DEATH RECEPTOR 5 INTERNALIZATION IS REQUIRED FOR LYSOSOMAL PERMEABILIZATION BY TRAIL IN MALIGNANT LIVER CELL LINES (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 1.
Published in final edited form as: Gastroenterology. 2009 Mar 9;136(7):2365–2376.e7. doi: 10.1053/j.gastro.2009.02.071
Abstract
Background & Aims
TRAIL cytotoxicity in hepatocellular carcinoma cells is mediated by lysosomal permeabilization. Our aims were to determine which TRAIL receptor, death receptor 4 or 5, mediates lysosomal permeabilization and assess whether receptor endocytosis followed by trafficking to lysosomes contributes in this process.
Methods
TRAIL ligand internalization in Huh-7 cells was examined by confocal microscopy using Flag-tagged TRAIL, whereas death receptor 4- and 5-enhanced green fluorescent protein (EGFP) internalization was assessed by total internal reflection microscopy. Clathrin-dependent endocytosis was inhibited by expressing dominant negative dynamin.
Results
Although Huh-7 cells express both TRAIL receptors, shRNA silencing of death receptor 5 but not death receptor 4, attenuated TRAIL-mediated lysosomal permeabilization and apoptosis The TRAIL:death receptor 5 complex underwent rapid cellular internalization upon ligand stimulation, whereas the TRAIL:death receptor 4 complex was not efficiently internalized. Death receptor 5-EGFP internalization was dependent upon a dileucine-based internalization motif. Endocytosis of the TRAIL:death receptor 5 complex was dynamin-dependent, and was required for rapid lysosomal permeabilization and apoptosis in multiple malignant hepatocellular and cholangiocarcinoma cell lines. Upon TRAIL treatment, death receptor 5 co-localized with lysosomes after internalization. Inhibition of death receptor 5 trafficking to lysosomes by Rab7 siRNA also reduced TRAIL-mediated lysosomal disruption and apoptosis.
Conclusions
TRAIL mediated endocytosis of death receptor 5 with trafficking to lysosomes contributes to lysosomal protease release into the cytosol and efficient apoptosis in malignant liver cell lines.
INTRODUCTION
Tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL), a potent death ligand that preferentially induces apoptosis in transformed cells, is under evaluation as an anti-cancer agent in humans1, 2. TRAIL initiates apoptosis signaling cascades by ligating two death receptors, death receptor 4 (DR4) (also referred to as TRAIL receptor 1, TNSF10A) and death receptor 5 (DR5) (also referred to as TRAIL receptor 2/KILLER/TRICK-2, TNSF10B) 3. Differences in signaling between the two receptors have not been identified to date, although DR4 selectively mediates apoptosis in chronic lymphocytic leukemia cells4 while DR5 appears to mediate TRAIL cytotoxicity in epithelial derived malignancies5.
TRAIL signaling in liver is especially important where receptor expression can suppress hepatocarcinogenesis6, in addition to its potential efficacy in targeting established tumors7, 8. In malignant hepatocellular and cholangiocarcinoma cells, TRAIL induces cell death via a pathway involving lysosomal permeabilization9, 10. Although the release of lysosomal cathepsins, especially cathepsin B, into the cytosol during apoptosis in these cell lines is well established11, the mechanisms by which this lysosomal permeabilization occurs remains incompletely understood. One possible mechanism that would place death inducing signaling complex (DISC) constituents in proximity with lysosomes would be TRAIL receptor internalization by endocytosis followed by vesicular trafficking to lysosomes. Consistent with this possibility, a link between receptor endocytosis and apoptosis has been well established for the death receptors Fas and TNF receptor 112, 13. TRAIL receptor internalization by a clathrin dependent mechanism involving the GTPase dynamin has also been reported in HeLa and BJAB cells, but is not required for apoptosis in these cell types14, 15. Whether TRAIL killing requires receptor internalization in other cells, particularly cells that rely on lysosomal permeabilization for apoptosis (e.g., hepatocellular carcinoma cells), has not been examined.
Internalization of membrane receptors is mediated by signals present within their cytosolic domains, usually a short, linear sequence of amino acids 16. Common internalization motifs for clathrin-mediated endocytosis include tryosine- and dileucine-based signals 13, 16, 17. The internalization signal(s) for TRAIL signals have not been explored, although both DR4 and DR5 contain classic dileucine-based internalization signals, [DE]XXXLL amino acid sequences between the transmembrane and death domains. The fate of TRAIL receptors following internalization has also not been well delineated. Internalized receptors can be delivered to early endosomes and subsequently transported through late endosomes to lysosomes18, 19. Alternatively, endocytosed receptors can be transported back to the plasma membrane, a process referred to as receptor recycling. Various steps in this intracellular trafficking of internalized membrane receptors are controlled by members of the Rab GTPase protein family, which consists of over 60 Rab proteins20. Among them, Rab 7 is known to regulate late steps in the endocytotic pathway, especially fusion of late endosomes with lysosomes 21, 22.
The current work addresses the role of TRAIL receptor internalization and the lysosomal pathway of apoptosis in liver cells. Using inhibitors of various steps along this pathway, our studies demonstrate that DR5 internalization and localization to the lysosomes occurs during the lysosomal pathway of apoptosis in transformed liver cells.
MATERIALS AND METHODS
Cell culture
The human hepatocellular carcinoma cell lines, Huh-7 and HNU 499, cholangiocarcinoma cell lines, Mz-ChA-1 and HuCCT-1, and human cervical cancer cell line, HeLa, were grown in DMEM supplemented with 10% fetal bovine serum, 100,000 units/L penicillin, 100 mg/L streptomycin, and 100 mg/L gentamicin.
Generation of stable transfectants expressing DR4 or DR5 short hairpin RNA
Short hairpin RNA (shRNA) for DR4 and DR5 was from Sigma Aldrich (St. Louis, MO) [MISSION short hairpin RNA lentiviral plasmid, targeting Nucleotides 1499–1519, Gene bank accession #NM 003844 for DR4-shRNA and Nucleotides 1531–1551, Gene bank accession # NM 003842 for DR5-shRNA (shRNA)]. Huh-7 cells were transfected using OptiMEM I (GIBCO-Invitrogen, Carlsbad, CA) containing 6 μL/mL Lipofectamine (Invitrogen), 1 μg/mL plasmid DNA and 6 μL/mL Plus reagent (Invitrogen). Forty-eight hours after transfection, fresh DMEM containing 1 μg/mL puromycin was added. Surviving clones were separated using cloning rings and individually cultured. DR4 and DR5 expression in the clones was assessed by immunoblot analysis.
Generation of EGFP- tagged DR4, DR5, and cathepsin B
The DR4 and DR5 coding sequences were amplified by RT-PCR with a forward primer containing the BamHI restriction site and the reverse primer containing the EcoRI restriction site. The PCR products were cloned into pCSC 23, which is derived from pCDNA3.1 (Gibco-Invitrogen, Carlsbad, CA). The CMV-driven DR4 and DR5 were then engineered in frame to generate DR4 and DR5 constructs fused to EGFP at their C-terminal cytoplasmic domains. All vectors were sequenced to confirm that the construct was in frame and no PCR artifacts introduced. Cathepsin B-EGFP was generated as previously described 24.
TRAIL Internalization
Huh-7 cells were pre-incubated for 30 minutes on ice in the presence of FLAG-TRAIL (400 ng/mL). FLAG-TRAIL was preoligomerized by incubating the epitope-tagged ligand with 2 μg/mL of M2 monoclonal anti-FLAG antibody (Sigma-Aldrich, St. Louis, MO). Cells were then incubated at 37°C for pre-selected time intervals. Further, internalization was blocked by aspirating the medium and rapidly replacing it with ice-cold PBS. In selected studies, cells were washed by PBS containing 0.2 M acetic acid plus 0.5 M NaCl for 2 times for 2 minutes followed by 1 time for 1 minute in order to eliminate cell surface FLAG-TRAIL. Next, cells were fixed with PBS containing 4% paraformaldehyde for 20 minutes at 37°C. After three washes with PBS, cells were permeabilized in PBS containing 0.2% Triton-X 100, washed again in PBS three times for 3 min, and subsequently blocked for 60 min at 37°C in blocking buffer consisting of 5% goat serum and 5% glycerol in PBS. Cells were then incubated with Alexa Fluor 488-conjugated anti-mouse IgG at a concentration of 1:1000 in blocking buffer for 1 hour at 37°C, washed again in PBS three times for 3 min, rinsed with H2O, and mounted using ProLong Gold Antifade Reagent (wiith DAPI) for examination by confocal fluorescence microscopy (Zeiss LSM 510, Carl Zeiss, Jena, Germany) employing excitation and emission wavelengths of 488 nm and 507 nm for Alexa Fluor 488, respectively, and 364nm and 385nm to 430 nm for DAPI staining, respectively. Internalized FLAG-TRAIL was quantified using image analysis software (Carl Zeiss Vision GmbH, Munich, Germany). Data were expressed as the average fluorescence intensity in the cell multiplied by the number of pixels above the background. At least 100 cells were imaged for each data point. In selected experiments, DR5 or DR4 internalization was examined by total internal reflection microscopy (TIRF) 25–27. Cells were transfected with respective DR4-EGFP or DR5-EGFP plasmid 48 hours before the experiment. Internalization assay was performed for 30 minutes after adding TRAIL using TIRF microscopy (Axiovert 200M, Zeiss). EGFP on the cell surface was quantified by manually counting discrete fluorescent foci per cell on respective TIRF microscopy images. Cells were localized on bright field images.
