1999;39:674–678. doi: 10.1046/j.1526-4610.1999.3909674.x. [DOI] [PubMed] [Google Scholar]
- 11.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 12.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 15.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 16.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 17.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 18.Obata K, Yamanaka H, Dai Y, et al. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 19.Obata K, Yamanaka H, Dai Y, et al. Activation of extracellular signal-regulated protein kinase in the dorsal root ganglion following inflammation near the nerve cell body. Neuroscience. 2004;126:1011–1021. doi: 10.1016/j.neuroscience.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Liverman CS, Brown J, Klein RM, Berman NE. Evidence of peripheral mechanisms for regulation of facial allodynia. Headache. 2007;47:789. [Google Scholar]
- 21.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 22.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 23.Kanarek RB, Mandillo S, Wiatr C. Chronic sucrose intake augments antinociception induced by injections of mu but not kappa opioid receptor agonists into the periaqueductal gray matter in male and female rats. Brain Res. 2001;920:97–105. doi: 10.1016/s0006-8993(01)03039-6. [DOI] [PubMed] [Google Scholar]
- 24.Anseloni VC, Weng HR, Terayama R, et al. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97:93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Ro JY. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci Lett. 2007;421:91–95. doi: 10.1016/j.neulet.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ro JY. Bite force measurement in awake rats: a behavioral model for persistent orofacial muscle pain and hyperalgesia. J Orofac Pain. 2005;19:159–167. [PubMed] [Google Scholar]
- 27.Oriowo MA, Landgren BM, Stenstrom B, Diczfalusy E. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception. 1980;21:415–424. doi: 10.1016/s0010-7824(80)80018-7. [DOI] [PubMed] [Google Scholar]
- 28.Ambalavanar R, Moritani M, Moutanni A, et al. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006;120:53–68. doi: 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa A, Ren K, Tsuboi Y, et al. A new model of experimental parotitis in rats and its implication for trigeminal nociception. Exp Brain Res. 2003;152:307–316. doi: 10.1007/s00221-003-1538-x. [DOI] [PubMed] [Google Scholar]
- 30.Takeda M, Tanimoto T, Ikeda M, et al. Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. J Neurophysiol. 2005;93:2723–2738. doi: 10.1152/jn.00631.2004. [DOI] [PubMed] [Google Scholar]
- 31.Meyers MJ, Sun J, Carlson KE, et al. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn J, Dina OA, Goswami C, et al. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 33.Puri V, Puri S, Svojanovsky SR, et al. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26:33–42. doi: 10.1111/j.1468-2982.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 34.Purves-Tyson TD, Keast JR. Rapid actions of estradiol on cyclic amp response-element binding protein phosphorylation in dorsal root ganglion neurons. Neuroscience. 2004;129:629–637. doi: 10.1016/j.neuroscience.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Duric V, McCarson KE. Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain. 2007;3:32. doi: 10.1186/1744-8069-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambalavanar R, Moutanni A, Dessem D. Inflammation of craniofacial muscle induces widespread mechanical allodynia. Neurosci Lett. 2006;399:249–254. doi: 10.1016/j.neulet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- 39.Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- 40.Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47:552–563. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- 42.Bereiter DA, Barker DJ. Hormone-induced enlargement of receptive fields in trigeminal mechanoreceptive neurons. I. Time course, hormone, sex and modality specificity. Brain Res. 1980;184:395–410. doi: 10.1016/0006-8993(80)90808-2. [DOI] [PubMed] [Google Scholar]
- 43.Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- 45.Albanito L, Madeo A, Lappano R, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 46.Vivacqua A, Bonofiglio D, Recchia AG, et al. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 47.Vivacqua A, Bonofiglio D, Albanito L, et al. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- 48.Obata K, Yamanaka H, Kobayashi K, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obata K, Yamanaka H, Dai Y, et al. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoubina EV, Smith PG. Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J Urol. 2003;169:382–385. doi: 10.1016/S0022-5347(05)64132-8. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- 52.Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]