Site directed mutagenesis of DR5-EGFP
DR5 contains a classic dileucine-based internalization signal (ESEHLL) between the transmembrane and death domains, amino acids 278–283 (Gene bank accession #NM 147187). Point mutations of the dileucines to alanines within this potential internalization motif was performed using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Sense and anti-sense primer used for the mutation was 5′-tcccccggggagtcagagcatgcagctgaaccggcagaagctgaaagg-3′ and 3′-agggggcccctcagtctcgtacgtcgacttggccgtcttcgactttcc-5′, respectively. The mutations were confirmed by DNA sequencing.
Wild type and mutant dynamin K44A adenovirus transduction
K44A expressing adenovirus was generated as previously described 28. Cells were transduced with PBS/1% bovine serum albumin containing 25 MOI (multiplicity of infection) of adenovirus that expresses either wild type dynamin, K44A GTPase dynamin mutant, or the empty vector. After 1 hour of incubation, complete culture medium was added. Transduction efficiency was assessed by immunofluorescence for the viral V-5 tag 28. Transduction efficiency using this approach was ≥95% for Huh-7 cells and HNU 499 cells, ≥75% for HuCCT and Mz-ChA-1 cells, and virtually 100% for HeLa cells.
Statistical analysis
All data represent at least three independent experiments and are expressed as means ± SE unless otherwise indicated. Differences between groups were compared by using an unpaired two-tailed t-test, and p values <0.05 were considered statistically significant.
RESULTS
DR5 rather than DR4 predominantly contributes to TRAIL-induced lysosomal disruption and apoptosis in Huh 7 cells
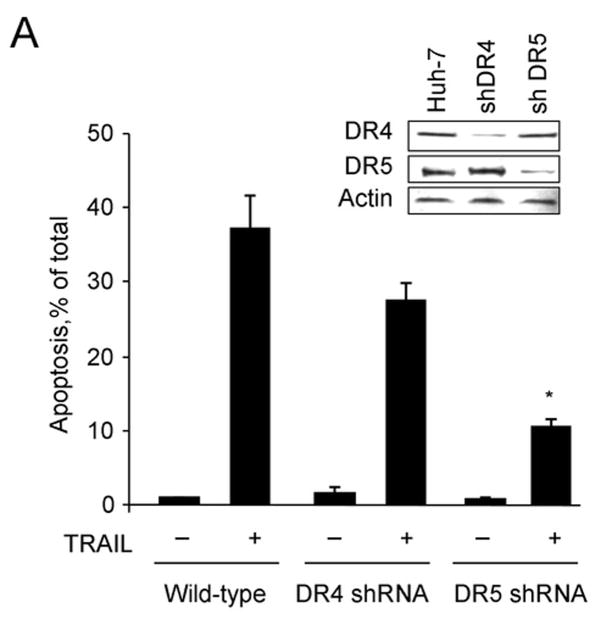
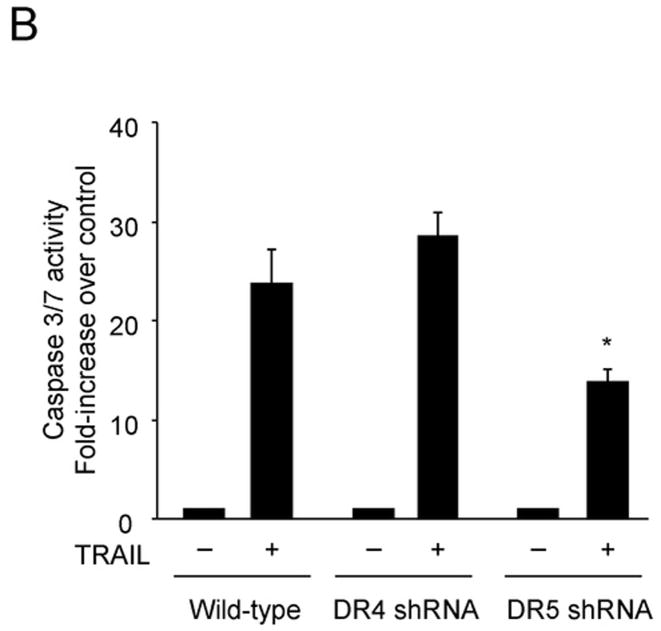
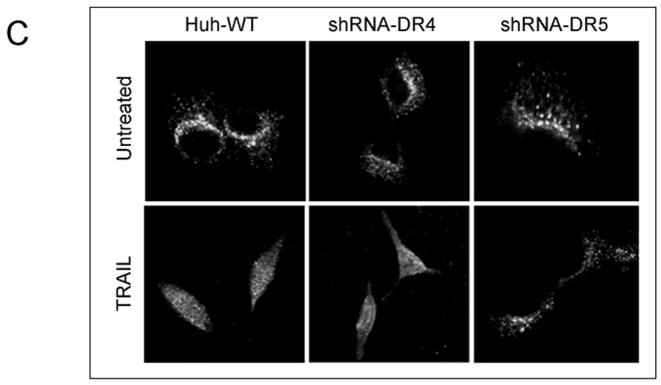
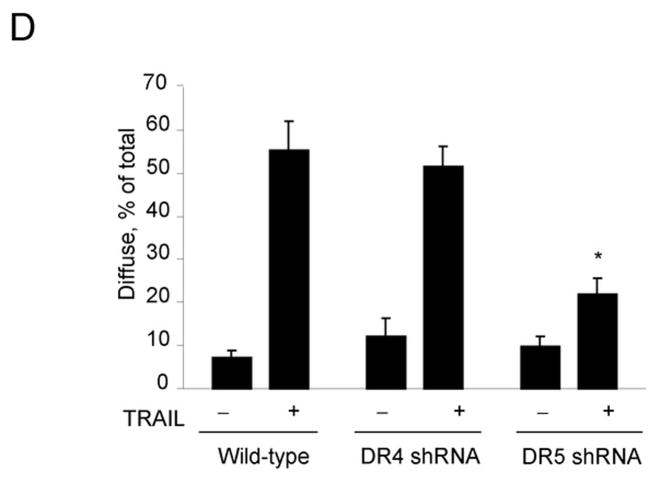
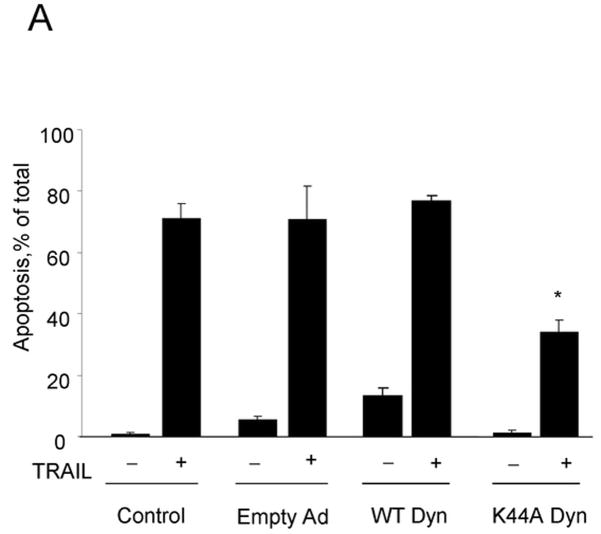
To examine the involvement of the two cognate TRAIL receptors, DR4 and DR5, for apoptosis in Huh-7 cells, shRNA technology was employed to selectively knockdown each receptor. The efficiency and specificity of shRNA targeted knock down of DR4 and DR5 were verified by immunoblot analysis (Fig. 1A). Knockdown of DR5 reduced TRAIL-mediated apoptosis as assessed by morphological and biochemical (caspase 3/7 activity) criteria (Fig. 1A,B). In contrast, DR4 shRNA had much less effect on TRAIL-induced apoptosis. Apoptosis was also more efficient with an agonistic DR5 monoclonal antibody than with a DR4 directed agonistic antibody (Supplemental Fig. 1). We next determined whether DR5 was essential for lysosomal permeabilization by TRAIL. As previously described by us 9, cathepsin B undergoes a redistribution from lysosomes into the cytosol (punctate immunofluorescence becomes diffuse) following incubation of Huh-7 cells with TRAIL (Fig. 1C). DR5 shRNA prevented this TRAIL-induced cathepsin B redistribution, whereas DR4 shRNA did not (Fig. 1C,D). Collectively, these data suggest that TRAIL mediated lysosomal permeabilization and apoptosis occurs largely by a DR5-dependent pathway in Huh-7 cells.
Figure 1. DR5 contributes more than DR4 to TRAIL-mediated lysosomal permeabilization and apoptosis.
(A) Huh-7 cells stably transfected with short hairpin RNA (shRNA) complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Error bars in this and subsequent figures depict ± SEM from 3 or more independent experiments. * p<0.05, TRAIL-treated DR5-shRNA vs. wt. Insert, immunoblotting showing effective knockdown of DR4 and DR5 on total cell lysates. (B) After cells were treated with TRAIL for 8 hours, activity of activity of effector caspases 3 and 7 was measured by a fluorogenic assay. Data are expressed as fold-increase of relative fluorescence units (RFLU) over control value (untreated cells), which was arbitrarily set to 1, and represent the mean ± SE. * p<0.05, TRAIL treated DR5-shRNA vs. wt. (C) Huh-7 cells stably transfected with shRNA targeting DR4 or DR5, or untransfected Huh-7 (wild-type, wt), were treated with 4 ng/mL TRAIL for 4 hours. Localization of cathepsin B was visualized by immunofluorescence by using a confocal microscope (64x), and (D) cells were scored for punctate or diffuse appearance of the antigen. * p<0.05, TRAIL treated DR5-shRNA vs. wt. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
The TRAIL:DR5 complex is internalized by a dileucine-based motif
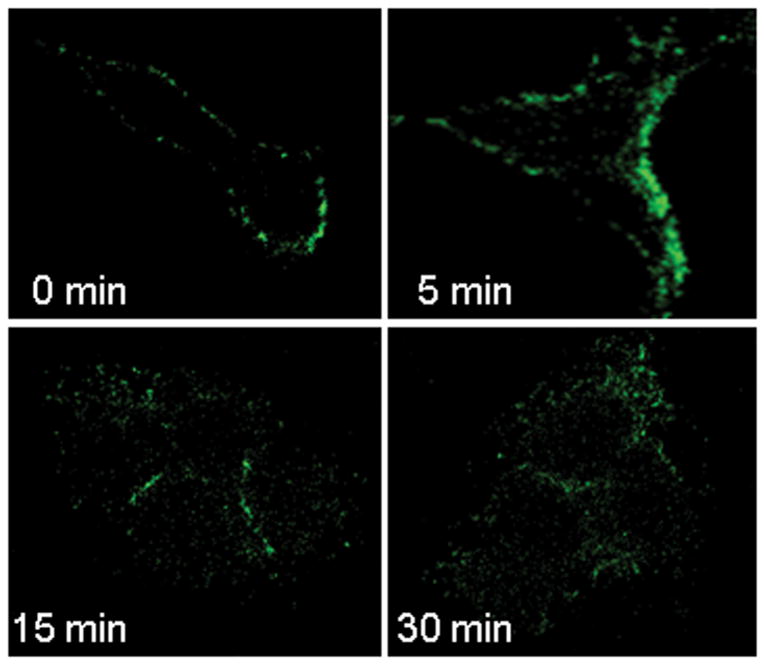
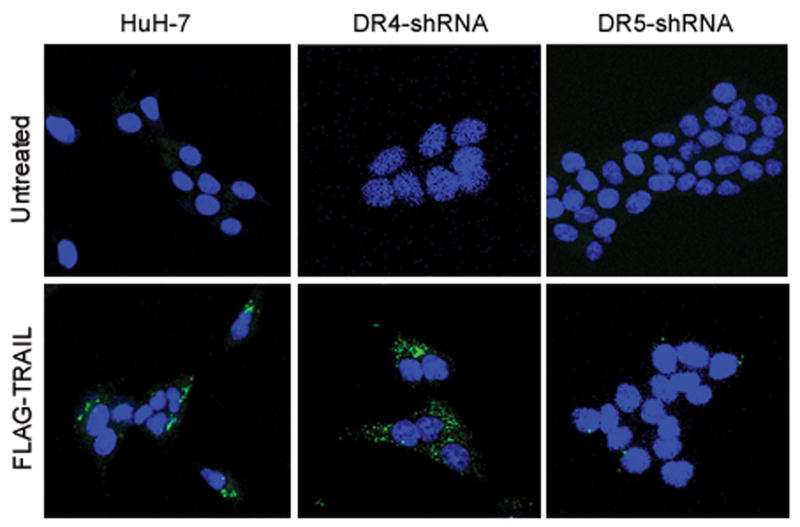
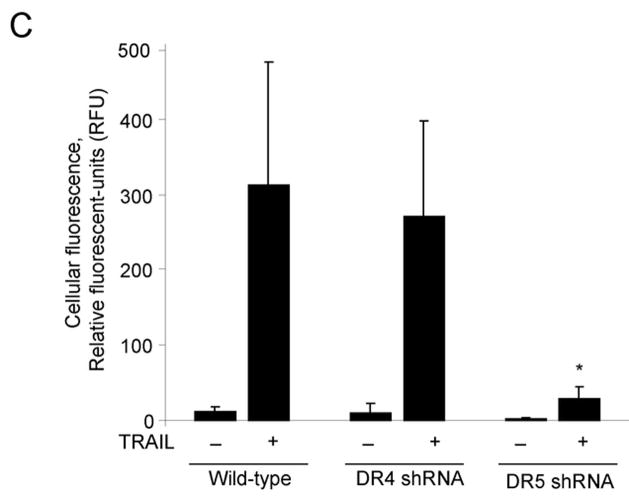
Next, we determined whether TRAIL:TRAIL receptor complexes undergo cellular internalization. Huh-7 cells were initially incubated with FLAG-tagged TRAIL at 4°C to allow ligand binding but block endocytosis. To achieve synchronized endocytosis, cells were rapidly warmed to 37°C. At 4 °C, FLAG-TRAIL was bound to the cell surface with little or no apparent cell internalization (Fig. 2A). Upon warming to 37°C, cellular TRAIL internalization was apparent within 5 minutes and progressed by 30 minutes (Fig. 2A). Internalized TRAIL (30 min) was more clearly observed when cells were acid-washed, which eliminates cell surface TRAIL, prior to fixation (Fig. 2B,C). Internalization of Flag-TRAIL was also observed in the DR4-shRNA transfected cells but was reduced in the cells transfected with DR5-shRNA (Fig. 2B,C). These results indicate that internalization of TRAIL predominantly occurs through DR5 rather than DR4.
Figure 2. TRAIL undergoes cellular internalization upon ligand stimulation.
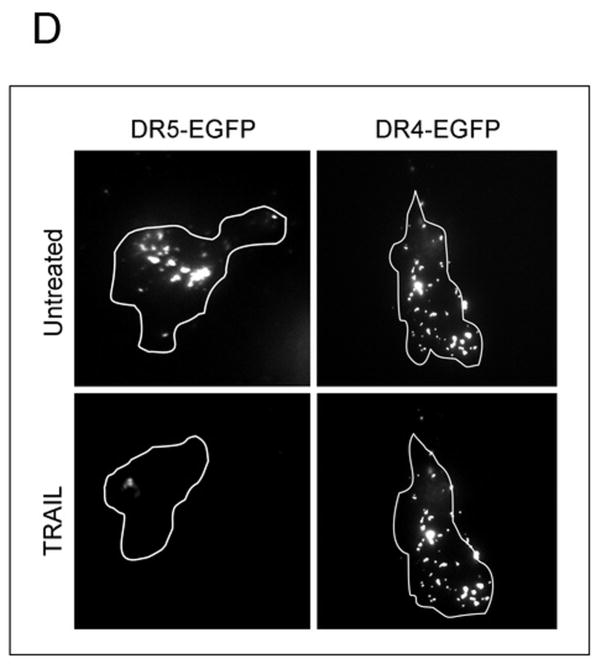
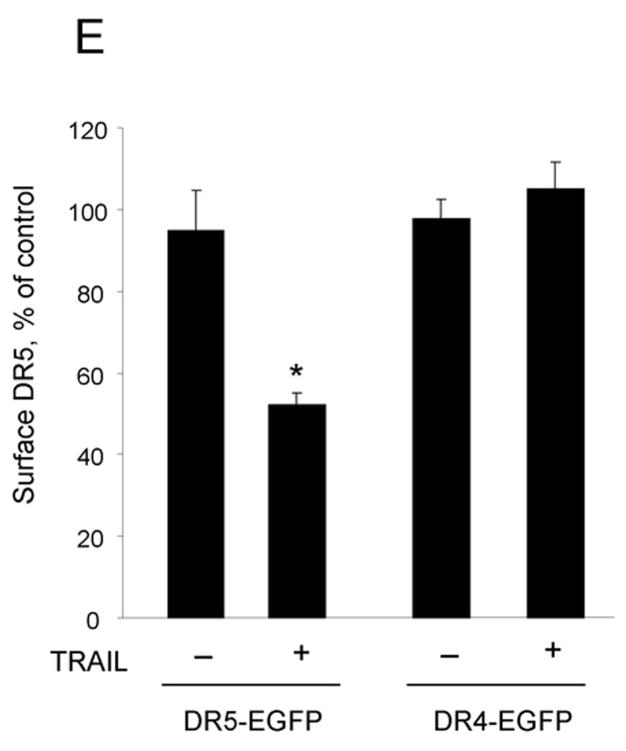
(A) Huh-7 cells were incubated with FLAG-TRAIL cross-linked by anti-FLAG M2 antibody for 30 minutes on ice, followed by incubation in 37 °C for the indicated time. Cells were then fixed, incubated with fluorescent secondary antibody, and analyzed by confocal microscopy. (B) In order to visualize only internalized TRAIL, Huh-7 cells stably transfected with shRNA complementary to DR4 or DR5, or untransfected Huh-7 (wild-type, wt) were treated with FLAG-TRAIL and mouse monoclonal M2 antibody as illustrated in panel A, then washed with 0.2 M acetic acid before fixation to remove the membrane-associated FLAG-TRAIL. Cells were then incubated with fluorescent anti-mouse IgG. Blue fluorescence, DAPI staining; green fluorescence, internalized FLAG-TRAIL. (C) Quantification of internalized FLAG-TRAIL. Cells were treated as illustrated in panel B. Data were expressed as the area of the cell multiplied by the average fluorescence intensity in the cell above the background. * p<0.01, TRAIL treated DR5-shRNA vs. TRAIL treated wt. (D) Cells were transiently transfected with a plasmid expressing enhanced green fluorescent protein (EGFP)-tagged DR5 (DR5-EGFP). Cells were observed on a heated microscope stage at 37°C with or without 20 ng/mL of TRAIL for 30 minutes. Disappearance of membrane DR5 was observed by total internal reflection fluorescence (TIRF) microscopy. (E) The percentage receptor remaining on the cell surface was assessed by quantification of discrete fluorescent foci. * p<0.01, TRAIL-treated DR5-EGFP vs. DR4-EGFP.
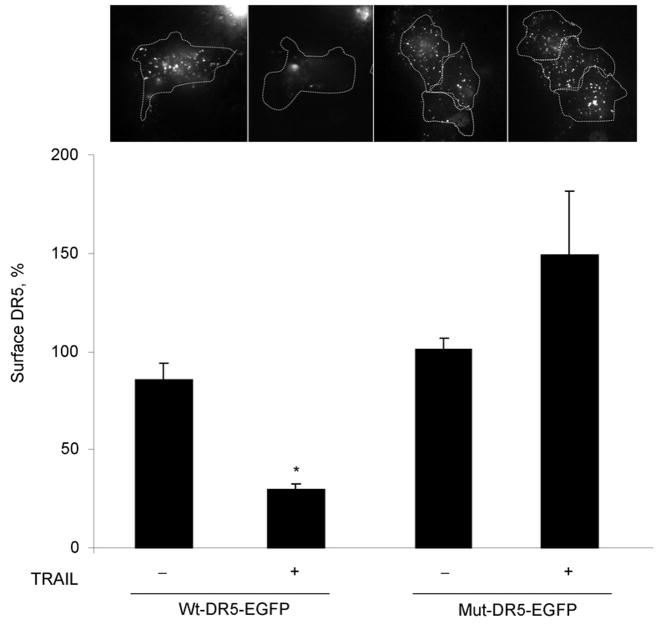
To complement the studies of TRAIL internalization, we also examined receptor internalization. For these studies we employed EGFP-tagged DR5 or DR4 and TIRF microscopy, which only images fluorescence within 100 nm of the cell surface contact area (i.e., contact area between cell and coverslip) 25 and is commonly used to monitor egress of fluorescent molecules from the plasma membrane 29. With this technique, loss of a fluorescence protein-tagged plasma membrane protein is consistent with a cellular internalization process 27. Under basal conditions DR5-EGFP was identified on the plasma membrane. Within 30 minutes of adding TRAIL, however, the amount of DR5-EGFP at the cell surface decreased, consistent with ligand-stimulated receptor internalization (Fig. 2D,E). On the other hand, the amount of DR4-EGFP observed on the plasma membrane did not change with TRAIL treatment (Fig. 2D,E). Most internalization signals for plasma membrane transmembrane proteins are within the cytosolic domains16. Analysis of the amino acid sequence between the transmembrane domain and death domain of DR5 revealed a classic dileucine internalization motif ESEHLL at aa-278 to aa-283 (Gene bank accession #NM 147187) 16, 17. Because substitution of both leucines to alanine abrogates cell internalization by this motif 30, 282L and 283L of DR5-EGFP were mutated to alanine employing site directed mutagenesis and internalization of this transmembrane receptor was examined by TIRF. Both wild-type-DR5-EGFP (wt-DR5-EGFP) and mutant DR5 (mut-DR5-EGFP) had comparable membrane expression of DR5 (Supplemental Fig. 2). Although internalization was induced within 30 minutes of TRAIL treatment in wt-DR5-EGFP transfected cells, the mut-DR5-EGFP failed to internalize (Fig. 3). Interestingly, fluorescent mut-DR5-GFP foci actually increased after TRAIL exposure indicating that clusters of TRAIL:mut-DR5 were continuously accumulating following ligand stimulation but were unable to be internalized. Collectively, these data indicate that theTRAIL:DR5 complex is preferentially internalized by the cell via a dileucine-based signal.
Figure 3.
Mutation of predicted internalization motif in DR5 blocks TRAIL mediated endocytosis of DR5. Huh-7 cells were transiently transfected with wild type (wt)- DR5-EGFP or mutant (mut)-DR5-EGFP for 48 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated wt-DR5-EGFP vs. TRAIL treated mut-DR5-EGFP.
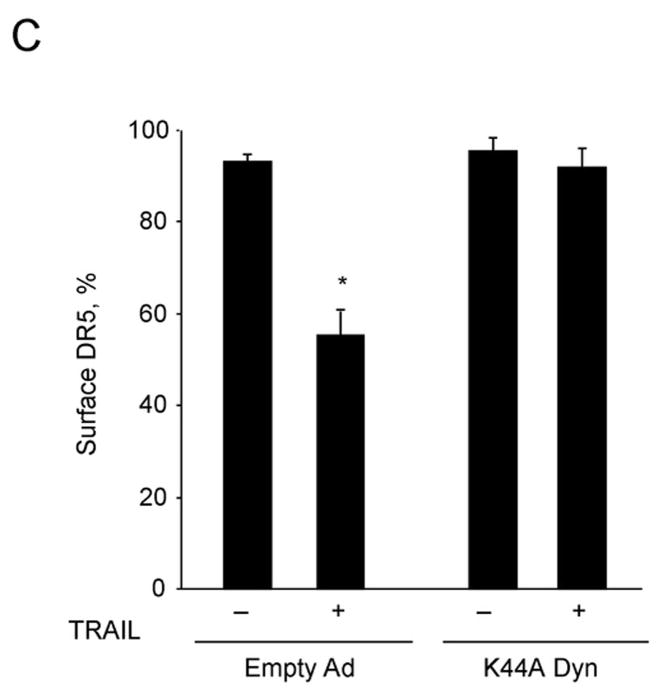
Dominant negative dynamin (K44A Dyn) inhibits TRAIL-DR5 internalization, lysosomal permeabilization and apoptosis
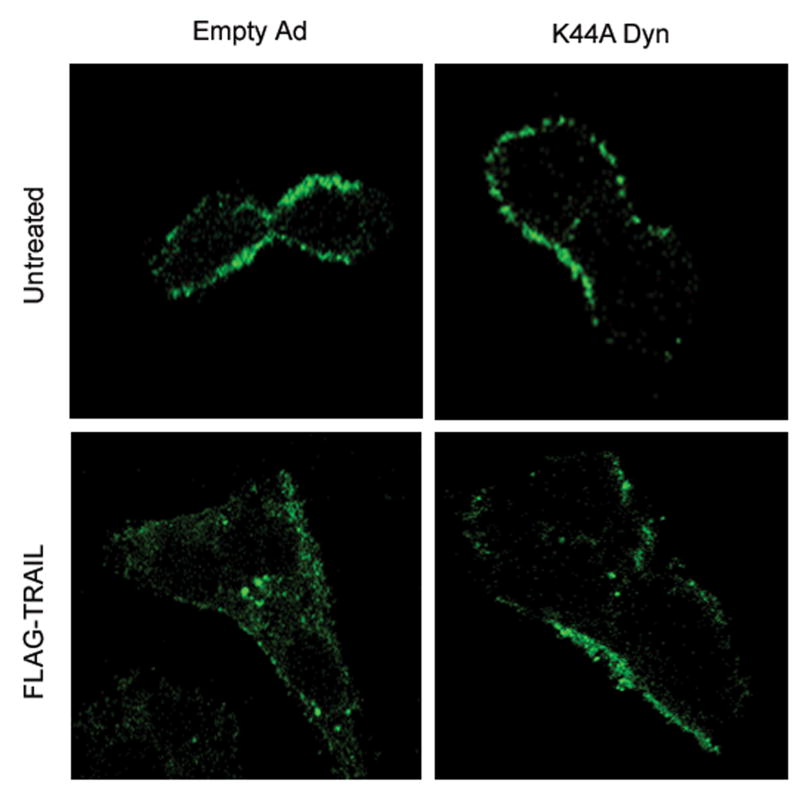
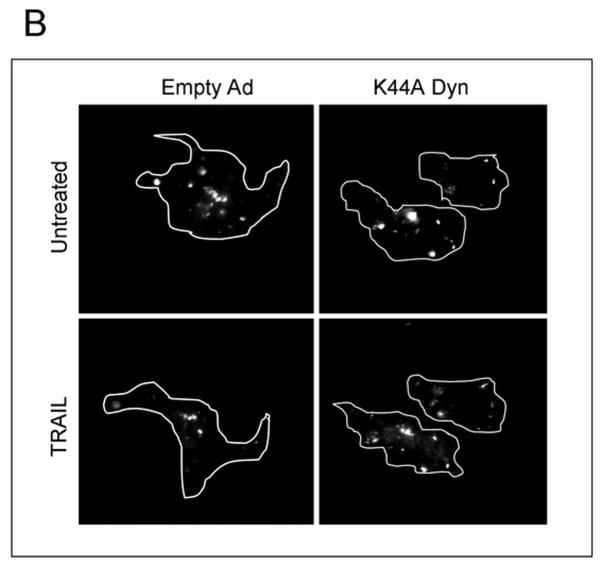
TRAIL has previously been reported to undergo endocytosis by a process that can be inhibited by expression of a K44A dominant negative dynamin mutant14, 15. Because dynamin not only mediates endocytosis, but also contributes to vesicle trafficking from the Golgi network, we first determined whether the K44A mutant reduces membrane localization of DR5 by flow cytometry. Transduction with dominant negative dynamin did not affect the cell membrane localization of DR5 in untreated cells (Supplemental Figure 3). However, the K44A mutant inhibited TRAIL internalization in Huh-7 cells (Fig. 4A). In addition, transduction with K44A mutant inhibited DR5-EGFP endocytosis, presenting loss of cell surface receptor fluorescence by TRAIL as assessed by TIRF microscopy (Fig. 4B,C). Thus, this K44A dynamin mutant inhibits both receptor and ligand internalization. Although components of the death inducing signaling complex can be released from the receptor into the cytosol, the observation that the transmembrane protein DR5-EGFP is internalized by a dynamin-dependent process indicates endocytosis is the mechanism of cellular uptake.
Figure 4.
(A) Huh-7 cells were transduced with either adenovirus encoding K44A Dyn or empty vector (empty Ad). Twenty-four hours after infection, cells were treated with FLAG-TRAIL (400 ng/mL), fixed, stained with anti-FLAG antibody and analyzed by confocal microscopy. (B) Twenty-four hours after transient transfection with DR5-EGFP, cells were transduced with either K44A Dyn or empty adenovirus for 24 hours. Cells were then treated with diluent or TRAIL (20 ng/mL) for 30 min at 37 °C on the heated stage of a TIRF microscope. (C) The percentage of surface receptor remaining at 30 min imaged by TIRF was assessed by quantification of fluorescent particles. *p< 0.05, TRAIL treated empty ad vs. K44A Dyn ad.
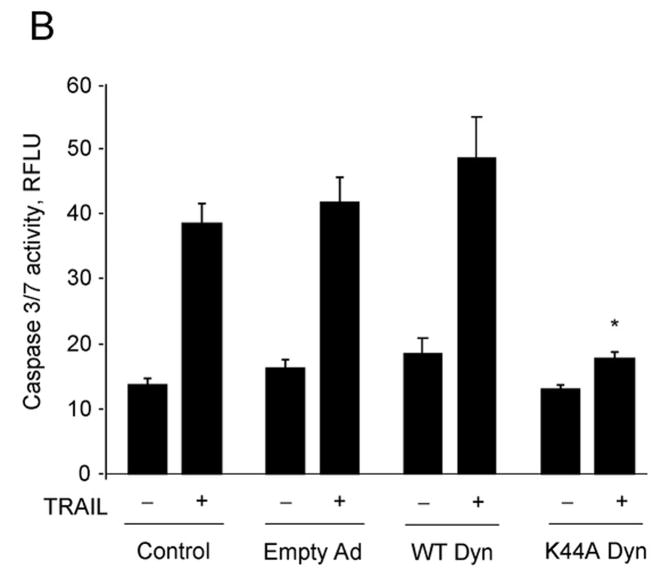
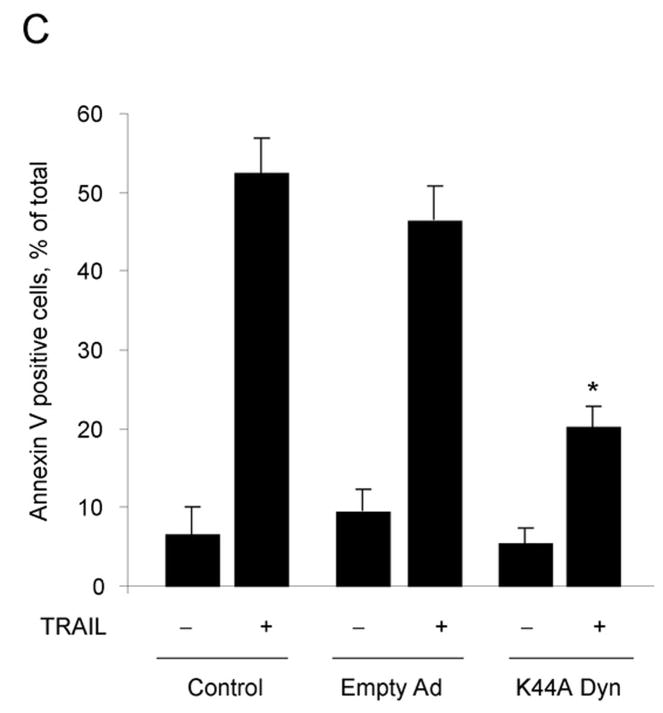
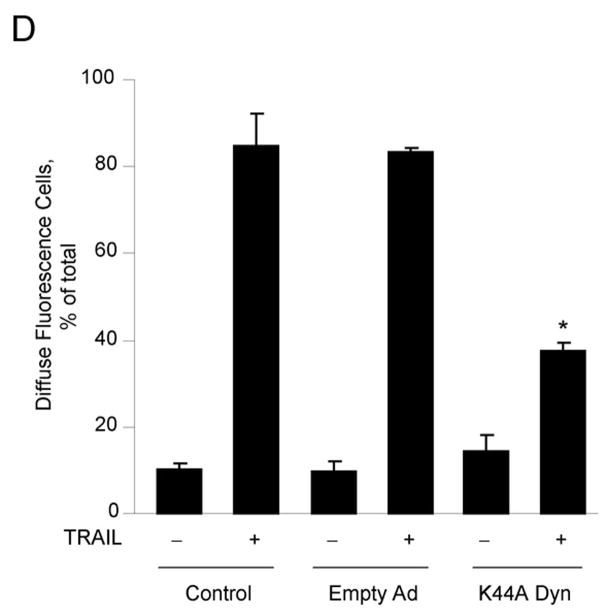
Next, we proceeded to determine whether internalization of the TRAIL:DR5 complex is required for lysosomal permeabilization and apoptosis. Compared to the cells transduced with empty adenovirus, the K44A dynamin adenovirus also inhibited TRAIL-induced apoptosis (Fig. 5A–C) whereas transduction with wild type dynamin had no effect (Fig. 5A, B). This inhibitory effect of K44A dynamin on apoptosis was also observed in HNU 499 cells, another HCC cell line, and the cholangiocarcinoma cell lines, HuCCT-1 and Mz-ChA-1 cells (supplemental Fig. 4A, B). Release of lysosomal cathepsin B into the cytosol by TRAIL treatment was also attenuated by transduction with the K44A dynamin mutant (Fig. 5D). These observation imply that dynamin-mediated TRAIL:DR5 endocytosis contributes toTRAIL-induced lysosomal disruption and apoptosis in malignant liver derived cell lines.
Figure 5. K44A dynamin inhibits TRAIL-mediated cathepsin B release from lysosomes and cytotoxicity in Huh-7 cells.
(A) Huh-7 cells infected with either wild type (WT) dynamin, K44A dynamin adenovirus, or empty adenovirus were incubated with TRAIL (4 ng/mL) for 8 hours. Apoptosis was assessed by morphological criteria after DAPI staining. Control: Not infected. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (B) Activation of effector caspases3 and 7 was measured by a fluorogenic assay. * p<0.05, TRAIL-treated K44A Ad. vs. empty Ad. (C) After cells were infected with adenovirus and treated with TRAIL as shown in Figure 5A, Annexin V study was performed using confocal microscopy. * p<0.01, TRAIL-treated K44A Ad. vs. empty Ad. (D) Beginning 24 h after transduction with K44A dynamin or empty adenovirus, Huh-7 cells were treated with TRAIL (4 ng/mL) for 4 hours. Cells were scored for punctate or diffuse appearance of cathepsin B as illustrated in Figure 2A. * p< 0. 05, TRAIL treated K44A Dyn Ad. vs. empty Ad. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
Prior studies in HeLa cells suggested that internalization of TRAIL is not required for TRAIL-induced apoptosis 14, 15. To reconcile these results with our observations, we determined whether the involvement of TRAIL internalization in cell death was cell type-specific. Consistent with prior results 14, 15, we observed that transduction with the K44A mutant did not reduce TRAIL mediated apoptosis in HeLa cells (Supplemental Fig. 5A,B). Lysosomal release of cathepsin D into the cytosol was not observed in HeLa cells during TRAIL exposure (Supplemental Fig. 5C,D). Thus, TRAIL mediated cytotoxicity in HeLa cells does not involve either TRAIL receptor uptake or lysosomal disruption.
DR5 is trafficked to lysosomes by Rab 7 dependent process
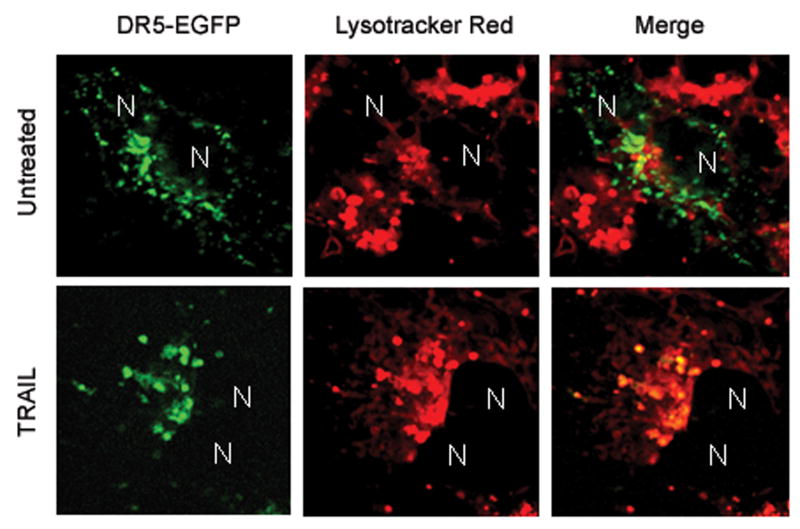
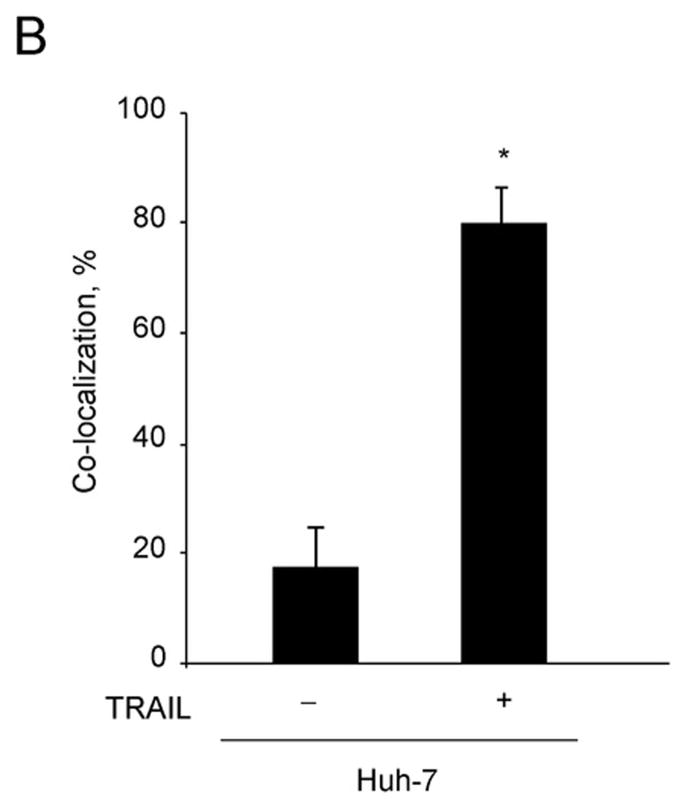
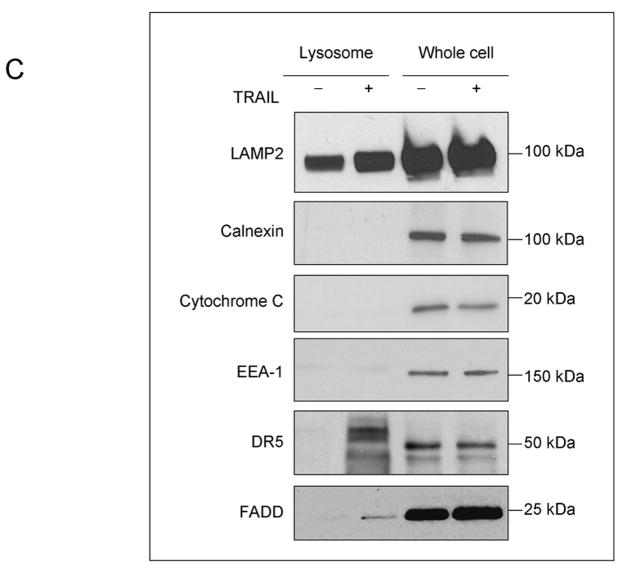
To determine whether DR5 co-localizes with lysosomes, Huh-7 cells transfected with DR5-EGFP were treated with TRAIL for 60 min and then observed by confocal microscopy. The lysosomal marker, Lysotracker Red, was employed to identify lysosomes. DR5-EGFP did not co-localize with Lysotracker Red in untreated cells. After 60 min of TRAIL treatment, however, DR5-EGFP co-localized with lysosomes (Fig. 6A,B). To further confirm this observation, lysosomes were purified from cultured Huh-7 cells. Immunoblot analysis demonstrated DR5 in lysosomal fractions from TRAIL-treated but not vehicle treated cells (Fig. 6C). FADD was also detected in TRAIL-treated lysosomes, consistent with presence of an active TRAIL receptor signaling complex in this cell fraction. Of interest, the upper 48kDa band ran with a slightly higher apparent molecular weight, suggesting possible post-translational modification.
Figure 6. DR5 becomes localized to the lysosomes upon TRAIL-treatment.
(A) Huh-7 cells were transfected with DR5-EGFP for 48 hours, then treated with 20 ng/mL TRAIL for 1 hour. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. (B) Co-localization is calculated as ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. * p<0.05, untreated Huh-7 cells vs. TRAIL treated Huh-7 cells. (C) After cells were treated with diluent or 20 ng/mL TRAIL for 1 hour, lysosomes were isolated. Collected lysosomes and whole-cell lysates were subjected to immunoblot analysis for indicated proteins. The purity of the lysosomal preparation was verified by the absence of the ER (calnexin), early endosome (EEA-1), and mitochondrial (cytochrome c oxidase) markers. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
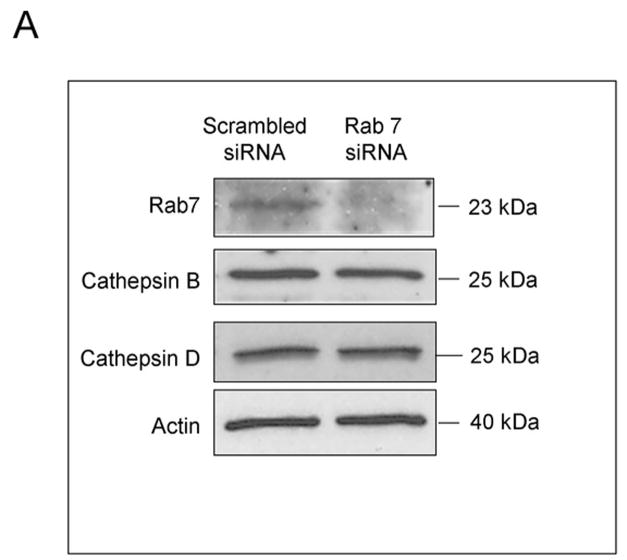
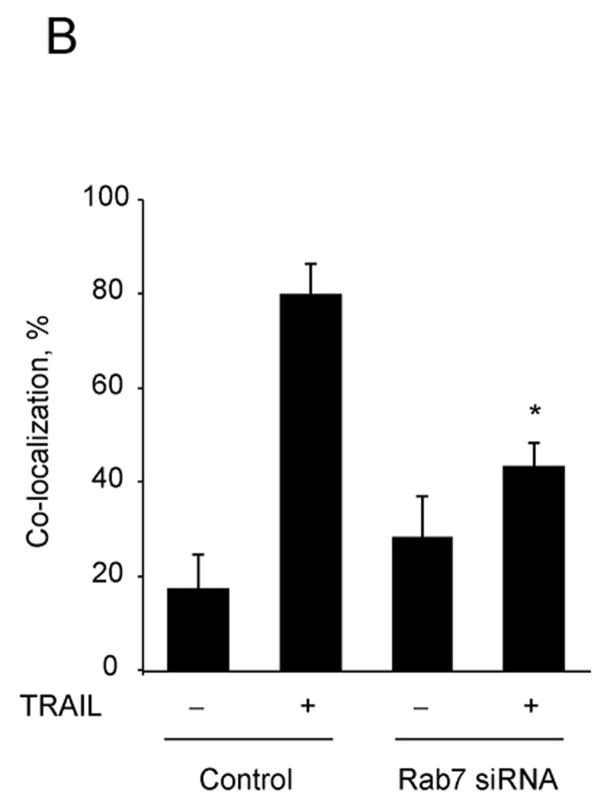
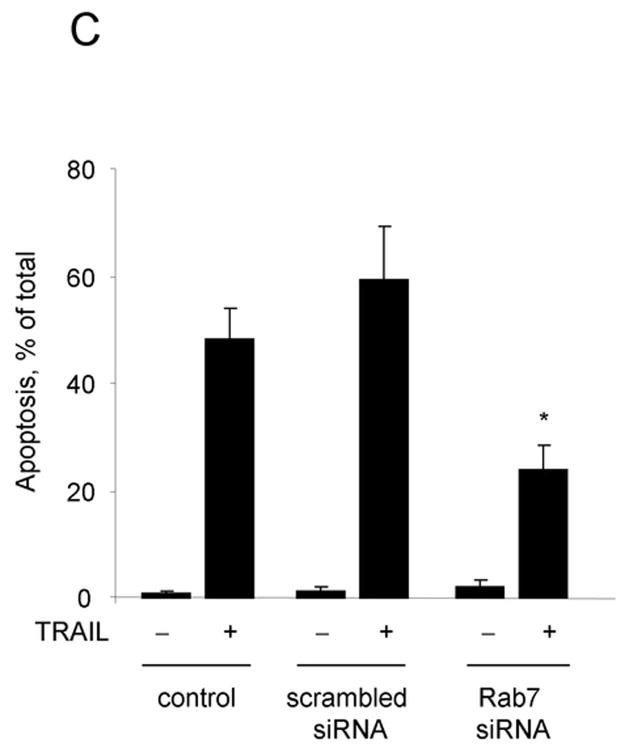
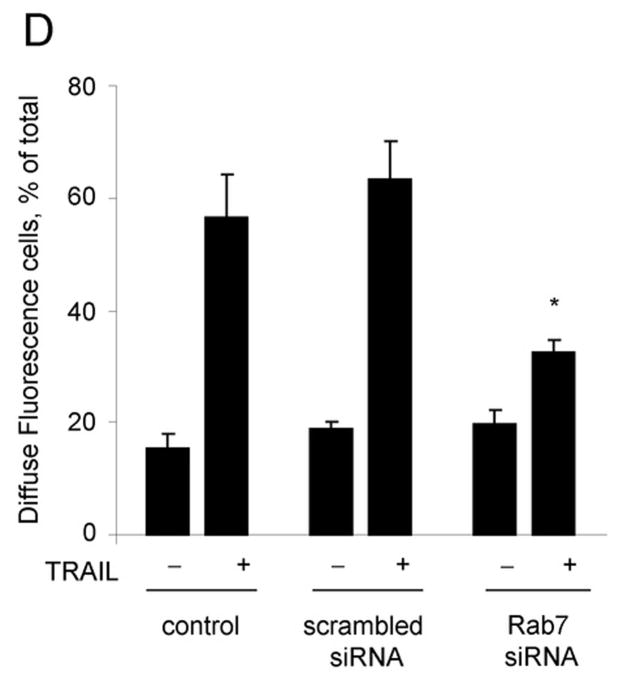
As Rab7 contributes to the trafficking of membrane receptors from early endosomes to lysosomes, we employed Rab7 siRNA to assess if DR5 trafficking to lysosomes is Rab7 dependent. Because Rab7 may also be required for transport of newly synthesized lysosomal proteins from the trans Golgi network (TGN) to lysosomes 31, we first determined if Rab7 knockdown reduces the expression of lysosomal cathepsins or cathepsin B trafficking to the lysosomes. siRNA targeted knock down of Rab7 did not alter the protein expression of lysosomal cathepsins (Fig. 7A) nor the co-localization of cathepsin B with Lysotracker Red (Supplemental Fig. 6A,B). However, the trafficking of DR5 to lysosomes was inhibited by targeted knockdown of Rab7 (Fig. 7D). Rab7 siRNA also significantly reduced TRAIL induced apoptosis and lysosomal permeabilization (Fig. 7C,D). These data suggest that trafficking of internalized DR5 to lysosomes is Rab7 dependent, and participates in TRAIL induced apoptosis of Huh-7 cells.
Figure 7. Rab7 is required for lysosomal trafficking of DR5 and apoptosis mediated by TRAIL.
(A) Huh-7 cells were transfected with Rab7 siRNA or scrambled siRNA for 48 hours. Cells were lysed and were subjected to immunoblot analysis for Rab7, cathepsin B, and cathepsin D. (B) Huh-7 cells were transfected with Rab7 siRNA. After 24 hours, cells were transfected with DR5-EGFP for another 24 hours. Cells were then treated with 4 ng/mL TRAIL for 60 minutes. Lysotracker Red was added 30 minutes before cells were observed by confocal microscopy. Co-localization was calculated as a ratio of co-localized DR5-EGFP and Lysotracker Red compared to total EGFP. *p<0.05, TRAIL-treated Rab7 siRNA vs. control. (C) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 8 hours. Apoptosis was assessed by nuclear morphologic changes using nuclear binding dye DAPI. (D) Untransfected (control) and Huh-7 cells transfected with Rab7 siRNA or scrambled siRNA were treated with TRAIL for 4 hours. Cellular compartmentation of cathepsin D was assesed by immunofluorescence and confocal microscope. Cells were scored as punctate or diffuse based on cellular localization of the antigen * p< 0.05, TRAIL-treated Rab7 siRNA transfected cells vs. scrambled siRNA transfected cells. Experimental procedures relevant to this figure are in the supplemental experimental materials and methods.
DISCUSSION
TRAIL initiates cytotoxic signals by ligating one of two cognate receptors, DR4 and DR5. In the current study, TRAIL-induced lysosomal permeabilization occurred predominantly by signaling via DR5, rather than DR4, in Huh-7 cells. These data suggest that DR5 and DR4 may signal differently in liver cells, that DR5 appears to be more efficient than DR4 in mediating apoptosis. The differential internalization of these two receptors is a potential explanation for these findings. Internalization of DR5 was dependent upon a classic dileucine-based motif within the cytoplasmic domain of the receptor. Although it did not internalize, mut-DR5-EGFP did cluster on the plasma membrane following TRAIL treatment, suggesting this mutant binds TRAIL and aggregates in a manner similar to the wild type receptor. This dileucine-based motif is known to interact with the clathrin endocytic machinery 16, and provides a molecular explanation for DR5 by a clathrin-dependent mechanism. Interestingly, DR4 also contains dileucine internalization motif within its cytoplasmic domain (aa 333 to aa 338, Gene bank accession #NM 003844). A ready explanation as to why DR5 and not DR4 is internalized by Huh-7 cells is not readily apparent, but may relate to preferential post-translational alterations of one receptor as compared to the other (e.g., phosphorylation, ubiquitination, etc). Additional studies will be required to explore these differences in internalization between the two receptors.
The TRAIL:DR5 complex is internalized and localizes to lysosomes within 60 minutes of TRAIL treatment. Consistent with a prior report 15, the endocytosis of this complex is dynamin-dependent. This DR5 internalization is required for cathepsin B release from lysosomes into the cytosol and subsequent killing of Huh-7 cells. Remarkably, the active death receptor complex is trafficked to lysosomes, as demonstrated by the TRAIL-dependent recovery of both DR5 and FADD with lysosomes. Additional experiments demonstrated that this trafficking to lysosome is Rab7-dependent. This is compatible with the established role for Rab7 in late endocytic trafficking of several other membrane receptors, including EGFR and angiotensin II type IA receptor22, 32, 33. Importantly, Rab7 siRNA inhibited not only the movement of DR5 to lysosomes, but also TRAIL mediated killing. These observations invite the speculation that Rab7-mediated trafficking of an active death receptor signaling complex to lysosomes contributes to permeabilization of this organelle and subsequent cell death.
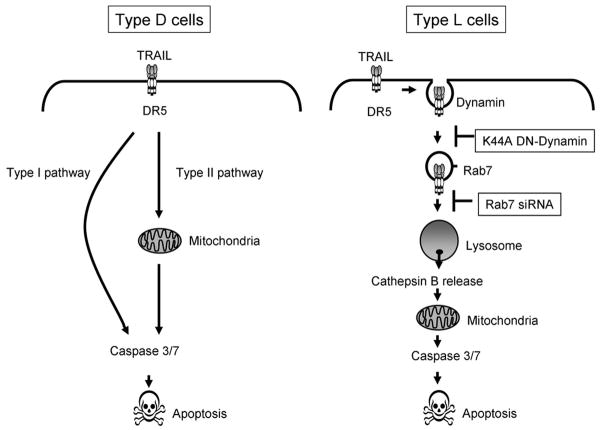
In the current study, inhibiting internalization of the TRAIL:DR5 complex in several malignant liver-derived cell lines diminished TRAIL-induced lysosomal permeabilization and cell death. In contrast, we observed robust TRAIL-induced apoptosis without lysosomal permeabilization in HeLa cells, in agreement with earlier reports14, 15. Likewise, transfection with the dominant negative K44A dynamin mutant did not block TRAIL-mediated apoptosis in HeLa cells. Just as Fas-mediated apoptosis is dependent on mitochondrial dysfunction and is protected by antiapoptotic Bcl-2 family members in hepatocytes (Type II cells) but not in lymphocytes (Type I cells) 34, 35, the present contrast between Huh-7 and HeLa Cells suggests that TRAIL-activated signaling also differs among different cells. Both type I (e.g., BJAB cells) and type II cells (e.g., primary salivary epithelial cells) have been reported for TRAIL signaling 14, 36, 37. In addition, we suggest that transformed liver cells be classified as ‘type L cells’ (for lysosomal cytotoxic signaling) because of their dependence on TRAIL internalization and lysosomal permeabilization for apoptosis induction. In contrast, cells such as HeLa cells should be classified as “type D cells” (for direct signaling) to reflect their lack of dependence on lysosomal permeabilization for cytotoxicity. This suggestion is consistent with the emerging concept that internalization plays a pivotal role in the signaling by death receptor in certain cell types 13, 38.
In prior studies, we have demonstrated that lysosomal permeabilization, in part requires, c-Jun-NH2-terminal-kinase (JNK) activation of the pro-apoptotic Bcl-2 protein Bim and Bax 10. In these studies, translocation of Bim and Bax to lysosomes was observed. The current study extends these observations by also identifying DR5 translocation to lysosomes. Based on these data, it is tempting to speculate that DR5 containing endosomes may also “pick up” the proapoptotic Bcl-proteins transporting them to lysosomes resulting in lysosomal permeabilization. Future studies will be required to identify the proteins associated with DR5 associated receptosomes as they are trafficked to lysosomes.
In summary, our studies indicate that, upon binding to TRAIL, DR5 is internalized by a dynamin dependent process and subsequently transported to lysosomes by a Rab7-dependent process. We hypothesize that this trafficking and targeting of the receptor complex brings the apoptotic machinery in proximity to lysosomes (Fig. 8). Then, in a caspase 8- and Bim-dependent manner, Bax is targeted to the lysosomes, inducing membrane permeabilization and apoptosis 10. Although the processes that regulate this pathway require further elucidation, this lysosomal pathway appears to play a crucial role in TRAIL-induced apoptosis of hepatocellular carcinoma cells.
Figure 8. Schematic representation of the proposed model for TRAIL-induced apoptosis through internalization of TRAIL ligand-complex and lysosomal permeabilization.
See text for details.
Supplementary Material
1
Acknowledgments
This work was supported by NIH Grant DK63947 to GJG, DK 44650 to MAM, CA69008 to SHK, HL86990 to VHS, DK 9875 to JLM and the Mayo Foundation.
The authors thank Erin Nystuen-Bungum for her excellent secretarial assistance and James Tarara for technical support for confocal microscopy. We also thank Hong Cao, Barbara Schroeder, Shaun G. Weller, Stacey A. Rizza, and Gary D. Bren, for useful discussions and expert technical assistance.
Footnotes
The authors report no conflict of interest regarding this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–90. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yagita H, Takeda K, Hayakawa Y, et al. TRAIL and its receptors as targets for cancer therapy. Cancer Sci. 2004;95:777–83. doi: 10.1111/j.1349-7006.2004.tb02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14:359–72. doi: 10.1038/sj.cr.7290236. [DOI] [PubMed] [Google Scholar]
- 4.MacFarlane M, Inoue S, Kohlhaas SL, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ. 2005;12:773–82. doi: 10.1038/sj.cdd.4401649. [DOI] [PubMed] [Google Scholar]
- 5.van der Sloot AM, Tur V, Szegezdi E, et al. Designed tumor necrosis factor-related apoptosis-inducing ligand variants initiating apoptosis exclusively via the DR5 receptor. Proc Natl Acad Sci U S A. 2006;103:8634–9. doi: 10.1073/pnas.0510187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–23. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 8.Finnberg N, El-Deiry WS. TRAIL death receptors as tumor suppressors and drug targets. Cell Cycle. 2008;7:1525–8. doi: 10.4161/cc.7.11.5975. [DOI] [PubMed] [Google Scholar]
- 9.Guicciardi ME, Bronk SF, Werneburg NW, et al. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1337–46. doi: 10.1152/ajpgi.00497.2006. [DOI] [PubMed] [Google Scholar]
- 10.Werneburg NW, Guicciardi ME, Bronk SF, et al. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282:28960–70. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 11.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–90. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 12.Lee KH, Feig C, Tchikov V, et al. The role of receptor internalization in CD95 signaling. Embo J. 2006;25:1009–23. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider-Brachert W, Tchikov V, Neumeyer J, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–28. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Kohlhaas SL, Craxton A, Sun XM, et al. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–41. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- 15.Austin CD, Lawrence DA, Peden AA, et al. Death-receptor activation halts clathrin-dependent endocytosis. Proc Natl Acad Sci U S A. 2006;103:10283–8. doi: 10.1073/pnas.0604044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich J, Hou X, Wegener AM, et al. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. Embo J. 1994;13:2156–66. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, Kirkpatrick D, Jiang X, et al. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–48. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Xia S, Dun XP, Hu PS, et al. Postendocytotic traffic of the galanin R1 receptor: a lysosomal signal motif on the cytoplasmic terminus. Proc Natl Acad Sci U S A. 2008;105:5609–13. doi: 10.1073/pnas.0801456105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucci C, Thomsen P, Nicoziani P, et al. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–80. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitelli R, Santillo M, Lattero D, et al. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–7. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 23.Hackbarth JS, Lee SH, Meng XW, et al. S-peptide epitope tagging for protein purification, expression monitoring, and localization in mammalian cells. Biotechniques. 2004;37:835–9. [PubMed] [Google Scholar]
- 24.Roberts LR, Kurosawa H, Bronk SF, et al. Cathepsin B contributes to bile salt-induced apoptosis of rat hepatocytes. Gastroenterology. 1997;113:1714–26. doi: 10.1053/gast.1997.v113.pm9352877. [DOI] [PubMed] [Google Scholar]
- 25.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–75. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 26.Shaw RM, Fay AJ, Puthenveedu MA, et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–60. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappoport JZ, Simon SM. Real-time analysis of clathrin-mediated endocytosis during cell migration. J Cell Sci. 2003;116:847–55. doi: 10.1242/jcs.00289. [DOI] [PubMed] [Google Scholar]
- 28.Kang-Decker N, Cao S, Chatterjee S, et al. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 29.Nagamatsu S. TIRF microscopy analysis of the mechanism of insulin exocytosis. Endocr J. 2006;53:433–40. doi: 10.1507/endocrj.kr-75. [DOI] [PubMed] [Google Scholar]
- 30.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–57. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 31.Press B, Feng Y, Hoflack B, et al. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J Cell Biol. 1998;140:1075–89. doi: 10.1083/jcb.140.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceresa BP, Bahr SJ. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem. 2006;281:1099–106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 33.Dale LB, Seachrist JL, Babwah AV, et al. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem. 2004;279:13110–8. doi: 10.1074/jbc.M313333200. [DOI] [PubMed] [Google Scholar]
- 34.Kurosawa H, Que FG, Roberts LR, et al. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–93. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- 35.Scaffidi C, Schmitz I, Zha J, et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–8. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura H, Kawakami A, Iwamoto N, et al. Rapid and significant induction of TRAIL-mediated type II cells in apoptosis of primary salivary epithelial cells in primary Sjogren’s syndrome. Apoptosis. 2008 doi: 10.1007/s10495-008-0261-2. [DOI] [PubMed] [Google Scholar]
- 37.Ozoren N, El-Deiry WS. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–7. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Algeciras-Schimnich A, Shen L, Barnhart BC, et al. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol. 2002;22:207–20. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1